Temporal Appearance of Enhanced Innate Anxiety in Alzheimer Model Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Behavioral Testing
2.3.1. Predator Odor Test Using 2-methyl-thiazoline (2MT)
2.3.2. Open-Field Test
2.4. Immunohistochemistry
2.5. Statistics
3. Results
3.1. Fox Odor Avoidance Behavior
3.2. Open-Field Behavior
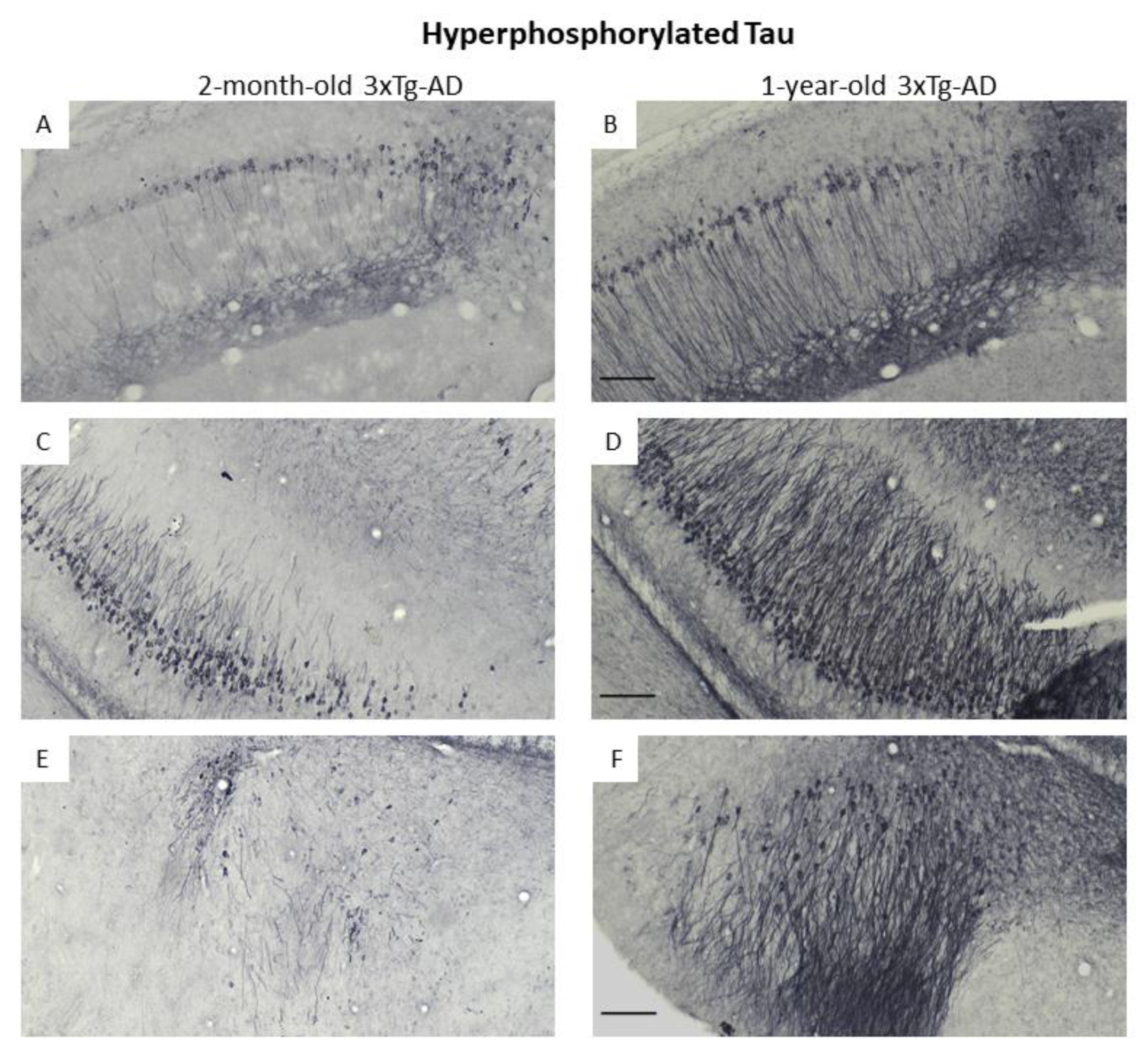
3.3. Immunohistochemical Confirmation of Temporal Appearance of the Histological Hallmarks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gimenez-Llort, L.; Alveal-Mellado, D. Digging Signatures in 13-Month-Old 3xTg-AD Mice for Alzheimer’s Disease and Its Disruption by Isolation Despite Social Life Since They Were Born. Front. Behav. Neurosci. 2020, 14, 611384. [Google Scholar] [CrossRef]
- Babulal, G.M.; Chen, L.; Doherty, J.M.; Murphy, S.A.; Johnson, A.M.; Roe, C.M. Longitudinal Changes in Anger, Anxiety, and Fatigue Are Associated with Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 87, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Nedelec, T.; Couvy-Duchesne, B.; Monnet, F.; Daly, T.; Ansart, M.; Gantzer, L.; Lekens, B.; Epelbaum, S.; Dufouil, C.; Durrleman, S. Identifying health conditions associated with Alzheimer’s disease up to 15 years before diagnosis: An agnostic study of French and British health records. Lancet. Digit. Health 2022, 4, e169–e178. [Google Scholar] [CrossRef] [PubMed]
- Hebda-Bauer, E.K.; Simmons, T.A.; Sugg, A.; Ural, E.; Stewart, J.A.; Beals, J.L.; Wei, Q.; Watson, S.J.; Akil, H. 3xTg-AD mice exhibit an activated central stress axis during early-stage pathology. J. Alzheimer’s Dis. 2013, 33, 407–422. [Google Scholar] [CrossRef]
- Zhao, Q.; Xiang, H.; Cai, Y.; Meng, S.S.; Zhang, Y.; Qiu, P. Systematic evaluation of the associations between mental disorders and dementia: An umbrella review of systematic reviews and meta-analyses. J. Affect. Disord. 2022, 307, 301–309. [Google Scholar] [CrossRef]
- Falgas, N.; Allen, I.E.; Spina, S.; Grant, H.; Pina Escudero, S.D.; Merrilees, J.; Gearhart, R.; Rosen, H.J.; Kramer, J.H.; Seeley, W.W.; et al. The severity of neuropsychiatric symptoms is higher in early-onset than late-onset Alzheimer’s disease. Eur. J. Neurol. 2022, 29, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Pairojana, T.; Phasuk, S.; Suresh, P.; Huang, S.P.; Pakaprot, N.; Chompoopong, S.; Hsieh, T.C.; Liu, I.Y. Age and gender differences for the behavioral phenotypes of 3xTg alzheimer’s disease mice. Brain Res. 2021, 1762, 147437. [Google Scholar] [CrossRef]
- Oddo, S.; Billings, L.; Kesslak, J.P.; Cribbs, D.H.; LaFerla, F.M. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron 2004, 43, 321–332. [Google Scholar] [CrossRef]
- Varkonyi, D.; Torok, B.; Sipos, E.; Fazekas, C.L.; Banrevi, K.; Correia, P.; Chaves, T.; Farkas, S.; Szabo, A.; Martinez-Bellver, S.; et al. Investigation of Anxiety- and Depressive-like Symptoms in 4- and 8-Month-Old Male Triple Transgenic Mouse Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 10816. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Feldon, J.; Yee, B.K. Age-dependent phenotypic characteristics of a triple transgenic mouse model of Alzheimer disease. Behav. Neurosci. 2008, 122, 733–747. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Sun, Y.; Li, R.; Brana, C.; Feldon, J.; Yee, B.K. Limited impact of social isolation on Alzheimer-like symptoms in a triple transgenic mouse model. Behav. Neurosci. 2009, 123, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Pietropaolo, S.; Feldon, J.; Yee, B.K. Environmental enrichment eliminates the anxiety phenotypes in a triple transgenic mouse model of Alzheimer’s disease. Cogn. Affect. Behav. Neurosci. 2014, 14, 996–1008. [Google Scholar] [CrossRef]
- Bruzsik, B.; Biro, L.; Sarosdi, K.R.; Zelena, D.; Sipos, E.; Szebik, H.; Torok, B.; Mikics, E.; Toth, M. Neurochemically distinct populations of the bed nucleus of stria terminalis modulate innate fear response to weak threat evoked by predator odor stimuli. Neurobiol. Stress 2021, 15, 100415. [Google Scholar] [CrossRef] [PubMed]
- Staples, L.G. Predator odor avoidance as a rodent model of anxiety: Learning-mediated consequences beyond the initial exposure. Neurobiol. Learn. Mem. 2010, 94, 435–445. [Google Scholar] [CrossRef]
- Le Moene, O.; Agmo, A. Responses to positive and aversive stimuli in estrous female rats housed in a seminatural environment: Effects of yohimbine and chlordiazepoxide. Pharmacol. Biochem. Behav. 2019, 179, 43–54. [Google Scholar] [CrossRef]
- Hebb, A.L.; Zacharko, R.M.; Dominguez, H.; Trudel, F.; Laforest, S.; Drolet, G. Odor-induced variation in anxiety-like behavior in mice is associated with discrete and differential effects on mesocorticolimbic cholecystokinin mRNA expression. Neuropsychopharmacology 2002, 27, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Flores Guzman, B.; Elizabeth Chaffey, T.; Hansika Palpagama, T.; Waters, S.; Boix, J.; Tate, W.P.; Peppercorn, K.; Dragunow, M.; Waldvogel, H.J.; Faull, R.L.M.; et al. The Interplay Between Beta-Amyloid 1-42 (Abeta1-42)-Induced Hippocampal Inflammatory Response, p-tau, Vascular Pathology, and Their Synergistic Contributions to Neuronal Death and Behavioral Deficits. Front. Mol. Neurosci. 2020, 13, 522073. [Google Scholar] [CrossRef]
- Wu, N.; Rao, X.; Gao, Y.; Wang, J.; Xu, F. Amyloid-beta deposition and olfactory dysfunction in an Alzheimer’s disease model. J. Alzheimer’s Dis. 2013, 37, 699–712. [Google Scholar] [CrossRef]
- Alvarado-Martinez, R.; Salgado-Puga, K.; Pena-Ortega, F. Amyloid beta inhibits olfactory bulb activity and the ability to smell. PLoS ONE 2013, 8, e75745. [Google Scholar] [CrossRef]
- Wesson, D.W.; Levy, E.; Nixon, R.A.; Wilson, D.A. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer’s disease mouse model. J. Neurosci. 2010, 30, 505–514. [Google Scholar] [CrossRef]
- Puzzo, D.; Privitera, L.; Leznik, E.; Fa, M.; Staniszewski, A.; Palmeri, A.; Arancio, O. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J. Neurosci. 2008, 28, 14537–14545. [Google Scholar] [CrossRef]
- Wesson, D.W.; Borkowski, A.H.; Landreth, G.E.; Nixon, R.A.; Levy, E.; Wilson, D.A. Sensory network dysfunction, behavioral impairments, and their reversibility in an Alzheimer’s beta-amyloidosis mouse model. J. Neurosci. 2011, 31, 15962–15971. [Google Scholar] [CrossRef] [PubMed]
- Palop, J.J.; Mucke, L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: From synapses toward neural networks. Nat. Neurosci. 2010, 13, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, W.S.; Pan, M.; Ossenkoppele, R.; Coesmans, M.; Gatchel, J.R.; Ismail, Z.; Lanctot, K.L.; Fischer, C.E.; Mortby, M.E.; van den Berg, E.; et al. Sex differences in neuropsychiatric symptoms in Alzheimer’s disease dementia: A meta-analysis. Alzheimer’s Res. Ther. 2022, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Vasiliadis, H.M.; Desjardins, F.; Roberge, P.; Grenier, S. Sex Differences in Anxiety Disorders in Older Adults. Curr. Psychiatry Rep. 2020, 22, 75. [Google Scholar] [CrossRef]
- Likhtik, E.; Stujenske, J.M.; Topiwala, M.A.; Harris, A.Z.; Gordon, J.A. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat. Neurosci. 2014, 17, 106–113. [Google Scholar] [CrossRef]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The Open Field Test. In Mood and Anxiety Related Phenotypes in Mice; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–20. [Google Scholar] [CrossRef]
- Wirth-Dzieciolowska, E.; Lipska, A.; Wesierska, M. Selection for body weight induces differences in exploratory behavior and learning in mice. Acta. Neurobiol. Exp. (Wars) 2005, 65, 243–253. [Google Scholar]
- Konkoly, J.; Kormos, V.; Gaszner, B.; Sandor, Z.; Kecskes, A.; Alomari, A.; Szilagyi, A.; Szilagyi, B.; Zelena, D.; Pinter, E. The Role of TRPA1 Channels in the Central Processing of Odours Contributing to the Behavioural Responses of Mice. Pharmaceuticals 2021, 14, 1336. [Google Scholar] [CrossRef]
- Trease, A.J.; George, J.W.; Roland, N.J.; Lichter, E.Z.; Emanuel, K.; Totusek, S.; Fox, H.S.; Stauch, K.L. Hyperphosphorylated Human Tau Accumulates at the Synapse, Localizing on Synaptic Mitochondrial Outer Membranes and Disrupting Respiration in a Mouse Model of Tauopathy. Front. Mol. Neurosci. 2022, 15, 852368. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, M.; Yin, X.; Chen, K.; Hu, Z.; Zhou, Q.; Cao, X.; Chen, Z.; Liu, D. The role of pathological tau in synaptic dysfunction in Alzheimer’s diseases. Transl. Neurodegener. 2021, 10, 45. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, H.; Yao, L.; Wei, Y. Olfactory impairment and the risk of cognitive decline and dementia in older adults: A meta-analysis. Braz. J. Otorhinolaryngol. 2021, 87, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.R., III; Carter, M.A.; Monroe, T.B. Narrative Review of Sensory Changes as a Biomarker for Alzheimer’s Disease. Biol. Res. Nurs. 2021, 23, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, P.; Schuurman, T.; Prinz, H. Loss of smell leads to dementia in mice: Is Alzheimer’s disease a degenerative disorder of the olfactory system? J. Protein. Chem. 1989, 8, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Mitrano, D.A.; Houle, S.E.; Pearce, P.; Quintanilla, R.M.; Lockhart, B.K.; Genovese, B.C.; Schendzielos, R.A.; Croushore, E.E.; Dymond, E.M.; Bogenpohl, J.W.; et al. Olfactory dysfunction in the 3xTg-AD model of Alzheimer’s disease. IBRO Neurosci. Rep. 2021, 10, 51–61. [Google Scholar] [CrossRef]
- Coronas-Samano, G.; Portillo, W.; Beltran Campos, V.; Medina-Aguirre, G.I.; Paredes, R.G.; Diaz-Cintra, S. Deficits in odor-guided behaviors in the transgenic 3xTg-AD female mouse model of Alzheimer’s disease. Brain Res. 2014, 1572, 18–25. [Google Scholar] [CrossRef]
- Nguyen, E.T.; Selmanovic, D.; Maltry, M.; Morano, R.; Franco-Villanueva, A.; Estrada, C.M.; Solomon, M.B. Endocrine stress responsivity and social memory in 3xTg-AD female and male mice: A tale of two experiments. Horm. Behav. 2020, 126, 104852. [Google Scholar] [CrossRef]
- Marin-Pardo, D.; Gimenez-Llort, L. Olfactory Signatures in the Food Finding Test in Mice With Normal and Alzheimer’s Disease-Pathological Aging With Special Concerns on the Effects of Social Isolation. Front. Neurosci. 2021, 15, 733984. [Google Scholar] [CrossRef]
- Sancheti, H.; Patil, I.; Kanamori, K.; Diaz Brinton, R.; Zhang, W.; Lin, A.L.; Cadenas, E. Hypermetabolic state in the 7-month-old triple transgenic mouse model of Alzheimer’s disease and the effect of lipoic acid: A 13C-NMR study. J. Cereb. Blood Flow. Metab. 2014, 34, 1749–1760. [Google Scholar] [CrossRef]
- Marchese, M.; Cowan, D.; Head, E.; Ma, D.; Karimi, K.; Ashthorpe, V.; Kapadia, M.; Zhao, H.; Davis, P.; Sakic, B. Autoimmune manifestations in the 3xTg-AD model of Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 39, 191–210. [Google Scholar] [CrossRef]
- Adlimoghaddam, A.; Snow, W.M.; Stortz, G.; Perez, C.; Djordjevic, J.; Goertzen, A.L.; Ko, J.H.; Albensi, B.C. Regional hypometabolism in the 3xTg mouse model of Alzheimer’s disease. Neurobiol. Dis. 2019, 127, 264–277. [Google Scholar] [CrossRef]
- Friesen, M.; Ziegler-Waldkirch, S.; Egenolf, M.; d’Errico, P.; Helm, C.; Mezo, C.; Dokalis, N.; Erny, D.; Katzmarski, N.; Coelho, R.; et al. Distinct Abeta pathology in the olfactory bulb and olfactory deficits in a mouse model of Abeta and alpha-syn co-pathology. Brain Pathol. 2022, 32, e13032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Xing, R.Z.; Luo, X.B.; Xu, H.; Chang, R.C.; Zou, L.Y.; Liu, J.J.; Yang, X.F. Anxiety-like behavior and dysregulation of miR-34a in triple transgenic mice of Alzheimer’s disease. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2853–2862. [Google Scholar] [PubMed]
- Ong-Palsson, E.; Njavro, J.R.; Wilson, Y.; Pigoni, M.; Schmidt, A.; Muller, S.A.; Meyer, M.; Hartmann, J.; Busche, M.A.; Gunnersen, J.M.; et al. The beta-Secretase Substrate Seizure 6-Like Protein (SEZ6L) Controls Motor Functions in Mice. Mol. Neurobiol. 2022, 59, 1183–1198. [Google Scholar] [CrossRef]
- Yu, N.Y.; Chang, S.H. Characterization of the fine motor problems in patients with cognitive dysfunction—A computerized handwriting analysis. Hum. Mov. Sci. 2019, 65, 71–79. [Google Scholar] [CrossRef]
- Liu, X.; Abudukeremu, A.; Jiang, Y.; Cao, Z.; Wu, M.; Sun, R.; Chen, Z.; Chen, Y.; Zhang, Y.; Wang, J. Fine or Gross Motor Index as a Simple Tool for Predicting Cognitive Impairment in Elderly People: Findings from The Irish Longitudinal Study on Ageing (TILDA). J. Alzheimer’s Dis. 2021, 83, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Langford-Smith, A.; Malinowska, M.; Langford-Smith, K.J.; Wegrzyn, G.; Jones, S.; Wynn, R.; Wraith, J.E.; Wilkinson, F.L.; Bigger, B.W. Hyperactive behaviour in the mouse model of mucopolysaccharidosis IIIB in the open field and home cage environments. Genes. Brain Behav. 2011, 10, 673–682. [Google Scholar] [CrossRef]
- Catuzzi, J.E.; Beck, K.D. Anxiety vulnerability in women: A two-hit hypothesis. Exp. Neurol. 2014, 259, 75–80. [Google Scholar] [CrossRef]
- Moser, J.S.; Moran, T.P.; Kneip, C.; Schroder, H.S.; Larson, M.J. Sex moderates the association between symptoms of anxiety, but not obsessive compulsive disorder, and error-monitoring brain activity: A meta-analytic review. Psychophysiology 2016, 53, 21–29. [Google Scholar] [CrossRef]
- Kosel, F.; Pelley, J.M.S.; Franklin, T.B. Behavioural and psychological symptoms of dementia in mouse models of Alzheimer’s disease-related pathology. Neurosci. Biobehav. Rev. 2020, 112, 634–647. [Google Scholar] [CrossRef]
- Hutton, C.P.; Lemon, J.A.; Sakic, B.; Rollo, C.D.; Boreham, D.R.; Fahnestock, M.; Wojtowicz, J.M.; Becker, S. Early Intervention with a Multi-Ingredient Dietary Supplement Improves Mood and Spatial Memory in a Triple Transgenic Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, 835–857. [Google Scholar] [CrossRef]
- Kapadia, M.; Mian, M.F.; Ma, D.; Hutton, C.P.; Azam, A.; Narkaj, K.; Cao, C.; Brown, B.; Michalski, B.; Morgan, D.; et al. Differential effects of chronic immunosuppression on behavioral, epigenetic, and Alzheimer’s disease-associated markers in 3xTg-AD mice. Alzheimer’s Res. Ther. 2021, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Baeta-Corral, R.; Defrin, R.; Pick, C.G.; Gimenez-Llort, L. Tail-flick test response in 3xTg-AD mice at early and advanced stages of disease. Neurosci. Lett. 2015, 600, 158–163. [Google Scholar] [CrossRef]
- Nie, L.; He, K.; Xie, F.; Xiao, S.; Li, S.; Xu, J.; Zhang, K.; Yang, C.; Zhou, L.; Liu, J.; et al. Loganin substantially ameliorates molecular deficits, pathologies and cognitive impairment in a mouse model of Alzheimer’s disease. Aging 2021, 13, 23739–23756. [Google Scholar] [CrossRef]
- Gloria, Y.; Ceyzeriat, K.; Tsartsalis, S.; Millet, P.; Tournier, B.B. Dopaminergic dysfunction in the 3xTg-AD mice model of Alzheimer’s disease. Sci. Rep. 2021, 11, 19412. [Google Scholar] [CrossRef]
- Sterniczuk, R.; Antle, M.C.; Laferla, F.M.; Dyck, R.H. Characterization of the 3xTg-AD mouse model of Alzheimer’s disease: Part 2. Behavioral and cognitive changes. Brain Res. 2010, 1348, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Xia, J.; Li, H.; Zhang, Z.; Yang, Y.; Huang, X.; He, Z.; Liu, J.; Yang, X. Ginsenoside Rg1 Ameliorates Behavioral Abnormalities and Modulates the Hippocampal Proteomic Change in Triple Transgenic Mice of Alzheimer’s Disease. Oxid. Med.Cell Longev. 2017, 2017, 6473506. [Google Scholar] [CrossRef]
- Qu, X.S.; Shi, H.; Cao, X.L.; Dong, X.F.; Li, T.; Jiao, J.J.; Qi, J.S.; Wu, M.N. Effects of adiponectin on the anxiety and memory impairment in triple transgenic Alzheimer’s disease model mice. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2017, 33, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Escrig, A.; Canal, C.; Sanchis, P.; Fernandez-Gayol, O.; Montilla, A.; Comes, G.; Molinero, A.; Giralt, M.; Gimenez-Llort, L.; Becker-Pauly, C.; et al. IL-6 trans-signaling in the brain influences the behavioral and physio-pathological phenotype of the Tg2576 and 3xTgAD mouse models of Alzheimer’s disease. Brain Behav. Immun. 2019, 82, 145–159. [Google Scholar] [CrossRef]
- Muntsant, A.; Gimenez-Llort, L. Impact of Social Isolation on the Behavioral, Functional Profiles, and Hippocampal Atrophy Asymmetry in Dementia in Times of Coronavirus Pandemic (COVID-19): A Translational Neuroscience Approach. Front. Psychiatry 2020, 11, 572583. [Google Scholar] [CrossRef]
- Nie, L.; Wei, G.; Peng, S.; Qu, Z.; Yang, Y.; Yang, Q.; Huang, X.; Liu, J.; Zhuang, Z.; Yang, X. Melatonin ameliorates anxiety and depression-like behaviors and modulates proteomic changes in triple transgenic mice of Alzheimer’s disease. Biofactors 2017, 43, 593–611. [Google Scholar] [CrossRef]
- Muntsant, A.; Jimenez-Altayo, F.; Puertas-Umbert, L.; Jimenez-Xarrie, E.; Vila, E.; Gimenez-Llort, L. Sex-Dependent End-of-Life Mental and Vascular Scenarios for Compensatory Mechanisms in Mice with Normal and AD-Neurodegenerative Aging. Biomedicines 2021, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Altayo, F.; Sanchez-Ventura, J.; Vila, E.; Gimenez-Llort, L. Crosstalk between Peripheral Small Vessel Properties and Anxious-like Profiles: Sex, Genotype, and Interaction Effects in Mice with Normal Aging and 3xTg-AD mice at Advanced Stages of Disease. J. Alzheimer’s Dis. 2018, 62, 1531–1538. [Google Scholar] [CrossRef]
- Muntsant, A.; Gimenez-Llort, L. Genotype Load Modulates Amyloid Burden and Anxiety-Like Patterns in Male 3xTg-AD Survivors despite Similar Neuro-Immunoendocrine, Synaptic and Cognitive Impairments. Biomedicines 2021, 9, 715. [Google Scholar] [CrossRef]
- Torres-Lista, V.; De la Fuente, M.; Gimenez-Llort, L. Survival Curves and Behavioral Profiles of Female 3xTg-AD Mice Surviving to 18-Months of Age as Compared to Mice with Normal Aging. J. Alzheimer’s Dis. Rep. 2017, 1, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Virgili, J.; Lebbadi, M.; Tremblay, C.; St-Amour, I.; Pierrisnard, C.; Faucher-Genest, A.; Emond, V.; Julien, C.; Calon, F. Characterization of a 3xTg-AD mouse model of Alzheimer’s disease with the senescence accelerated mouse prone 8 (SAMP8) background. Synapse 2018, 72, e22025. [Google Scholar] [CrossRef]
- Garcia-Mesa, Y.; Lopez-Ramos, J.C.; Gimenez-Llort, L.; Revilla, S.; Guerra, R.; Gruart, A.; Laferla, F.M.; Cristofol, R.; Delgado-Garcia, J.M.; Sanfeliu, C. Physical exercise protects against Alzheimer’s disease in 3xTg-AD mice. J. Alzheimer’s Dis. 2011, 24, 421–454. [Google Scholar] [CrossRef]
- Garcia-Mesa, Y.; Gimenez-Llort, L.; Lopez, L.C.; Venegas, C.; Cristofol, R.; Escames, G.; Acuna-Castroviejo, D.; Sanfeliu, C. Melatonin plus physical exercise are highly neuroprotective in the 3xTg-AD mouse. Neurobiol. Aging 2012, 33, 1124.e1113–1129. [Google Scholar] [CrossRef] [PubMed]
- Guillemaud, O.; Ceyzeriat, K.; Saint-Georges, T.; Cambon, K.; Petit, F.; Ben Haim, L.; Carrillo-de Sauvage, M.A.; Guillermier, M.; Bernier, S.; Herard, A.S.; et al. Complex roles for reactive astrocytes in the triple transgenic mouse model of Alzheimer disease. Neurobiol. Aging 2020, 90, 135–146. [Google Scholar] [CrossRef]
- Fertan, E.; Stover, K.R.J.; Brant, M.G.; Stafford, P.M.; Kelly, B.; Diez-Cecilia, E.; Wong, A.A.; Weaver, D.F.; Brown, R.E. Effects of the Novel IDO Inhibitor DWG-1036 on the Behavior of Male and Female 3xTg-AD Mice. Front. Pharmacol. 2019, 10, 1044. [Google Scholar] [CrossRef]
- Baeta-Corral, R.; Johansson, B.; Gimenez-Llort, L. Long-term Treatment with Low-Dose Caffeine Worsens BPSD-Like Profile in 3xTg-AD Mice Model of Alzheimer’s Disease and Affects Mice with Normal Aging. Front. Pharmacol. 2018, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- St-Amour, I.; Pare, I.; Tremblay, C.; Coulombe, K.; Bazin, R.; Calon, F. IVIg protects the 3xTg-AD mouse model of Alzheimer’s disease from memory deficit and Abeta pathology. J. Neuroinflammation 2014, 11, 54. [Google Scholar] [CrossRef]
- Corpas, R.; Grinan-Ferre, C.; Rodriguez-Farre, E.; Pallas, M.; Sanfeliu, C. Resveratrol Induces Brain Resilience Against Alzheimer Neurodegeneration Through Proteostasis Enhancement. Mol. Neurobiol. 2019, 56, 1502–1516. [Google Scholar] [CrossRef]
- Tournissac, M.; Vu, T.M.; Vrabic, N.; Hozer, C.; Tremblay, C.; Melancon, K.; Planel, E.; Pifferi, F.; Calon, F. Repurposing beta-3 adrenergic receptor agonists for Alzheimer’s disease: Beneficial effects in a mouse model. Alzheimer’s Res. Ther. 2021, 13, 103. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mesa, Y.; Colie, S.; Corpas, R.; Cristofol, R.; Comellas, F.; Nebreda, A.R.; Gimenez-Llort, L.; Sanfeliu, C. Oxidative Stress Is a Central Target for Physical Exercise Neuroprotection Against Pathological Brain Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Bories, C.; Arsenault, D.; Lemire, M.; Tremblay, C.; De Koninck, Y.; Calon, F. Transgenic autoinhibition of p21-activated kinase exacerbates synaptic impairments and fronto-dependent behavioral deficits in an animal model of Alzheimer’s disease. Aging 2017, 9, 1386–1403. [Google Scholar] [CrossRef]
- Corpas, R.; Grinan-Ferre, C.; Palomera-Avalos, V.; Porquet, D.; Garcia de Frutos, P.; Franciscato Cozzolino, S.M.; Rodriguez-Farre, E.; Pallas, M.; Sanfeliu, C.; Cardoso, B.R. Melatonin induces mechanisms of brain resilience against neurodegeneration. J. Pineal. Res. 2018, 65, e12515. [Google Scholar] [CrossRef] [PubMed]
- Torres-Lista, V.; Lopez-Pousa, S.; Gimenez-Llort, L. Marble-burying is enhanced in 3xTg-AD mice, can be reversed by risperidone and it is modulable by handling. Behav. Process. 2015, 116, 69–74. [Google Scholar] [CrossRef]
- Santana-Santana, M.; Bayascas, J.R.; Gimenez-Llort, L. Sex-Dependent Signatures, Time Frames and Longitudinal Fine-Tuning of the Marble Burying Test in Normal and AD-Pathological Aging Mice. Biomedicines 2021, 9, 994. [Google Scholar] [CrossRef]
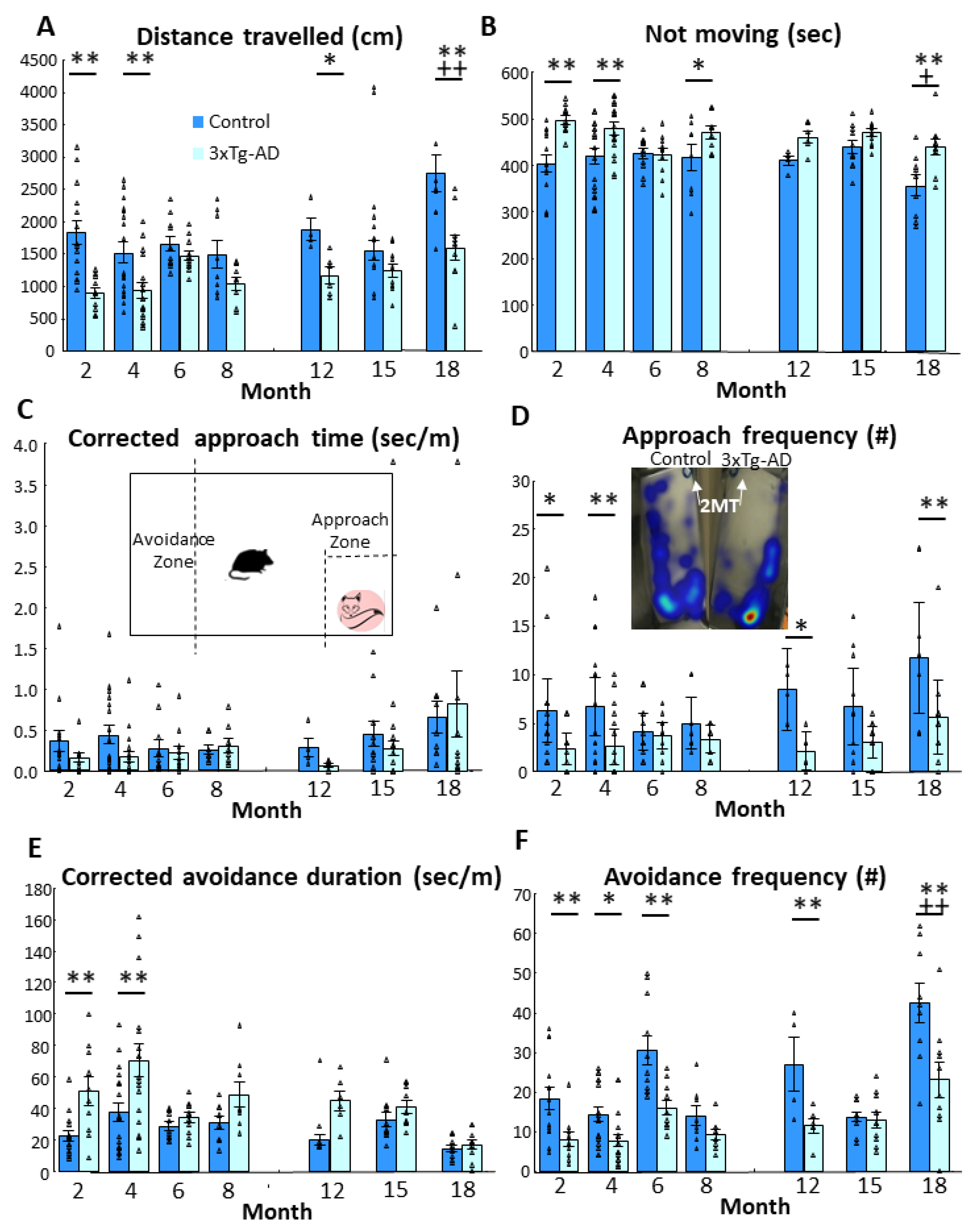

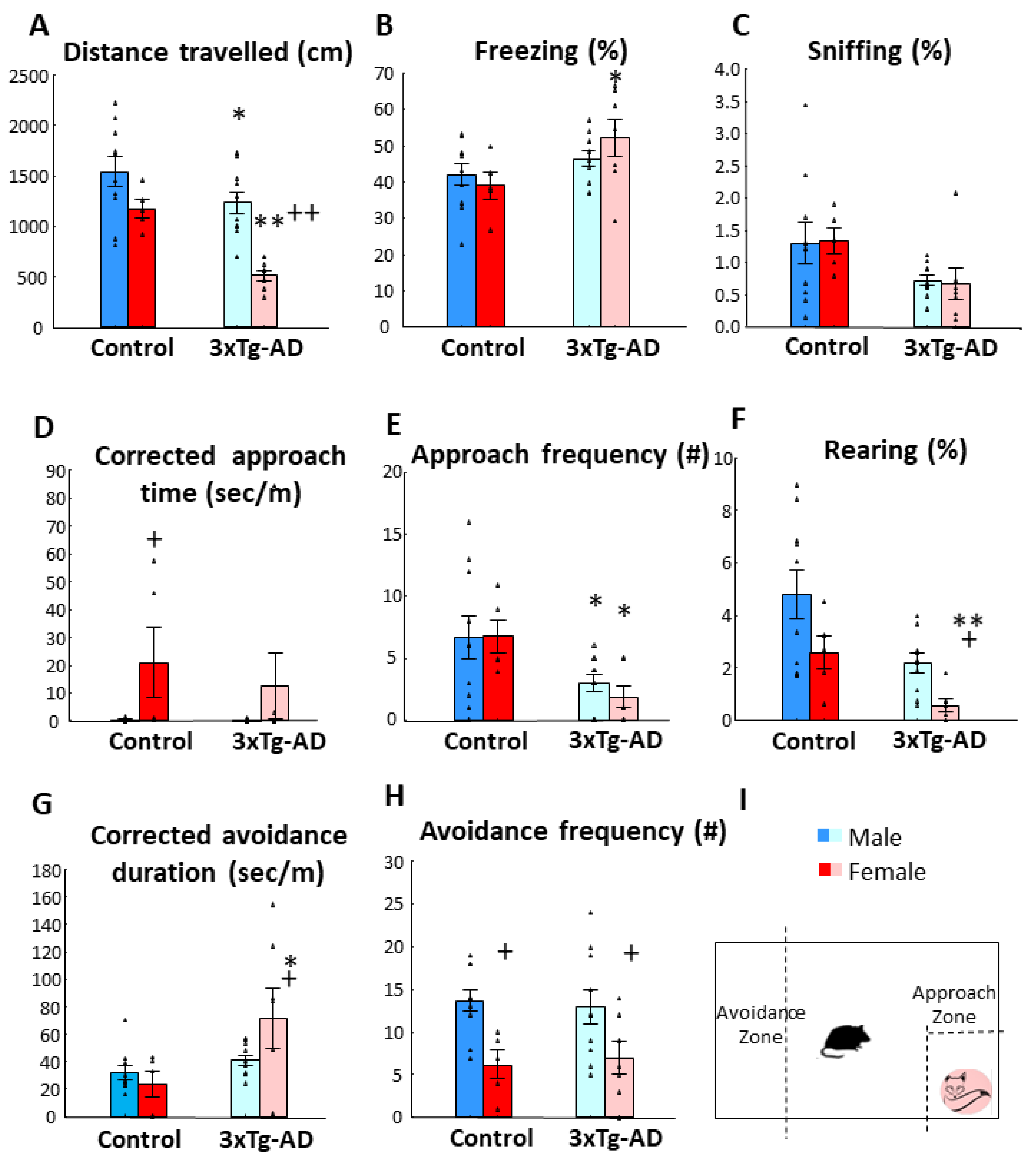
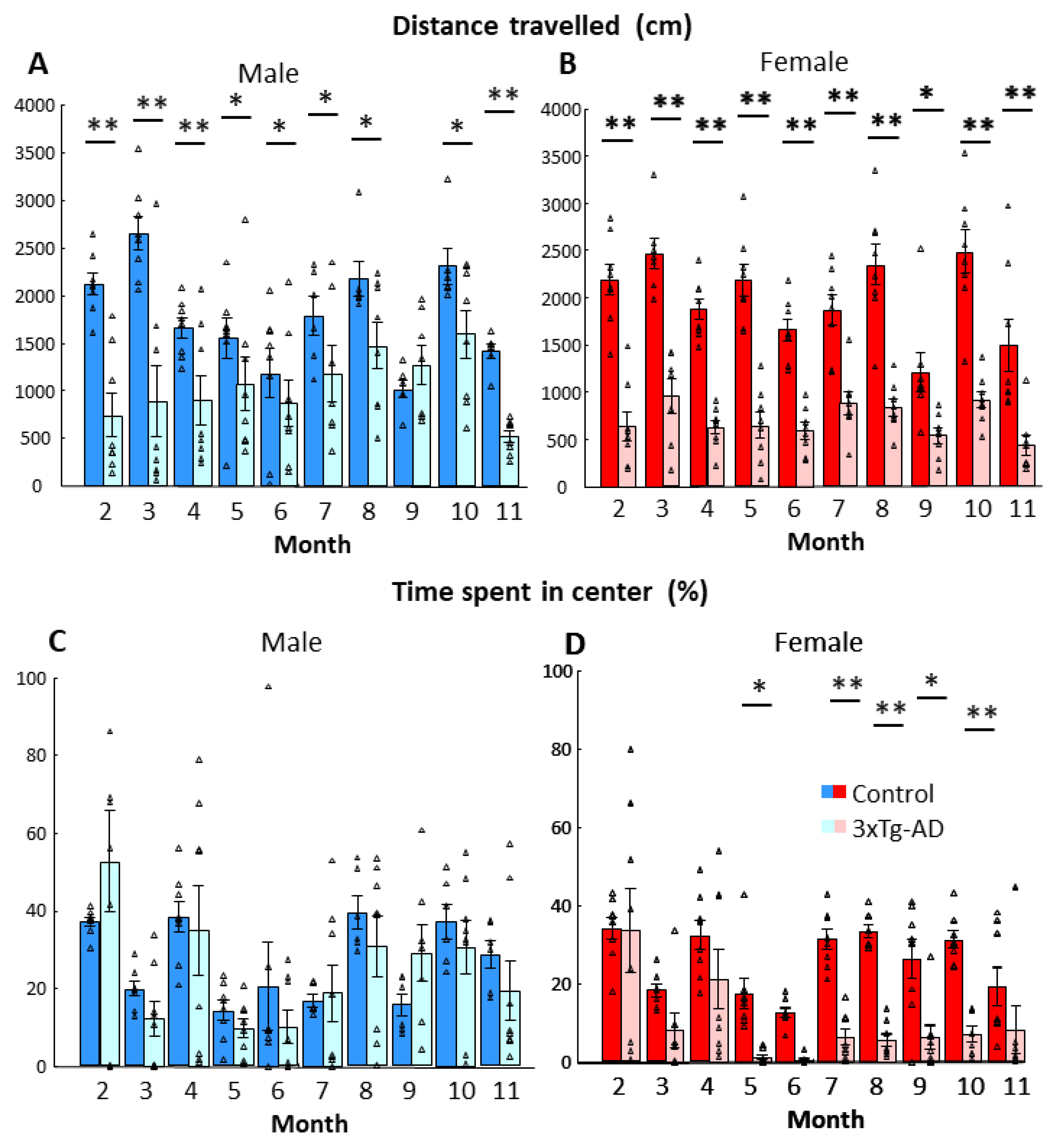


| Parameters | Genotype | Age | Genotype × Age | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Distance | 42.844 | 0.000 | 7.446 | 0.000 | 2.098 | 0.057 |
| Not moving (s) | 29.025 | 0.000 | 2.618 | 0.019 | 1.784 | 0.107 |
| Approach duration | 5.047 | 0.026 | 4.425 | 0.004 | 0.396 | 0.880 |
| Corrected approach duration | 1.431 | 0.233 | 2.598 | 0.020 | 0.559 | 0.762 |
| Approach frequency | 23.080 | 0.000 | 2.662 | 0.018 | 1.051 | 0.395 |
| Avoidance duration | 3.594 | 0.060 | 4.289 | 0.000 | 1.102 | 0.364 |
| Corrected duration avoidance | 19.306 | 0.000 | 6.812 | 0.000 | 1.750 | 0.114 |
| Avoidance frequency | 42.238 | 0.000 | 16.709 | 0.000 | 2.604 | 0.020 |
| Parameters | Genotype | Sex | Genotype x Sex | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Distance | 20.596 | 0.000 | 1.041 | 0.314 | 0.743 | 0.394 |
| Not moving (s) | 0.716 | 0.403 | 0.685 | 0.413 | 3.258 | 0.079 |
| Approach duration | 0.490 | 0.488 | 0.031 | 0.859 | 1.721 | 0.197 |
| Corrected approach duration | 0.004 | 0.945 | 0.271 | 0.605 | 1.267 | 0.267 |
| Approach frequency | 3.930 | 0.055 | 0.153 | 0.697 | 0.682 | 0.414 |
| Avoidance duration | 0.112 | 0.738 | 0.143 | 0.706 | 2.048 | 0.161 |
| Corrected duration avoidance | 6.061 | 0.018 | 0.178 | 0.675 | 1.707 | 0.199 |
| Avoidance frequency | 9.338 | 0.004 | 2.076 | 0.158 | 0.202 | 0.655 |
| Freezing duration | 7.464 | 0.009 | 0.090 | 0.765 | 0.866 | 0.358 |
| Freezing frequency | 4.116 | 0.050 | 14.862 | 0.000 | 0.882 | 0.354 |
| Sniffing duration | 2.004 | 0.165 | 0.354 | 0.555 | 3.181 | 0.083 |
| Sniffing frequency | 5.349 | 0.027 | 1.036 | 0.316 | 3.221 | 0.081 |
| Rearing duration | 1.342 | 0.254 | 0.414 | 0.523 | 3.971 | 0.054 |
| Rearing frequency | 3.408 | 0.073 | 0.324 | 0.573 | 2.704 | 0.109 |
| Parameters | Genotype | Sex | Genotype × Sex | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Distance | 14.351 | 0.000 | 18.199 | 0.000 | 1.890 | 0.180 |
| Not moving (s) | 17.604 | 0.000 | 17.633 | 0.000 | 3.181 | 0.085 |
| Approach duration | 2.119 | 0.156 | 7.446 | 0.010 | 1.833 | 0.186 |
| Corrected approach duration | 0.427 | 0.518 | 6.033 | 0.020 | 0.392 | 0.535 |
| Approach frequency | 10.092 | 0.036 | 0.146 | 0.704 | 0.208 | 0.651 |
| Avoidance duration | 0.547 | 0.465 | 4.197 | 0.049 | 0.034 | 0.853 |
| Corrected duration avoidance | 6.479 | 0.016 | 0.944 | 0.339 | 2.995 | 0.094 |
| Avoidance frequency | 0.000 | 0.978 | 13.149 | 0.001 | 0.162 | 0.690 |
| Freezing duration | 6.091 | 0.019 | 0.007 | 0.977 | 0.030 | 0.861 |
| Freezing frequency | 0.743 | 0.396 | 7.030 | 0.013 | 3.189 | 0.085 |
| Sniffing duration | 10.068 | 0.003 | 5.476 | 0.026 | 0.001 | 0.972 |
| Sniffing frequency | 9.042 | 0.006 | 0.489 | 0.490 | 0.292 | 0.593 |
| Rearing duration | 5.803 | 0.022 | 0.118 | 0.733 | 1.433 | 0.241 |
| Rearing frequency | 10.068 | 0.004 | 5.476 | 0.027 | 0.001 | 0.972 |
| Degree of Freedom | F | p | |
|---|---|---|---|
| Distance travelled (cm) | |||
| Genotype | 1, 23 | 53.972 | 0.000 |
| Sex | 1, 23 | 1.017 | 0.324 |
| Genotype × Sex | 1, 23 | 3.567 | 0.072 |
| Time | 9, 207 | 20.267 | 0.000 |
| Genotype × Time | 9, 207 | 7.736 | 0.000 |
| Sex × Time | 9, 207 | 0.451 | 0.905 |
| Genotype × Sex × Time | 9, 207 | 2.822 | 0.004 |
| Time spent in centrum (%) | |||
| Genotype | 1, 23 | 5.662 | 0.026 |
| Sex | 1, 23 | 7.561 | 0.011 |
| Genotype × Sex | 1, 23 | 4.317 | 0.049 |
| Time | 9, 207 | 13.458 | 0.000 |
| Genotype × Time | 9, 207 | 1.508 | 0.147 |
| Sex × Time | 9, 207 | 0.410 | 0.186 |
| Genotype × Sex × Time | 9, 207 | 1.382 | 0.198 |
| Body weight (g) | |||
| Genotype | 1,26 | 1.554 | 0.224 |
| Sex | 1,26 | 57.509 | 0.000 |
| Genotype × Sex | 1,26 | 27.872 | 0.000 |
| Time | 9234 | 120.606 | 0.000 |
| Genotype × Time | 9234 | 0.694 | 0.714 |
| Sex × Time | 9234 | 1.429 | 0.176 |
| Genotype × Sex × Time | 9234 | 5.782 | 0.000 |
| Degree of Freedom | F | p | |
|---|---|---|---|
| Distance travelled (cm) | |||
| Genotype | 1, 26 | 43.003 | 0.000 |
| Sex | 1, 26 | 1.940 | 0.175 |
| Genotype × Sex | 1, 26 | 0.030 | 0.865 |
| Time | 5, 130 | 0.963 | 0.443 |
| Genotype × Time | 5, 130 | 0.425 | 0.831 |
| Sex × Time | 5, 130 | 1.781 | 0.101 |
| Genotype × Sex × Time | 5, 130 | 1.031 | 0.402 |
| Time spent in centrum (%) | |||
| Genotype | 1, 26 | 8.939 | 0.007 |
| Sex | 1, 26 | 7.856 | 0.010 |
| Genotype × Sex | 1, 26 | 0.572 | 0.457 |
| Time | 5, 130 | 11.730 | 0.000 |
| Genotype × Time | 5, 130 | 5.880 | 0.000 |
| Sex × Time | 5, 130 | 1.753 | 0.129 |
| Genotype × Sex × Time | 5, 130 | 1.610 | 0.163 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, A.; Farkas, S.; Fazekas, C.; Correia, P.; Chaves, T.; Sipos, E.; Makkai, B.; Török, B.; Zelena, D. Temporal Appearance of Enhanced Innate Anxiety in Alzheimer Model Mice. Biomedicines 2023, 11, 262. https://doi.org/10.3390/biomedicines11020262
Szabó A, Farkas S, Fazekas C, Correia P, Chaves T, Sipos E, Makkai B, Török B, Zelena D. Temporal Appearance of Enhanced Innate Anxiety in Alzheimer Model Mice. Biomedicines. 2023; 11(2):262. https://doi.org/10.3390/biomedicines11020262
Chicago/Turabian StyleSzabó, Adrienn, Szidónia Farkas, Csilla Fazekas, Pedro Correia, Tiago Chaves, Eszter Sipos, Bernadett Makkai, Bibiána Török, and Dóra Zelena. 2023. "Temporal Appearance of Enhanced Innate Anxiety in Alzheimer Model Mice" Biomedicines 11, no. 2: 262. https://doi.org/10.3390/biomedicines11020262
APA StyleSzabó, A., Farkas, S., Fazekas, C., Correia, P., Chaves, T., Sipos, E., Makkai, B., Török, B., & Zelena, D. (2023). Temporal Appearance of Enhanced Innate Anxiety in Alzheimer Model Mice. Biomedicines, 11(2), 262. https://doi.org/10.3390/biomedicines11020262






