Analysis of PD-L1 and CD3 Expression in Glioblastoma Patients and Correlation with Outcome: A Single Center Report
Abstract
1. Introduction
2. Patients and Materials
2.1. Cremona Retrospective Cohort
2.2. Immunohistochemical Staining
2.3. Evaluation of Immunohistochemistry
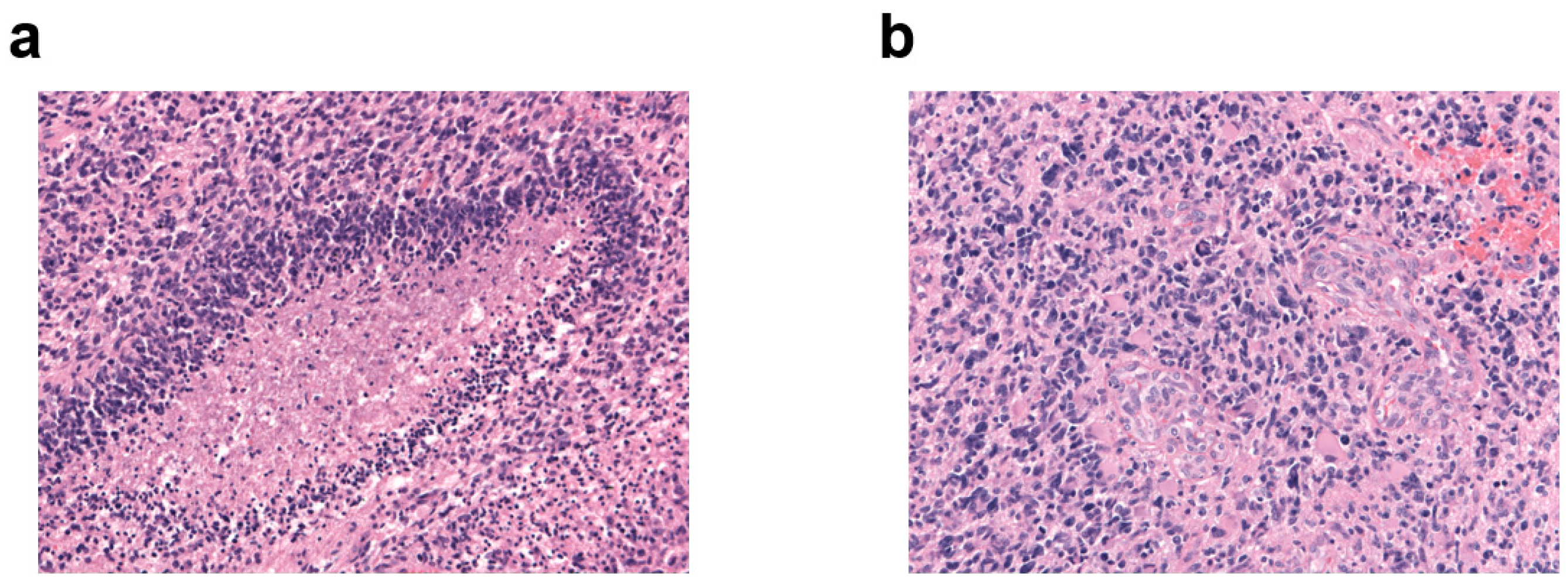
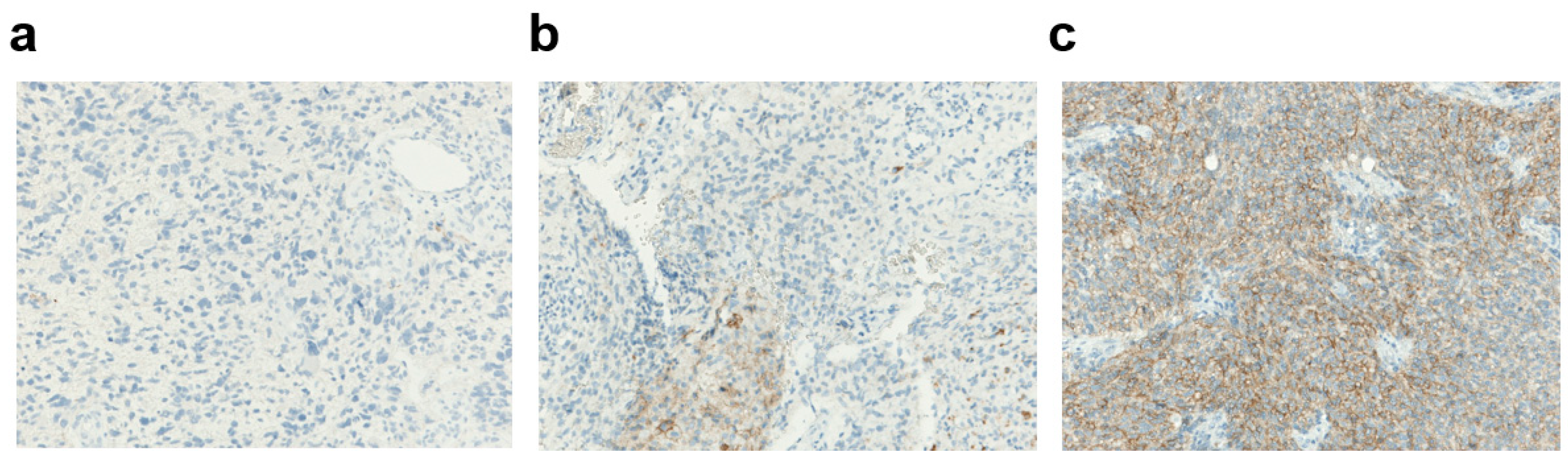
2.3.1. PD-L1 Expression
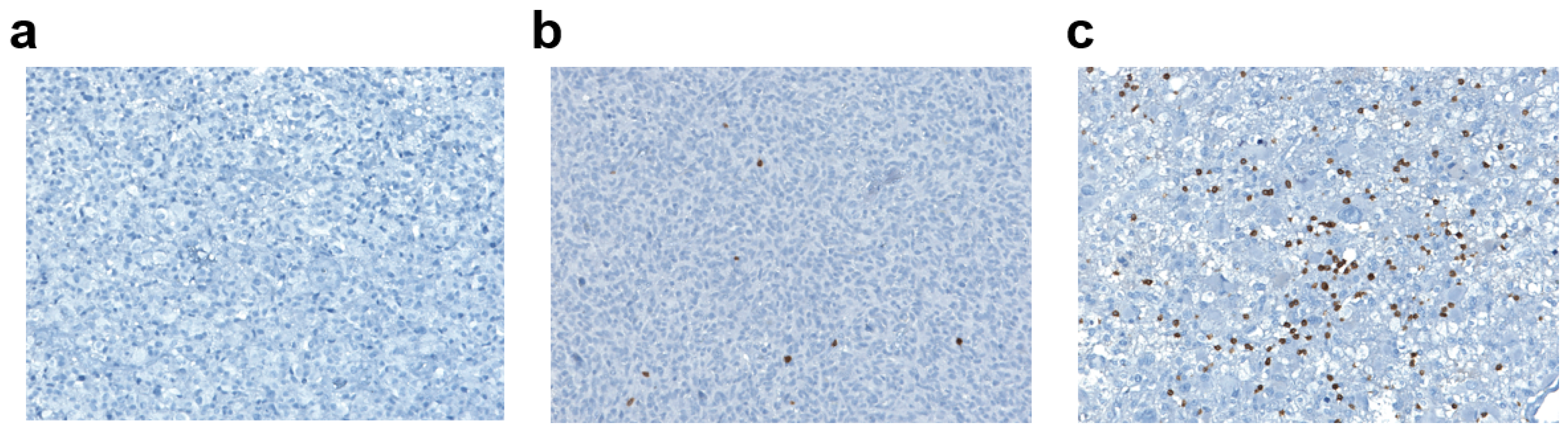
2.3.2. Tumor-Infiltrating Lymphocyte Density
2.4. MGMT and MIB-1
2.5. p53
2.6. Statistical Analysis
3. Results
3.1. Cremona Retrospective Cohort
3.1.1. Patients
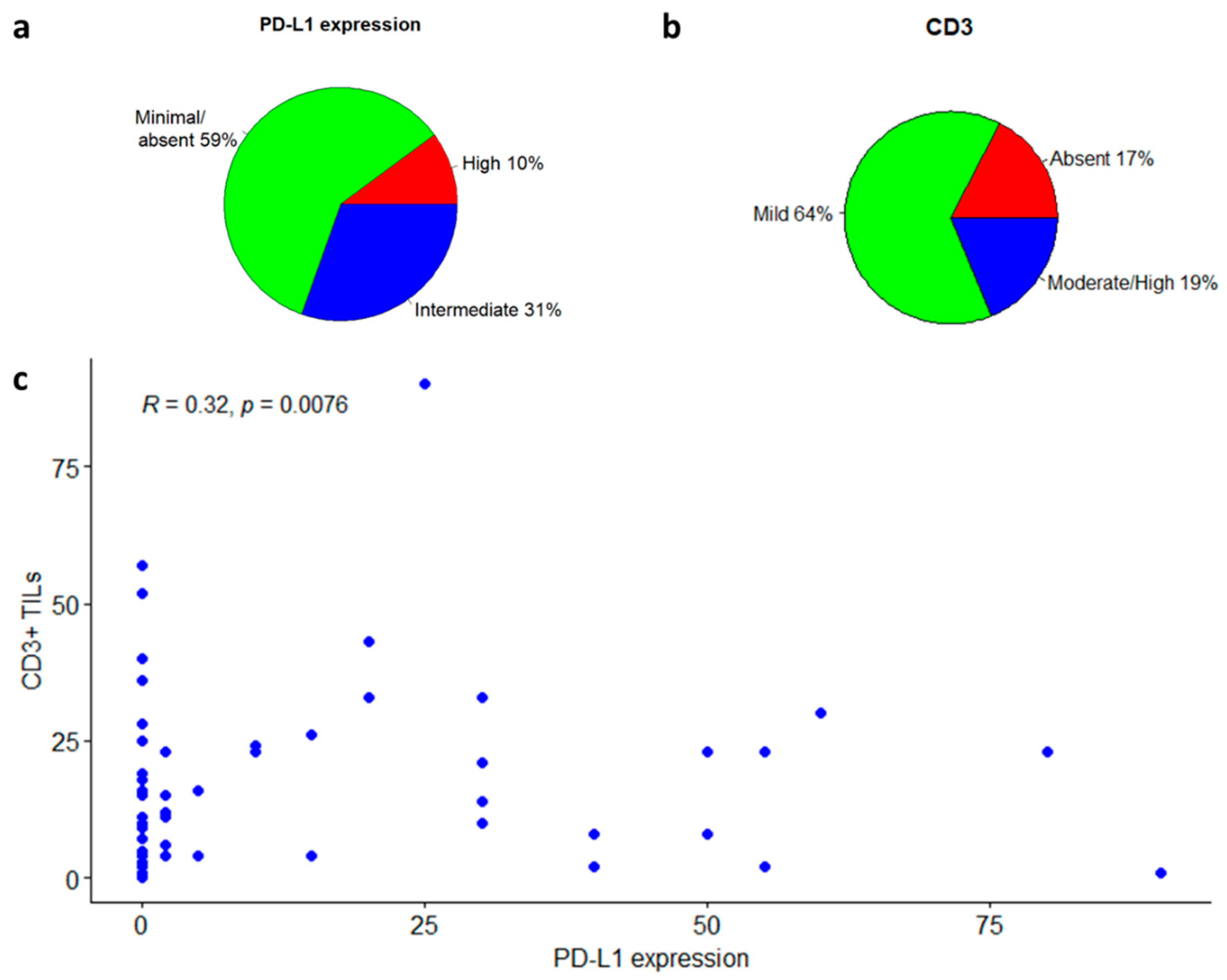
3.1.2. PD-L1 Expression
3.1.3. Tumor-Infiltrating Lymphocytes
3.2. Survival Analyses
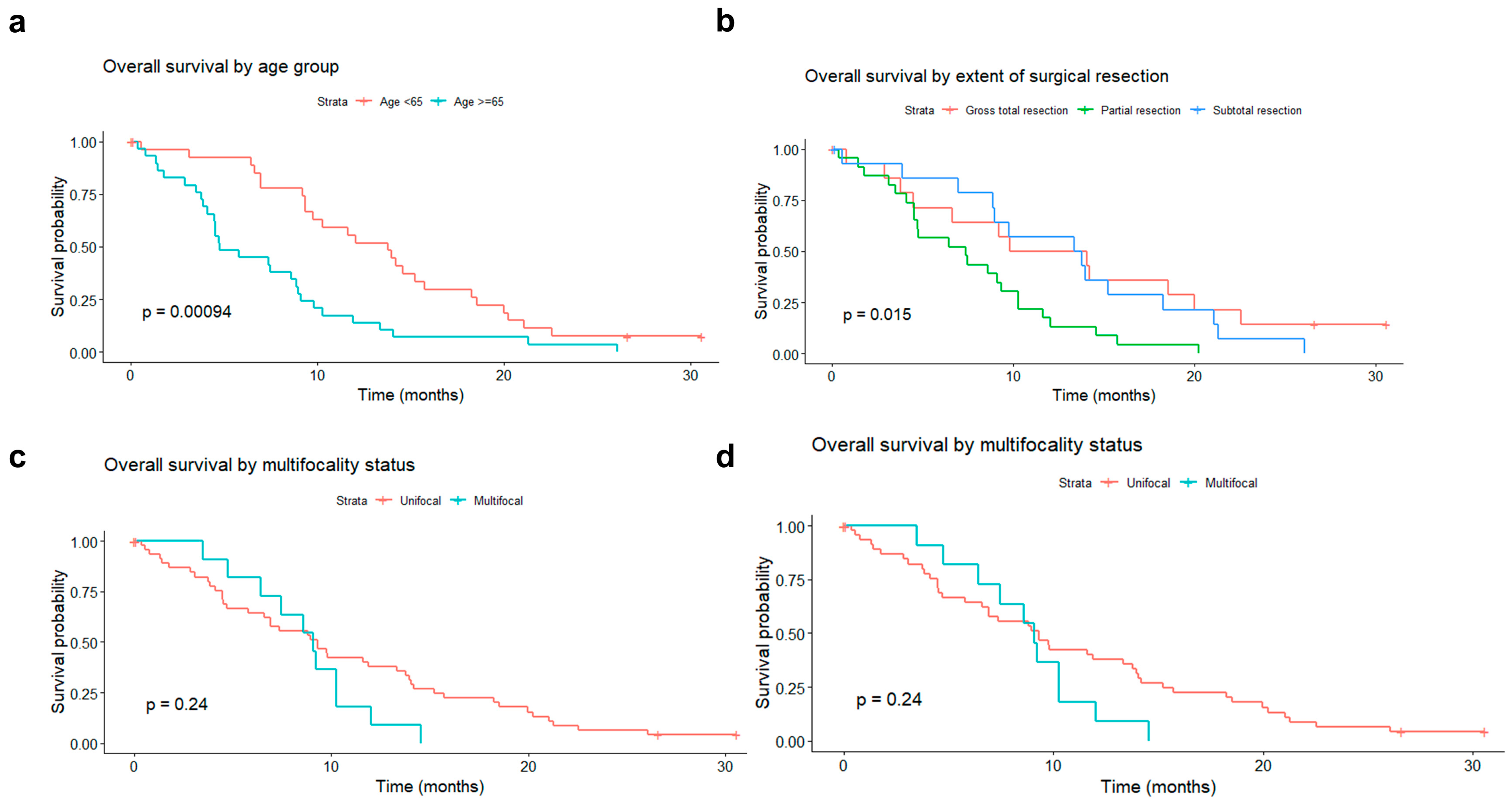
3.2.1. Association of Patient and Tumor Features with Survival
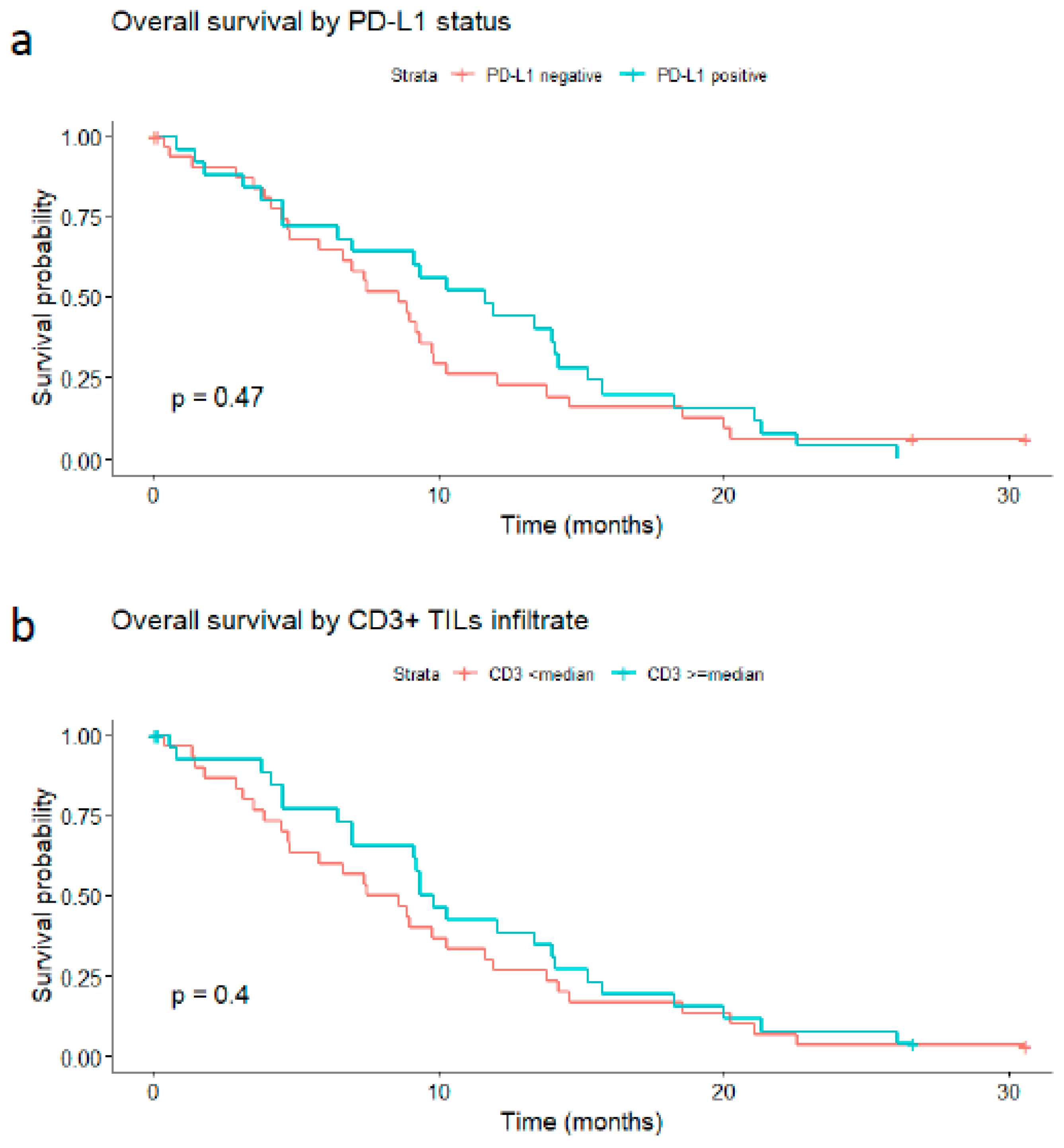
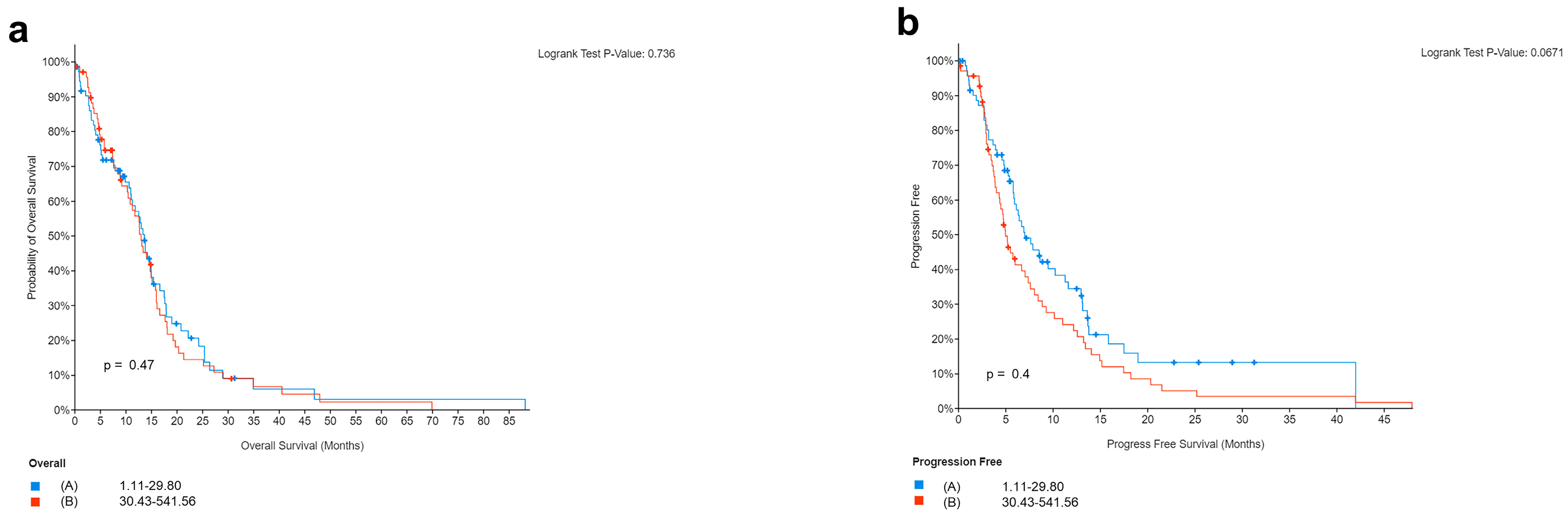
3.2.2. Survival Analyses in Relation to PD-L1 or CD3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tejaswi Kanderi, V.G. Glioblastoma Multiforme. PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/32644380/ (accessed on 1 December 2022).
- Ma, R.; Taphoorn, M.J.B.; Plaha, P. Advances in the management of glioblastoma. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Saran, F.; Chinot, O.L.; Henriksson, R.; Mason, W.; Wick, W.; Cloughesy, T.; Dhar, S.; Pozzi, E.; Garcia, J.; Nishikawa, R. Bevacizumab, temozolomide, and radiotherapy for newly diagnosed glioblastoma: Comprehensive safety results during and after first-line therapy. Neuro. Oncol. 2016, 18, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Kukreja, L.; Li, C.J.; Ezhilan, S.; Iyer, V.R.; Kuo, J.S. Emerging Epigenetic Therapies for Brain Tumors. Neuromolecular Med. 2022, 24, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Tang, L.; Li, X.; Fan, F.; Liu, Z. Immunotherapy for glioma: Current management and future application. Cancer Lett. 2020, 476, 1–12. [Google Scholar] [CrossRef]
- Chuntova, P.; Chow, F.; Watchmaker, P.B.; Galvez, M.; Heimberger, A.B.; Newell, E.W.; Diaz, A.; Depinho, R.A.; Li, M.O.; Wherry, E.J.; et al. Unique challenges for glioblastoma immunotherapy-discussions across neuro-oncology and non-neuro-oncology experts in cancer immunology. Meeting Report from the 2019 SNO Immuno-Oncology Think Tank. Neuro. Oncol. 2021, 23, 356–375. [Google Scholar] [CrossRef]
- Jackson, C.M.; Choi, J.; Lim, M. Mechanisms of immunotherapy resistance: Lessons from glioblastoma. Nat. Immunol. 2019, 20, 1100–1109. [Google Scholar] [CrossRef]
- Nduom, E.K.; Wei, J.; Yaghi, N.K.; Huang, N.; Kong, L.Y.; Gabrusiewicz, K.; Ling, X.; Zhou, S.; Ivan, C.; Chen, J.Q.; et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro. Oncol. 2016, 18, 195–205. [Google Scholar] [CrossRef]
- Mcdermott, D.F.; Atkins, M.B. PD-1 as a potential target in cancer therapy. Cancer Med. 2013, 2, 662. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA. Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Taylor, O.G.; Brzozowski, J.S.; Skelding, K.A. Glioblastoma multiforme: An overview of emerging therapeutic targets. Front. Oncol. 2019, 9, 963. [Google Scholar] [CrossRef]
- McGrail, D.J.; Pilié, P.G.; Rashid, N.U.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.B.; Lim, B.; et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Rotte, A.; Sahasranaman, S.; Budha, N. Targeting TIGIT for Immunotherapy of Cancer: Update on Clinical Development. Biomedicines 2021, 9, 1277. [Google Scholar] [CrossRef] [PubMed]
- Twomey, J.D.; Zhang, B. Cancer Immunotherapy Update: FDA-Approved Checkpoint Inhibitors and Companion Diagnostics. AAPS J. 2021, 23, 39. [Google Scholar] [CrossRef]
- McGranahan, T.; Therkelsen, K.E.; Ahmad, S.; Nagpal, S. Current State of Immunotherapy for Treatment of Glioblastoma. Curr. Treat. Options Oncol. 2019, 20, 24. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727. [Google Scholar] [PubMed]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: Mechanisms of action, efficacy, and limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Swain, C.A.; Shevde, L.A. Informing the new developments and future of cancer immunotherapy: Future of cancer immunotherapy. Cancer Metastasis Rev. 2021, 40, 549–562. [Google Scholar] [CrossRef]
- Majd, N.K.; Dasgupta, P.R.; de Groot, J.F. Immunotherapy for Neuro-oncology. Adv. Exp. Med. Biol. 2021, 1342, 233–258. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, Y.; Ren, X.; Wan, D. Prognostic value of programmed death ligand 1 (PD-L1) in glioblastoma: A systematic review, meta-analysis and validation based on dataset. Bioengineered 2021, 12, 10366–10378. [Google Scholar] [CrossRef]
- El Samman, D.M.; El Mahdy, M.M.; Cousha, H.S.; El Rahman Kamar, Z.A.; Mohamed, K.A.K.; Gabal, H.H.A. Immunohistochemical expression of programmed death-ligand 1 and CD8 in glioblastomas. J. Pathol. Transl. Med. 2021, 55, 388–397. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Kiesel, B.; Widhalm, G.; Rajky, O.; Ricken, G.; Wohrer, A.; Dieckmann, K.; Filipits, M.; Brandstetter, A.; Weller, M.; et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro. Oncol. 2015, 17, 1064–1075. [Google Scholar] [CrossRef]
- Sener Dede, D.; Tatli Dogan, H.; Asan, F.B.; Gumuskaya Ocal, B.; Ayik, S.; Sendur, M.A.N.; Ulas, A.; Bilgin, B.; Hizal, M.; Akinci, M.B.; et al. The association of programmed death-ligand 1 (PD-L1), programmed cell death (PD-1), tumor infiltrating lymphocytes(TILs) and isocitrate dehydrogenase (IDH-1) mutation in glioblastome multiforme(GBM). Ann. Oncol. 2017, 28, v110. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef]
- Dahlin, A.M.; Henriksson, M.L.; Van Guelpen, B.; Stenling, R.; Öberg, Å.; Rutegrd, J.; Palmqvist, R. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod. Pathol. 2011, 24, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Generali, D.; Fox, S.B.; Berruti, A.; Brizzi, M.P.; Campo, L.; Bonardi, S.; Wigfield, S.M.; Bruzzi, P.; Bersiga, A.; Allevi, G.; et al. Role of carbonic anhydrase IX expression in prediction of the efficacy and outcome of primary epirubicin/tamoxifen therapy for breast cancer. Endocr. Relat. Cancer 2006, 13, 921–930. [Google Scholar] [CrossRef]
- Taube, J.M.; Klein, A.; Brahmer, J.R.; Xu, H.; Pan, X.; Kim, J.H.; Chen, L.; Pardoll, D.M.; Topalian, S.L.; Anders, R.A. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 2014, 20, 5064–5074. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Kudchadkar, R.R.; Yu, B.; Gallenstein, D.; Horak, C.E.; Inzunza, H.D.; Zhao, X.; Martinez, A.J.; Wang, W.; Gibney, G.; et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J. Clin. Oncol. 2013, 31, 4311–4318. [Google Scholar] [CrossRef] [PubMed]
- Velcheti, V.; Schalper, K.A.; Carvajal, D.E.; Anagnostou, V.K.; Syrigos, K.N.; Sznol, M.; Herbst, R.S.; Gettinger, S.N.; Chen, L.; Rimm, D.L. Programmed death ligand-1 expression in non-small cell lung cancer. Lab. Investig. 2014, 94, 107–116. [Google Scholar] [CrossRef]
- Zeng, J.; See, A.P.; Phallen, J.; Jackson, C.M.; Belcaid, Z.; Ruzevick, J.; Durham, N.; Meyer, C.; Harris, T.J.; Albesiano, E.; et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 343–349. [Google Scholar] [CrossRef]
- Wainwright, D.A.; Chang, A.L.; Dey, M.; Balyasnikova, I.V.; Kim, C.K.; Tobias, A.; Cheng, Y.; Kim, J.W.; Qiao, J.; Zhang, L.; et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin. Cancer Res. 2014, 20, 5290–5301. [Google Scholar] [CrossRef]
- Queirolo, P.; Spagnolo, F.; Ascierto, P.A.; Simeone, E.; Marchetti, P.; Scoppola, A.; Del Vecchio, M.; Di Guardo, L.; Maio, M.; Di Giacomo, A.M.; et al. Efficacy and safety of ipilimumab in patients with advanced melanoma and brain metastases. J. Neurooncol. 2014, 118, 109. [Google Scholar] [CrossRef] [PubMed]
- Margolin, K.; Ernstoff, M.S.; Hamid, O.; Lawrence, D.; McDermott, D.; Puzanov, I.; Wolchok, J.D.; Clark, J.I.; Sznol, M.; Logan, T.F.; et al. Ipilimumab in patients with melanoma and brain metastases: An open-label, phase 2 trial. Lancet. Oncol. 2012, 13, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Kmiecik, J.; Poli, A.; Brons, N.H.C.; Waha, A.; Eide, G.E.; Enger, P.Ø.; Zimmer, J.; Chekenya, M. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J. Neuroimmunol. 2013, 264, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Koshkaki, H.R.; Minasi, S.; Ugolini, A.; Trevisi, G.; Napoletano, C.; Zizzari, I.G.; Gessi, M.; Giangaspero, F.; Mangiola, A.; Nuti, M.; et al. Immunohistochemical Characterization of Immune Infiltrate in Tumor Microenvironment of Glioblastoma. J. Pers. Med. 2020, 10, 112. [Google Scholar] [CrossRef]
- Kather, J.N.; Suarez-Carmona, M.; Charoentong, P.; Weis, C.A.; Hirsch, D.; Bankhead, P.; Horning, M.; Ferber, D.; Kel, I.; Herpel, E.; et al. Topography of cancer-associated immune cells in human solid tumors. Elife 2018, 7, e36967. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Ma, X. Prognostic value of tumor-infiltrating lymphocytes in gliomas: A Systematic Review. Res. Pap. 2020. [Google Scholar] [CrossRef]
- Orrego, E.; Castaneda, C.A.; Castillo, M.; Bernabe, L.A.; Casavilca, S.; Chakravarti, A.; Meng, W.; Garcia-Corrochano, P.; Villa-Robles, M.R.; Zevallos, R.; et al. Distribution of tumor-infiltrating immune cells in glioblastoma. CNS Oncol. 2018, 7, CNS21. [Google Scholar] [CrossRef]
- Sayour, E.J.; McLendon, P.; McLendon, R.; De Leon, G.; Reynolds, R.; Kresak, J.; Sampson, J.H.; Mitchell, D.A. Increased proportion of FoxP3+ regulatory T cells in tumor infiltrating lymphocytes is associated with tumor recurrence and reduced survival in patients with glioblastoma. Cancer Immunol. Immunother. 2015, 64, 419–427. [Google Scholar] [CrossRef]
- Barar, J. Targeting Tumor Microenvironment: The Key Role of Immune System. Bioimpacts 2012, 2, 1. [Google Scholar] [CrossRef]
- Li, F.; Li, C.; Cai, X.; Xie, Z.; Zhou, L.; Cheng, B.; Zhong, R.; Xiong, S.; Li, J.; Chen, Z.; et al. The association between CD8+ tumor-infiltrating lymphocytes and the clinical outcome of cancer immunotherapy: A systematic review and meta-analysis. EClinicalMedicine 2021, 41, 101134. [Google Scholar] [CrossRef]
- Lohr, J.; Ratliff, T.; Huppertz, A.; Ge, Y.; Dictus, C.; Ahmadi, R.; Grau, S.; Hiraoka, N.; Eckstein, V.; Ecker, R.C.; et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-β. Clin. Cancer Res. 2011, 17, 4296–4308. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, H.; Chen, B.; Liu, X.; Zhang, S.; Zong, Z.; Gao, M. PD-L1-Mediated Immunosuppression in Glioblastoma Is Associated With the Infiltration and M2-Polarization of Tumor-Associated Macrophages. Front. Immunol. 2020, 11, 588552. [Google Scholar] [CrossRef] [PubMed]
- Lynes, J.P.; Nwankwo, A.K.; Sur, H.P.; Sanchez, V.E.; Sarpong, K.A.; Ariyo, O.I.; Dominah, G.A.; Nduom, E.K. Biomarkers for immunotherapy for treatment of glioblastoma. J. Immunother. Cancer 2020, 8, e000348. [Google Scholar] [CrossRef]
- Chen, R.Q.; Liu, F.; Qiu, X.Y.; Chen, X.Q. The Prognostic and Therapeutic Value of PD-L1 in Glioma. Front. Pharmacol. 2019, 9, 1503. [Google Scholar] [CrossRef]
- Hao, C.; Chen, G.; Zhao, H.; Li, Y.; Chen, J.; Zhang, H.; Li, S.; Zhao, Y.; Chen, F.; Li, W.; et al. PD-L1 expression in glioblastoma, the clinical and prognostic significance: A systematic literature review and meta-analysis. Front. Oncol. 2020, 10, 1015. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, R.J.; Verbaan, D.; Lamba, S.; Zanon, C.; Jeuken, J.W.M.; Boots-Sprenger, S.H.E.; Wesseling, P.; Hulsebos, T.J.M.; Troost, D.; Van Tilborg, A.A.; et al. The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro. Oncol. 2014, 16, 1263–1273. [Google Scholar] [CrossRef]
- Hartmann, C.; Hentschel, B.; Wick, W.; Capper, D.; Felsberg, J.; Simon, M.; Westphal, M.; Schackert, G.; Meyermann, R.; Pietsch, T.; et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: Implications for classification of gliomas. Acta Neuropathol. 2010, 120, 707–718. [Google Scholar] [CrossRef]
- Bleeker, F.E.; Molenaar, R.J.; Leenstra, S. Recent advances in the molecular understanding of glioblastoma. J. Neuro-Oncology 2012 1081 2012, 108, 11–27. [Google Scholar] [CrossRef]
- Songtao, Q.; Lei, Y.; Si, G.; Yanqing, D.; Huixia, H.; Xuelin, Z.; Lanxiao, W.; Fei, Y. IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer Sci. 2012, 103, 269–273. [Google Scholar] [CrossRef]
- Zhang, P.; Xia, Q.; Liu, L.; Li, S.; Dong, L. Current Opinion on Molecular Characterization for GBM Classification in Guiding Clinical Diagnosis, Prognosis, and Therapy. Front. Mol. Biosci. 2020, 7, 562798. [Google Scholar] [CrossRef]
- Mansouri, A.; Karamchandani, J.; Das, S. Molecular Genetics of Secondary Glioblastoma. Exon Publ. 2017, 27–42. [Google Scholar] [CrossRef]
- Mansouri, A.; Hachem, L.D.; Mansouri, S.; Nassiri, F.; Laperriere, N.J.; Xia, D.; Lindeman, N.I.; Wen, P.Y.; Chakravarti, A.; Mehta, M.P.; et al. MGMT promoter methylation status testing to guide therapy for glioblastoma: Refining the approach based on emerging evidence and current challenges. Neuro. Oncol. 2019, 21, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; Thorsson, V.; et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell 2018, 173, 291–304.e6. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
| Feature | Primary Tumors | Relapse | Overall |
|---|---|---|---|
| N. (%) * | N. (%) * | N. (%) * | |
| Total number | 58 | 11 | 69 |
| Age: median (range) | 66 (41–81) | 57 (45–78) | 64 (41–81) |
| Sex | |||
| Female | 22 (38) | 6 (55) | 28 (41) |
| Male | 36 (62) | 5 (45) | 41 (59) |
| Type of resection | |||
| Gross total resection | 15 (28) | 3 (43) | 18 (30) |
| Subtotal resection | 15 (28) | 1 (14) | 16 (27) |
| Partial resection | 23 (43) | 3 (43) | 26 (43) |
| N.A. | 5 (-) | 4 (-) | 9 (-) |
| Alive at the end of follow up | |||
| Yes | 4 (7) | 1 (9) | 5 (7) |
| No | 54 (93) | 10 (91) | 64 (93) |
| Histotype | |||
| Glioblastoma | 56 (97) | 10 (91) | 66 (96) |
| Gliosarcoma | 2 (3) | 1 (9) | 3 (4) |
| Hemisphere | |||
| Right | 32 (55) | 5 (46) | 37 (54) |
| Left | 26 (45) | 4 (36) | 30 (43) |
| Other ** | 0 (0) | 2 (18) | 2 (3) |
| Multifocal | |||
| No | 47 (81) | 9 (82) | 56 (81) |
| Yes | 11 (19) | 2 (18) | 13 (19) |
| IDH1 mutated | 0 (0) | 0 (0) | 0 (0) |
| MGMT promoter | |||
| Methylated | 21 (38) | 7 (78) | 28 (43) |
| Unmethylated | 35 (62) | 2 (22) | 37 (57) |
| N.A. | 4 (-) | 2 (-) | 4 (-) |
| Mib1: median (range) | 35% (4–80%) | 43% (10–60%) | 35% (4–80%) |
| P53 | |||
| Negative | 7 (12) | 4 (36) | 11 (16) |
| Intermediate | 40 (69) | 4 (36) | 44 (64) |
| Positive | 11 (19) | 3 (27) | 14 (20) |
| P53 | |||
| Wild type | 40 (69) | 4 (36) | 44 (64) |
| Mutated | 18 (31) | 7 (64) | 25 (36) |
| CD3 | |||
| Absent | 10 (17) | 2 (18) | 12 (17) |
| Mild | 37 (64) | 7 (64) | 44 (64) |
| Moderate/High | 11 (19) | 2 (18) | 13 (19) |
| CD3+ TILs count: median (range) | 8 (0–90) | 11 (1–57) | 9 (0–90) |
| PD-L1 | |||
| <1% | 33 (57) | 8 (73) | 41 (59) |
| ≥1% | 25 (43) | 3 (27) | 28 (41) |
| Variable | Hazard Ratio | 95% Confidence Interval | p-Value * |
|---|---|---|---|
| Age | 1.121 | 1.029–1.220 | 0.009 |
| tt(Age) | 0.963 | 0.927–0.9996 | 0.048 |
| Sex | |||
| Female | 1 | ||
| Male | 0.891 | 0.511–1.554 | 0.685 |
| Type of surgery | |||
| GTR | 1 | ||
| Subtotal | 1.255 | 0.576–2.733 | 0.568 |
| Partial | 2.675 | 1.263–5.667 | 0.01 |
| Hemisphere | |||
| Right | 1 | ||
| Left | 0.72 | 0.419–1.235 | 0.233 |
| Multifocality | |||
| No | 1 | ||
| Yes | 0.061 | 0.002–1.492 | 0.086 |
| tt(Multifocality) | 5.564 | 1.227–25.23 | 0.026 |
| MGMT | |||
| Methylated | 1 | ||
| Unmethylated | 0.485 | 0.131–1.797 | 0.279 |
| tt(MGMT) | 1.868 | 1.009–3.458 | 0.047 |
| MIB1 | 0.996 | 0.976–1.016 | 0.674 |
| p53 | |||
| Wild type | 1 | ||
| Mutated | 0.994 | 0.553–1.785 | 0.983 |
| PD-L1 | |||
| Negative | 1 | ||
| Positive | 0.8197 | 0.478–1.407 | 0.471 |
| CD3 | |||
| <median | 1 | ||
| ≥median | 0.741 | 0.433–1.268 | 0.274 |
| Variable | Hazard Ratio | 95% Confidence Interval | p-Value * |
|---|---|---|---|
| Age | 1.111 | 1.013–1.219 | 0.025 |
| tt(Age) | 0.97 | 0.931–1.010 | 0.143 |
| Type of surgery | |||
| GTR | 1 | ||
| Subtotal | 1.28 | 0.551–2.972 | 0.566 |
| Partial | 2.749 | 1.135–6.661 | 0.025 |
| Multifocality | |||
| No | 1 | ||
| Yes | 0.052 | 0.002–1.208 | 0.065 |
| tt(Multifocality) | 4.335 | 0.983–19.114 | 0.053 |
| MGMT | |||
| Methylated | 1 | ||
| Unmethylated | 0.652 | 0.158–2.695 | 0.555 |
| tt(MGMT) | 1.651 | 0.847–3.218 | 0.141 |
| ClinicalTrials.gov Identifier | Treatment | PHASE OF TRIAL | Primary End Point | Summary of Results |
|---|---|---|---|---|
| NCT02017717 | Nivolumab vs. bevacizumab | III | OS | Median OS 9.5 months vs. 9.8 months |
| NCT02617589 | Nivolumab vs. Temozolomide + radiation therapy | III | OS | Median OS 13.4months vs. 14.88 months |
| NCT02667587 | Temozolomide + radiation therapy + nivolumab or placebo | III | PFS and OS | No survival advantage over placebo |
| NCT03743662 | Nivolumab with radiation therapy and bevacizumab | II | OS | Ongoing study |
| NCT02550249 | Neoadjuvant nivolumab | II | Efficacy and safety | Median OS 7.3 months |
| NCT04396860 | Lpilimumab and nivolumab + radiation therapy | II/III | PFS and OS | Ongoing study |
| NCT04145115 | Lpilimumab + nivolumab | II | ORR | Ongoing study |
| NCT02337491 | Pembrolizumab with or without bevacizumab | II | MTD, DLT and PFS | Median OS 8.8 months together vs. 10.3 months for pembrolizumab alone |
| NCT02336165 | Durvalumab monotherapy, with bevacizumab or with radiaotherapy | II | OS and PFS | Ongoing study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobhani, N.; Bouchè, V.; Aldegheri, G.; Rocca, A.; D’Angelo, A.; Giudici, F.; Bottin, C.; Donofrio, C.A.; Pinamonti, M.; Ferrari, B.; et al. Analysis of PD-L1 and CD3 Expression in Glioblastoma Patients and Correlation with Outcome: A Single Center Report. Biomedicines 2023, 11, 311. https://doi.org/10.3390/biomedicines11020311
Sobhani N, Bouchè V, Aldegheri G, Rocca A, D’Angelo A, Giudici F, Bottin C, Donofrio CA, Pinamonti M, Ferrari B, et al. Analysis of PD-L1 and CD3 Expression in Glioblastoma Patients and Correlation with Outcome: A Single Center Report. Biomedicines. 2023; 11(2):311. https://doi.org/10.3390/biomedicines11020311
Chicago/Turabian StyleSobhani, Navid, Victoria Bouchè, Giovanni Aldegheri, Andrea Rocca, Alberto D’Angelo, Fabiola Giudici, Cristina Bottin, Carmine Antonio Donofrio, Maurizio Pinamonti, Benvenuto Ferrari, and et al. 2023. "Analysis of PD-L1 and CD3 Expression in Glioblastoma Patients and Correlation with Outcome: A Single Center Report" Biomedicines 11, no. 2: 311. https://doi.org/10.3390/biomedicines11020311
APA StyleSobhani, N., Bouchè, V., Aldegheri, G., Rocca, A., D’Angelo, A., Giudici, F., Bottin, C., Donofrio, C. A., Pinamonti, M., Ferrari, B., Panni, S., Cominetti, M., Aliaga, J., Ungari, M., Fioravanti, A., Zanconati, F., & Generali, D. (2023). Analysis of PD-L1 and CD3 Expression in Glioblastoma Patients and Correlation with Outcome: A Single Center Report. Biomedicines, 11(2), 311. https://doi.org/10.3390/biomedicines11020311












