Which Epidemiological Characteristics Drive Decision Making in the Management of Patients with Vestibular Schwannoma?
Abstract
1. Introduction
2. Materials and Methods
- (a)
- (wait and scan):
- -
- Includes all patients indicated for observation who remained untreated until 12/2021.
- -
- Follow-up consists of repeated magnetic resonance imaging (MRI), usually 6 months after initial diagnosis and then once annually for at least 3 years. The subsequent lengthening of the interval between follow-ups depends on the morphological and functional stability of the tumor, the age, and the preferences of the patients.
- (b)
- SRT (stereoradiotherapy):
- -
- Includes patients indicated for stereotactic irradiation mainly, but not exclusively, using the Leksell Gamma knife (LGN). The interval between initial diagnosis and treatment should be less than 6 months.
- (c)
- Surgery:
- -
- Includes patients indicated for surgery with an interval between the initial diagnosis and treatment of less than 6 months.
- (d)
- WaS—Surgery:
- -
- Includes patients indicated for observation who were later indicated for surgery. The minimum interval between initial diagnosis and surgery was 6 months, with at least one control MRI performed during this interval.
- (e)
- WaS—SRT:
- -
- Includes patients indicated for observation who were later irradiated. The minimum interval between diagnosis and surgery was 6 months, with at least one control MRI performed.
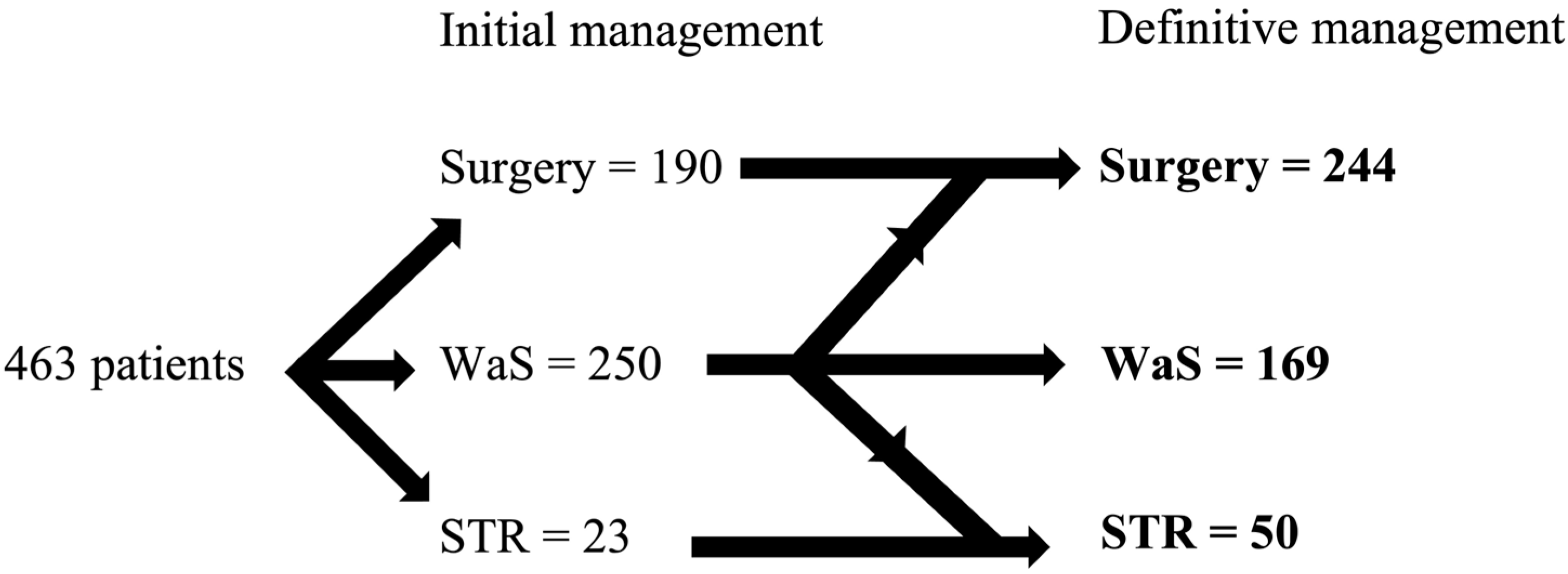
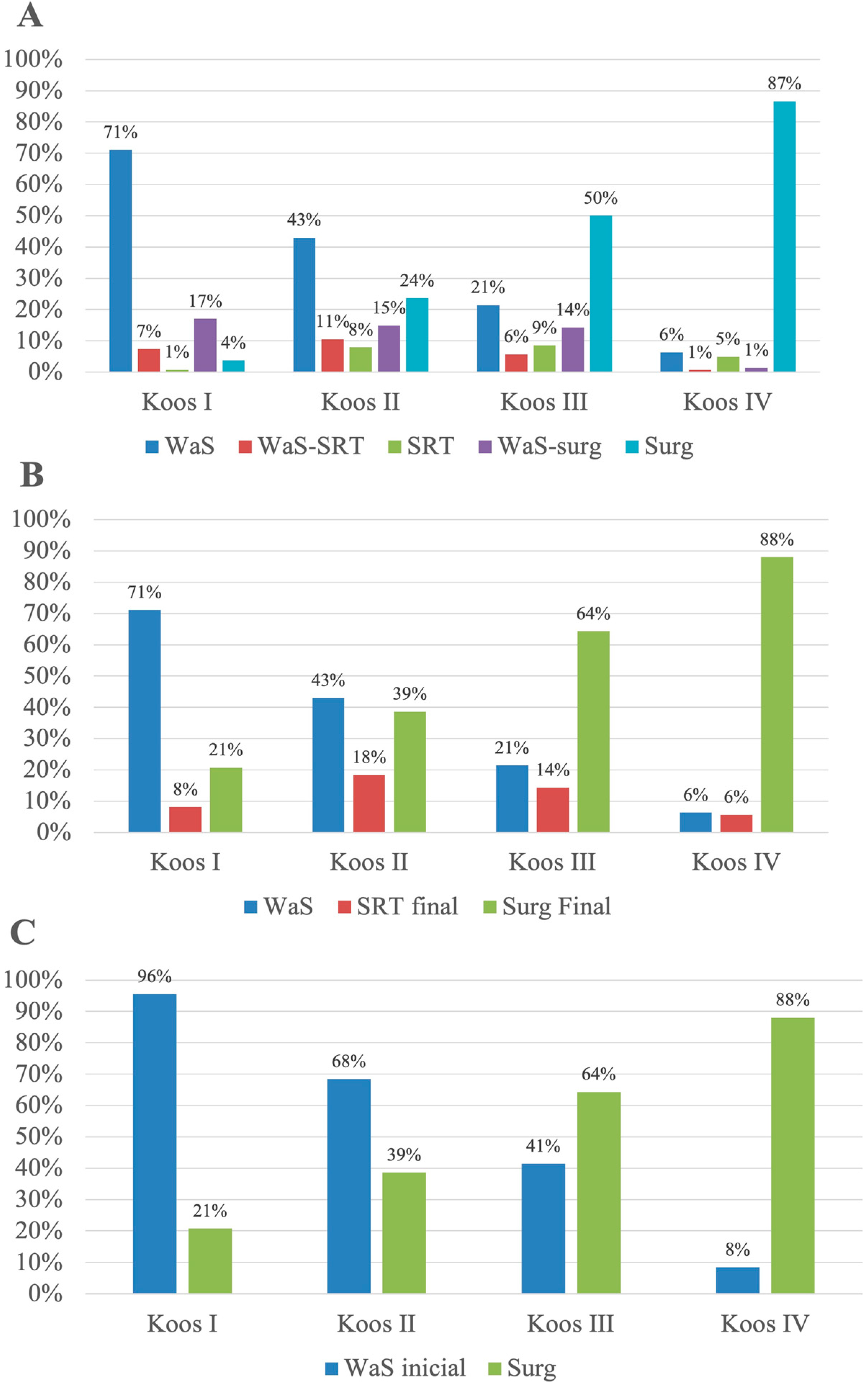
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reddy, C.E.; Lewis-Jones, H.G.; Javadpour, M.; Ryland, I.; Lesser, T.H. Conservative management of vestibular schwannomas of 15 to 31 mm intracranial diameter. J. Laryngol. Otol. 2014, 128, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Fik, Z.; Lazak, J.; Hruba, S.; Cada, Z.; Zverrna, E.; Betka, J. Hearing improvement after vestibular schwannoma surgery in the era of the hearing preservation rule—Case report and literature review. Biomed. Pap. 2022, 166, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Waterval, J.; Kania, R.; Somers, T. EAONO Position Statement on Vestibular Schwannoma: Imaging Assessment. What are the Indications for Performing a Screening MRI Scan for a Potential Vestibular Schwannoma? J. Int. Adv. Otol. 2018, 14, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, J.P.; Lohse, C.M.; Grossardt, B.R.; Lane, J.I.; Carlson, M.L. Rising Incidence of Sporadic Vestibular Schwannoma: True Biological Shift Versus Simply Greater Detection. Otol. Neurotol. 2020, 41, 813–847. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, J.P.; Nassiri, A.M.; Habermann, E.B.; Lohse, C.M.; Holton, S.J.; Carlson, M.L. Underreporting of Vestibular Schwannoma Incidence Within National Brain Tumor and Cancer Registries in the United States. Otol. Neurotol. 2021, 42, e758–e763. [Google Scholar] [CrossRef] [PubMed]
- Goldbrunner, R.; Weller, M.; Regis, J.; Lund-Johansen, M.; Stavrinou, P.; Reuss, D.; Evans, D.G.; Lefranc, F.; Sallabanda, K.; Falini, A.; et al. EANO guideline on the diagnosis and treatment of vestibular schwannoma. Neuro-Oncology 2020, 22, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Caye-Thomasen, P.; Stangerup, S.-E. Spontaneous tumour shrinkage in 1261 observed patients with sporadic vestibular schwannoma. J. Laryngol. Otol. 2013, 127, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Thielhelm, T.P.; Goncalves, S.; Welford, S.M.; Mellon, E.A.; Cohen, E.R.; Nourbakhsh, A.; Fernandez-Valle, C.; Telischi, F.; Ivan, M.E.; Dinh, C.T. Understanding the Radiobiology of Vestibular Schwannomas to Overcome Radiation Resistance. Cancers 2021, 13, 4575. [Google Scholar] [CrossRef] [PubMed]
- Betka, J.; Zverina, E.; Lisy, J.; Chovanec, M.; Kluh, J.; Kraus, J. Vestibulární schwannom [Vestibular schwannoma]. Otorinolaryng Foniat 2008, 57, 221–225. [Google Scholar]
- Roehm, P.C.; Gantz, B.J. Management of Acoustic Neuromas in Patients 65 Years or Older. Otol. Neurotol. 2007, 28, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, J.; Tos, M.; Sanna, M.; Moffat, D.A.; Monsell, E.M.; Berliner, K.I. New and Modified Reporting Systems from the Consensus Meeting on Systems for Reporting Results in Vestibular Schwannoma. Otol. Neurotol. 2003, 24, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Thakker, A.; Gupta, K.K. Vestibular Schwannoma: What We Know and Where We are Heading. Head Neck Pathol. 2020, 14, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- D’Haese, S.; Parmentier, H.; Keppler, H.; Van Vooren, S.; Van Driessche, V.; Bauters, W.; Van Roost, D.; Dhooge, I. Vestibular schwannoma: Natural growth and possible predictive factors. Acta Oto-Laryngol. 2019, 139, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.; Skilbeck, C.; Saeed, S.; Bradford, R. Expectant Management of Vestibular Schwannoma: A Retrospective Multivariate Analysis of Tumor Growth and Outcome. Skull Base 2011, 21, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Kleijwegt, M.; Bettink, F.; Malessy, M.; Putter, H.; van der Mey, A. Clinical Predictors Leading to Change of Initial Conservative Treatment of 836 Vestibular Schwannomas. J. Neurol. Surg. Part B Skull Base 2020, 81, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Reznitsky, M.; Petersen, M.; West, N.; Stangerup, S.-E.; Cayé-Thomasen, P. The natural history of vestibular schwannoma growth—Prospective 40-year data from an unselected national cohort. Neuro-Oncology 2021, 23, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Sethi, M.; Borsetto, D.; Cho, Y.; Gair, J.; Gamazo, N.; Jefferies, S.; Joannides, A.; Mannion, R.; Helmy, A.; Axon, P.; et al. The Conditional Probability of Vestibular Schwannoma Growth at Different Time Points After Initial Stability on an Observational Protocol. Otol. Neurotol. 2020, 41, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, M.A.; Hannink, G.; Steens, S.C.A.; Mulder, J.J.S.; Rovers, M.M.; Kunst, H.P.M. Development of a model to predict vestibular schwannoma growth: An opportunity to introduce new wait and scan strategies. Clin. Otolaryngol. 2021, 46, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Borsetto, D.; Gair, J.; Kenyon, O.; Das, T.; Donnelly, N.; Axon, P.; Macfarlane, R.; Mannion, R.; Scoffings, D.; Bance, M.; et al. When Should We Stop Scanning Older Patients with Vestibular Schwannomas? J. Neurol. Surg. Part B Skull Base 2019, 80, 333–337. [Google Scholar] [CrossRef] [PubMed]
| Koos | n | INT | n |
|---|---|---|---|
| I | 137 | 0 | 137 |
| II | 114 | 1 | 103 |
| III | 70 | 2 | 98 |
| IV | 142 | 3 | 58 |
| 4 | 31 | ||
| 5 | 9 | ||
| NA | 27 |
| Modality | WaS–STR | STR | WaS–Surgery | Surgery | WaS | |
|---|---|---|---|---|---|---|
| Age | 60.2 | 62.8 | 47.8 | 48.3 | 59.4 | |
| WaS–STR | 60.2 | >0.05 | <0.001 | <0.001 | >0.05 | |
| STR | 62.8 | >0.05 | <0.001 | <0.001 | >0.05 | |
| WaS–surgery | 47.8 | <0.001 | <0.001 | >0.05 | <0.001 | |
| Surgery | 48.3 | <0.001 | <0.001 | >0.05 | <0.001 | |
| WaS | 59.4 | >0.05 | >0.05 | <0.001 | <0.001 |
| Modality | WaS–STR | STR | WaS–Surgery | Surgery | WaS | |
|---|---|---|---|---|---|---|
| Tumor size (mm) | 12.6 | 18.1 | 12.0 | 24.6 | 11.0 | |
| WaS–STR | 12.6 | >0.05 | >0.05 | <0.001 | >0.05 | |
| STR | 18.1 | >0.05 | <0.05 | <0.01 | <0.05 | |
| WaS–surgery | 12.0 | >0.05 | <0.05 | <0.001 | >0.05 | |
| Surgery | 24.6 | <0.001 | <0.01 | <0.001 | <0.001 | |
| WaS | 11.0 | >0.05 | <0.05 | >0.05 | <0.001 |
| Age | Gender | Initial Size | ||||
|---|---|---|---|---|---|---|
| 0–59 | 60+ | F | M | 0–10 mm | 11+ mm | |
| Average growth (mm) | 1.3 | 0.90 | 1.1 | 1.1 | 0.98 | 1.2 |
| % of growing | 36% | 26% | 32% | 29% | 25% | 35% |
| Average growth (mm) | 3.7 | 3.5 | 3.4 | 3.8 | 4.0 | 3.4 |
| 0–59 | 60+ | |
|---|---|---|
| Koos 1 | 27% | 34% |
| Koos 2 | 25% | 24% |
| Koos 3 | 14% | 17% |
| Koos 4 | 35% | 23% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fík, Z.; Vlasák, A.; Zvěřina, E.; Sýba, J.; Lazák, J.; Peterková, L.; Koucký, V.; Betka, J. Which Epidemiological Characteristics Drive Decision Making in the Management of Patients with Vestibular Schwannoma? Biomedicines 2023, 11, 340. https://doi.org/10.3390/biomedicines11020340
Fík Z, Vlasák A, Zvěřina E, Sýba J, Lazák J, Peterková L, Koucký V, Betka J. Which Epidemiological Characteristics Drive Decision Making in the Management of Patients with Vestibular Schwannoma? Biomedicines. 2023; 11(2):340. https://doi.org/10.3390/biomedicines11020340
Chicago/Turabian StyleFík, Zdeněk, Aleš Vlasák, Eduard Zvěřina, Jaroslav Sýba, Jan Lazák, Lenka Peterková, Vladimír Koucký, and Jan Betka. 2023. "Which Epidemiological Characteristics Drive Decision Making in the Management of Patients with Vestibular Schwannoma?" Biomedicines 11, no. 2: 340. https://doi.org/10.3390/biomedicines11020340
APA StyleFík, Z., Vlasák, A., Zvěřina, E., Sýba, J., Lazák, J., Peterková, L., Koucký, V., & Betka, J. (2023). Which Epidemiological Characteristics Drive Decision Making in the Management of Patients with Vestibular Schwannoma? Biomedicines, 11(2), 340. https://doi.org/10.3390/biomedicines11020340






