Indole Alkaloids from Psychoactive Mushrooms: Chemical and Pharmacological Potential as Psychotherapeutic Agents
Abstract
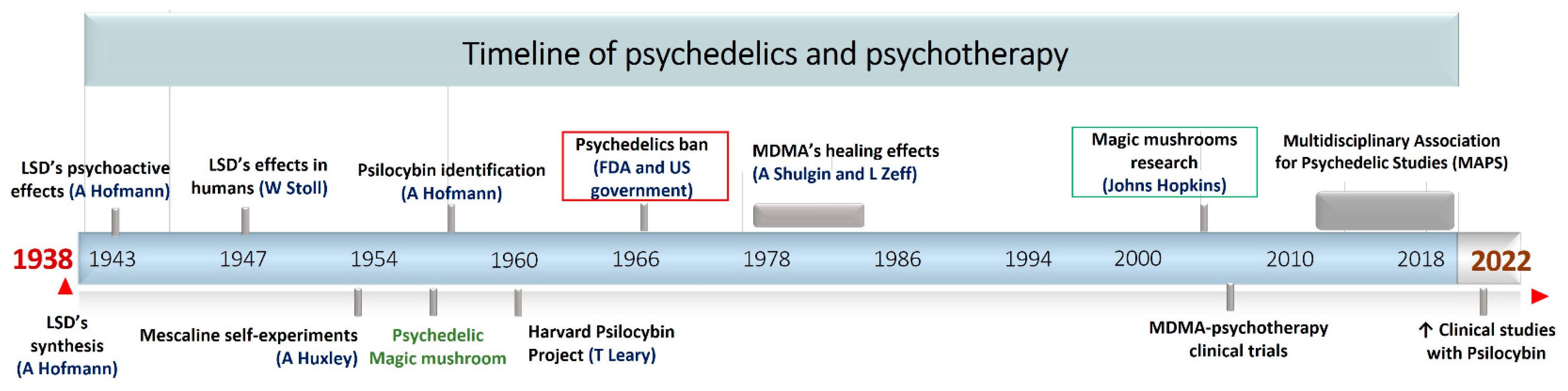
:1. Introduction
2. Ethnomycology and Historical Overview of Psychoactive Mushrooms around the World
3. Distribution of Psychoactive Mushrooms and Chemotaxonomic Relevance of Indole Alkaloids
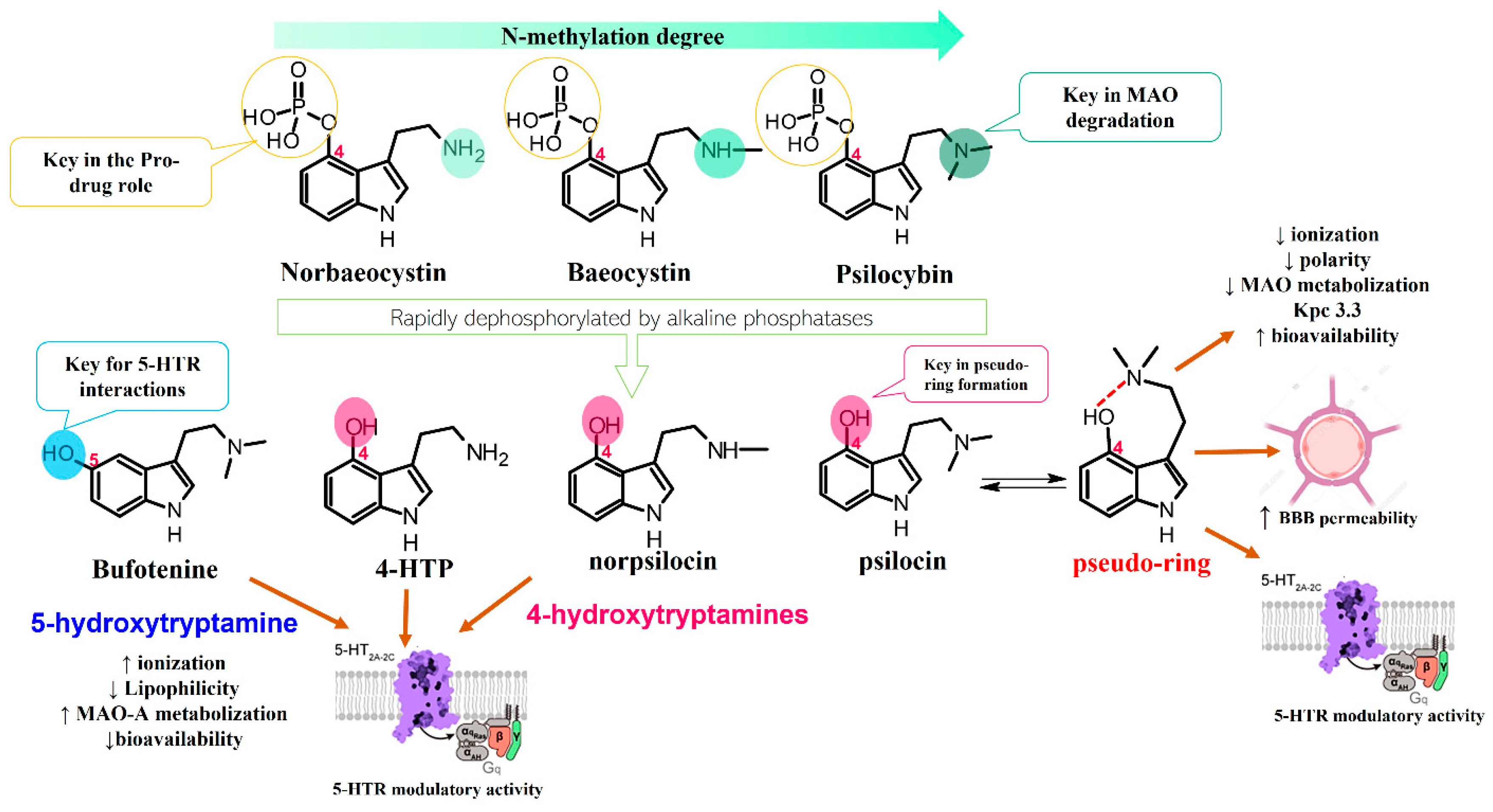
4. Chemical Features of Mushroom-Derived Indole Alkaloids
4.1. Diversity and Biosynthesis of Mushroom-Derived Indole Alkaloids
4.2. Chemical Characteristics, Extraction Methods, and Alternative Production Techniques
4.3. Analytical Methods and Alternative Strategies of Production
Alternative Strategies of Production
5. Psychopharmacological Potential of Mushroom-Derived Indoles
5.1. Preclinical Research
5.2. Ongoing Clinical Trials
Safety Profile
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, N.J.; Donovan, M.C.O. The Genetics of Neuropsychiatric Disorders. Brain Neurosci. Adv. 2018, 2, 2398212818799271. [Google Scholar] [CrossRef] [PubMed]
- Cristino, A.S.; Williams, S.M.; Hawi, Z.; An, J.; Bellgrove, M.A.; Schwartz, C.E.; Costa, L.F.; Claudianos, C. Neurodevelopmental and Neuropsychiatric Disorders Represent an Interconnected Molecular System. Mol. Psychiatry 2014, 19, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Leichsenring, F.; Steinert, C.; Ioannidis, J.P.A. Toward a Paradigm Shift in Treatment and Research of Mental Disorders. Psychol. Med. 2022, 49, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Charlson, F.; Van Ommeren, M.; Flaxman, A.; Cornett, J.; Whiteford, H.; Saxena, S. New WHO Prevalence Estimates of Mental Disorders in Conflict Settings: A Systematic Review and Meta-Analysis. Lancet 2019, 394, 240–248. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Mental Disorders Collaborators. Global, Regional, and National Burden of 12 Mental Disorders in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef]
- Raffagnato, A.; Iannattone, S.; Tascini, B.; Venchiarutti, M.; Broggio, A.; Zanato, S.; Traverso, A.; Mascoli, C.; Manganiello, A.; Miscioscia, M.; et al. The COVID-19 Pandemic: A Longitudinal Study on the Emotional-Behavioral Sequelae for Children and Adolescents with Neuropsychiatric Disorders and Their Families. Int. J. Environ. Res. Public Health 2021, 18, 9880. [Google Scholar] [CrossRef]
- Ramirez, J.M.; Muñoz, A.; Vaz-leal, F.J. Cognitive Function and Neuropsychiatric Disorders after COVID-19: A Long Term Social and Clinical Problem? BioMed 2022, 2, 50–59. [Google Scholar] [CrossRef]
- Winkler, P.; Formanek, T.; Mlada, K.; Kagstrom, A.; Mohrova, Z.; Mohr, P.; Csemy, L. Increase in Prevalence of Current Mental Disorders in the Context of COVID-19: Analysis of Repeated Nationwide Cross-Sectional Surveys. Epidemiol. Psychiatr. Sci. 2020, 29, E173. [Google Scholar] [CrossRef]
- Orv, G.H.; Judprqwh, Q.; Gonçalves, C.; Noris-garcía, E.; Rivero, N.P.; Brigida, A.L.; Schultz, S.; Siniscalco, D.; Jorge, R.; García, G. Impact of SARS-CoV-2 on Neuropsychiatric Disorders. World J. Psychiatry 2021, 11, 347–354. [Google Scholar] [CrossRef]
- Sartori, S.B.; Singewald, N. Novel Pharmacological Targets in Drug Development for the Treatment of Anxiety and Anxiety-Related Disorders. Pharmacol. Ther. 2019, 204, 107402. [Google Scholar] [CrossRef]
- Fornaro, M.; Anastasia, A.; Novello, S.; Fusco, A.; Pariano, R.; De Berardis, D.; Solmi, M.; Veronese, N.; Stubbs, B.; Vieta, E.; et al. The Emergence of Loss of Efficacy during Antidepressant Drug Treatment for Major Depressive Disorder: An Integrative Review of Evidence, Mechanisms, and Clinical Implications. Pharmacol. Res. 2019, 139, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, C.; Gibson, K.; Read, J.; Cowan, O.; Dehar, T. Long-Term Antidepressant Use: Patient Perspectives of Benefits and Adverse Effects. Patient Prefer. Adherence 2016, 10, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.C.; Leucht, S.; Davis, J.M. Maximizing Response to First-Line Antipsychotics in Schizophrenia: A Review Focused on Finding from Meta-Analysis. Psychopharmacology 2019, 236, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Jollans, L.; Whelan, R. Neuromarkers for Mental Disorders: Harnessing Population Neuroscience. Front. Psychiatry 2018, 9, 242. [Google Scholar] [CrossRef]
- Nestler, E.J.; Hyman, S.E. Animal Models of Neuropsychiatric Disorders. Nat. Neurosci. 2010, 13, 1161–1169. [Google Scholar] [CrossRef]
- Tang, S.W.; Tang, W.H. Opportunities in Novel Psychotropic Drug Design from Natural Compounds. Int. J. Neuropsychopharmacol. 2019, 22, 601–607. [Google Scholar] [CrossRef]
- Wang, Y.S.; Shen, C.Y.; Jiang, J.G. Antidepressant Active Ingredients from Herbs and Nutraceuticals Used in TCM: Pharmacological Mechanisms and Prospects for Drug Discovery. Pharmacol. Res. 2019, 150, 104520. [Google Scholar] [CrossRef]
- Thornton, J.M.; Laskowski, R.A.; Borkakoti, N. Psychedelic Therapy: A Roadmap for Wider Acceptance and Utilization. Nat. Med. 2021, 27, 1666–1669. [Google Scholar] [CrossRef]
- Peritore, C.S. The Promise of Psychedelic Research. Futur. Drug Discov. 2022, 22, FDD70. [Google Scholar] [CrossRef]
- Wieczorek, P.P.; Witkowska, D.; Jasicka-Misiak, I.; Poliwoda, A.; Oterman, M.; Zielińska, K. Bioactive Alkaloids of Hallucinogenic Mushrooms. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; Volume 46, pp. 133–168. ISBN 9780444634627. [Google Scholar]
- Zafar, M. Bioactive Alkaloids from Fungi: Psilocybin. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–4242. ISBN 9783642221446. [Google Scholar]
- Matsushima, Y.; Eguchi, F.; Kikukawa, T.; Matsuda, T. Historical Overview of Psychoactive Mushrooms. Inflamm. Regen. 2009, 29, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Guerra-Doce, E. Psychoactive Substances in Prehistoric Times: Examining the Archaeological Evidence. Time Mind 2015, 8, 91–112. [Google Scholar] [CrossRef]
- Miller, M.J.; Albarracin-Jordan, J.; Moore, C.; Capriles, J.M. Chemical Evidence for the Use of Multiple Psychotropic Plants in a 1,000-Year-Old Ritual Bundle from South America. Proc. Natl. Acad. Sci. USA 2019, 166, 11207–11212. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.M.; Carvalho, F.; de Bastos, M.L.; Guedes de Pinho, P.; Carvalho, M. The Hallucinogenic World of Tryptamines: An Updated Review. Arch. Toxicol. 2015, 89, 1151–1173. [Google Scholar] [CrossRef] [PubMed]
- Guzman, G. Hallucinogenic Mushrooms in Mexico: An Overview. Econ. Bot. 2008, 62, 404–412. [Google Scholar] [CrossRef]
- Michelot, D.; Melendez-Howell, L.M. Amanita Muscaria: Chemistry, Biology, Toxicology, and Ethnomycology. Mycol. Res. 2003, 107, 131–146. [Google Scholar] [CrossRef]
- Ubel, W.R.; Arora, D. A Study of Cultural Bias in Field Guide Determinations of Mushroom Edibility Using the Iconic Mushroom, Amanita Muscaria, as an Example 1. Econ. Bot. 2008, 62, 223–243. [Google Scholar] [CrossRef]
- Gholami-Shabani, M.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. Natural Product Synthesis by Fungi: Recent Trends and Future Prospects. In Recent Advancement in White Biotechnology through Fungi; Springer: Berlin/Heidelberg, Germany, 2019; pp. 195–228. ISBN 9783030148461. [Google Scholar]
- Patocka, J.; Wu, R.; Nepovimova, E.; Valis, M.; Wu, W.; Kuca, K. Chemistry and Toxicology of Major Bioactive Substances in Inocybe Mushrooms. Int. J. Mol. Sci. 2021, 22, 2218. [Google Scholar] [CrossRef]
- Sanford, J.H. Japan’s “Laughing Mushrooms”. Econ. Bot. 1972, 26, 174–181. [Google Scholar] [CrossRef]
- Reschke, K.; Popa, F.; Yang, Z.L.; Kost, G. Diversity and Taxonomy of Tricholoma Species from Yunnan, China, and Notes on Species from Europe and North America. Mycologia 2018, 110, 1081–1109. [Google Scholar] [CrossRef]
- Fuentes, J.J.; Fonseca, F.; Elices, M.; Farré, M.; Torrens, M. Therapeutic Use of LSD in Psychiatry: A Systematic Review of Randomized-Controlled Clinical Trials. Front. Psychiatry 2020, 10, 943. [Google Scholar] [CrossRef] [Green Version]
- Doblin, R.E.; Christiansen, M.; Jerome, L.; Burge, B. The Past and Future of Psychedelic Science: An Introduction to This Issue. J. Psychoactive Drugs 2019, 51, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Wurst, M.; Kysilka, R.; Flieger, M. Psychoactive Tryptamines from Basidiomycetes. Folia Microbiol. 2002, 47, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Maclean, K.A.; Johnson, M.W.; Griffiths, R.R. Mystical Experiences Occasioned by the Hallucinogen Psilocybin Lead to Increases in the Personality Domain of Openness. J. Psychopharmacol. 2011, 25, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Tupper, K.W.; Wood, E.; Yensen, R.; Johnson, M.W. Psychedelic Medicine: A Re-Emerging Therapeutic Paradigm. Can. Med. J. 2015, 187, 1054–1059. [Google Scholar] [CrossRef]
- Doss, M.K.; Smith, G.S.; Pova, M.; Rosenberg, M.D.; Sepeda, N.D.; Davis, A.K.; Finan, P.H.; Pekar, J.J.; Barker, P.B.; Grif, R.R.; et al. Psilocybin Therapy Increases Cognitive and Neural Flexibility in Patients with Major Depressive Disorder. Transl. Psychiatry 2021, 11, 574. [Google Scholar] [CrossRef]
- Gukasyan, N.; Davis, A.K.; Barrett, F.S.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Griffiths, R.R. Efficacy and Safety of Psilocybin-Assisted Treatment for Major Depressive Disorder: Prospective 12-Month Follow-Up. Psychopharmacology 2022, 36, 151–158. [Google Scholar] [CrossRef]
- Yaden, D.B.; Nayak, S.M.; Gukasyan, N. The Potential of Psychedelics for End of Life and Palliative Care The Potential of Psychedelics for End of Life and Palliative Care. Curr. Top. Behav. Neurosci. 2021, 56, 169–184. [Google Scholar] [CrossRef]
- Doss, M.K.; Madden, M.B.; Gaddis, A.; Nebel, M.B.; Griffiths, R.R.; Mathur, B.N.; Barrett, F.S. Models of Psychedelic Drug Action: Modulation of Cortical-Subcortical Circuits. Brain 2022, 145, 441–456. [Google Scholar] [CrossRef]
- Guzmán, G.; Allen, J.W.; Gartz, J. A Worldwide Geographical Distribution of the Neurotropic Fungi, an Analysis and Discussion. Ann. Mus. Civ. Rovereto 1998, 14, 189–280. [Google Scholar]
- Florea, S.; Panaccione, D.G.; Schardl, C.L. Ergot Alkaloids of the Family Clavicipitaceae. Phytopathology 2017, 107, 504–518. [Google Scholar] [CrossRef]
- Fattorusso, E.; Taglialatela-Scafati, O. Modern Alkaloids: Structure, Isolation, Synthesis, and Biology; Fattorusso, E., Taglialatela-Scafati, O., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 9783527312238. [Google Scholar]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 9780470741689. [Google Scholar]
- Alberti, F.; Kaleem, S.; Weaver, J.A. Recent Developments of Tools for Genome and Metabolome Studies in Basidiomycete Fungi and Their Application to Natural Product Research. Biol. Open 2020, 9, bio056010. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Feng, T.; Liu, J. N-Containing Compounds of Macromycetes. Nat. Prod. Rep. 2011, 28, 783–808. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Gavia, D.J.; Tang, Y. Biosynthesis of Fungal Indole Alkaloids. Nat. Prod. Rep. 2014, 31, 1474–1487. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Chooi, Y.; Lu, Y.; Lin, H. Biosynthesis of Bioactive Natural Products from Basidiomycota. Org. Biomol. Chem. 2019, 17, 1027–1036. [Google Scholar] [CrossRef]
- Blei, F.; Dçrner, S.; Fricke, J.; Baldeweg, F.; Trottmann, F.; Komor, A.; Meyer, F.; Hertweck, C.; Hoffmeister, D. Simultaneous Production of Psilocybin and a Cocktail of B-Carboline Monoamine Oxidase Inhibitors in “Magic” Mushrooms. Chemistry 2020, 26, 729–734. [Google Scholar] [CrossRef]
- Fraley, A.E.; Sherman, D. Enzyme Evolution in Fungal Indole Alkaloid Biosynthesis. FEBS J. 2020, 287, 1381–1402. [Google Scholar] [CrossRef]
- Chen, J.J.; Han, M.Y.; Gong, T.; Yang, J.L.; Zhu, P. Recent Progress in Ergot Alkaloid Research. RSC Adv. 2017, 7, 27384–27396. [Google Scholar] [CrossRef]
- Jakubczyk, D.; Cheng, J.Z.; O’Connor, S.E. Biosynthesis of the Ergot Alkaloids. Nat. Prod. Rep. 2014, 31, 1328–1338. [Google Scholar] [CrossRef]
- Omar, F.; Tareq, A.M.; Alqahtani, A.M.; Dhama, K.; Sayeed, M.A.; Emran, T.B.; Simal-gandara, J. Plant-Based Indole Alkaloids: A Comprehensive Overview from a Pharmacological Perspective. Molecules 2021, 26, 2297. [Google Scholar] [CrossRef]
- Homer, J.A.; Sperry, J. Mushroom-Derived Indole Alkaloids. J. Nat. Prod. 2017, 80, 2178–2187. [Google Scholar] [CrossRef]
- Gotvaldová, K.; Hájková, K.; Borovic, J.; Jurok, R.; Cihlá, P.; Kucha, M. Stability of Psilocybin and Its Four Analogs in the Biomass of the Psychotropic Mushroom Psilocybe Cubensis. Drug Test. Anal. 2021, 13, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Lenz, C.; Wick, J.; Braga, D.; Lackner, G.; Garcia, M.; Hertweck, C.; Gressler, M.; Hoffmeister, D. Injury-Triggered Blueing Reactions of Psilocybe “Magic” Mushrooms. Angew. Chem. Int. Ed. 2020, 59, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Lenz, C.; Dörner, S.; Sherwood, A.; Hoffmeister, D. Structure Elucidation and Spectroscopic Analysis of Chromophores Produced by Oxidative Psilocin Dimerization. Chem. Eur. J. 2021, 27, 12166–12171. [Google Scholar] [CrossRef] [PubMed]
- Gartz, J. Extraction and Analysis of Indole Derivatives from Fungal Biomass. J. Basic Microbiol. 1994, 34, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Musshoff, F.; Madea, B.; Beike, J. Hallucinogenic Mushrooms on the German Market—Simple Instructions for Examination and Identification. Forensic Sci. Int. 2000, 113, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, K.; Kanamori, T.; Iwata, Y.; Ohmae, Y.; Sugita, R.; Inoue, H.; Kishi, T. Morphological and Chemical Analysis of Magic Mushrooms in Japan. Forensic Sci. Int. 2003, 138, 85–90. [Google Scholar] [CrossRef]
- Jensen, N.; Gartz, J.; Laatsch, H. Aeruginascin, a Trimethylammonium Analogue of Psilocybin from the Hallucinogenic Mushroom Inocybe Aeruginascens. Planta Med. 2006, 72, 665–666. [Google Scholar] [CrossRef]
- Beutler, J.A.; Marderosian, A.H. Der Chemical Variation in Amanita. J. Nat. Prod. 1981, 44, 422–431. [Google Scholar] [CrossRef]
- Pérez-Silva, E.; Alfonso, R.M.A. Chromatographic and Taxonomic Evaluation of Amanita Citrina (Agaricales). Mycologia 1983, 75, 1030–1035. [Google Scholar] [CrossRef]
- Teichert, A.; Schmidt, J.; Porzel, A.; Arnold, N.; Wessjohann, L. Brunneins A–C, B-Carboline Alkaloids from Cortinarius Brunneus. J. Nat. Prod. 2007, 70, 1529–1531. [Google Scholar] [CrossRef]
- Young, C.A.; Schardl, C.L.; Panaccione, D.G.; Florea, S.; Takach, J.; Charlton, N.; Moore, N.; Webb, J.; Jaromezyk, J. Genetics, Genomics and Evolution of Ergot Alkaloid Diversity. Toxins 2015, 7, 1273–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crews, C. Analysis of Ergot Alkaloids. Toxins 2015, 7, 2024–2050. [Google Scholar] [CrossRef]
- Sherwood, A.M.; Halberstadt, A.L.; Klein, A.K.; Mccorvy, J.D.; Kaylo, K.W.; Kargbo, R.B.; Meisenheimer, P. Synthesis and Biological Evaluation of Tryptamines Found in Hallucinogenic Mushrooms: Norbaeocystin, Baeocystin, Norpsilocin, and Aeruginascin. J. Nat. Med. 2020, 83, 461–467. [Google Scholar] [CrossRef]
- Costa, T.O.G.; Morales, R.A.V.; Brito, J.P.; Gordo, M.; Pinto, A.C.; Bloch, C., Jr.; Gal, E.; Ota, R. Occurrence of Bufotenin in the Osteocephalus Genus (Anura: Hylidae). Toxicon 2005, 46, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Peng, W.; Wang, Z.; Xu, A. β-Carboline Alkaloids: Biochemical and Pharmacological Functions. Curr. Med. Chem. 2007, 14, 479–500. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, R.J.R.; Lamsho, M.; Gottfried, S.; Spiteller, M.; Spiteller, P. HR-MALDI-MS Imaging Assisted Screening of β—Carboline Alkaloids Discovered from Mycena Metata. J. Nat. Prod. 2013, 76, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Toyo’oka, T.; Kato, M.; Fukushima, T.; Shirota, O.; Goda, Y. Determination of Psilocybin in Hallucinogenic Mushrooms by Reversed-Phase Liquid Chromatography with Fluorescence Detection. Talanta 2005, 66, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Diana, J.; Mavungu, D.; Malysheva, S.V.; Sanders, M.; Larionova, D.; Robbens, J.; Dubruel, P.; Van Peteghem, C.; Saeger, S. De Development and Validation of a New LC–MS/MS Method for the Simultaneous Determination of Six Major Ergot Alkaloids and Their Corresponding Epimers. Application to Some Food and Feed Commodities. Food Chem. 2012, 135, 292–303. [Google Scholar] [CrossRef]
- Piechowska, P.; Zawirska-wojtasiak, R.; Mildner-Szkudlarz, S. Bioactive β-Carbolines in Food: A Review. Nutrients 2019, 11, 814. [Google Scholar] [CrossRef]
- Caesar, L.K.; Kellogg, J.J.; Kvalheim, O.M.; Cech, N.B. Opportunities and Limitations for Untargeted Mass Spectrometry Metabolomics to Identify Biologically Active Constituents in Complex Natural Product Mixtures. J. Nat. Prod. 2019, 82, 469–484. [Google Scholar] [CrossRef]
- Chang, S.T.; Wasser, S.P. Current and Future Research Trends in Agricultural and Biomedical Applications of Medicinal Mushrooms and Mushroom Products (Review). Int. J. Med. Mushrooms 2018, 20, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Fricke, J.; Lenz, C.; Wick, J.; Blei, F.; Hoffmeister, D. Production Options for Psilocybin: Making of the Magic. Chem. A Eur. J. 2019, 25, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jia, Y. Ergot Alkaloids: Synthetic Approaches to Lysergic Acid and Clavine Alkaloids. Nat. Prod. Rep. 2017, 34, 411–432. [Google Scholar] [CrossRef]
- Rathnayake, U.; Garner, P. Asymmetric Synthesis of Lysergic Acid via an Intramolecular (3 + 2) Dipolar Cycloaddition/Ring-Expansion Sequence. Org. Lett. 2021, 23, 6756–6759. [Google Scholar] [CrossRef] [PubMed]
- Hulvová, H.; Galuszka, P.; Frébortová, J.; Frébort, I. Parasitic Fungus Claviceps as a Source for Biotechnological Production of Ergot Alkaloids. Biotechnol. Adv. 2013, 31, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Fricke, J.; Kargbo, R.; Regestein, L.; Lenz, C.; Peschel, G.; Rosenbaum, M.A.; Sherwood, A.; Hoffmeister, D. Scalable Hybrid Synthetic/Biocatalytic Route to Psilocybin. Chem. A Eur. J. 2020, 26, 8281–8285. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.; Toyang, N.; Steele, B.; Valentine, H.; Grant, J.; Ali, A.; Ngwa, W.; Gordon, L. The Therapeutic Potential of Psilocybin. Molecules 2021, 26, 2948. [Google Scholar] [CrossRef]
- Artin, H.; Zisook, S.; Ramanathan, D. How Do Serotonergic Psychedelics Treat Depression: The Potential Role of Neuroplasticity. World J. Psychiatry 2021, 11, 201–214. [Google Scholar] [CrossRef]
- Zeiss, R.; Gahr, M.; Graf, H. Rediscovering Psilocybin as an Antidepressive Treatment Strategy. Pharmaceuticals 2021, 14, 985. [Google Scholar] [CrossRef]
- Ling, S.; Ceban, F.; Lui, L.M.W.; Lee, Y.; Teopiz, K.M.; Rodrigues, N.B.; Lipsitz, O.; Gill, H.; Subramaniapillai, M.; Mansur, R.B.; et al. Molecular Mechanisms of Psilocybin and Implications for the Treatment of Depression. CNS Drugs 2021, 36, 17–30. [Google Scholar] [CrossRef]
- Sard, H.; Kumaran, G.; Morency, C.; Roth, B.L.; Toth, B.A.; He, P.; Shuster, L. SAR of Psilocybin Analogs: Discovery of a Selective 5-HT2C Agonist. Bioorg. Med. Chem. Lett. 2005, 15, 4555–4559. [Google Scholar] [CrossRef] [PubMed]
- Glatfelter, G.C.; Pham, D.N.K.; Walther, D.; Golen, J.A.; Chadeayne, A.R.; Baumann, M.H.; Manke, D.R. Synthesis, Structural Characterization, and Pharmacological Activity of Novel Quaternary Salts of 4—Substituted Tryptamines. ACS Omega 2022, 7, 24888–24894. [Google Scholar] [CrossRef] [PubMed]
- Mcbride, M.C. Bufotenine: Toward an Understanding of Possible Psychoactive Mechanisms. J. Psychoact. Drugs 2011, 32, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Vigerelli, H.; Sciani, J.M.; Eula, M.A.C.; Sato, L.A.; Antoniazzi, M.M.; Jared, C.; Pimenta, D.C. Biological Effects and Biodistribution of Bufotenine on Mice. Biomed Res. Int. 2018, 2018, 1032638. [Google Scholar] [CrossRef]
- Ferraz, C.A.A.; de Oliveira Júnior, R.G.; Picot, L.; da Silva Almeida, J.R.G.; Nunes, X.P. Pre-Clinical Investigations of β-Carboline Alkaloids as Antidepressant Agents: A Systematic Review. Fitoterapia 2019, 137, 104196. [Google Scholar] [CrossRef]
- Adell, A.; Biggs, T.A.; Myers, R.D. Action of Harman (1-Methyl-β-Carboline) on the Brain: Body Temperature and in Vivo Efflux of 5-HT from Hippocampus of the Rat. Neuropharmacology 1996, 35, 1101–1107. [Google Scholar] [CrossRef]
- Aricioglu, F.; Altunbas, H. Harmane Induces Anxiolysis and Antidepressant-Like Effects in Rats. Ann. N. Y. Acad. Sci. 2003, 1009, 196–201. [Google Scholar] [CrossRef]
- Smith, K.L.; Ford, G.K.; Jessop, D.S.; Finn, D.P. Behavioural, Neurochemical and Neuroendocrine Effects of the Endogenous β-Carboline Harmane in Fear-Conditioned Rats. J. Psychopharmacol. 2013, 27, 162–170. [Google Scholar] [CrossRef]
- Farzin, D.; Mansouri, N. Antidepressant-like Effect of Harmane and Other β-Carbolines in the Mouse Forced Swim Test. Eur. Neuropsychopharmacol. 2006, 16, 324–328. [Google Scholar] [CrossRef]
- Liu, F.; Wu, J.; Gong, Y.; Wang, P.; Zhu, L.; Tong, L.; Chen, X.; Ling, Y.; Huang, C. Harmine Produces Antidepressant-like Effects via Restoration of Astrocytic Functions. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 258–267. [Google Scholar] [CrossRef]
- Fortunato, J.J.; Réus, G.Z.; Kirsch, T.R.; Stringari, R.B.; Fries, G.R.; Kapczinski, F.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Quevedo, J. Effects of β-Carboline Harmine on Behavioral and Physiological Parameters Observed in the Chronic Mild Stress Model: Further Evidence of Antidepressant Properties. Brain Res. Bull. 2010, 81, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-ghiri, M.; Nasehi, M.; Zarrindast, M. Anxiolytic and Antidepressant e Ff Ects of ACPA and Harmaline Co-Treatment. Behav. Brain Res. 2019, 364, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sharma, V.; Manikyam, H.; Krishna, A. Ergot Alkaloids: A Review on Therapeutic Applications. Eur. J. Med. Plants 2016, 14, 1–17. [Google Scholar] [CrossRef]
- Tfelt-Hansen, P.; Saxena, P.R.; Dahlöf, C.; Pascual, J.; Láinez, M.; Henry, P.; Diener, H.C.; Schoenen, J.; Ferrari, M.D.; Goadsby, P.J. Ergotamine in the Acute Treatment of Migraine. A Review and European Consensus. Brain 2000, 123, 9–18. [Google Scholar] [CrossRef]
- Hesselgrave, N.; Troppoli, T.A.; Wulff, A.B.; Cole, A.B. Harnessing Psilocybin: Antidepressant-like Behavioral and Synaptic Actions of Psilocybin Are Independent of 5-HT2R Activation in Mice. Proc. Natl. Acad. Sci. USA 2021, 118, e2022489118. [Google Scholar] [CrossRef]
- Odland, A.U.; Kristensen, J.L.; Andreasen, J.T. Investigating the Role of 5-HT2A and 5-HT2C Receptor Activation in the Effects of Psilocybin, DOI, and Citalopram on Marble Burying in Mice. Behav. Brain Res. 2021, 401, 113093. [Google Scholar] [CrossRef]
- Erkizia-santamaría, I.; Alles-pascual, R.; Horrillo, I.; Meana, J.J.; Ortega, J.E. Serotonin 5-HT2A, 5-HT2c and 5-HT1A Receptor Involvement in the Acute Effects of Psilocybin in Mice. In Vitro Pharmacological Profile and Modulation of Thermoregulation and Head-Twich Response. Biomed. Pharmacother. 2022, 154, 113612. [Google Scholar] [CrossRef]
- López-Gimenez, J.; González-Maeso, J. Hallucinogens and Serotonin 5-HT2A Receptor-Mediated Signaling Pathways. Curr. Top. Behav. Neurosci. 2018, 36, 45–73. [Google Scholar] [CrossRef]
- Zhang, G.; Stackman, R. The Role of Serotonin 5-HT 2A Receptors in Memory and Cognition. Front. Pharmacol. 2015, 6, 225. [Google Scholar] [CrossRef]
- Shao, L.; Liao, C.; Gregg, I.; Davoudian, P.A.; Savalia, N.K.; Delagarza, K.; Kwan, A.C.; Shao, L.; Liao, C.; Gregg, I.; et al. Report Psilocybin Induces Rapid and Persistent Growth of Dendritic Spines in Frontal Cortex in Vivo Ll Report Psilocybin Induces Rapid and Persistent Growth of Dendritic Spines in Frontal Cortex in Vivo. Neuron 2021, 109, 2535–2544.e4. [Google Scholar] [CrossRef]
- Raval, N.R.; Johansen, A.; Donovan, L.L.; Ros, F.; Ozenne, B.; Hansen, H.D.; Knudsen, G.M. A Single Dose of Psilocybin Increases Synaptic Density and Decreases 5-HT2A Receptor Density in the Pig Brain. Int. J. Mol. Sci. 2021, 22, 835. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.K.; Chatha, M.; Laskowski, L.J.; Anderson, E.I.; Brandt, S.D.; Chapman, S.J.; Mccorvy, J.D.; Halberstadt, A.L. Investigation of the Structure−Activity Relationships of Psilocybin Analogues. ACS Pharmacol. Transl. Sci. 2021, 4, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.M.; Anas, N.A.; Sen, A.K.; Hinegardner-hendricks, J.D.; Dell, P.J.O.; Gibbons, W.J.; Flower, J.E.; Mcmurray, M.S.; Jones, J.A. Development of an E. Coli-Based Norbaeocystin Production Platform and Evaluation of Behavioral Effects in Rats. Metab. Eng. Commun. 2022, 14, e00196. [Google Scholar] [CrossRef] [PubMed]
- Lenz, C.; Dörner, S.; Trottmann, F.; Hertweck, C.; Sherwood, A.; Hoffmeister, D. Assessment of Bioactivity-Modulating Pseudo-Ring Formation in Psilocin and Related Tryptamines. ChemBioChem 2022, 23, e202200183. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R.W.; Snoddy, H.D.; Perry, K.W. Tissue Distribution, Metabolism and Effects of Bufotenine Administered to Rats. Neuropharmacology 1995, 34, 799–804. [Google Scholar] [CrossRef]
- Abu Ghazaleh, H.; Lalies, M.D.; Nutt, D.J.; Hudson, A.L. The Modulatory Action of Harmane on Serotonergic Neurotransmission in Rat Brain. Brain Res. 2015, 1597, 57–64. [Google Scholar] [CrossRef]
- Hussain, G.; Rasul, A.; Anwar, H.; Aziz, N.; Razzaq, A.; Wei, W.; Ali, M.; Li, J.; Li, X. Role of Plant Derived Alkaloids and Their Mechanism in Neurodegenerative Disorders. Int. J. Biol. Sci. 2018, 14, 341–357. [Google Scholar] [CrossRef]
- Finberg, J.; Rabey, J. Inhibitors of MAO-A and MAO-B in Psychiatry and Neurology. Front. Pharmacol. 2016, 7, 340. [Google Scholar] [CrossRef]
- Liechti, M.E. Modern Clinical Research on LSD. Neuropsychopharmacology 2017, 42, 2114–2127. [Google Scholar] [CrossRef]
- Brown, R.T.; Nicholas, C.R.; Cozzi, N.V.; Gassman, M.C.; Cooper, K.M.; Muller, D.; Thomas, C.D.; Hetzel, S.J.; Henriquez, K.M.; Ribaudo, A.S.; et al. Pharmacokinetics of Escalating Doses of Oral Psilocybin in Healthy Adults. Clin. Pharmacokinet. 2017, 56, 1543–1554. [Google Scholar] [CrossRef]
- Grimm, O.; Kraehenmann, R.; Preller, K.H.; Seifritz, E.; Vollenweider, F.X. Psilocybin Modulates Functional Connectivity of the Amygdala during Emotional Face Discrimination. Eur. Neuropsychopharmacol. 2018, 28, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin Produces Substantial and Sustained Decreases in Depression and Anxiety in Patients with Life-Threatening Cancer: A Randomized Double-Blind Trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef] [PubMed]
- Bogenschutz, M.P.; Ross, S.; Bhatt, S.; Baron, T.; Forcehimes, A.A.; Laska, E.; Mennenga, S.E.; Donnell, K.O.; Owens, L.T.; Podrebarac, S.; et al. Percentage of Heavy Drinking Days Following Psilocybin-Assisted Psychotherapy vs Placebo in the Treatment of Adult Patients With Alcohol Use Disorder A Randomized Clinical Trial. JAMA Psychiatry 2022, 79, 953–962. [Google Scholar] [CrossRef] [PubMed]



| Scientific Name | Common Name | Community (Country) | Uses | Ref. |
|---|---|---|---|---|
| Amanita muscaria | Miskwedo | Ojibwa and Algonquin (North America) | Used in sacred ancestral ceremonies and for the treatment of mental and spiritual ailments, supplying states of happiness and relaxation. | [27,28] |
| Cordyceps sinensis | Chong xia cao | Tibet (China) | In traditional Chinese and Tibetan medicine, this fungus was recognized for its aphrodisiacal properties. In addition, alcoholic extracts of this mushroom are used for mood improvement and as energy drinks because of its ginseng-like effects. | [29] |
| Gymnopilus purpuratus | Badiadimurobuni The ear of the spirit | Amazonian natives (Brazil) | In the XVII century, Jesuits reported that tribes of central Amazonia in Brazil, Peru, and Ecuador prepared Gymnopilus-based inebriating beverages for shamanic and healing purposes. | [21] |
| Inocybe aeruginascens | Fibrous head | No specified (Hungary) | This mushroom is also recognized in different Europe countries as the “good trip”, because after its consumption people describe an exceptionally pleasant sensation with sparkling fantasies, experience of a flying soul and euphoric feelings. | [30] |
| Panaeolus papilionaceus | Waraitake or odoritake Laughing mushroom | (Japan) | Reports of recreative use date back to the 11th century; traditional reports indicate that Waraitake consumers became particularly happy, dancing, singing, and laughing compulsively, and this behavior was similar to drunk states. | [31] |
| Psilocybe aztecorum | Apipitzin | Nahuatls/Popocatépetl (México) | Popularly known as “rainwater child”, one of the main species used in central Mexico indigenous civilizations for entheogenic purposes. | [26] |
| Psilocybe caerulescens and P. mexicana | Tenanácatl | Nahuatls (Mexico) | Sacred mushroom used in religious and sacred ceremonies. | [26] |
| Tricholoma muscarium | No reported | No specified (Japan) | This is an important edible agaric mushroom with significant economic value in Japan. The Tricholoma species have an important ecological role because of the ectomycorrhizal formation with different plan families, being considered markers of conservation value measurements. | [32] |
| Group | Psychoactive Markers | Scaffold | Example | Psychotropic Mechanism | Representative Mushrooms Genus |
|---|---|---|---|---|---|
| 1 | Tryptamine or indole alkaloids | 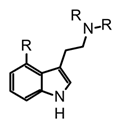 | 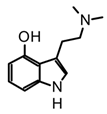 psilocin | Agonist of different serotonin receptors (5HT2a-1a-2c) and ion channels | Psilocybe, Panaeolus, Gymnopilus, Copelandia, Agrocybe, Hyboloma, Galerina, Gerronema, Pluteus, Inocybe, Conocybe, Panaeolina |
| 2 | Isoxazole amino acids | 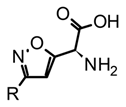 |  muscimol | Agonists of the ionotropic GABAA receptor | Amanita |
| 3 | Ergot alkaloids-Indole type |  |  lysergic acid | Multitarget action. Partial agonist of 5-HT2, adrenergic (α1A/2), and dopamine (D2) receptors | Claviceps and Cordyceps |
| 4 | Indole-type alkaloids | 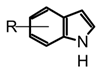 Not fully identified | Chemical studies are still required | Not elucidated yet | Boletus, Heimiella, Russula and some gasteromycetes |
| Indole Type | Alkaloid | Fungal Sources | Extraction Methods | Ref. |
|---|---|---|---|---|
| Tryptamines |  psilocybin | Psilocybe, Panaeolus, Gymnopilus, Copelandia, Agrocybe, Hyboloma, Galerina, Gerronema, Pluteus, Inocybe, Conocybe, Panaeolia. | Dry fungal biomass in the dark and at temperatures below 25 °C. Maceration or UAE with methanol or methanol with 0.5% (v/v) acetic acid at 25 °C, avoiding direct light. Homogenize samples during extraction using a vortex at 13 xG for 2 h. Re-extraction of the biomass using methanol under the conditions of temperature, agitation, and light as previously described. | [35,56,59,60,61] |
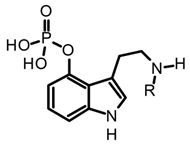 R=H norbaeocystin R=CH3 baeocystin | Conocybe cyanopus, C. smith, Panaeolus cyanescens, Inocybe spp., Psilocybe spp. | [35] | ||
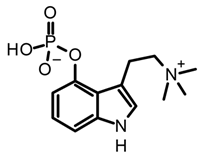 aeruginasin | Inocybe aeruginascens | [56,62] | ||
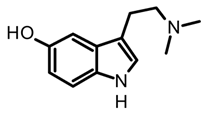 bufotenine | Amanita citrina, A. porphyria, A. rubescens | Dried or fresh fungal biomass. Soxhlet extraction with methanol or maceration with 50% methanol with mechanical homogenization. | [35,63,64] | |
| β-carbolines | 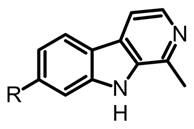 R=H harmane R=OH harmol R=OCH3 harmine | Psilocybe mexicana, P. cyanescens, P. semilanceata and P. cubensis | Lyophilized and grounded fungal biomass saturated with HCl 0.1 M. Extracted with dichloromethane (1:1 v/v). The aqueous phases basified pH 12 with NaOH and are extracted with dichloromethane. | [50] |
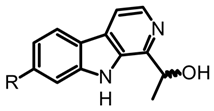 (R)-cordysinin C (S)-cordysinin D | Cordyceps sinensis and P. mexicana | [50] | ||
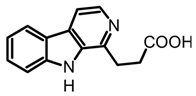 β-Carboline-1-propanoic acid | Boletus curtisii and Cortinarius brunneus | Lyophilized fruiting bodies extracted with aqueous MeOH (80%) using an ultrasound bath for 1h at room temperature. The resultant extract concentrated to dryness in a vacuum. | [55,65] | |
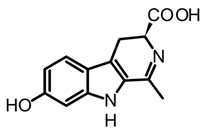 brunnein A | Agrocybe sp. and C. brunneus | Lyophilized and powdered fruiting bodies. Maceration with methanol under mechanical homogenization (1 h, 60 °C and 175 rpm). | [47,65] | |
| Ergot | 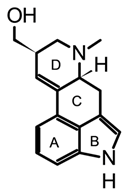 lysergol | Claviceps spp. | Grounded and homogenized samples. Maceration with organic basified solvents (DCM, chloroform, acetonitrile) using solvent ratios from 1:3 to 1:10 (w/v). Mechanical homogenization through vortexing or shaking (30–90 min) Organic extracts could be partitioned and pre-concentrated using solid-phase extraction (SPE). | [52,66,67] |
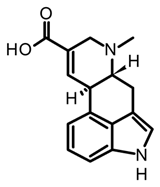 paspalic acid | C. paspali | |||
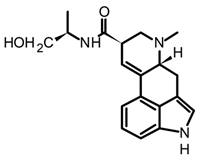 ergonovine | C. purpurea |
| Indole Type | Sample (Alkaloids Detected) | Analytical Technique | Method | Ref. |
|---|---|---|---|---|
| Tryptamines | P. cubensis and Copelandia spp. (Psilocin, psilocybin) | HPLC-DAD | Symmetry RP C18 column (150 × 2.1 mm, 5 µm). MP 10 mM ammonium formate buffer (pH 3.5) and ACN (95:5, v/v), at a flowrate of 0.2 mL/min for 20 min. Detection at 220 nm. | [61] |
| P. cubensis (Psilocin, baeocystin, psilocybin, aeruginascin) | HPLC-ESI-MS | Zorbax Eclipse Plus RP C18 column (100 × 2.1 mm, 1.8 μm). MP 10 mmolL−1 ammonium formate with 0.1% (v/v) formic acid and 10 mmolL−1 ammonium formate with 0.1% (v/v) formic acid in methanol. Flow rate 0.25 mL min−1 for 7 min. For MS detection, a triple quadrupole 6460 spectrometer was used with positive ESI ionization in the dynamic multiple reaction monitoring (dMRM) acquisition mode. | [56] | |
| P. mexicana (psilocin, baeocystin, psilocybin) | HPLC-ESI-HRMS | RP C18 column (250 × 4.6 mm, 3 μm) with MP 0.1% C18 column TFA in water and ACN in gradient mode. Flow rate of 0.4 mLmin−1. Detection with HRMS using an exact Orbitrap spectrometer and electrospray ionization in positive mode. | [50] | |
| Psilocybe spp. and Panaeolus cyanescens (psilocybin) | HPLC-FL | C18 column (150mm × 4.6 mm, 3 μm). MP 50mM ammonium acetate (AcONH4)–CH3CN (73:27). Isocratic elution and rate flow 1.0 mLmin−1. FL detector at 39 nm (excitation at 321 nm). | [72] | |
| β-carbolines | P. mexicana (Harmane, harmine, harmol) | HPLC-ESI-MS | RP C18 column (250 × 2.1 mm, 10 mm ID). MP 0.1% TFA in water and ACN. Flow 2 mLmin−1 with linear gradient with an increase from 10 to 100% ACN for 20 min. | [50] |
| Mycena metata (Metatacarbolines) | HR-MALDI-MS | MALDI-MS imaging of the caps saturated solution in 80% MeOH and 1% TFA, ImagePrep device (Bruker Daltonics). | [71] | |
| Cortinarius brunneus (Brunneins) | HPLC-ESI-MS | RP C18 column (ODS 150 × 2.0 mm i.di, 5 μm). MP water and ACN with FA 0.2% (10:90). Using an isocratic mode for 15 min with flow 0.5 mLmin−1. | [65] | |
| Ergot | C. purpurea (Lysergic, isolysergic, and paspalic acids) | CE-UV | P/ACE 2200 CE system with capillary (37 cm × 50 μm ID, 360 μm). The voltage applied was 25 kV, time for the separation was 12 min, and UV detection at 214 nm. | [67] |
| C. purpurea (Ergometrine, ergotamine, ergosine, ergocryptine) | LC-MS/MS | RP C18 column (150 × 2.1 mm, 3.5 μm). MP water/0.2 M ammonium bicarbonate and methanol/0.2 M ammonium bicarbonate pH 10 at a flow rate of 0.15 mL/min in gradient mode. Detection with MS using triple quadrupole mass spectrometer with positive electrospray ionization. | [73] |
| Type | Alkaloid | In Vitro Studies (Target Activity = _ nM) | In Vivo Studies (Preclinical Research) | Therapeutic Effects (Preclinical) | Ref. |
|---|---|---|---|---|---|
| Tryptamine | Psilocybin | h5-HT2A EC50 = 3475 m5-HT2B EC50 = 74 m5-HT2C EC50 = 506 5-HT1A Ki > 10,000 5-HT1B Ki > 10,000 5-HT1D Ki = 2119 5-HT1E Ki = 194.8 5-HT5 Ki = 6181 5-HT6 Ki = 413.5 5-HT7 Ki = 579.9 | Antidepressant-like behavioral activity in chronically stressed mice with a single injection of psilocybin (1 mg/kg). Anti-compulsive-like behavior using the marble burying test in mice after treatment with a single injection of psilocybin (1 or 2 mg/kg). A single dose of psilocybin (1 mg/kg) improves stress-related behavioral deficits in mice, stimulating the growth of dendritic spines in the frontal cortex and promoting excitatory neurotransmission. A single intravenous dose of psilocybin (0.8 mg/kg) showed important changes in the pig brain, promoting synaptogenesis, increasing hippocampus density, and decreasing 5-HT2AR intensity. Psilocybin shown to down-regulate the functional connectivity within dopamine (DA)-associated striatal networks and increase the functional connectivity in cortical areas. A murine study showed that psilocin increases the concentrations of extracellular dopamine and serotonin in the mesoaccumbens and mesocortical pathways. | Antidepressant Anti-anhedonic Anxiolytic Anti-compulsive Cortical activation Promote synaptogenesis ↑ Neural density Potency neural circuitry ↑ Neuroplasticity | [67,80,83,84,85,86,87,88] |
| Psilocin | h5-HT2A EC50 = 4.3 m5-HT2A EC50 = 9.9 m5-HT2B EC50 = 58 m5-HT2C EC50 = 30 5-HT1A Ki = 49.0 5-HT1B Ki = 219.6 5-HT1D Ki = 36.4 5-HT1E Ki = 52.2 5-HT5 Ki = 83.7 5-HT6 Ki = 57.0 5-HT7 Ki = 3.5 α2A Ki = 1379 α2B Ki = 1894 D3 Ki = 2645 | ||||
| Baeocystin/Norpsilocin | h5-HT2A EC50 = 8.4 m5-HT2A EC50 = 19.0 (For norpsilocin) | Mice were treated with different intravenous doses of baeocystin, and the mouse head-twitch response was measured for 20 min. No significative psychotropic-like effects were detected at doses between 0.03 to 3 mg/kg. | No reported | [68] | |
| Aeruginascin metabolite * | h5-HT1D Ki = 486 h5-HT2B Ki = 128 DAT Ki = 792 | No in vivo studies reported | [87] | ||
| Bufotenine | 5-HT2A Ki = 15 5-HT2C Ki = 145 5-HT1A IC50 = 55 5-HT1B IC50 = 29 5-HT3 Ki = 34 | An effective dose of 0.63 mg/day administered in mice did not cause significant physiological and behavioral effects on the animals. Bufotenine concentrations after injection (100 mg/kg) were slightly higher in the hypothalamus than in the cortex; its effects were exerted predominantly on the peripheral nervous system. | [88,89] | ||
| β-Carbolines | Harmane | MAO-A IC50 = 340 MAO-A Ki = 220 MAO-B IC50 > 10,000 MAO-B Ki = 57,000 5-HT2A Ki = 268 5-HT2C Ki = 2490 | The systemic administration of harmane (5–20 mg/kg) in male Sprague-Dawley rats enhanced 5-HT in a dose-dependent manner. Acute intraperitoneal administration of harmane (5–20 mg/kg) in male adult rats showed antidepressant and anxiolytic effects. Intraperitoneal injection of harmane (2.5 and 10 mg/kg) in rats demonstrated potential sedative effects, also increasing corticosterone, serotonin, and noradrenaline concentrations in different regions of the brain | Antidepressant Anxiolytic | [90,91,92,93,94] |
| Harmine | MAO-A IC50 = 8.7 MAO-A Ki = 5.0 MAO-B IC50 > 10,000 5-HT2A Ki = 397 5-HT2C Ki = 5340 | Mice treated with an intraperitoneal injection of harmine (20 mg/kg) for 10 days showed a significative reduction of depressive-like behaviors by ↓ of brain-derived neurotrophic factor (BDNF), ↑ the protein expression of the glutamate transporter. Male adult Wistar rats exposed to a chronic mild stress protocol were treated with harmine (15 mg/kg) for 7 days, which reversed anhedonia behavior and induced changes in the adrenal gland weight. | Antidepressant Anti-anhedonic Promote neurogenesis Stimulate neuroplasticity Repair astrocytic functions | [90,94,95,96] | |
| Harmaline | MAO-A IC50 = 11.8 MAO-A Ki = 48 MAO-B IC50 > 10,000 5-HT2A Ki = 5010 5-HT2C Ki = 9430 | Intraperitoneal injection of harmaline (2.5 and 5 mg/kg) produced an anxiogenic-like response in male NMRI mice, whereas 10 mg/kg of this carboline induced antidepressant-like behavior in a forced swim test. | Antidepressant Anxiolytic | [90,97] | |
| Ergot | Ergotamine | 5-HT2A Ki = 20.4 5-HT2B Ki = 6.76 5-HT2C EC50 = 7.94 5-HT1A Ki = 12.9 5-HT1B Ki = 13.2 5-HT1D Ki = 4.36 5-HT1E Ki = 602 5-HT1F Ki = 169 5-HT6 Ki = 57.0 α1A Ki = 10 α2 Ki = 6.3 D2 Ki = 3.16 | Extensive studies in animals related with the vasoconstrictor effect; however, no reports related to neuropsychiatric disorders were found. | Anti-migraine | [98,99] |
| Intervention | Title | Conditions | Phase/ Status | |
|---|---|---|---|---|
| Tryptamines | Psilocybin (25 mg) | The safety and efficacy of psilocybin in participants with Type 2 bipolar disorder (BP-II) Depression | Bipolar disorder, Depression | Phase 2/recruiting |
| Psilocybin (10 mg in 1st session and 25 mg in 2nd session) | Psilocybin Therapy for Depression and Anxiety in Parkinson’s Disease (PDP) | Parkinson’s, Depression, Anxiety | Phase 2/recruiting | |
| Psilocybin (Two sessions) | Psychopharmacology of Psilocybin in Cancer Patients | Depressive symptoms Anxiety Cancer | Phase 2/ completed | |
| Psilocybin (0.25 mg/kg) | Efficacy of Psilocybin in OCD: a Double-Blind, Placebo-Controlled Study | Obsessive-compulsive Disorder | Phase 1/recruiting | |
| Psilocybin (100 or 300 µg/kg) | Psilocybin for Treatment of Obsessive-Compulsive Disorder (PSILOCD) | Obsessive-compulsive Disorder | Phase 1/in course | |
| Psilocybin (25 mg) | Psilocybin for Treatment-Resistant Depression | Depression | Phase 2/in course | |
| Psilocybin (Single dose) | The Safety and Efficacy Of Psilocybin as an Adjunctive Therapy in Participants with Treatment Resistant Depression | Resistant Depression | Phase 2/ completed | |
| Psilocybin (25 mg) | The Safety and Efficacy of Psilocybin in Patients with Treatment-resistant Depression and Chronic Suicidal Ideation | Resistant Depression Suicidal behavior | Phase 2/recruiting | |
| Psilocybin (25 mg) | A Study of Psilocybin for Major Depressive Disorder (MDD) | MDD | Phase 2/in course | |
| Psilocybin (0.215mg/kg) | Clinical, Neurocognitive, and Emotional Effects of Psilocybin in Depressed Patients—Proof of Concept | Depressive Disorder | Phase 2/ completed | |
| Psilocybin (25 mg, 2 sessions) | Psilocybin for Depression in People with Mild Cognitive Impairment or Early Alzheimer’s Disease | Depressive Symptoms Alzheimer Disease Mild Cognitive Impairment | Phase 2/recruiting | |
| Psilocybin (25 mg) | Psilocybin Treatment of Major Depressive Disorder with Co-occurring Alcohol Use Disorder | MDD Alcohol Use Disorder | Phase 2/recruiting | |
| Psilocybin (25 and 30 mg) | Psilocybin-Enhanced Psychotherapy for Methamphetamine Use Disorder | Amphetamine-Related Disorders | Phase 2/recruiting | |
| Psilocybin (30 mg) | Psilocybin-facilitated Smoking Cessation Treatment: A Pilot Study | Nicotine Dependence | NA | |
| Psilocybin (25 mg) | A Double-Blind Trial of Psilocybin-Assisted Treatment of Alcohol Dependence | Alcohol Dependence | Phase 2/ completed | |
| Psilocybin (25 mg) | Standardized Natural Psilocybin-assisted Psychotherapy for Tapering of Opioid Medication | Opioid Dependence Chronic Pain | Phase 2/not recruiting yet | |
| Carboline | Harmine Harmine + DMT | Neurodynamics of Prosocial Emotional Processing Following Serotonergic Stimulation With N,N-Dimethyltryptamine (DMT) and Harmine in Healthy Subjects | Emotional and Mood disorders | Phase 2/recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plazas, E.; Faraone, N. Indole Alkaloids from Psychoactive Mushrooms: Chemical and Pharmacological Potential as Psychotherapeutic Agents. Biomedicines 2023, 11, 461. https://doi.org/10.3390/biomedicines11020461
Plazas E, Faraone N. Indole Alkaloids from Psychoactive Mushrooms: Chemical and Pharmacological Potential as Psychotherapeutic Agents. Biomedicines. 2023; 11(2):461. https://doi.org/10.3390/biomedicines11020461
Chicago/Turabian StylePlazas, Erika, and Nicoletta Faraone. 2023. "Indole Alkaloids from Psychoactive Mushrooms: Chemical and Pharmacological Potential as Psychotherapeutic Agents" Biomedicines 11, no. 2: 461. https://doi.org/10.3390/biomedicines11020461
APA StylePlazas, E., & Faraone, N. (2023). Indole Alkaloids from Psychoactive Mushrooms: Chemical and Pharmacological Potential as Psychotherapeutic Agents. Biomedicines, 11(2), 461. https://doi.org/10.3390/biomedicines11020461






