Abstract
Obesity is a cancer progression risk factor; excessive adipocytes increase adipokine secretion. Visfatin, a novel adipokine highly expressed in cancer patients, is related to breast cancer risk. The modulation of nicotinamide adenine dinucleotide (NAD+) metabolism and the induction of a tumorigenic environment plays a vital role in cancer progression. Among cancer cell types, cancer stem-like cells (CSCs) with self-renewal and chemotherapy-resistance abilities could modulate tumor progression and cancer recurrence ability. In this study, we focused on visfatin’s modulation effect on stemness-related properties using the high-malignancy breast cancer cell line MDA-MB-231 in in vitro and in vivo studies. Visfatin treatment significantly increased both the sphere number and sphere diameter and increased the protein expression of NANOG homeobox (NANOG), sex-determining region Y-box 2 (SOX2), and octamer-binding transcription factor 4 (OCT4), as well as SIRT1 protein levels. The serum angiogenesis marker VEGF and extracellular nicotinamide phosphoribosyl transferase (NAMPT, visfatin) were induced after visfatin treatment, increasing the stemness and angiogenesis environment, which were significantly reduced by the visfatin inhibitor FK866. Our results demonstrate that the visfatin-activated SIRT–SOX2 axis promotes triple-negative breast cancer stemness and enriches the tumorigenic microenvironment.
1. Introduction
Breast cancer is considered the most common cancer worldwide [1]. Among breast cancer types, triple-negative breast cancer lacks estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression [2]. It is characterized by poor prognosis and high drug resistance, resulting in low survival rates. Therefore, it is important to find new therapeutic targets for this cancer.
Obesity is one of the risk factors of breast cancer progression because it leads to an increase in secreted factors [3]. An elevated BMI increases the risk factors for breast cancer, including inflammatory effects and metastasis ability [4]. Obese cancer patients present with larger tumor sizes and higher tumor grades [5]. The elevation of adipokine levels plays an important role in cancer progression [6]. Adipokines induce cancer progression and the epithelial to mesenchymal transition [7] by modulating angiogenesis, invasion, and chemoresistance [8]. The novel adipokine visfatin, also known as nicotinamide phosphoribosyl transferase (NAMPT), shows increased levels in obesity, gynecological diseases, and breast cancer patients [9,10]. The intracellular form of NAMPT is a rate-limited enzyme in NAD+ biosynthesis, while the serum visfatin level (extracellular form) is a potential indicator of poor prognosis [11]. An increase in NAD+ is related to tumor drug resistance and improvements in DNA repair progression [12]. Among all breast cancer types, MDA-MB-231 showed the highest intracellular and extracellular NAMPT expression [13]. This expression may by elevated by adipose-derived stem cell secretion [14] and tumor-associated macrophages [13], with the supportive environment and intracellular NAMPT expression providing a malignant environment [15].
Since NAD+ metabolism is composed of cADP-ribose synthases, ADP-ribosyltransferases and sirtuins such as Sirtuin 1 (SIRT1) are elevated in response to visfatin in cancer stem cells (CSCs) [16]. This results in enhanced self-renewal and cancer metastasis abilities [10] through the octamer-binding transcription factor 4 (OCT4)–SIRT1–p53 axis [11]. Cancer stem-like cells, a subset of tumor cells with self-renewal and chemotherapy resistance abilities, could modulate tumor progression and cancer recurrence ability [17]. The activation of transcription factors is an important regulator of stemness. NANOG homeobox (NANOG), sex determining region Y-box 2 (SOX2), and OCT4, common regulators of stemness, could mediate cancer proliferation and metastasis, which are associated with poor overall survival and advanced disease stage [18]. Our study aimed to determine visfatin’s role in breast cancer stemness progression and the novel therapeutic strategy of visfatin inhibition.
2. Materials and Methods
2.1. Cell Culture
MDA-MB-231 human breast cancer cells were purchased from the Bioresource Collection and Research Center (BCRC, Hsinchu, Taiwan). DMEM/F12 medium was used for cell culturing (CAISSON, Taichung City, Taiwan) supplemented with 10% fetal bovine serum (FBS; CORNING, Manassas, VA, USA), 1% antibiotic-antimycotic solution (CORNING), sodium bicarbonate (2.438 g/L; BioShop, Burlington, ON, Canada), and 4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES; 5.986 g/L; BioShop) in a humidified incubator (37 °C, 5% CO2). Visfatin was obtained from Peprotech (#130-09, Rehovot, Israel), and its inhibitor was obtained from Cayman (Ann Arbor, MI, USA).
2.2. MTT Assay
We cultured 3 × 103 cells in 96-well plates. After 24 h of serum-free starvation, they were treated with different dosages of visfatin for 24 and 48 h. After treatment, 5 mg/mL of MTT (3-(4,5-dimethyl thiazol)-2,5-diphenyltetrazolium bromide (Abcam, Cambridge, MA, USA) was diluted to 1 mg/mL with culture medium and kept in a 37 °C CO2 incubator for 3 h until a purple crystal formed. We added 100 μL of DMSO to dissolve the crystal. An ELISA reader (Molecular Devices, San Jose, CA, USA) was used to detect the absorbance at the wavelengths of 570 and 630 nm.
2.3. Sphere Formation Assay
MDA-MB-231 cells were cultured in 6-well plates (1 × 105) and treated with visfatin for 24 or 48 h. Then, they were harvested and suspended as pellets, followed by counting using trypan blue. Then, 500 cells/well were cultured in 96-well ultra-low attachment plates (Corning, Shanghai, China, 3474) in tumor sphere medium (1 × B27 (Life Technologies, Carlsbad, CA, USA), 20 ng/mL epidermal growth factor (Enzo, Beijing, China), 10 ng/mL basic fibroblast growth factor (Sciencell, Carlsbad, CA, USA), 5 μg/ml insulin (Life Technologies, Carlsbad, CA, USA), and 0.4% bovine serum albumin (Sigma-Aldrich, St. Louis, MO, USA) for 7 days. Microscopy was used to capture the sphere formation, and ImageJ software was used to analyze the diameter [19].
2.4. Xenograft Animal Model
Five-week-old female Balb/c nude mice (BioLASCO, Taipei, Taiwan) were housed under a 12 h light/12 h dark cycle in a pathogen-free environment with food and water available ad libitum. After 1 week of adaptation, MDA-MB-231-GFP cells (2 × 106 in 100 μL PBS/mice) were injected subcutaneously into the right flank of the mice. After 1 week, the animals were randomly divided into three groups (n = 5/group) and treated with either visfatin (2 ng/g) or FK866 (4 mg/kg) intraperitoneal injections for 56 days or were untreated controls [9]. We used calipers and calculated the tumor size using the formula 0.5 × length × width2. All animal studies were conducted according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Taipei Medical University (IACUC Approval No. 2019-0034).
2.5. Western Blot Analysis
Tumor samples (about 0.1 g) were homogenized with lysis buffer containing a protease inhibitor (Roche, Basel, Switzerland) and a phosphatase inhibitor (Roche) using TissueLyser II (Qiagen, Chatsworth, CA, USA) and centrifuged at 12,000× g for 30 min at 4 °C. A BCA kit (T-Pro Biotechnology, New Taipei City, Taiwan) was used to measure the tissue and serum protein concentration. We used 10–15% SDS-polyacrylamide gel electrophoresis (PAGE) for protein separation and then transferred the samples onto Immobilon-P polyvinylidene fluoride (PVDF) membranes (0.22 µm) for 125 min at 95 V. Then, we used blocking buffer (5% BSA) for 1 h at room temperature and incubated the samples with primary antibodies against NAMPT (Proteintech, Rehovot, Israel), NANOG (Proteintech), OCT4 (Proteintech), SOX2 (Proteintech), SIRT1 (Cell signaling, Boston, MA, USA), VEGF (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Proteintech, Rehovot, Israel) overnight at 4 °C. After washing 3 times, the samples were incubated with anti-rabbit/mouse IgG coupled with alkaline phosphatase (1: 10,000) for 2 h. We used an ECL chemiluminescent kit to visualize the antibody–antigen interaction and detected the signal with an eBlot Touch Imager (eBlot Photoelectric Technology, Shanghai, China) [20]. The relative intensity was measured using ImageJ software (NIH, Bethesda, MD, USA).
2.6. Statistical Analysis
Data are expressed as mean ± standard error of the mean (SEM), and GraphPad Prism version 8.0 (GraphPad, San Diego, CA, USA) was used for the statistical analyses. We utilized Student’s t-test, one-way analysis of variance (ANOVA), and Tukey’s post hoc test. p < 0.05 was considered statistically significant.
3. Results
3.1. Effect of Visfatin on Cell Viability
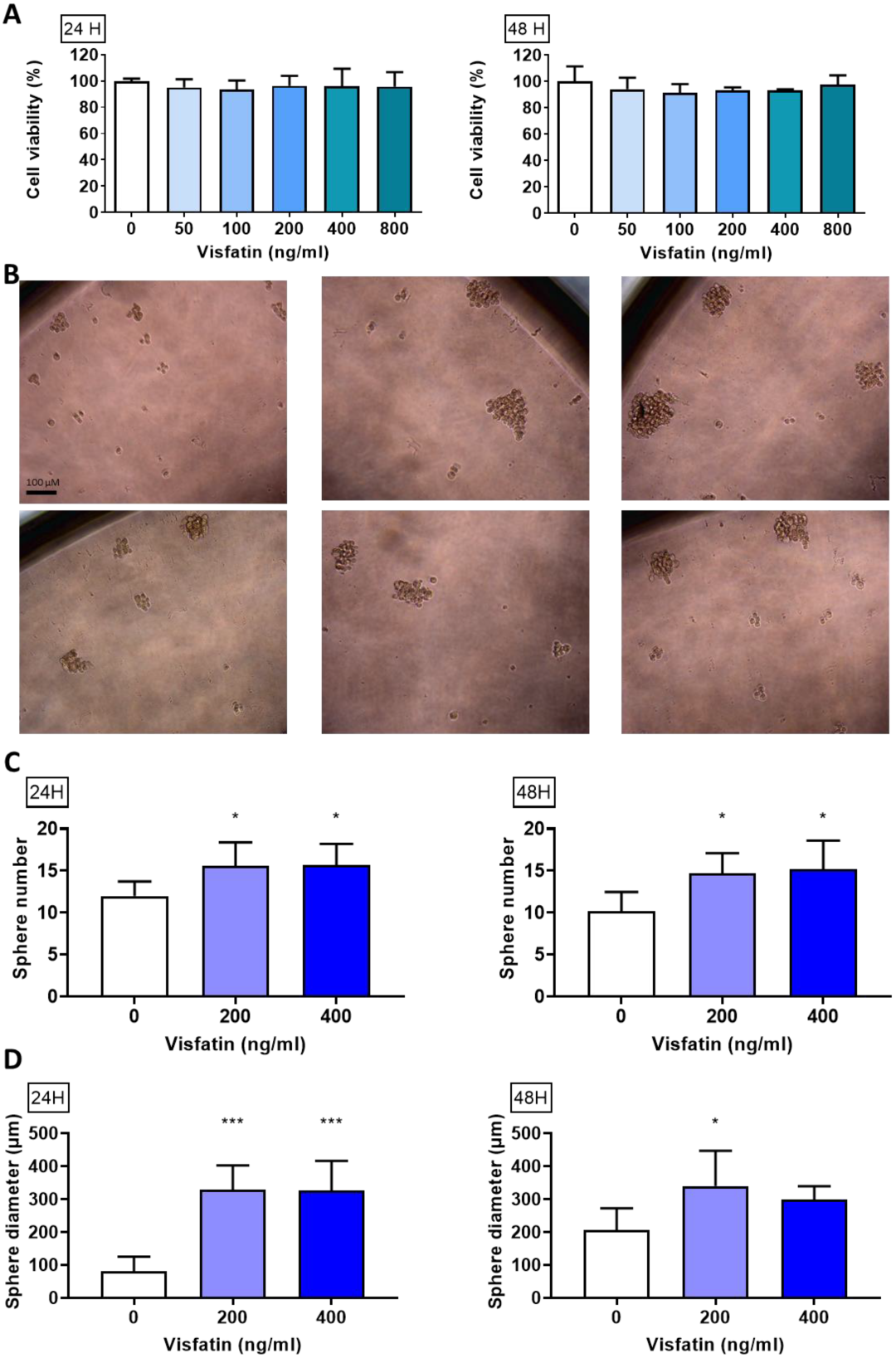
To explore visfatin’s role in breast cancer cell viability, an MTT assay was used after the 24 or 48 h treatments. There were no statistically significant changes after visfatin treatment, indicating that short-term exposure does not alter proliferation (Figure 1A).
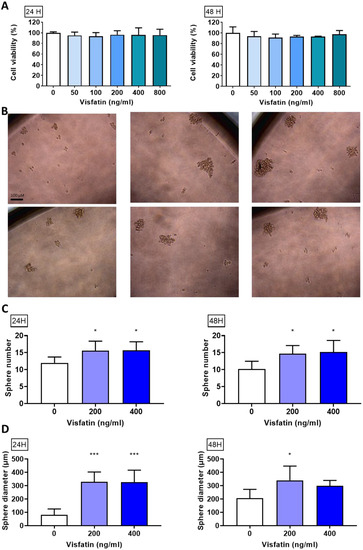
Figure 1.
Effect of visfatin on sphere formation in MDA−MB−231. (A) Cell viability of MDA−MB−231 after visfatin treatment. (B) Sphere morphology after culturing in sphere formation medium for 7 days. (C) Sphere number and (D) sphere diameter measured using ImageJ. Data are presented as mean ± SD. * p < 0.05 and *** p < 0.001 as compared with control group.
3.2. Effect of Visfatin Exposure on Sphere Formation in MDA-MB-231
To evaluate whether visfatin could activate stemness ability and self-renewal in breast cancer, MDA-MB-231 cells were treated with visfatin (0, 200, or 400 ng/mL) for 24 or 48 h (Figure 1B) while using a sphere-forming assay and calculate the sphere number (Figure 1C) and diameter (Figure 1D). Our results showed that visfatin significantly increased the sphere formation ability.
3.3. Body Weight and Tumor Size Changes in Visfatin-Exposed Xenograft Animal Model
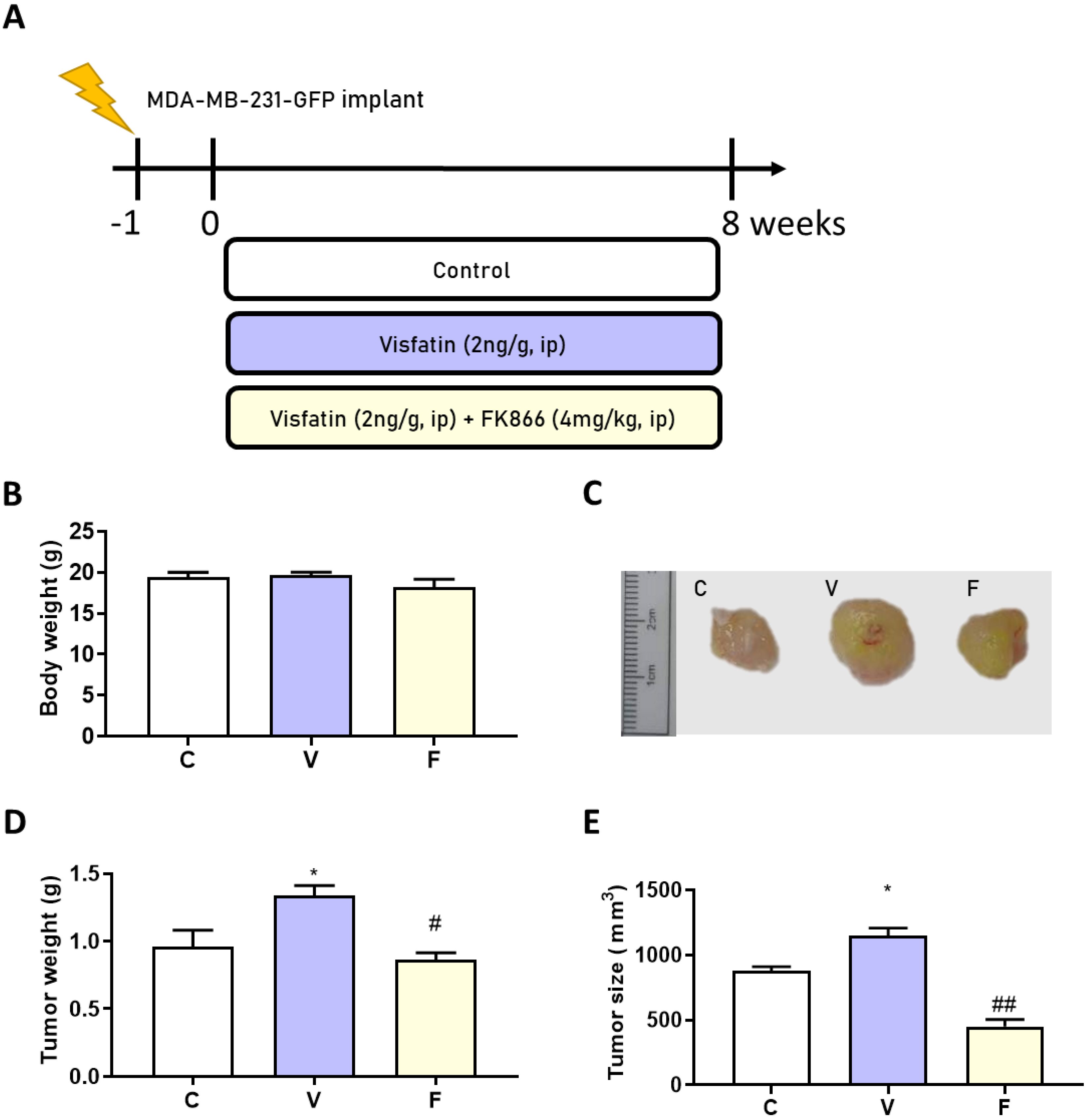
To confirm the in vitro results of visfatin-induced tumor-initiating capabilities in vivo, we injected the MDA-MB-231-GFP cell line (2 × 106 cells/100 μL) into Balb/c nude mice. After 1 week, either visfatin alone or visfatin with its inhibitor were given intraperitoneally for 8 weeks (Figure 2A). No significant body weight change was observed between groups (Figure 2B). Interestingly, visfatin treatment significantly increased both tumor size and tumor weight, while treatment with its inhibitor displayed a significant reduction in tumor size and weight (Figure 2C–E).
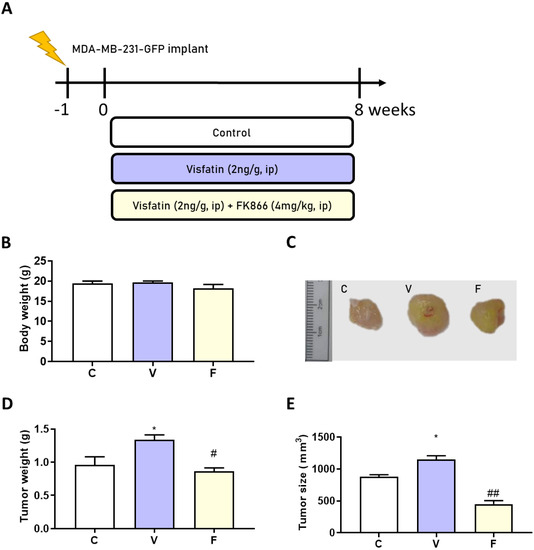
Figure 2.
Effects of visfatin and its inhibitor FK866 on an MDA−MB−231−GFP xenograft animal model. (A) Flow chart of model induction. (B) Change in body weight. (C) Tumor morphology. (D) Tumor weight change and (E) tumor size change after 56 days’ treatment. Data are presented as mean ± SEM. C, control group. V, visfatin−induced group. F, visfatin + FK866 inhibitor group. * p < 0.05 compared to C. # p < 0.05 and ## p < 0.01 compared to V.
3.4. Stemness-Related Protein Expression in Visfatin-Exposed Xenograft Animal Model
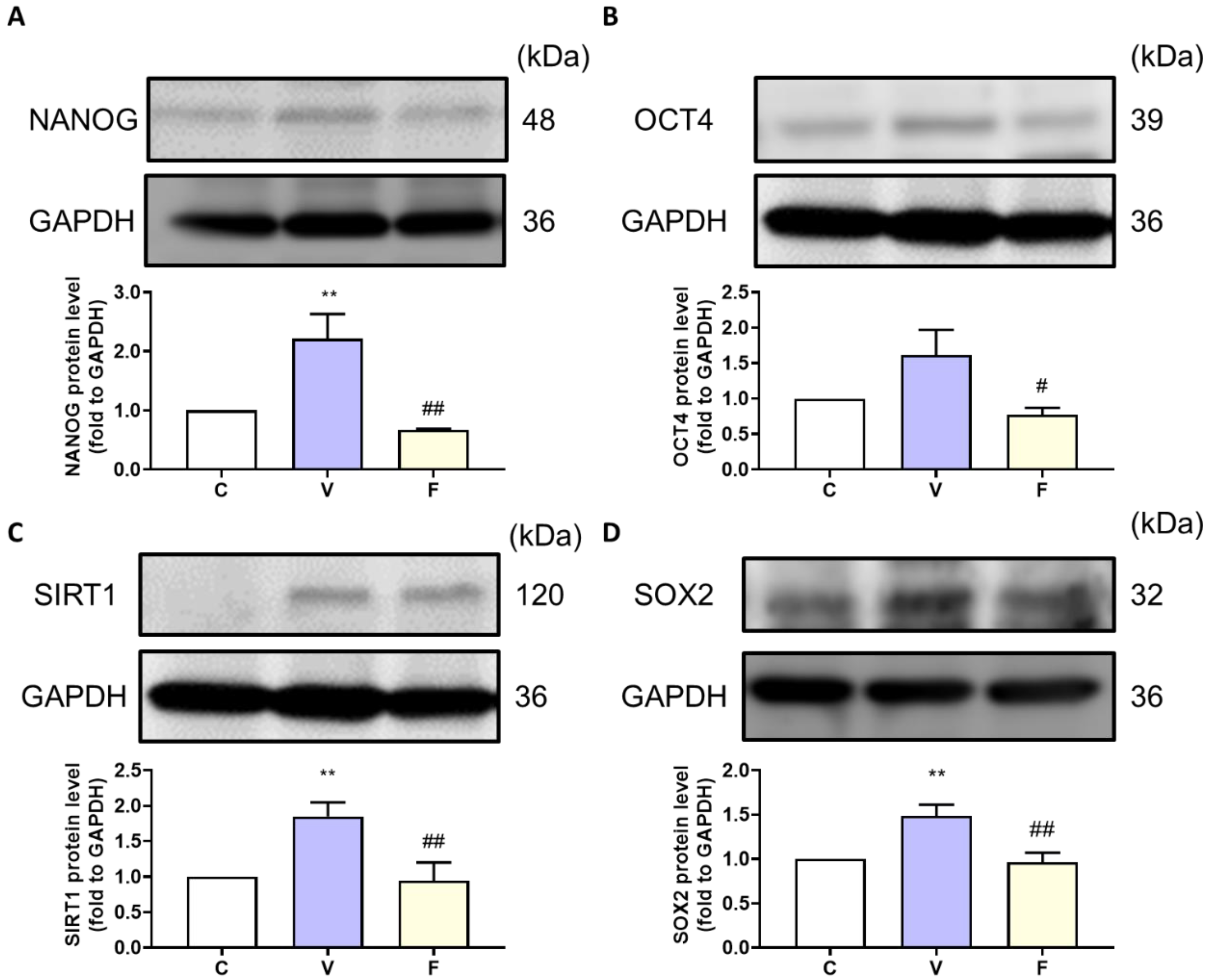
With an increase in tumor weight, we evaluated the stemness markers NANOG, OCT4, and the SIRT1–SOX2 axis in the tumor. Stemness-related protein expression was measured by Western blot analysis. The visfatin-treated group showed induction of stemness-related protein expression, while the visfatin inhibitor FK866 significantly decreased the NANOG, OCT4, SIRT1, and SOX2 protein expression (Figure 3). These results suggest that visfatin, through an increase in NAD+, upregulates SIRT1 levels and activates the SIRT–SOX2 axis to modulate stemness progression [21,22].
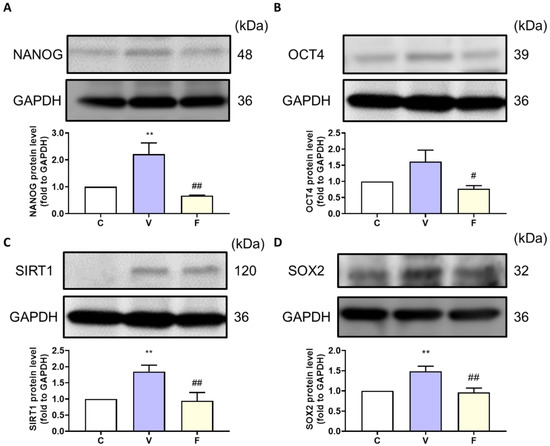
Figure 3.
Effect of visfatin on stemness-related protein expression in xenograft tumor tissue. The tumor stemness-related expression of (A) NANOG, (B) OCT4, (C) SIRT1, and (D) SOX2 proteins. Data are presented as mean ± SEM. C, control group. V, visfatin-induced group. F, visfatin + FK866 inhibitor group. ** p < 0.01 compared to C. # p < 0.05 and ## p < 0.01 compared to V.
3.5. Extracellular NAMPT and Serum Angiogenesis Marker Changes in Visfatin-Exposed Xenograft Animal Model
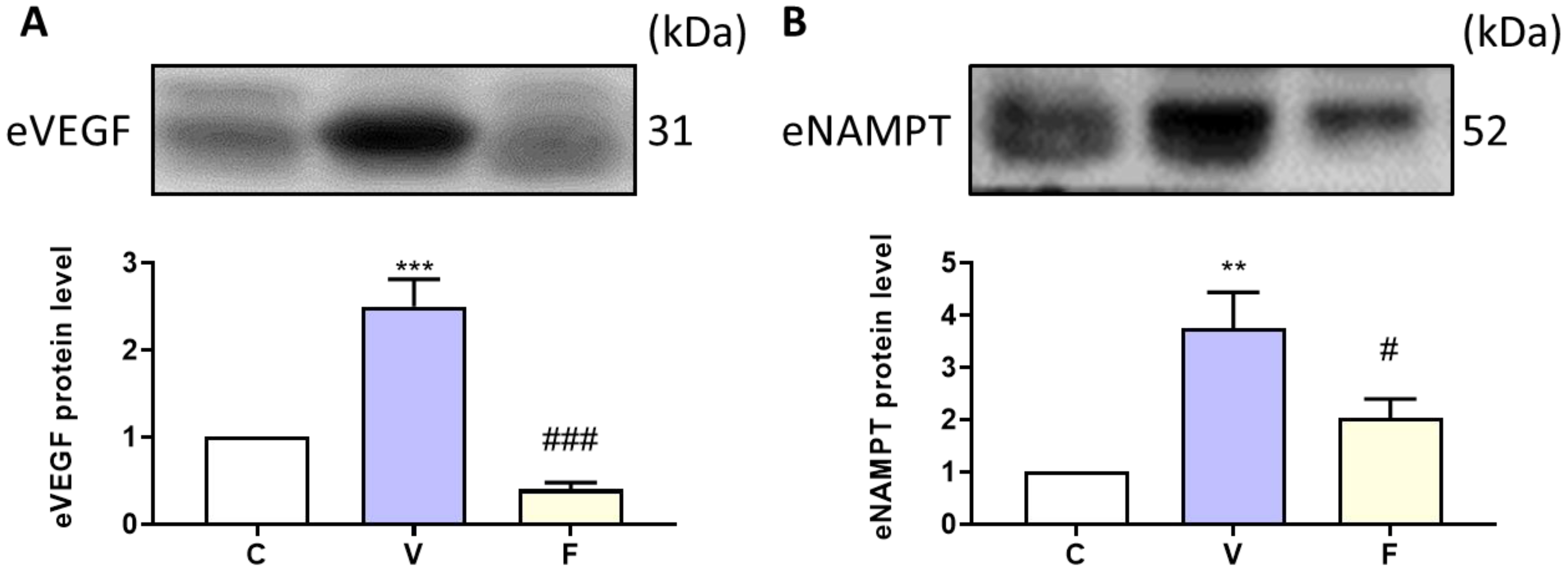
To explore the effect of visfatin and its inhibitor on extracellular NAMPT and the angiogenesis marker in serum, we used Western blot analysis to measure protein expression. Our results showed that visfatin induction significantly increased serum VEGF and NAMPT levels, while the inhibition of visfatin significantly decreased the cancer-stemness-rich microenvironment (Figure 4).
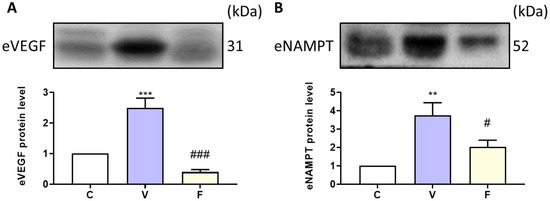
Figure 4.
Effect of visfatin on serum angiogenesis marker and extracellular NAMPT expression. The protein expressions of (A) VEGF and (B) eNAMPT were analyzed by Western blot. Data are presented as mean ± SEM. C, control group. V, visfatin-induced group. F, visfatin + FK866 inhibitor group. ** p < 0.01 and *** p < 0.001 compared to C. # p < 0.05 and ### p < 0.001 compared to V.
4. Discussion
This work presented the first insights into visfatin’s role in stemness-related markers in a breast cancer animal model with the induction of OCT4, NANOG, and the SIRT1–SOX2 axis. Treatment with the visfatin inhibitor FK866 significantly reversed the induced stemness and decreased the tumor size.
Obesity is one of the known risk factors for breast cancer and is related to worse prognosis and overall survival [23]. Every 5 kg/m2 increase in BMI increases breast cancer risk by 2% [24]. In addition, the efficacy of chemotherapy is significantly lower in obese breast cancer patients [25]. The growth of adipocytes increases the secretion levels of cytokines and adipokines, making adipokines one of major contributors to breast cancer progression [26]. Among various adipokines, adiponectin, leptin, resistin, and visfatin are considered to have the greatest relevance to obesity-related cancer [6]. Visfatin, as a relatively newly discovered adipokine, has not been fully explored in the context of tumor progression. Several studies have revealed that the serum visfatin level is highly associated with breast cancer incidence [27]. Additionally, its specificity may qualify it as a diagnostic indicator for breast cancer [28]. In colorectal cancer, visfatin-induced stemness was shown to be related to stem cell signaling transduction and radiotherapy resistance, with a positive correlation being found between stemness-related marker expression and NAMPT expression [29].
Visfatin (NAMPT)’s role in tumorigenesis is attributed to its identity as a rate-limiting enzyme in the salvage pathway [30]. NAMPT’s management involves the recycling of nicotinamide (NAM), where nicotinamide riboside (NR) converts it to nicotinamide mononucleotide (NMN). NMN is enzymatically converted to nicotinamide adenine dinucleotide (NAD+). High NAD+ levels have been observed in CSCs and could modulate sirtuin function, especially SIRT1 activity, which regulates SOX2-related stemness expression [31]. A reduction in NAD+ levels causes self-renewal to decrease and activates apoptosis [32]. Our data suggest that the visfatin-treated tissues showed significantly higher NANOG, OCT4, and SOX2 stemness-related protein expression, with the excessive NAD+ production activating SIRT1 expression to enhance SOX2 expression. Moreover, the inhibition of NAMPT through treatment with the visfatin inhibitor FK866 significantly decreased stemness expression and reduced tumor size.
The secreted form of NAMPT (eNAMPT, extracellular NAMPT) has been reported to reflect cytokine function and is associated with cancer and inflammatory disease incidence [33]. Recent studies have revealed that circulating serum eNAMPT was increased in all cancer patients and may be a potential therapeutic target [34]. eNAMPT could increase colony formation ability [35]. However, there is no solid evidence in breast cancer studies to date. Additionally, how eNAMPT is secreted and the modulation between iNAMPT (intracellular NAMPT) and eNAMPT is still not clear. In this study, we measured eNAMPT protein expression and visfatin treatment significantly increased eNAMPT protein expression. FK866 decreased its expression, which is consistent with the pattern of stemness-related protein expression.
Nutrient supply is an important factor to support cancer progression [36]. Therefore, angiogenesis improves tumor growth and migration, as creating a new vascular system enhances the nutrition supply [37]. Adipocyte-derived angiogenic adipokines include VEGF as one of the angiogenesis factors that enriches the tumorigenic microenvironment [36] and increases the number of CSCs [38]. In our study, visfatin treatment increased the serum VEGF level.
Altogether, our data suggest that in breast cancer, the adipokine visfatin increased cancer sphere formation and stemness-related protein expression with SIRT1 modulation and also increased eNAMPT and VEGF levels with subsequent angiogenesis enhancement and enrichment of the tumorigenic microenvironment. Further studies could focus on the signaling pathways that could modulate eNAMPT as a potential novel therapeutic target. The limited sample size makes it difficult to predict the modulatory effect of NAMPT, and further studies should enlarge the sample size to predict the effects of NAMPT and stemness-related genes on tumor size with a mathematical model [39].
5. Conclusions
Exposure to the adipokine visfatin may activate the SIRT1-SOX2 axis and stemness progression in breast cancer, as visfatin treatment increased sphere formation and tumor size by activating stemness-related protein expression and increasing angiogenesis, providing a malignant environment for breast cancer progression. The inhibition of visfatin may provide a new therapeutic direction for the treatment of adipokine-related microenvironments in CSCs.
Author Contributions
The work presented here was carried out in collaboration among all authors. Y.-F.C.: methodology, writing—original draft preparation, software; K.-C.H., H.-Y.C. (Hsin-Yuan Chen), T.-C.H., Y.-H.S. and T.-M.S.: methodology, software, validation; resources; C.-P.C., H.-Y.C. (Hsin-Yi Chang), Y.-J.H. and K.-L.W.: resources; M.A.: reviewing and editing; S.-M.H.: conceptualization, resources, supervision, reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the grants MOST108-2320-B-214-001, MOST109-2314-B-038-059, MOST109-2628-B-038-015, MOST109-2320-B-254-001, MOST109-2314-B-214-004, MOST110-2314-B-038-158, and MOST110-2628-B-038-018 from the Ministry of Science and Technology, Taiwan, NSTC111-2811-B-038-022 and 111-2628-B-038-019 from the National Science and Technology Council, Taiwan and supported by the Research Center for Food and Cosmetic Safety (ZRRPF3M0081), Chang Gung University of Science and Technology.
Institutional Review Board Statement
All animal studies were conducted according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Taipei Medical University (Permit No.: LAC-2019-0034).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sinha, S.; Bhatia, R.; Narasimamurthy, M.; Rayne, S.; Grover, S. Epidemiology of Breast Cancer Presentation in Botswana, South Africa, and the United States. J. Surg. Res. 2022, 279, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Anders, C.; Carey, L.A. Understanding and treating triple-negative breast cancer. Oncology 2008, 22, 1233–1239, Discussion 1239–1240+1243. [Google Scholar] [PubMed]
- Brown, K.A.; Simpson, E.R. Obesity and breast cancer: Progress to understanding the relationship. Cancer Res. 2010, 70, 4–7. [Google Scholar] [CrossRef]
- Kolb, R.; Zhang, W. Obesity and Breast Cancer: A Case of Inflamed Adipose Tissue. Cancers 2020, 12, 1686. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Thun, M.J. Obesity and cancer. Oncogene 2004, 23, 6365–6378. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Hung, A.C.; Lo, S.; Yuan, S.F. Adipocytokines visfatin and resistin in breast cancer: Clinical relevance, biological mechanisms, and therapeutic potential. Cancer Lett. 2021, 498, 229–239. [Google Scholar] [CrossRef]
- Muehlberg, F.L.; Song, Y.H.; Krohn, A.; Pinilla, S.P.; Droll, L.H.; Leng, X.; Seidensticker, M.; Ricke, J.; Altman, A.M.; Devarajan, E.; et al. Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis 2009, 30, 589–597. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Piperigkou, Z.; Karamanos, N.K.; Zolota, V. Altered Adipokine Expression in Tumor Microenvironment Promotes Development of Triple Negative Breast Cancer. Cancers 2022, 14, 4139. [Google Scholar] [CrossRef]
- Chiang, Y.-F.; Chen, H.-Y.; Huang, K.-C.; Lin, P.-H.; Hsia, S.-M. Dietary Antioxidant Trans-Cinnamaldehyde Reduced Visfatin-Induced Breast Cancer Progression: In Vivo and In Vitro Study. Antioxidants 2019, 8, 625. [Google Scholar] [CrossRef]
- Schüler-Toprak, S.; Ortmann, O.; Buechler, C.; Treeck, O. The Complex Roles of Adipokines in Polycystic Ovary Syndrome and Endometriosis. Biomedicines 2022, 10, 2503. [Google Scholar] [CrossRef]
- Assiri, A.M.; Kamel, H.F. Evaluation of diagnostic and predictive value of serum adipokines: Leptin, resistin and visfatin in postmenopausal breast cancer. Obes. Res. Clin. Pract. 2016, 10, 442–453. [Google Scholar] [CrossRef]
- Gujar, A.D.; Le, S.; Mao, D.D.; Dadey, D.Y.; Turski, A.; Sasaki, Y.; Aum, D.; Luo, J.; Dahiya, S.; Yuan, L.; et al. An NAD+-dependent transcriptional program governs self-renewal and radiation resistance in glioblastoma. Proc. Natl. Acad. Sci. USA 2016, 113, E8247–E8256. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Chen, H.D.; Lo, S.; Chen, Y.K.; Huang, Y.C.; Hu, S.C.; Hsieh, Y.C.; Hung, A.C.; Hou, M.F.; Yuan, S.F. Visfatin Enhances Breast Cancer Progression through CXCL1 Induction in Tumor-Associated Macrophages. Cancers 2020, 12, 3256. [Google Scholar] [CrossRef]
- Huang, J.Y.; Wang, Y.Y.; Lo, S.; Tseng, L.M.; Chen, D.R.; Wu, Y.C.; Hou, M.F.; Yuan, S.F. Visfatin Mediates Malignant Behaviors through Adipose-Derived Stem Cells Intermediary in Breast Cancer. Cancers 2019, 12, 29. [Google Scholar] [CrossRef]
- Sat-Muñoz, D.; Martínez-Herrera, B.E.; Quiroga-Morales, L.A.; Trujillo-Hernández, B.; González-Rodríguez, J.A.; Gutiérrez-Rodríguez, L.X.; Leal-Cortés, C.A.; Portilla-de-Buen, E.; Rubio-Jurado, B.; Salazar-Páramo, M.; et al. Adipocytokines and Insulin Resistance: Their Role as Benign Breast Disease and Breast Cancer Risk Factors in a High-Prevalence Overweight-Obesity Group of Women over 40 Years Old. Int. J. Environ. Res. Public Health 2022, 19, 6093. [Google Scholar] [CrossRef]
- Verdin, E. NAD⁺ in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef]
- Shi, L.; Tang, X.; Qian, M.; Liu, Z.; Meng, F.; Fu, L.; Wang, Z.; Zhu, W.G.; Huang, J.D.; Zhou, Z.; et al. A SIRT1-centered circuitry regulates breast cancer stemness and metastasis. Oncogene 2018, 37, 6299–6315. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, J.; Yang, H. OCT4, SOX2, and NANOG positive expression correlates with poor differentiation, advanced disease stages, and worse overall survival in HER2+ breast cancer patients. Onco Targets Ther. 2018, 11, 7873–7881. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Chen, H.; Lo, P.-K. In vitro Tumorsphere Formation Assays. Bio-protocol 2013, 3, e325. [Google Scholar] [CrossRef]
- Chiang, Y.F.; Chung, C.P.; Lin, J.H.; Chiang, W.; Chen, H.Y.; Ali, M.; Shih, Y.H.; Wang, K.L.; Huang, T.C.; Chang, H.Y.; et al. Adlay Seed (Coix lacryma-jobi L. var. Ma-yuen Stapf.) Ethanolic Extract Fractions and Subfractions Induce Cell Cycle Arrest and Apoptosis in Human Breast and Cervical Cancer Cell Lines. Molecules 2022, 27, 3984. [Google Scholar] [CrossRef]
- Behrouzfar, K.; Alaee, M.; Nourbakhsh, M.; Gholinejad, Z.; Golestani, A. Extracellular NAMPT/visfatin causes p53 deacetylation via NAD production and SIRT1 activation in breast cancer cells. Cell Biochem. Funct. 2017, 35, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.Q.; Li, H.X.; Hou, X.J.; Huang, M.Y.; Zhu, Z.M.; Wei, L.X.; Tang, C.X. Capsaicin suppresses hepatocarcinogenesis by inhibiting the stemness of hepatic progenitor cells via SIRT1/SOX2 signaling pathway. Cancer Med. 2022, 11, 4283–4296. [Google Scholar] [CrossRef] [PubMed]
- Abrahamson, P.E.; Gammon, M.D.; Lund, M.J.; Flagg, E.W.; Porter, P.L.; Stevens, J.; Swanson, C.A.; Brinton, L.A.; Eley, J.W.; Coates, R.J. General and abdominal obesity and survival among young women with breast cancer. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, W.; Dai, Z.; Wang, M.; Tian, T.; Liu, X.; Kang, H.; Guan, H.; Zhang, S.; Dai, Z. Association between body mass index and breast cancer risk: Evidence based on a dose-response meta-analysis. Cancer Manag. Res. 2018, 10, 143–151. [Google Scholar] [CrossRef]
- Lee, K.; Kruper, L.; Dieli-Conwright, C.M.; Mortimer, J.E. The Impact of Obesity on Breast Cancer Diagnosis and Treatment. Curr. Oncol. Rep. 2019, 21, 41. [Google Scholar] [CrossRef]
- Khan, S.; Shukla, S.; Sinha, S.; Meeran, S.M. Role of adipokines and cytokines in obesity-associated breast cancer: Therapeutic targets. Cytokine Growth Factor Rev. 2013, 24, 503–513. [Google Scholar] [CrossRef]
- Assiri, A.M.; Kamel, H.F.; Hassanien, M.F. Resistin, visfatin, adiponectin, and leptin: Risk of breast cancer in pre- and postmenopausal saudi females and their possible diagnostic and predictive implications as novel biomarkers. Dis. Markers 2015, 2015, 253519. [Google Scholar] [CrossRef]
- Dalamaga, M.; Karmaniolas, K.; Papadavid, E.; Pelekanos, N.; Sotiropoulos, G.; Lekka, A. Elevated serum visfatin/nicotinamide phosphoribosyl-transferase levels are associated with risk of postmenopausal breast cancer independently from adiponectin, leptin, and anthropometric and metabolic parameters. Menopause 2011, 18, 1198–1204. [Google Scholar] [CrossRef]
- Lucena-Cacace, A.; Otero-Albiol, D.; Jiménez-García, M.P.; Muñoz-Galvan, S.; Carnero, A. NAMPT Is a Potent Oncogene in Colon Cancer Progression that Modulates Cancer Stem Cell Properties and Resistance to Therapy through Sirt1 and PARP. Clin. Cancer. Res. 2018, 24, 1202–1215. [Google Scholar] [CrossRef]
- Srivastava, S. Emerging therapeutic roles for NAD+ metabolism in mitochondrial and age-related disorders. Clin. Transl. Med. 2016, 5, 25. [Google Scholar] [CrossRef]
- Jensen, M.M.; Erichsen, K.D.; Johnbeck, C.B.; Björkling, F.; Madsen, J.; Bzorek, M.; Jensen, P.B.; Højgaard, L.; Sehested, M.; Kjær, A. [18F]FLT and [18F]FDG PET for non-invasive treatment monitoring of the nicotinamide phosphoribosyltransferase inhibitor APO866 in human xenografts. PLoS ONE 2013, 8, e53410. [Google Scholar] [CrossRef]
- Sharif, T.; Martell, E.; Dai, C.; Kennedy, B.E.; Murphy, P.; Clements, D.R.; Kim, Y.; Lee, P.W.; Gujar, S.A. Autophagic homeostasis is required for the pluripotency of cancer stem cells. Autophagy 2017, 13, 264–284. [Google Scholar] [CrossRef]
- Grolla, A.A.; Travelli, C.; Genazzani, A.A.; Sethi, J.K. Extracellular nicotinamide phosphoribosyltransferase, a new cancer metabokine. Br. J. Pharmacol. 2016, 173, 2182–2194. [Google Scholar] [CrossRef]
- Dalamaga, M.; Archondakis, S.; Sotiropoulos, G.; Karmaniolas, K.; Pelekanos, N.; Papadavid, E.; Lekka, A. Could serum visfatin be a potential biomarker for postmenopausal breast cancer? Maturitas 2012, 71, 301–308. [Google Scholar] [CrossRef]
- Samal, B.; Sun, Y.; Stearns, G.; Xie, C.; Suggs, S.; McNiece, I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol. Cell. Biol. 1994, 14, 1431–1437. [Google Scholar] [CrossRef]
- Vona-Davis, L.; Rose, D.P. Angiogenesis, adipokines and breast cancer. Cytokine Growth Factor Rev. 2009, 20, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F. Angiogenesis: Update 2005. J. Thromb. Haemost. 2005, 3, 1835–1842. [Google Scholar] [CrossRef]
- Seton-Rogers, S. Cancer stem cells. VEGF promotes stemness. Nat. Rev. Cancer 2011, 11, 831. [Google Scholar] [CrossRef]
- Nave, O. Adding features from the mathematical model of breast cancer to predict the tumour size. Int. J. Comput. Math. Comput. Syst. Theory 2020, 5, 159–174. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).