Confirmation of CD19+ B-Lymphocyte Depletion Prior to Intake of the Second Dose of Ocrelizumab in Multiple Sclerosis Patients
Abstract
1. Introduction
1.1. Pathogenesis of Multiple Sclerosis
1.2. Mechanism of Action of Disease-Modifying Ocrelizumab Drug
2. Materials and Methods
2.1. Participants
2.2. Collection of Data and Study Procedure
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamout, B.I.; Alroughani, R. Multiple sclerosis. Semin. Neurol. 2018, 38, 212–225. [Google Scholar] [CrossRef]
- Kobelt, G.; Thompson, A.; Berg, J.; Gannedahl, M.; Eriksson, J.; MSCOI Study Group. European Multiple Sclerosis Platform.e New insights into the burden and costs of multiple sclerosis in Europe. Mult. Scler. J. 2017, 23, 1123–1136. [Google Scholar] [CrossRef]
- Cellerino, M.; Boffa, G.; Lapucci, C.; Tazza, F.; Sbragia, E.; Mancuso, E.; Bruschi, N.; Minguzzi, S.; Ivaldi, F.; Poirè, I.; et al. Predictors of ocrelizumab effectiveness in patients with multiple sclerosis. Neurother. J. Am. Soc. Exp. Neurother. 2021, 18, 2579–2588. [Google Scholar] [CrossRef]
- The Multiple Sclerosis International Federation. Atlas of MS—3rd Edition, Part 2: Clinical Management of Multiple Sclerosis around the World. UK: The Multiple Sclerosis International Federation. 2021. Available online: https://www.msif.org/wp-content/uploads/2021/05/Atlas-3rd-Edition-clinical-management-report-EN-5-5-21.pdf (accessed on 23 May 2022).
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Tarlinton, R.E.; Martynova, E.; Rizvanov, A.A.; Khaiboullina, S.; Verma, S. Role of viruses in the pathogenesis of multiple sclerosis. Viruses 2020, 12, e643. [Google Scholar] [CrossRef]
- Moutsianas, L.; Jostins, L.; Beecham, A.H.; Dilthey, A.T.; K Xifara, D.K.; Ban, M.; Shah, T.S.; Patsopoulos, N.A.; Alfredsson, L.; Anderson, C.A.; et al. Class II HLA interactions modulate genetic risk for multiple sclerosis. Nat. Genet. 2015, 47, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Williamson, D.; Ashley-Koch, A. HLA-DR15 haplotype and multiple sclerosis: A HuGE review. Am. J. Epidemiol. 2007, 165, 1097–1109. [Google Scholar] [CrossRef]
- Russi, A.E.; Ebel, M.E.; Yang, Y.; Brown, M.A. Male-specific IL-33 expression regulates sex-dimorphic EAE susceptibility. Proc. Natl. Acad. Sci. USA 2018, 115, e15201529. [Google Scholar] [CrossRef]
- Karussis, D. The diagnosis of multiple sclerosis and the various related demyelinating syndromes: A critical review. J. Autoimmun. 2014, 48–49, 134142. [Google Scholar] [CrossRef] [PubMed]
- Trapp, B.D.; Peterson, J.; Ransohoff, R.M.; Mörk, S.; Bö, L. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 1998, 338, 278–285. [Google Scholar] [CrossRef]
- Prineas, J.W.; Kwon, E.E.; Cho, E.S.; Sharer, L.R.; Barnett, M.H.; Oleszak, E.L.; Hoffman, B.; Morgan, B.P. Immunopathology of secondary-progressive multiple sclerosis. Ann. Neurol. 2001, 50, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Haruki Koike, H.; Katsuno, M. Macrophages and Autoantibodies in Demyelinating Diseases. Cells 2021, 10, 844. [Google Scholar] [CrossRef]
- Gjelstrup, M.C.; Stilund, M.; Petersen, T.; Møller, H.J.; Petersen, E.L.; Christensen, T. Subsets of activated monocytes and markers of inflammation in incipient and progressed multiple sclerosis. Immunol. Cell. Biol. 2018, 96, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Raveney, B.J.E.; Sato, W.; Takewaki, D.; Zhang, C.; Kanazawa, T.; Lin, Y.; Okamoto, T.; Araki, M.; Kimura, Y.; Sato, N.; et al. Involvement of cytotoxic Eomes-expressing CD4+ T cells in secondary progressive multiple sclerosis. Proc. Natl. Acad. Sci. USA 2021, 118, e2021818118. [Google Scholar] [CrossRef]
- Machado-Santos, J.; Saji, E.; Tröscher, A.R.; Paunovic, M.; Liblau, R.; Gabriely, G.; Bien, C.G.; Bauer, J.; Lassmann, H. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain 2018, 141, 2066–2082. [Google Scholar] [CrossRef]
- Lazibat, I.; Rubinić Majdak, M.; Županić, S. Multiple sclerosis: New aspects of Immunopathogenesis. Acta Clin. Croat. 2018, 57, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Martin-Blondel, G.; Mars, L.T.; Liblau, R.S. Role of CD8 T cell subsets in the pathogenesis of multiple sclerosis. FEBS Lett. 2011, 585, 3758–3763. [Google Scholar] [CrossRef]
- Wagner, C.A.; Roqué, P.J.; Mileur, T.R.; Liggitt, D.; Goverman, J.M. Myelin-specific CD8+ T cells exacerbate brain inflammation in CNS autoimmunity. J. Clin. Investig. 2020, 130, 203–213. [Google Scholar] [CrossRef]
- Dalakas, M.C. B cells as therapeutic targets in autoimmune neurological disorders. Nat. Clin. Pract. Neurol. 2008, 4, 557–567. [Google Scholar] [CrossRef]
- Perdaens, O.; van Pesch, V. Molecular mechanisms of immunosenescene and inflammaging: Relevance to the immunopathogenesis and treatment of multiple sclerosis. Front. Neurol. 2022, 12, 811518. [Google Scholar] [CrossRef]
- Mulero, P.; Midaglia, L.; Montalban, X. Ocrelizumab: A new milestone in multiple sclerosis therapy. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418773025. [Google Scholar] [CrossRef] [PubMed]
- MedlinePlus Medical Encyclopedia. CSF Oligoclonal Banding. Available online: https://medlineplus.gov/ency/article/003631.htm (accessed on 12 May 2022).
- Chanvillard, C.; Jacolik, R.F.; Infante-Duarte, C.; Nayak, R.C. The role of natural killer cells in multiple sclerosis and their therapeutic implications. Front. Immunol. 2013, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Al-Falahi, Y.; Sand, K.L.; Knudsen, E.; Damaj, B.B.; Rolin, J.; Maghazachi, A.A. Splenic natural killer cell activity in two models of experimental neurodegenerative diseases. J. Cell. Mol. Med. 2009, 13, 2693–26703. [Google Scholar] [CrossRef]
- Zhang, B.; Yamamura, T.; Kondo, T.; Fujiwara, M.; Tabira, T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J. Exp. Med. 1997, 186, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Shi, F.D.; Jung, S.; Pien, G.C.; Wang, J.; Salazar-Mather, T.P.; He, T.T.; Weaver, J.T.; Ljunggren, H.G.; Biron, C.A.; et al. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 896–905. [Google Scholar] [CrossRef]
- Pagenstecher, A.; Lassmann, S.; Carson, M.J.; Kincaid, C.L.; Stalder, A.K.; Campbell, I.L. Astrocyte-targeted expression of IL-12 induces active cellular immune responses in the central nervous system and modulates experimental allergic encephalomyelitis. J. Immunol. Baltim. 2000, 164, 4481–4492. [Google Scholar] [CrossRef]
- Shi, F.D.; Takeda, K.; Akira, S.; Sarvetnick, N.; Ljunggren, H.G. IL-18 directs autoreactive T cells and promotes autodestruction in the central nervous system via induction of IFN-gamma by NK cells. J. Immunol. 2000, 165, 3099–3104. [Google Scholar] [CrossRef]
- Al-Ani, M.; Elemam, N.M.; Hundt, J.E.; Maghazachi, A.A. Drugs for multiple sclerosis activate natural killer cells: Do they protect against COVID-19 infection? Infect. Drug. Resist. 2020, 13, 3243–3254. [Google Scholar] [CrossRef]
- Perini, P.; Wadhwa, M.; Buttarello, M.; Meager, A.; Facchinetti, A.; Thorpe, R.; Biasi, G.; Gallo, P. Effect of IFNbeta and anti-IFNbeta antibodies on NK cells in multiple sclerosis patients. J. Neuroimmunol. 2000, 105, 91–95. [Google Scholar] [CrossRef] [PubMed]
- EMA—European Medicines Agency. European Medicines Agency. Ocrevus, INN-Ocrelizumab. Available online: https://www.ema.europa.eu/en/documents/product-information/ocrevus-epar-product-information_hr.pdf (accessed on 14 June 2022).
- Abbadessa, G.; Miele, G.; Cavalla, P.; Valentino, P.; Marfia, G.A.; Signoriello, E.; Landi, D.; Bosa, C.; Vercellino, M.; De Martino, A.; et al. CD19 cell count at baseline predicts B cell repopulation at 6 and 12 months in multiple sclerosis patients treated with ocrelizumab. Int. J. Environ. Res. Public Health 2021, 18, 8163. [Google Scholar] [CrossRef]
- Cencioni, M.T.; Mattoscio, M.; Magliozzi, R.; Bar-Or, A.; Muraro, P.A. B cells in multiple sclerosis-from targeted depletion to immune reconstitution therapies. Nat. Rev. Neurol. 2021, 17, 399–414. [Google Scholar] [CrossRef]
- Lamb, Y.N. Ocrelizumab: A review in multiple sclerosis. Drugs 2022, 82, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Brill, L.; Rechtman, A.; Zveik, O.; Haham, N.; Oiknine-Djian, E.; Wolf, D.G.; Levin, N.; Raposo, C.; Vaknin-Dembinsky, A. Humoral and T-cell response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab. J. Am. Med. Assoc. Neurol. 2021, 78, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Havrdová, E.; Arnold, D.L.; Bar-Or, A.; Comi, G.; Hartung, H.P.; Kappos, L.; Lublin, F.; Selmaj, K.; Traboulsee, A.; Belachew, S.; et al. No evidence of disease activity (NEDA) analysis by epochs in patients with relapsing multiple sclerosis treated with ocrelizumab vs interferon beta-1a. Mult. Scler. J. Exp. Transl. Clin. 2018, 4, 2055217318760642. [Google Scholar] [CrossRef]
- Gibiansky, E.; Petry, C.; Mercier, F.; Günther, A.; Herman, A.; Kappos, L.; Hauser, S.; Yamamoto, Y.; Wang, Q.; Model, F.; et al. Ocrelizumab in relapsing and primary progressive multiple sclerosis: Pharmacokinetic and pharmacodynamic analyses of OPERA I, OPERA II and ORATORIO. Br. J. Clin. Pharmacol. 2021, 87, 2511–2520. [Google Scholar] [CrossRef] [PubMed]
- Margoni, M.; Preziosa, P.; Filippi, M.; Rocca, M.A. Anti-CD20 therapies for multiple sclerosis: Current status and future perspectives. J. Neurol. 2022, 269, 1316–1334. [Google Scholar] [CrossRef]
- Capasso, N.; Nozzolillo, A.; Scalia, G.; Lanzillo, R.; Carotenuto, A.; De Angelis, M.; Petruzzo, M.; Saccà, F.; Russo, C.V.; Brescia Morra, V.; et al. Ocrelizumab depletes T-lymphocytes more than rituximab in multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 49, 102802. [Google Scholar] [CrossRef]
- Gingele, S.; Jacobus, T.L.; Konen, F.F.; Hümmert, M.W.; Sühs, K.W.; Schwenkenbecher, P.; Ahlbrecht, J.; Möhn, N.; Müschen, L.H.; Bönig, L.; et al. Ocrelizumab depletes CD20+ T cells in multiple sclerosis patients. Cells 2018, 8, 12. [Google Scholar] [CrossRef]
- Quendt, C.; Ochs, J.; Häusser-Kinzel, S.; Häusler, D.; Weber, M.S. Proinflammatory CD20+ T cells are differentially affected by multiple sclerosis therapeutics. Ann. Neurol. 2021, 90, 834–839. [Google Scholar] [CrossRef]
- Cross, A.; Bennett, J.; von Büdingen, H.C.; Carruthers, R.; Edwards, K.; Fallis, R.; Fiore, D.; Gelfand, J.; Giacomini, P.; Greenberg, G.; et al. Ocrelizumab treatment reduced levels of neurofilament light chain and numbers of B cells in the cerebrospinal fluid of patients with relapsing multiple sclerosis in the OBOE study. Neurology 2019, 92, 15. [Google Scholar]
- Bar-Or, A.; Bennett, J.; Budingen, H.V.; Carruthers, R.; Edwards, K.; Fallis, R.; Fiore, D.; Gelfand, J.; Giacomini, P.; Greenberg, B.; et al. B Cells, T Cells and inflammatory CSF biomarkers in primary progressive MS and relapsing MS in the OBOE (ocrelizumab biomarker outcome evaluation) trial. Neurology 2020, 94, 1635. [Google Scholar]
- Hauser, S.L.; Bar-Or, A.; Comi, G.; Giovannoni, G.; Hartung, H.P.; Hemmer, B.; Lublin, F.; Montalban, X.; Rammohan, K.W.; Selmaj, K.; et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N. Engl. J. Med. 2017, 376, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, C.R.; Rossi, N.D.; Capra, R. Ocrelizumab for the treatment of multiple sclerosis: Safety, efficacy, and pharmacology. Ther. Clin. Risk Manag. 2021, 17, 765–776. [Google Scholar] [CrossRef]
- Marrodan, M.; Laviano, J.; Oneto, S.; Reino, F.M.; Delorme, R.; Fornillo, F.; Férnandez, J.; Correale, J. Rituximab- and ocrelizumab-induced early- and late-onset neutropenia in a multiple sclerosis patient. Neurol. Sci. 2021, 42, 3893–3895. [Google Scholar] [CrossRef] [PubMed]
- Dale, D.C. How I diagnose and treat neutropenia. Curr. Opin. Hematol. 2016, 23, 1–4. [Google Scholar] [CrossRef]
- Baird-Gunning, J.; Yun, J.; Stevenson, W.; Ng, K. Severe delayed-onset neutropenia induced by ocrelizumab. Neurohospitalist 2021, 11, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Proschmann, U.; Katja Akgün, K.; Ziemssen, T. Lymphocyte Counts and Multiple Sclerosis Therapeutics: Between Mechanisms of Action and Treatment-Limiting Side Effects. Cells 2021, 10, 3177. [Google Scholar] [CrossRef] [PubMed]
- Abbadessa, G.; Maida, E.; Miele, G.; Lavorgna, L.; Marfia, G.A.; Valentino, P.; De Martino, A.; Cavalla, P.; Bonavita, S. Lymphopenia in multiple sclerosis patients treated with ocrelizumab is associated with an effect on CD8 T cells. Mult. Scler. Relat. Disord. 2022, 60, 103740. [Google Scholar] [CrossRef] [PubMed]
- Perriguey, M.; Maarouf, A.; Jan-Patrick Stellmann, J.P.; Rico, A.; Boutiere, C.; Demortiere, S.; Durozard, P.; Pelletier, J.; Audoin, B. Hypogammaglobulinemia and Infections in Patients with Multiple Sclerosis Treated with Rituximab. Neurol. Neuroimmunol. Neuroinflamm. 2021, 9, e1115. [Google Scholar] [CrossRef]
- Habek, M.; Piskač, D.; Gabelić, T.; Barun, B.; Adamec, I.; Krbot Skorić, M. Hypogammaglobulinemia, infections and COVID-19 in people with multiple sclerosis treated with ocrelizumab. Mult. Scler. Relat. Disord. 2022, 62, 103798. [Google Scholar] [CrossRef] [PubMed]
- Sempere, A.P.; Berenguer-Ruiz, L.; Borrego-Soriano, I.; Burgos-San Jose, A.; Concepcion-Aramendia, L.; Volar, L.; Aragones, M.; Palazón-Bru, A. Ocrelizumab in multiple sclerosis: A real-world study from Spain. Front. Neurol. 2020, 11, 592304. [Google Scholar] [CrossRef] [PubMed]
- Demir, S.; Atmaca, M.M.; Togrol, R.E. The first cure experience of a clinic: Approach to the patient to start ocrelizumab. Noro. Psikiyatr. Ars. 2019, 58, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Rich, R.R.; Fleisher, T.A.; Shearer, W.T.; Schroeder, H.; Frew, A.J.; Weyand, C.M. Clinical Immunology: Principles and Practice, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Fernández-Velasco, J.I.; Kuhle, J.; Monreal, E.; Meca-Lallana, V.; Meca-Lallana, J.; Izquierdo, G.; Gascón-Giménez, F.; Sainz de la Maza, S.; Walo-Delgado, P.E.; Maceski, A.; et al. Effect of ocrelizumab in blood leukocytes of patients with primary progressive MS. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e940. [Google Scholar] [CrossRef] [PubMed]
- LOINC. CD3-CD16+CD56+ (Natural killer) cells/100 Cells in Blood. Available online: https://loinc.org/loinc/8112-5/ (accessed on 12 July 2022).
- Hauser, S.L.; Kappos, L.; Xavier Montalban, X.; Craveiro, L.; Chognot, C.; Hughes, R.; Koendgen, H.; Pasquarelli, N.; Pradhan, A.; Prajapati, K.; et al. Safety of Ocrelizumab in Patients with Relapsing and Primary Progressive Multiple Sclerosis. Neurology 2021, 97, e1546–e1559. [Google Scholar] [CrossRef] [PubMed]
- Lanzillo, R.; Carotenuto, A.; Signoriello, E.; Iodice, R.; Miele, G.; Bisecco, A.; Maniscalco, G.T.; Sinisi, L.; Romano, F.; Di Gregorio, M.; et al. Prognostic Markers of Ocrelizumab Effectiveness in Multiple Sclerosis: A Real World Observational Multicenter Study. J. Clin. Med. 2022, 11, 2081. [Google Scholar] [CrossRef] [PubMed]
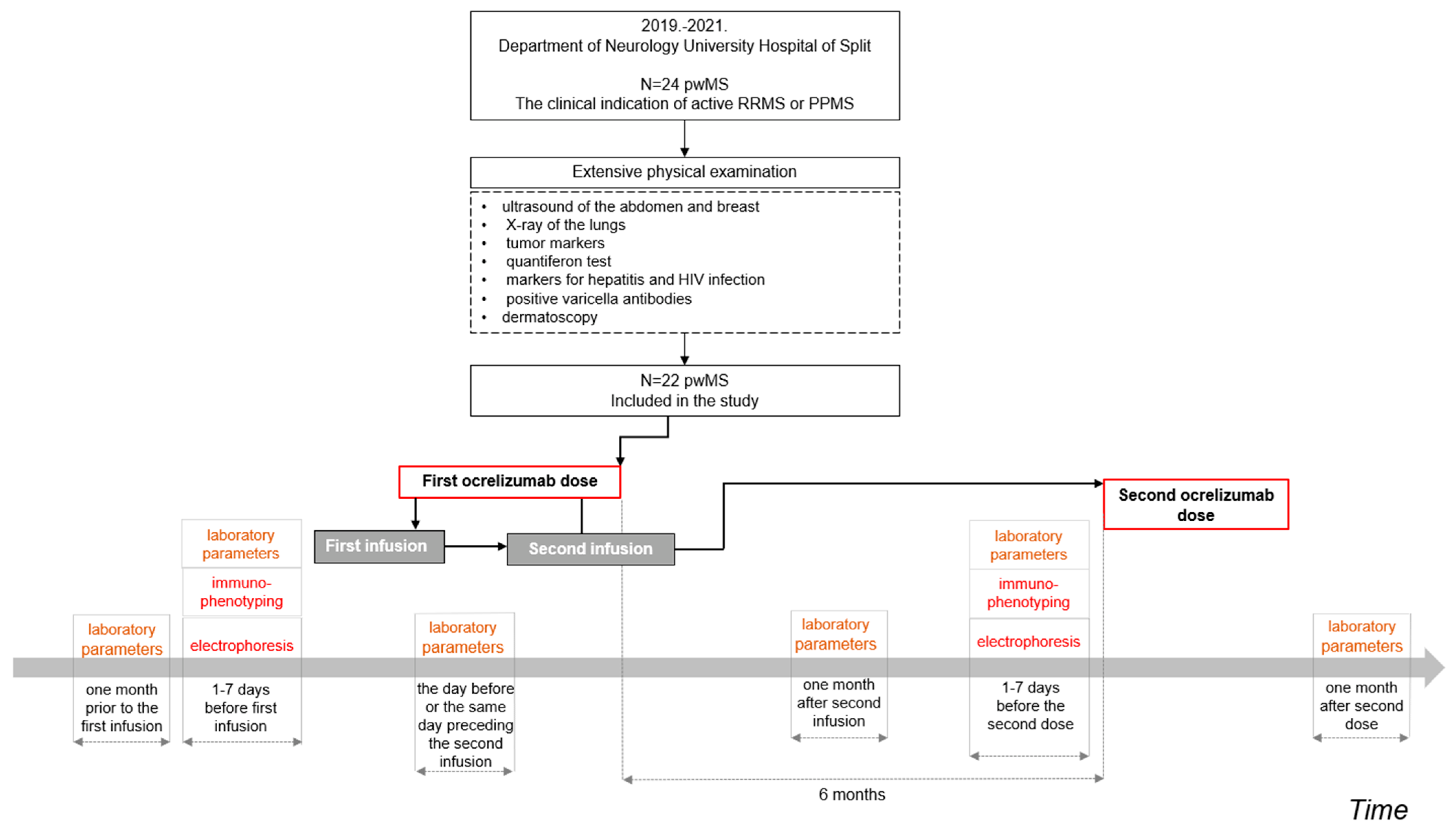
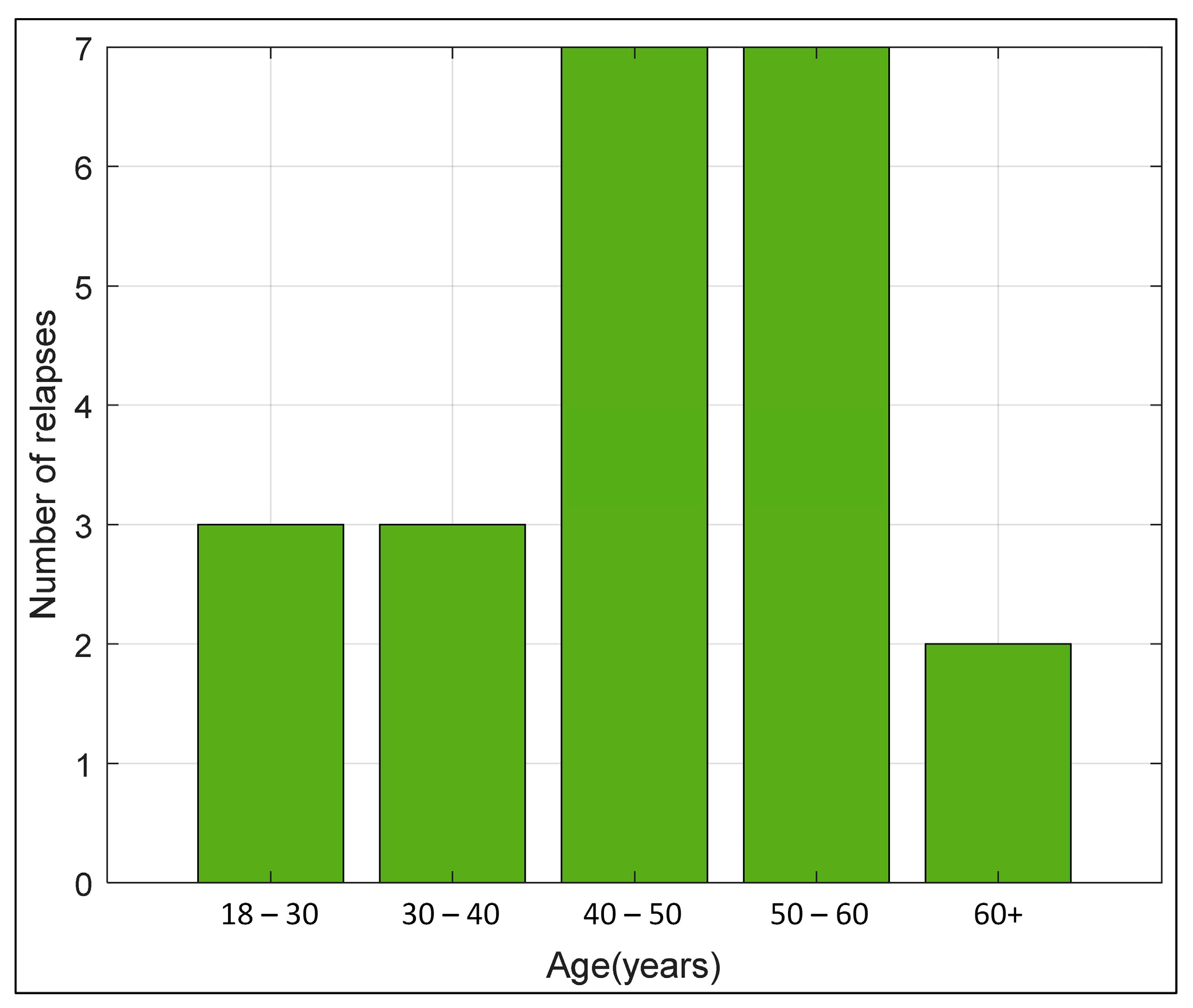
Age of patients at the start of ocrelizumab administration (years) | n = 22 46.50 IQR (38. 53) min-max (21–67) |
EDSS score before the first administration of ocrelizumab EDSS score before the second administration of ocrelizumab | n = 22 4.00 (2.63–5.38) 4.00(2.65–5.38) |
Duration of RRMS disease at the first administration of ocrelizumab (years) | n = 9 9 ± 3.46 |
Duration of PPMS at the first administration of ocrelizumab (years) | n = 8 4.25 ± 4.1 |
| Variable | Before Administration of the First Ocrelizumab Infusion | One Month after the Second Ocrelizumab Infusion | p * |
|---|---|---|---|
| Leukocytes (×109/L) | (n = 21) 6.00 (4.90–7.00) | (n = 20) 5.75 (5.25–7.20) | 0.938 |
| Erythrocytes (×1012/L) | (n = 21) 4.80 (4.31–4.93) | (n = 20) 4.78 (4.42–4.91) | 0.927 |
| Hemoglobin (g/L) | (n = 21) 139.00 (128.00–149.00) | (n = 20) 138.00 (130.75–148.25) | 0.927 |
| Thrombocytes (×109/L) | (n = 21) 233.00 (221.00–284.00) | (n = 20) 251.50 (215.75–279.50) | 0.764 |
| Neutrophils (×109/L) | (n = 21) 3.70 (2.68–4.47) | (n = 20) 3.40 (3.20–4.98) | 0.676 |
| Lymphocytes (×109/L) | (n = 21) 1.59 (1.24–2.10) | (n = 20) 1.36 (1.18–1.71) | 0.100 |
| Monocytes (×109/L) | (n = 21) 0.41 (0.33–0.53) | (n = 20) 0.46 (0.40–0.57) | 0.347 |
| Eosinophils (×109/L) | (n = 21) 0.14 (0.10–0.22) | (n = 20) 0.13 (0.09–0.20) | 0.657 |
| Basophils (×109/L) | (n = 21) 0.04 (0.03–0.05) | (n = 20) 0.04 (0.03–0.05) | 0.594 |
| AST (U/L) | (n = 19) 19.00 (18.00–21.50) | (n = 20) 20.00 (16.75–23.00) | 0.592 |
| ALT (U/L) | (n = 19) 20.00 (17.50–28.00) | (n = 20) 19.50 (16.00–24.25) | 0.663 |
| GGTc (U/L) | (n = 20) 15.00 (12.00–22.00) | (n = 20) 15.00 (12.75–21.00) | 0.881 |
| ALP (U/L) | (n = 7) 61.00 (49.00–74.00) | (n = 8) 59.50 (51.00–73.50) | 0.862 |
| LDH (U/L) | (n = 13) 158.00 (142.00–168.00) | (n = 11) 178.00 (149.50–181.50) | 0.401 |
| Electrophoresis | Before Administration of the First Dose of Ocrelizumab (n = 16) | Before Administration of the Second Dose of Ocrelizumab (n = 15) | p * |
|---|---|---|---|
| Albumin (g/L) | 43.70 (41.14–44.30) | 42.14 (41.20–43.15) | 0.265 |
| Alpha-1-globulins (g/L) | 2.68 (2.50–3.00) | 2.80 (2.69–2.98) | 0.635 |
| Alpha-2-globulins (g/L) | 6.51 (6.19–7.34) | 6.60 (6.30.6.94) | 0.797 |
| Beta-globulins (g/L) | 7.20 (6.90–8.13) | 7.42 (7.02–7.89) | 0.751 |
| Gamma -globulins (g/L) | 9.78 (8.64–10.88) | 9.59 (8.55–10.48) | 0.89 |
| Albumin/globulins | 1.63 (1.53–1.71) | 1.62 (1.50–1.71) | 0.736 |
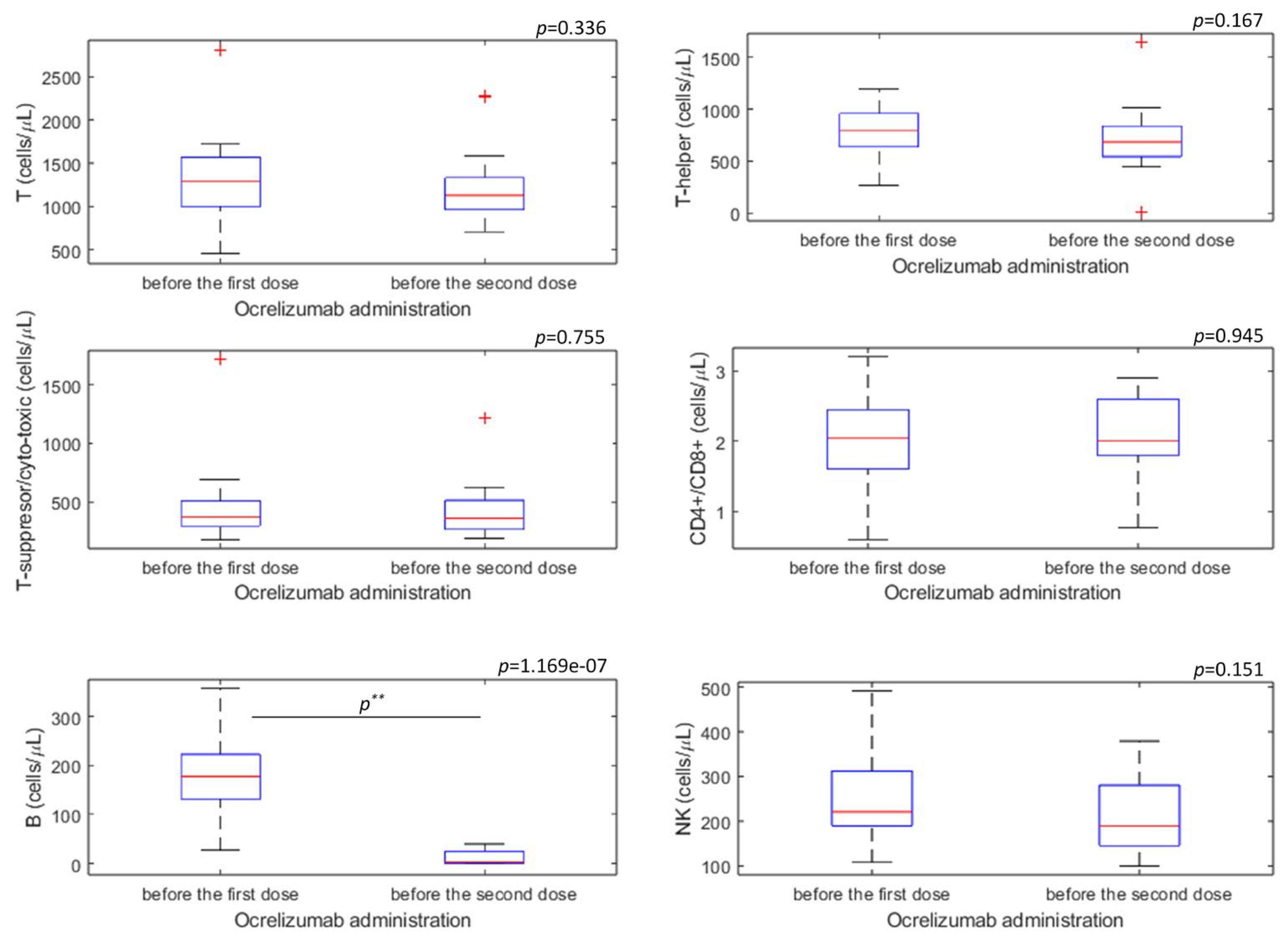
| Immunophenotyping of Lymphocytes | Cell Marker | Before Administration of the First Dose of Ocrelizumab (n = 17) | Before Administration of the Second Dose of Ocrelizumab (n = 19) | p * |
|---|---|---|---|---|
| T (cell/µL) | CD3+ | 1287.00 (1004.00–1560.75) | 1129.50 (972.00–1329.25) | 0.336 |
| T-helper (cell/µL) | CD3+CD4+ | 790.00 (656.50–937.50) | 685.00 (560.00–829.50) | 0.167 |
| T-suppressor/cytotoxic(cell/µL) | CD3+CD8+ | 367.50 (298.25–509.00) | 357.00 (264.50–495.50) | 0.755 |
| Ratio CD4+/CD8+ | CD4+/CD8+ | 2.05 (1.60–2.38) | 2.00 (1.80–2.60) | 0.946 |
| B (cell/µL) | CD19+ | 177.50 (134.50–220.25) | 3.50 (1.00–23.25) | <0.001 * |
| NK (cell/µL) | CD3− CD16+ 56+ | 221.00 (189.75–299.00) | 189.00 (146.25–266.75) | 0.151 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radmilo, M.; Pavelin, S.; Vujović, I.; Šoda, J.; Rogić Vidaković, M. Confirmation of CD19+ B-Lymphocyte Depletion Prior to Intake of the Second Dose of Ocrelizumab in Multiple Sclerosis Patients. Biomedicines 2023, 11, 353. https://doi.org/10.3390/biomedicines11020353
Radmilo M, Pavelin S, Vujović I, Šoda J, Rogić Vidaković M. Confirmation of CD19+ B-Lymphocyte Depletion Prior to Intake of the Second Dose of Ocrelizumab in Multiple Sclerosis Patients. Biomedicines. 2023; 11(2):353. https://doi.org/10.3390/biomedicines11020353
Chicago/Turabian StyleRadmilo, Marija, Sanda Pavelin, Igor Vujović, Joško Šoda, and Maja Rogić Vidaković. 2023. "Confirmation of CD19+ B-Lymphocyte Depletion Prior to Intake of the Second Dose of Ocrelizumab in Multiple Sclerosis Patients" Biomedicines 11, no. 2: 353. https://doi.org/10.3390/biomedicines11020353
APA StyleRadmilo, M., Pavelin, S., Vujović, I., Šoda, J., & Rogić Vidaković, M. (2023). Confirmation of CD19+ B-Lymphocyte Depletion Prior to Intake of the Second Dose of Ocrelizumab in Multiple Sclerosis Patients. Biomedicines, 11(2), 353. https://doi.org/10.3390/biomedicines11020353








