Contralateral Selectivity of Upper-Limb Motor Pools via Targeted Stimulation of the Cervical Spinal Cord
Abstract
1. Introduction
2. Materials and Methods
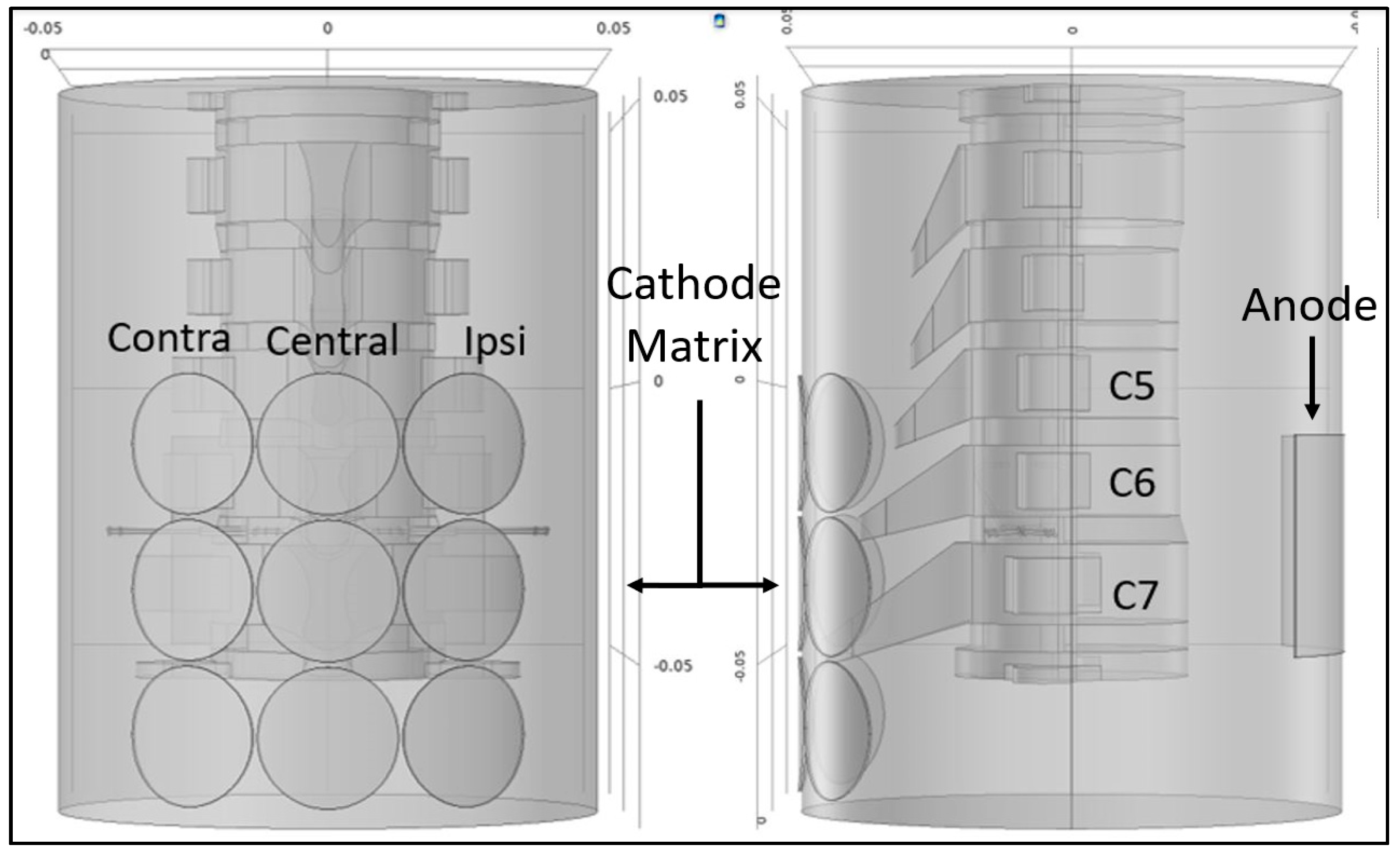
2.1. Study Design
2.2. Protocol
2.3. Electromyography (EMG)
2.4. Data Processing
2.5. Statistical Analysis
3. Results
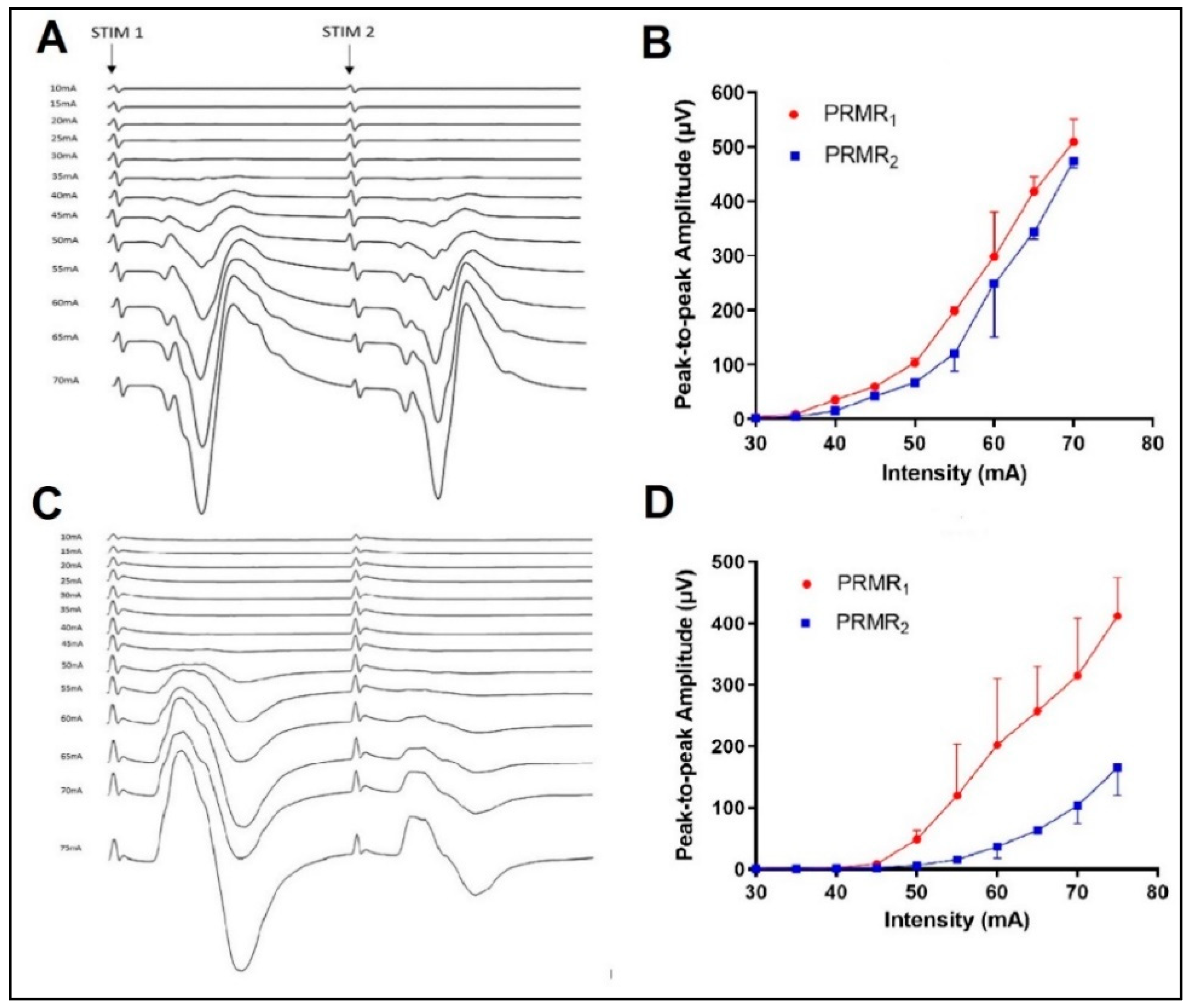
3.1. Test–Retest Reliability of Stimulus Characteristics
3.2. Effects of Spinal Level on PRMR1
3.3. Effects of Stimulus Intensity and Lateral Location on PRMR1
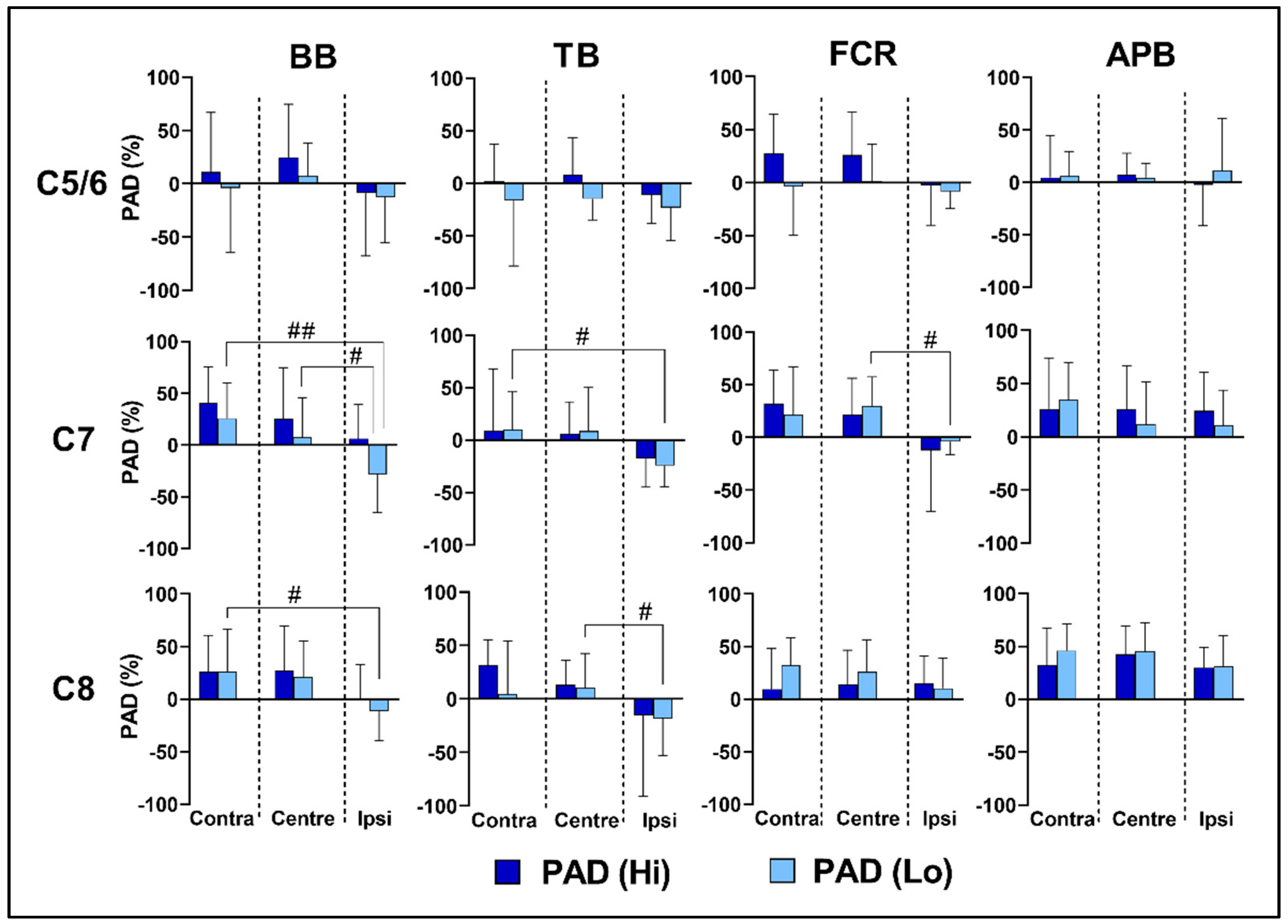
3.4. Effects of Spinal Level on Reflex Transmission
3.5. Effects of Lateral Location and Intensity on Reflex Transmission
3.6. Tolerability and Cardiovascular Response
4. Discussion
4.1. Selectivity of Upper-Limb Motor Pools
4.2. Reflex Transmission of tSCS
4.3. Effects of Intensity on PRMR1 and PAD
4.4. Test–Retest Reliability of Stimulation Indices
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wagner, F.B.; Mignardot, J.B.; Goff-Mignardot, L.; Camille, G.; Demesmaeker, R.; Komi, S.; Capogrosso, M.; Rowald, A.; Seáñez, I.; Caban, M.; et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018, 563, 65–71. [Google Scholar] [CrossRef]
- Sayenko, D.G.; Rath, M.; Ferguson, A.R.; Burdick, J.W.; Havton, L.A.; Edgerton, V.R.; Gerasimenko, Y.P. Self-Assisted Standing Enabled by Non-Invasive Spinal Stimulation after Spinal Cord Injury. J. Neurotrauma 2019, 36, 1435–1450. [Google Scholar] [CrossRef]
- Keller, A.; Singh, G.; Sommerfeld, J.H.; King, M.; Parikh, P.; Ugiliweneza, B.; D’Amico, J.; Gerasimenko, Y.; Behrman, A.L. Noninvasive spinal stimulation safely enables upright posture in children with spinal cord injury. Nat. Commun. 2021, 12, 5850. [Google Scholar] [CrossRef] [PubMed]
- Freyvert, Y.; Yong, N.A.; Morikawa, E.; Zdunowski, S.; Sarino, M.E.; Gerasimenko, Y.; Edgerton, V.R.; Lu, D.C. Engaging cervical spinal circuitry with non-invasive spinal stimulation and buspirone to restore hand function in chronic motor complete patients. Sci. Rep. 2018, 8, 15546. [Google Scholar] [CrossRef]
- Farina, D.; Jensen, W.; Akay, M. Introduction to Neural Engineering for Motor Rehabilitation; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 40. [Google Scholar]
- Gad, P.; Lee, S.; Terrafranca, N.; Zhong, H.; Turner, A.; Gerasimenko, Y.; Edgerton, V.R. Non-Invasive Activation of Cervical Spinal Networks after Severe Paralysis. J. Neurotrauma 2018, 35, 2145–2158. [Google Scholar] [CrossRef]
- Zhang, F.; Momeni, K.; Ramanujam, A.; Ravi, M.; Carnahan, J.; Kirshblum, S.; Forrest, G.F. Cervical Spinal Cord Transcutaneous Stimulation Improves Upper Extremity and Hand Function in People with Complete Tetraplegia: A Case Study. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 3167–3174. [Google Scholar] [CrossRef]
- Inanici, F.; Brighton, L.N.; Samejima, S.; Hofstetter, C.P.; Moritz, C.T. Transcutaneous Spinal Cord Stimulation Restores Hand and Arm Function after Spinal Cord Injury. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 310–319. [Google Scholar] [CrossRef]
- Danner, S.M.; Hofstoetter, U.S.; Ladenbauer, J.; Rattay, F.; Minassian, K. Can the human lumbar posterior columns be stimulated by transcutaneous spinal cord stimulation? A modeling study. Artif. Organs 2011, 35, 257–262. [Google Scholar] [CrossRef]
- Courtine, G.; Harkema, S.J.; Dy, C.J.; Gerasimenko, Y.P.; Dyhre-Poulsen, P. Modulation of multisegmental monosynaptic responses in a variety of leg muscles during walking and running in humans. J. Physiol. 2007, 582, 1125–1139. [Google Scholar] [CrossRef] [PubMed]
- Minassian, K.; Persy, I.; Rattay, F.; Dimitrijevic, M.R.; Hofer, C.; Kern, H. Posterior root-muscle reflexes elicited by transcutaneous stimulation of the human lumbosacral cord. Muscle Nerve 2007, 35, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Minassian, K.; Hofstoetter, U.S. Spinal Cord Stimulation and Augmentative Control Strategies for Leg Movement after Spinal Paralysis in Humans. CNS Neurosci. Ther. 2016, 22, 262–270. [Google Scholar] [CrossRef]
- Wu, Y.K.; Levine, J.M.; Wecht, J.R.; Maher, M.T.; LiMonta, J.M.; Saeed, S.; Santiago, T.M.; Bailey, E.; Kastuar, S.; Guber, K.S.; et al. Posteroanterior cervical transcutaneous spinal stimulation targets ventral and dorsal nerve roots. Clin. Neurophysiol. 2020, 131, 451–460. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, R.M.; Sasaki, A.; Sayenko, D.G.; Masugi, Y.; Nomura, T.; Nakazawa, K.; Milosevic, M. Selectivity and excitability of upper-limb muscle activation during cervical transcutaneous spinal cord stimulation in humans. J. Appl. Physiol. 2021, 131, 746–759. [Google Scholar] [CrossRef]
- Sayenko, D.G.; Atkinson, D.A.; Dy, C.J.; Gurley, K.M.; Smith, V.L.; Angeli, C.; Harkema, S.J.; Edgerton, V.R.; Gerasimenko, Y.P. Spinal segment-specific transcutaneous stimulation differentially shapes activation pattern among motor pools in humans. J. Appl. Physiol. 2015, 118, 1364–1374. [Google Scholar] [CrossRef]
- Calvert, J.S.; Manson, G.A.; Grahn, P.J.; Sayenko, D.G. Preferential activation of spinal sensorimotor networks via lateralized transcutaneous spinal stimulation in neurologically intact humans. J. Neurophysiol. 2019, 122, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, M.; Masugi, Y.; Sasaki, A.; Sayenko, D.G.; Nakazawa, K. On the reflex mechanisms of cervical transcutaneous spinal cord stimulation in human subjects. J. Neurophysiol. 2019, 121, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63 (Suppl. S11), S240–S252. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Mathur, S.; Eng, J.J.; MacIntyre, D.L. Reliability of surface EMG during sustained contractions of the quadriceps. J. Electromyogr. Kinesiol. 2005, 15, 102–110. [Google Scholar] [CrossRef]
- Formento, E.; Minassian, K.; Wagner, F.; Mignardot, J.B.; Le Goff-Mignardot, C.G.; Rowald, A.; Bloch, J.; Micera, S.; Capogrosso, M.; Courtine, G. Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nat. Neurosci. 2018, 21, 1728–1741. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Steele, A.G.; Varghese, B.; Martin, C.A.; Scheffler, M.S.; Markley, R.L.; Lo, Y.-K.; Sayenko, D.G. Cervical transcutaneous spinal stimulation for spinal motor mapping. iScience 2022, 25, 105037. [Google Scholar] [CrossRef]
- Hernández-Labrado, G.R.; Polo, J.L.; López-Dolado, E.; Collazos-Castro, J.E. Spinal cord direct current stimulation: Finite element analysis of the electric field and current density. Med. Biol. Eng. Comput. 2011, 49, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Mendez, A.; Islam, R.; Latypov, T.; Basa, P.; Joseph, O.J.; Knudsen, B.; Siddiqui, A.M.; Summer, P.; Staehnke, L.J.; Grahn, P.J.; et al. Segment-Specific Orientation of the Dorsal and Ventral Roots for Precise Therapeutic Targeting of Human Spinal Cord. Mayo Clin. Proc. 2021, 96, 1426–1437. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Freundl, B.; Binder, H.; Minassian, K. Recovery cycles of posterior root-muscle reflexes evoked by transcutaneous spinal cord stimulation and of the H reflex in individuals with intact and injured spinal cord. PLoS ONE 2019, 14, e0227057. [Google Scholar] [CrossRef] [PubMed]
- Cowan, J.M.; Day, B.L.; Marsden, C.; Rothwell, J.C. The effect of percutaneous motor cortex stimulation on H reflexes in muscles of the arm and leg in intact man. J. Physiol. 1986, 377, 333–347. [Google Scholar] [CrossRef]
- Ziemann, U.; Ilić, T.V.; Alle, H.; Meintzschel, F. Estimated magnitude and interactions of cortico-motoneuronal and Ia afferent input to spinal motoneurones of the human hand. Neurosci. Lett. 2004, 364, 48–52. [Google Scholar] [CrossRef]
- Brouwer, B.; Ashby, P. Corticospinal projections to lower limb motoneurons in man. Exp. Brain Res. 1992, 89, 649–654. [Google Scholar] [CrossRef]
- Roy, F.D.; Bosgra, D.; Stein, R.B. Interaction of transcutaneous spinal stimulation and transcranial magnetic stimulation in human leg muscles. Exp. Brain Res. 2014, 232, 1717–1728. [Google Scholar] [CrossRef] [PubMed]
- Wecht, J.R.; Savage, W.M.; Famodimu, G.O.; Mendez, G.A.; Levine, J.M.; Maher, M.T.; Weir, J.P.; Wecht, J.M.; Carmel, J.B.; Wu, Y.K.; et al. Posteroanterior Cervical Transcutaneous Spinal Cord Stimulation: Interactions with Cortical and Peripheral Nerve Stimulation. J. Clin. Med. 2021, 10, 5304. [Google Scholar] [CrossRef] [PubMed]
- Péréon, Y.; Guihéneuc, P. Late facilitations of motor evoked potentials by contralateral mixed nerve stimulation. Electroencephalogr. Clin. Neurophysiol. 1995, 97, 126–130. [Google Scholar] [CrossRef]

| RMT (mA) | Visit 1 | Visit 2 | ICC | TEM | MDC95 | ±95% LOAs |
|---|---|---|---|---|---|---|
| BB | 42 (6) | 42 (10) | 0.72 | 4 | 11 | −12/+12 |
| TB | 40 (8) | 41 (9) | 0.70 | 5 | 14 | −16/16 |
| FCR | 45 (10) | 45 (9) | 0.63 | 6 | 17 | −14/13 |
| APB | 39 (8) | 41 (10) | 0.77 | 4 | 11 | −14/10 |
| PADmax (%) | ||||||
| BB | 80 (10) | 73 (21) | 0.24 | 11 | 30 | −32/46 |
| TB | 60 (14) | 50 (30) | 0.10 | 22 | 61 | −52/71 |
| FCR | 61 (16) | 57 (25) | 0.23 | 18 | 50 | −47/56 |
| APB | 50 (23) | 61 (17) | 0.48 | 15 | 42 | −48/26 |
| XD (mA) | ||||||
| BB | 54 (11) | 51 (8) | 0.61 | 6 | 17 | −13/19 |
| TB | 48 (10) | 49 (11) | 0.78 | 5 | 14 | −14/13 |
| FCR | 50 (12) | 48 (10) | 0.59 | 7 | 19 | −17/22 |
| APB | 49 (14) | 47 (17) | 0.61 | 9 | 25 | −25/29 |
| PRMR1 | PAD | |||||||
|---|---|---|---|---|---|---|---|---|
| BB | TB | FCR | APB | BB | TB | FCR | APB | |
| Lateral Effect | F = 4.9 p = 0.012 η2 = 0.29 | - | F = 4.9 p = 0.035 η2 = 0.28 | F = 8.9 p = 0.001 η2 = 0.43 | F = 9.3 p = 0.001 η2 = 0.44 | F = 5.4 p = 0.012 η2 = 0.31 | F = 17.4 p = 0.000 η2 = 0.59 | - |
| Spinal Level | F = 39.7 p = 0.000 η2 = 0.77 | F = 43.4 p = 0.000 η2 = 0.79 | F = 39.8 p = 0.000 η2 = 0.77 | F = 107.0; p = 0.000 η2 = 0.90 | - | - | - | F = 7.7 p = 0.003 η2 = 0.39 |
| Intensity | F = 65.6 p = 0.000 η2 = 0.85 | F = 44.9 p = 0.000 η2 = 0.79 | F = 65.6 p = 0.000 η2 = 0.85 | F = 145.6 p = 0.000 η2 = 0.92 | F = 6.5 p = 0.026 η2 = 0.35 | F = 22.6 p = 0.000 η2 = 0.65 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fleming, N.; Taylor, C.; Etzelmueller, M.; Gill, C.; O'Keeffe, C.; Mahony, N.; Reilly, R.B. Contralateral Selectivity of Upper-Limb Motor Pools via Targeted Stimulation of the Cervical Spinal Cord. Biomedicines 2023, 11, 332. https://doi.org/10.3390/biomedicines11020332
Fleming N, Taylor C, Etzelmueller M, Gill C, O'Keeffe C, Mahony N, Reilly RB. Contralateral Selectivity of Upper-Limb Motor Pools via Targeted Stimulation of the Cervical Spinal Cord. Biomedicines. 2023; 11(2):332. https://doi.org/10.3390/biomedicines11020332
Chicago/Turabian StyleFleming, Neil, Clare Taylor, Mark Etzelmueller, Conor Gill, Clodagh O'Keeffe, Nicholas Mahony, and Richard B. Reilly. 2023. "Contralateral Selectivity of Upper-Limb Motor Pools via Targeted Stimulation of the Cervical Spinal Cord" Biomedicines 11, no. 2: 332. https://doi.org/10.3390/biomedicines11020332
APA StyleFleming, N., Taylor, C., Etzelmueller, M., Gill, C., O'Keeffe, C., Mahony, N., & Reilly, R. B. (2023). Contralateral Selectivity of Upper-Limb Motor Pools via Targeted Stimulation of the Cervical Spinal Cord. Biomedicines, 11(2), 332. https://doi.org/10.3390/biomedicines11020332







