Does TRODAT-1 SPECT Uptake Correlate with Cerebrospinal Fluid α-Synuclein Levels in Mid-Stage Parkinson’s Disease?
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Outcomes
2.2.1. 99mTc-TRODAT-1-SPECT Imaging
2.2.2. CSF α-Synuclein Expression
2.2.3. Montreal Cognitive Assessment (MoCA) Score
2.2.4. Neurological Assessment
3. Results
3.1. General Description of Individuals with PD
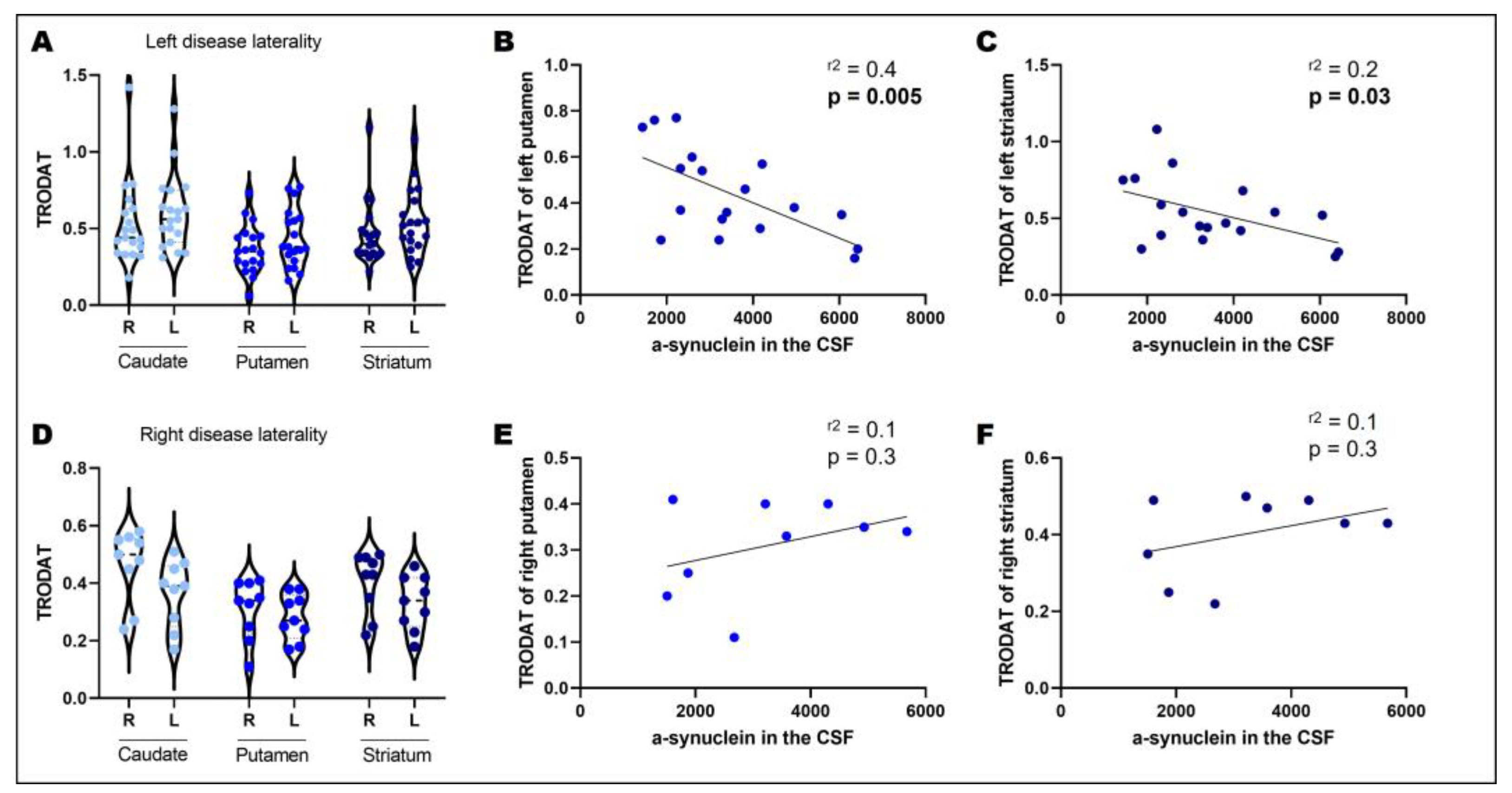
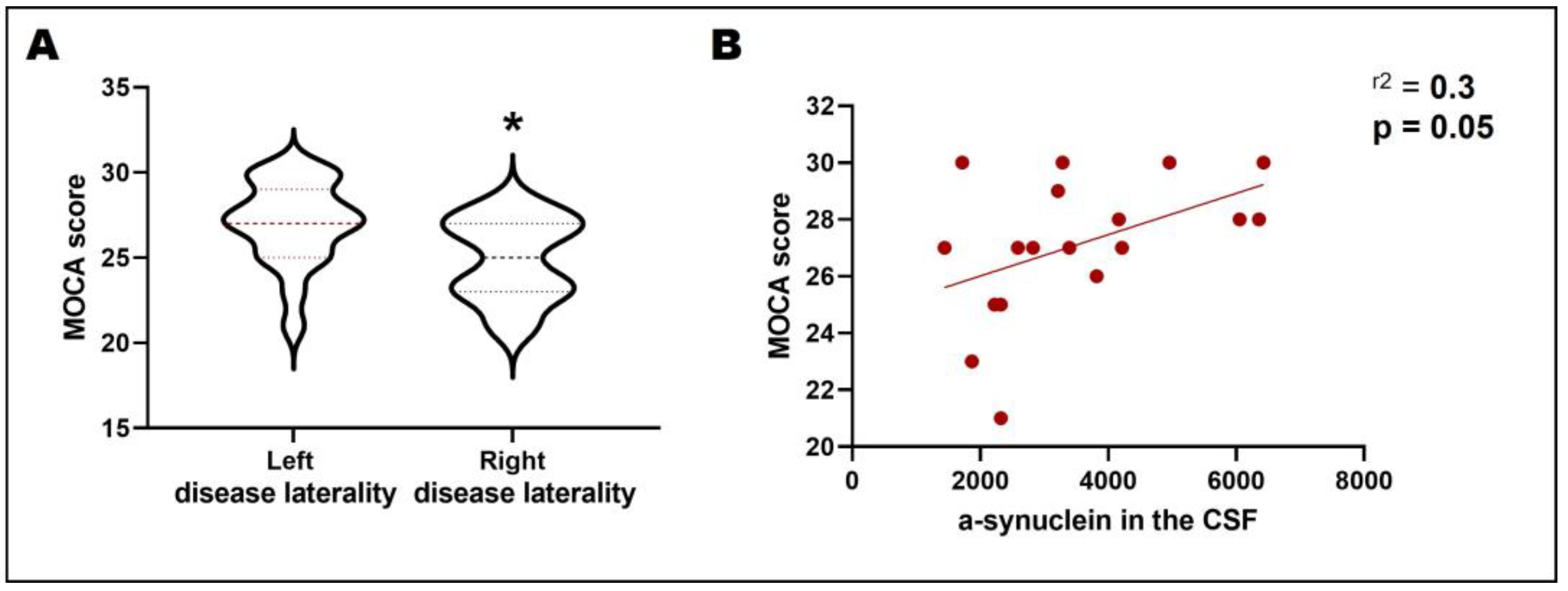
3.2. Disease Laterality, Dopamine Transporter Uptake, and CSF α-Synuclein
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2016 Parkinson’s Disease Collaborators. Global, Regional, and National Burden of Parkinson’s Disease, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Hornykiewicz, O.; Kish, S.J. Biochemical Pathophysiology of Parkinson’s Disease. Adv. Neurol. 1987, 45, 19–34. [Google Scholar] [PubMed]
- Obeso, J.A.; Rodríguez-Oroz, M.C.; Rodríguez, M.; Lanciego, J.L.; Artieda, J.; Gonzalo, N.; Olanow, C.W. Pathophysiology of the Basal Ganglia in Parkinson’s Disease. Trends Neurosci. 2000, 23, S8–S19. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Schapira, A.H.V. Non-Motor Symptoms of Parkinson’s Disease: Dopaminergic Pathophysiology and Treatment. Lancet Neurol. 2009, 8, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, Progression and Mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef]
- Miller-Patterson, C.; Buesa, R.; McLaughlin, N.; Jones, R.; Akbar, U.; Friedman, J.H. Motor Asymmetry over Time in Parkinson’s Disease. J. Neurol. Sci. 2018, 393, 14–17. [Google Scholar] [CrossRef]
- Kempster, P.A.; Gibb, W.R.; Stern, G.M.; Lees, A.J. Asymmetry of Substantia Nigra Neuronal Loss in Parkinson’s Disease and Its Relevance to the Mechanism of Levodopa Related Motor Fluctuations. J. Neurol. Neurosurg. Psychiatry 1989, 52, 72–76. [Google Scholar] [CrossRef]
- Zhu, S.; Zhong, M.; Bai, Y.; Wu, Z.; Gu, R.; Jiang, X.; Shen, B.; Zhu, J.; Pan, Y.; Dong, J.; et al. The Association between Clinical Characteristics and Motor Symptom Laterality in Patients with Parkinson’s Disease. Front. Neurol. 2021, 12, 663232. [Google Scholar] [CrossRef]
- Cubo, E.; Martín, P.M.; Martin-Gonzalez, J.A.; Rodríguez-Blázquez, C.; Kulisevsky, J. ELEP Group Members Motor Laterality Asymmetry and Nonmotor Symptoms in Parkinson’s Disease. Mov. Disord. 2010, 25, 70–75. [Google Scholar] [CrossRef]
- Tomer, R.; Levin, B.E.; Weiner, W.J. Side of Onset of Motor Symptoms Influences Cognition in Parkinson’s Disease. Ann. Neurol. 1993, 34, 579–584. [Google Scholar] [CrossRef]
- Tomer, R.; Aharon-Peretz, J.; Tsitrinbaum, Z. Dopamine Asymmetry Interacts with Medication to Affect Cognition in Parkinson’s Disease. Neuropsychologia 2007, 45, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Gibb, W.R.; Lees, A.J. The Relevance of the Lewy Body to the Pathogenesis of Idiopathic Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 1988, 51, 745–752. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-Synuclein in Lewy Bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Masliah, E.; Rockenstein, E.; Veinbergs, I.; Mallory, M.; Hashimoto, M.; Takeda, A.; Sagara, Y.; Sisk, A.; Mucke, L. Dopaminergic Loss and Inclusion Body Formation in Alpha-Synuclein Mice: Implications for Neurodegenerative Disorders. Science 2000, 287, 1265–1269. [Google Scholar] [CrossRef]
- Gao, L.; Tang, H.; Nie, K.; Wang, L.; Zhao, J.; Gan, R.; Huang, J.; Zhu, R.; Feng, S.; Duan, Z.; et al. Cerebrospinal Fluid Alpha-Synuclein as a Biomarker for Parkinson’s Disease Diagnosis: A Systematic Review and Meta-Analysis. Int. J. Neurosci. 2015, 125, 645–654. [Google Scholar] [CrossRef]
- Eusebi, P.; Giannandrea, D.; Biscetti, L.; Abraha, I.; Chiasserini, D.; Orso, M.; Calabresi, P.; Parnetti, L. Diagnostic Utility of Cerebrospinal Fluid α-Synuclein in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Mov. Disord. 2017, 32, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Peplow, P.V.; Martinez, B.; Gennarelli, T.A. Trends in Biomarkers of Neurodegenerative Diseases. In Neurodegenerative Diseases Biomarkers: Towards Translating Research to Clinical Practice; Peplow, P.V., Martinez, B., Gennarelli, T.A., Eds.; Neuromethods; Springer: New York, NY, USA, 2022; pp. 551–557. ISBN 978-1-07-161712-0. [Google Scholar]
- Aarsland, D.; Batzu, L.; Halliday, G.M.; Geurtsen, G.J.; Ballard, C.; Ray Chaudhuri, K.; Weintraub, D. Author Correction: Parkinson Disease-Associated Cognitive Impairment. Nat. Rev. Dis. Prim. 2021, 7, 53. [Google Scholar] [CrossRef]
- Suwijn, S.R.; van Boheemen, C.J.; de Haan, R.J.; Tissingh, G.; Booij, J.; de Bie, R.M. The Diagnostic Accuracy of Dopamine Transporter SPECT Imaging to Detect Nigrostriatal Cell Loss in Patients with Parkinson’s Disease or Clinically Uncertain Parkinsonism: A Systematic Review. EJNMMI Res. 2015, 5, 12. [Google Scholar] [CrossRef]
- Deng, X.; Li, F.-J.; Tang, C.-Y.; Zhang, J.; Zhu, L.; Zhou, M.-H.; Zhang, X.; Gong, H.-H.; Xiao, X.-Z.; Xu, R.-S. The Cortical Surface Correlates of Clinical Manifestations in the Mid-Stage Sporadic Parkinson’s Disease. Neurosci. Lett. 2016, 633, 125–133. [Google Scholar] [CrossRef]
- Hoops, S.; Nazem, S.; Siderowf, A.D.; Duda, J.E.; Xie, S.X.; Stern, M.B.; Weintraub, D. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 2009, 73, 1738–1745. [Google Scholar] [CrossRef]
- Adwani, S.; Yadav, R.; Kumar, K.; Chandra, S.R.; Pal, P.K. Neuropsychological Profile in Early Parkinson’s Disease: Comparison between Patients with Right Side Onset versus Left Side Onset of Motor Symptoms. Ann. Indian Acad. Neurol. 2016, 19, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Amick, M.M.; Grace, J.; Chou, K.L. Body Side of Motor Symptom Onset in Parkinson’s Disease Is Associated with Memory Performance. J. Int. Neuropsychol. Soc. 2006, 12, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Katzen, H.L.; Levin, B.E.; Weiner, W. Side and Type of Motor Symptom Influence Cognition in Parkinson’s Disease. Mov. Disord. 2006, 21, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.R.; Held, U.; Valko, P.O.; Wienecke, M.; Waldvogel, D. Body Side and Predominant Motor Features at the Onset of Parkinson’s Disease Are Linked to Motor and Nonmotor Progression. Mov. Disord. 2014, 29, 207–213. [Google Scholar] [CrossRef]
- Vancea, R.; Simonyan, K.; Petracca, M.; Brys, M.; Di Rocco, A.; Ghilardi, M.F.; Inglese, M. Cognitive Performance in Mid-Stage Parkinson’s Disease: Functional Connectivity under Chronic Antiparkinson Treatment. Brain Imaging Behav. 2019, 13, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Emamzadeh, F.N.; Surguchov, A. Parkinson’s Disease: Biomarkers, Treatment, and Risk Factors. Front. Neurosci. 2018, 12, 612. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Mid-stage PD | Impairment balance |
| Hoehn and Yahr score: 2.0–3.0 | Swallowing |
| After 12 h of off medication | Speech disorders |
| Anatomical anormalities | |
| Pre-existing, uncontrolled medical conditions | |
| Gender | ||
| Female (n, %) | 10 | 35.7% |
| Male (n, %) | 18 | 64.3% |
| Age | ||
| Mean (SD) | 57.86 | 8.84 |
| Median (Min–Max) | 58.57 | 41–76 |
| Disease duration | ||
| Mean (SD) | 10.18 | 4.47 |
| Median (Min–Max) | 10 | 1–20 |
| Disease severity | ||
| Mean (SD) | 42.86 | 8.52 |
| Median (Min–Max) | 40.5 | 25–57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coutinho, A.M.; Ghilardi, M.G.; Campos, A.C.P.; Etchebehere, E.; Fonoff, F.C.; Cury, R.G.; Pagano, R.L.; Martinez, R.C.R.; Fonoff, E.T. Does TRODAT-1 SPECT Uptake Correlate with Cerebrospinal Fluid α-Synuclein Levels in Mid-Stage Parkinson’s Disease? Biomedicines 2023, 11, 296. https://doi.org/10.3390/biomedicines11020296
Coutinho AM, Ghilardi MG, Campos ACP, Etchebehere E, Fonoff FC, Cury RG, Pagano RL, Martinez RCR, Fonoff ET. Does TRODAT-1 SPECT Uptake Correlate with Cerebrospinal Fluid α-Synuclein Levels in Mid-Stage Parkinson’s Disease? Biomedicines. 2023; 11(2):296. https://doi.org/10.3390/biomedicines11020296
Chicago/Turabian StyleCoutinho, Artur M., Maria Gabriela Ghilardi, Ana Carolina P. Campos, Elba Etchebehere, Fernanda C. Fonoff, Rubens G. Cury, Rosana L. Pagano, Raquel C. R. Martinez, and Erich T. Fonoff. 2023. "Does TRODAT-1 SPECT Uptake Correlate with Cerebrospinal Fluid α-Synuclein Levels in Mid-Stage Parkinson’s Disease?" Biomedicines 11, no. 2: 296. https://doi.org/10.3390/biomedicines11020296
APA StyleCoutinho, A. M., Ghilardi, M. G., Campos, A. C. P., Etchebehere, E., Fonoff, F. C., Cury, R. G., Pagano, R. L., Martinez, R. C. R., & Fonoff, E. T. (2023). Does TRODAT-1 SPECT Uptake Correlate with Cerebrospinal Fluid α-Synuclein Levels in Mid-Stage Parkinson’s Disease? Biomedicines, 11(2), 296. https://doi.org/10.3390/biomedicines11020296





