Unraveling Down Syndrome: From Genetic Anomaly to Artificial Intelligence-Enhanced Diagnosis
Abstract
1. Introduction
2. Diagnostics
2.1. Prenatal Diagnostics
2.2. Artificial Intelligence (AI)-Based Diagnosis
2.2.1. Facial Recognition
2.2.2. Genetic Screening
2.2.3. Analysis of Medical Data
2.2.4. Support for Prenatal Diagnosis
2.2.5. Decision Support Systems for Healthcare
3. Cognitive Challenges in Down Syndrome
3.1. Speech, Mental Abilities, and Memory Retention
3.2. Processing Speed, Inhibition, and Attention
3.3. Short-Term Auditory Memory
3.4. Organization, Spatial Cognition, and Self-Monitoring
3.5. Learning and Long-Term Memory
3.6. Associated Conditions and Disorders
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kazemi, M.; Salehi, M.; Kheirollahi, M. Down Syndrome: Current Status, Challenges and Future Perspectives. Int. J. Mol. Cell. Med. 2016, 5, 125–133. [Google Scholar]
- Akhtar, F.; Bokhari, S.R.A. Down Syndrome. In StatPearls; Disclosure: Syed Rizwan Bokhari Declares No Relevant Financial Relationships with Ineligible Companies; Treasure Island (FL) Ineligible Companies: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Ataman, A.D.; Vatanoglu-Lutz, E.E.; Yildirim, G. Medicine in stamps: History of Down syndrome through philately. J. Turk. Ger. Gynecol. Assoc. 2012, 13, 267–269. [Google Scholar] [CrossRef]
- Down, J. Observations on an ethnic classification of idiots. Clin. Lect. Rep. Lond. Hosp. 1866, 3, 259–262. [Google Scholar]
- Gardiner, K.; Herault, Y.; Lott, I.T.; Antonarakis, S.E.; Reeves, R.H.; Dierssen, M. Down syndrome: From understanding the neurobiology to therapy. J. Neurosci. 2010, 30, 14943–14945. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.D.; Lewis, H.C.; Hill, A.A.; Pandey, A.; Jackson, L.P.; Cabral, J.M.; Smith, K.P.; Liggett, L.A.; Gomez, E.B.; Galbraith, M.D.; et al. Trisomy 21 consistently activates the interferon response. Elife 2016, 5, e16220. [Google Scholar] [CrossRef] [PubMed]
- Asim, A.; Kumar, A.; Muthuswamy, S.; Jain, S.; Agarwal, S. “Down syndrome: An insight of the disease”. J. Biomed. Sci. 2015, 22, 41. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.J. Down Syndrome. N. Engl. J. Med. 2020, 382, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, S.E.; Skotko, B.G.; Rafii, M.S.; Strydom, A.; Pape, S.E.; Bianchi, D.W.; Sherman, S.L.; Reeves, R.H. Down syndrome. Nat. Rev. Dis. Primers 2020, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Barca, D.; Tarta-Arsene, O.; Dica, A.; Iliescu, C.; Budisteanu, M.; Motoescu, C.; Butoianu, N.; Craiu, D. Intellectual disability and epilepsy in down syndrome. Maedica 2014, 9, 344–350. [Google Scholar]
- Kvarnung, M.; Nordgren, A. Intellectual Disability & Rare Disorders: A Diagnostic Challenge. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 39–54. [Google Scholar] [CrossRef]
- Murthy, S.K.; Malhotra, A.K.; Mani, S.; Shara, M.E.; Al-Rowaished, E.E.; Naveed, S.; Alkhayat, A.I.; Alali, M.T. Incidence of Down syndrome in Dubai, UAE. Med. Princ. Pract. 2007, 16, 25–28. [Google Scholar] [CrossRef]
- Cereda, A.; Carey, J.C. The trisomy 18 syndrome. Orphanet J. Rare Dis. 2012, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Down Syndrome; 2023. Available online: https://www.cdc.gov/ncbddd/birthdefects/downsyndrome.html (accessed on 22 November 2023).
- Pota, P.; Grammatopoulou, V.; Torti, E.; Braddock, S.; Batanian, J.R. Instability of isochromosome 4p in a child with pure trisomy 4p syndrome features and entire 4q-arm translocation. Cytogenet. Genome Res. 2014, 144, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Vijay, S.; Sarojam, S.; Raveendran, S.; Syamala, V.; Leelakumari, S.; Narayanan, G.; Hariharan, S. Recurrent isochromosome 21 and multiple abnormalities in a patient suspected of having acute myeloid leukemia with eosinophilic differentiation—A rare case from South India. Chin. J. Cancer 2012, 31, 45–50. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hamanoue, H. Genetic Counseling: Chromosomal Structural Rearrangements. In Fetal Morph Functional Diagnosis; Springer: Singapore, 2020; pp. 271–296. [Google Scholar] [CrossRef]
- Lana-Elola, E.; Watson-Scales, S.D.; Fisher, E.M.; Tybulewicz, V.L. Down syndrome: Searching for the genetic culprits. Dis. Model. Mech. 2011, 4, 586–595. [Google Scholar] [CrossRef]
- Abukhaled, Y.; Hatab, K.; Awadhalla, M.; Hamdan, H. Understanding the genetic mechanisms and cognitive impairments in Down syndrome: Towards a holistic approach. J. Neurol. 2023. [Google Scholar] [CrossRef]
- Sharmin, F.; Begum, S.; Jahan, I.; Parvin, R.; Biswas, D.C. Down syndrome with Disorder of Sex development (DSD): A Rare Presentation. Bangladesh J. Child Health 2020, 44, 48–51. [Google Scholar] [CrossRef]
- Ministry of Health. The Clinical Assessment and Management of Children, Young People and Adults with Down Syndrome Recommended Clinical Practice; Ministry of Health: Wellington, New Zealand, 2001. [Google Scholar]
- Hendrix, J.A.; Amon, A.; Abbeduto, L.; Agiovlasitis, S.; Alsaied, T.; Anderson, H.A.; Bain, L.J.; Baumer, N.; Bhattacharyya, A.; Bogunovic, D.; et al. Opportunities, barriers, and recommendations in down syndrome research. Transl. Sci. Rare Dis. 2021, 5, 99–129. [Google Scholar] [CrossRef]
- Baksh, R.A.; Pape, S.E.; Chan, L.F.; Aslam, A.A.; Gulliford, M.C.; Strydom, A.; Consortium, G.-D. Multiple morbidity across the lifespan in people with Down syndrome or intellectual disabilities: A population-based cohort study using electronic health records. Lancet Public Health 2023, 8, e453–e462. [Google Scholar] [CrossRef]
- Varshney, K.; Iriowen, R.; Morrell, K.; Pillay, P.; Fossi, A.; Stephens, M.M. Disparities and outcomes of patients living with Down Syndrome undergoing healthcare transitions from pediatric to adult care: A scoping review. Am. J. Med. Genet. A 2022, 188, 2293–2302. [Google Scholar] [CrossRef]
- Chicoine, B.; Rivelli, A.; Fitzpatrick, V.; Chicoine, L.; Jia, G.; Rzhetsky, A. Prevalence of Common Disease Conditions in a Large Cohort of Individuals with Down Syndrome in the United States. J. Patient Cent. Res. Rev. 2021, 8, 86–97. [Google Scholar] [CrossRef]
- Klosowska, A.; Kuchta, A.; Cwiklinska, A.; Salaga-Zaleska, K.; Jankowski, M.; Klosowski, P.; Manski, A.; Zwiefka, M.; Anikiej-Wiczenbach, P.; Wierzba, J. Relationship between growth and intelligence quotient in children with Down syndrome. Transl. Pediatr. 2022, 11, 505–513. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, C.; Lee, A.S.; Gibbon, F.E.; van Bysterveldt, A.K.; Hart, N.J. Parent-mediated interventions for promoting communication and language development in young children with Down syndrome. Cochrane Database Syst. Rev. 2018, 10, CD012089. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Qiang, R.; Cheng, H.; Wang, A.; Chen, H.; Pan, Z. Analysis of the Global Disease Burden of Down Syndrome Using YLDs, YLLs, and DALYs Based on the Global Burden of Disease 2019 Data. Front. Pediatr. 2022, 10, 882722. [Google Scholar] [CrossRef] [PubMed]
- Davenport, T.; Kalakota, R. The potential for artificial intelligence in healthcare. Future Healthc. J. 2019, 6, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, J.; Munir, U.; Nori, A.; Williams, B. Artificial intelligence in healthcare: Transforming the practice of medicine. Future Healthc. J. 2021, 8, e188–e194. [Google Scholar] [CrossRef] [PubMed]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing healthcare: The role of artificial intelligence in clinical practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef] [PubMed]
- Alzubi, J.; Nayyar, A.; Kumar, A. Machine Learning from Theory to Algorithms: An Overview. J. Phys. Conf. Ser. 2018, 1142, 012012. [Google Scholar] [CrossRef]
- Briganti, G. Artificial intelligence: An introduction for clinicians. Rev. Mal. Respir. 2023, 40, 308–313. [Google Scholar] [CrossRef]
- Choi, R.Y.; Coyner, A.S.; Kalpathy-Cramer, J.; Chiang, M.F.; Campbell, J.P. Introduction to Machine Learning, Neural Networks, and Deep Learning. Transl. Vis. Sci. Technol. 2020, 9, 14. [Google Scholar]
- Deo, R.C. Machine Learning in Medicine. Circulation 2015, 132, 1920–1930. [Google Scholar] [CrossRef]
- Zhang, H.-G.; Jiang, Y.-T.; Dai, S.-D.; Li, L.; Hu, X.-N.; Liu, R.-Z. Application of intelligent algorithms in Down syndrome screening during second trimester pregnancy. World J. Clin. Cases 2021, 9, 4573–4584. [Google Scholar] [CrossRef] [PubMed]
- He, F.L.; Lin, B.; Mou, K.; Jin, L.Z.; Liu, J.T. A machine learning model for the prediction of down syndrome in second trimester antenatal screening. Clin. Chim. Acta 2021, 521, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Nawi, A.; Ismail, A.; Abdullah, S. The Impact on Family among Down syndrome Children with Early Intervention. Iran. J. Public Health 2013, 42, 996–1006. [Google Scholar] [PubMed]
- NDSS. Early Interventions. Available online: https://ndss.org/resources/early-intervention (accessed on 8 August 2023).
- Guralnick, M.J. Effectiveness of early intervention for vulnerable children: A developmental perspective. Am. J. Ment. Retard. 1998, 102, 319–345. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.Y.T.; Lee, C.P.; Leung, K.Y.; Lau, E.; Tang, M.H.Y. Human Chorionic Gonadotropin and Plasma Protein-A in Alpha0-Thalassemia Pregnancies. Obstet. Gynecol. 2006, 108, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.L.; Blakemore, K.J. A historical and practical review of first trimester aneuploidy screening. Semin. Fetal Neonatal Med. 2014, 19, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Shiefa, S.; Amargandhi, M.; Bhupendra, J.; Moulali, S.; Kristine, T. First Trimester Maternal Serum Screening Using Biochemical Markers PAPP-A and Free beta-hCG for Down Syndrome, Patau Syndrome and Edward Syndrome. Indian J. Clin. Biochem. 2013, 28, 3–12. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; He, F.; Zhong, K.; Yuan, S.; Wang, Z. Proficiency testing of maternal serum prenatal screening in second trimester in China, 2015. Biochem. Med. 2017, 27, 114–121. [Google Scholar] [CrossRef]
- Canick, J.A.; MacRae, A.R. Second trimester serum markers. Semin. Perinatol. 2005, 29, 203–208. [Google Scholar] [CrossRef]
- Ren, F.; Hu, Y.U.; Zhou, H.; Zhu, W.Y.; Jia, L.I.; Xu, J.J.; Xue, J. Second trimester maternal serum triple screening marker levels in normal twin and singleton pregnancies. Biomed. Rep. 2016, 4, 475–478. [Google Scholar] [CrossRef]
- Wald, N.J.; Bestwick, J.P.; Huttly, W.J. Improvements in antenatal screening for Down’s syndrome. J. Med. Screen. 2013, 20, 7–14. [Google Scholar] [CrossRef]
- Malone, F.D.; Canick, J.A.; Ball, R.H.; Nyberg, D.A.; Comstock, C.H.; Bukowski, R.; Berkowitz, R.L.; Gross, S.J.; Dugoff, L.; Craigo, S.D.; et al. First-trimester or second-trimester screening, or both, for Down’s syndrome. N. Engl. J. Med. 2005, 353, 2001–2011. [Google Scholar] [CrossRef]
- Zournatzi, V.; Daniilidis, A.; Karidas, C.; Tantanasis, T.; Loufopoulos, A.; Tzafettas, J. A prospective two years study of first trimester screening for Down syndrome. Hippokratia 2008, 12, 28–32. [Google Scholar] [PubMed]
- Gori, C.; Cocchi, G.; Corvaglia, L.T.; Ramacieri, G.; Pulina, F.; Sperti, G.; Cagnazzo, V.; Catapano, F.; Strippoli, P.; Cordelli, D.M.; et al. Down Syndrome: How to communicate the diagnosis. Ital. J. Pediatr. 2023, 49, 18. [Google Scholar] [CrossRef]
- Ho, L. Current status of the early childhood developmental intervention ecosystem in Singapore. Singap. Med. J. 2021, 62, S43–S52. [Google Scholar] [CrossRef]
- Paterick, T.E.; Patel, N.; Tajik, A.J.; Chandrasekaran, K. Improving health outcomes through patient education and partnerships with patients. Bayl. Univ. Med. Cent. Proc. 2017, 30, 112–113. [Google Scholar] [CrossRef]
- Wills, J. Health literacy: New packaging for health education or radical movement? Int. J. Public Health 2009, 54, 3–4. [Google Scholar] [CrossRef]
- Nutbeam, D. The evolving concept of health literacy. Soc. Sci. Med. 2008, 67, 2072–2078. [Google Scholar] [CrossRef]
- Bohnstedt, C.; Stenmarker, M.; Olersbacken, L.; Schmidt, L.; Larsen, H.B.; Schmiegelow, K.; Hansson, H. Participation, challenges and needs in children with down syndrome during cancer treatment at hospital: A qualitative study of parents’ experiences. Front. Rehabil. Sci. 2023, 4, 1099516. [Google Scholar] [CrossRef] [PubMed]
- Fuca, E.; Galassi, P.; Costanzo, F.; Vicari, S. Parental perspectives on the quality of life of children with Down syndrome. Front. Psychiatry 2022, 13, 957876. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, S.A.; Mossa, M.S.; Al Nuaimi, G.A.; Al Khateri, F.A. Down syndrome: Knowledge and attitudes among future healthcare providers. J. Taibah Univ. Med. Sci. 2023, 18, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Telman, G.; Sosnowska-Sienkiewicz, P.; Strauss, E.; Mazela, J.; Mankowski, P.; Januszkiewicz-Lewandowska, D. Why Is Health Care for Children with Down Syndrome So Crucial from the First Days of Life? A Retrospective Cohort Study Emphasized Transient Abnormal Myelopoiesis (TAM) Syndrome at Three Centers. Int. J. Environ. Res. Public Health 2022, 19, 9774. [Google Scholar] [CrossRef] [PubMed]
- Gadsboll, K.; Petersen, O.B.; Gatinois, V.; Strange, H.; Jacobsson, B.; Wapner, R.; Vermeesch, J.R.; Group, N.I.-m.S.; Vogel, I. Current use of noninvasive prenatal testing in Europe, Australia and the USA: A graphical presentation. Acta Obstet. Gynecol. Scand. 2020, 99, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Zalel, Y.; Zemet, R.; Kivilevitch, Z. The added value of detailed early anomaly scan in fetuses with increased nuchal translucency. Prenat. Diagn. 2017, 37, 235–243. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, S.; Han, J.; Zhen, L.; Yang, X.; Li, R.; Zhang, Y.; Jing, X.; Li, F.; Liu, H. Prenatal diagnosis of ultrasound soft markers in a single medical center of mainland China. Mol. Cytogenet. 2023, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Hasina, Z.; Wang, C.C. Prenatal and Postnatal Therapies for Down’s Syndrome and Associated Developmental Anomalies and Degenerative Deficits: A Systematic Review of Guidelines and Trials. Front. Med. 2022, 9, 910424. [Google Scholar] [CrossRef]
- Inglis, A.; Hippman, C.; Austin, J.C. Prenatal testing for Down syndrome: The perspectives of parents of individuals with Down syndrome. Am. J. Med. Genet. A 2012, 158A, 743–750. [Google Scholar] [CrossRef]
- Channell, M.M.; Mattie, L.J.; Hamilton, D.R.; Capone, G.T.; Mahone, E.M.; Sherman, S.L.; Rosser, T.C.; Reeves, R.H.; Kalb, L.G.; Down Syndrome Cognition, P. Capturing cognitive and behavioral variability among individuals with Down syndrome: A latent profile analysis. J. Neurodev. Disord. 2021, 13, 16. [Google Scholar] [CrossRef]
- Klein, J.A.; Haydar, T.F. Neurodevelopment in Down syndrome: Concordance in humans and models. Front. Cell Neurosci. 2022, 16, 941855. [Google Scholar] [CrossRef]
- Windsperger, K.; Hoehl, S. Development of Down Syndrome Research Over the Last Decades-What Healthcare and Education Professionals Need to Know. Front. Psychiatry 2021, 12, 749046. [Google Scholar] [CrossRef]
- Onnivello, S.; Pulina, F.; Locatelli, C.; Marcolin, C.; Ramacieri, G.; Antonaros, F.; Vione, B.; Caracausi, M.; Lanfranchi, S. Cognitive profiles in children and adolescents with Down syndrome. Sci. Rep. 2022, 12, 1936. [Google Scholar] [CrossRef] [PubMed]
- Zdravkova, K.; Krasniqi, V.; Dalipi, F.; Ferati, M. Cutting-edge communication and learning assistive technologies for disabled children: An artificial intelligence perspective. Front. Artif. Intell. 2022, 5, 970430. [Google Scholar] [CrossRef]
- Thomas, M.S.C.; Alfageme, O.O.; D’Souza, H.; Patkee, P.A.; Rutherford, M.A.; Mok, K.Y.; Hardy, J.; Karmiloff-Smith, A.; Consortium, L. A multi-level developmental approach to exploring individual differences in Down syndrome: Genes, brain, behaviour, and environment. Res. Dev. Disabil. 2020, 104, 103638. [Google Scholar] [CrossRef]
- Stoll, K.; Jackson, J. Supporting Patient Autonomy and Informed Decision-Making in Prenatal Genetic Testing. Cold Spring Harb. Perspect. Med. 2020, 10, a036509. [Google Scholar] [CrossRef]
- Stoll, C.; Dott, B.; Alembik, Y.; Roth, M.-P. Associated congenital anomalies among cases with Down syndrome. Eur. J. Med. Genet. 2015, 58, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Lawson, K.L.; Pierson, R.A. Maternal decisions regarding prenatal diagnosis: Rational choices or sensible decisions? J. Obstet. Gynaecol. Can. 2007, 29, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Skotko, B.G.; Macklin, E.A.; Muselli, M.; Voelz, L.; McDonough, M.E.; Davidson, E.; Allareddy, V.; Jayaratne, Y.S.; Bruun, R.; Ching, N.; et al. A predictive model for obstructive sleep apnea and Down syndrome. Am. J. Med. Genet. A 2017, 173, 889–896. [Google Scholar] [CrossRef]
- Baldo, F.; Piovesan, A.; Rakvin, M.; Ramacieri, G.; Locatelli, C.; Lanfranchi, S.; Onnivello, S.; Pulina, F.; Caracausi, M.; Antonaros, F.; et al. Machine learning based analysis for intellectual disability in Down syndrome. Heliyon 2023, 9, e19444. [Google Scholar] [CrossRef]
- Qin, B.; Liang, L.; Wu, J.; Quan, Q.; Wang, Z.; Li, D. Automatic Identification of Down Syndrome Using Facial Images with Deep Convolutional Neural Network. Diagnostics 2020, 10, 487. [Google Scholar] [CrossRef]
- Srisraluang, W.; Rojnueangnit, K. Facial recognition accuracy in photographs of Thai neonates with Down syndrome among physicians and the Face2Gene application. Am. J. Med. Genet. Part A 2021, 185, 3701–3705. [Google Scholar] [CrossRef]
- Jamshidnezhad, A.; Hosseini, S.M.; Mohammadi-Asl, J.; Mahmudi, M. An intelligent prenatal screening system for the prediction of Trisomy-21. Inform. Med. Unlocked 2021, 24, 100625. [Google Scholar] [CrossRef]
- Reshi, A.A.; Rustam, F.; Mehmood, A.; Alhossan, A.; Alrabiah, Z.; Ahmad, A.; Alsuwailem, H.; Choi, G.S. An Efficient CNN Model for COVID-19 Disease Detection Based on X-Ray Image Classification. Complexity 2021, 2021, 6621607. [Google Scholar] [CrossRef]
- Koivu, K.; Korpimäki, T.; Kivelä, P.; Pahikkala, T.; Sairanen, M. Evaluation of machine learning algorithms for improved risk assessment for Down’s syndrome. Comput. Biol. Med. 2018, 98, 1–7. [Google Scholar] [CrossRef]
- Reshi, A.A.; Ashraf, I.; Rustam, F.; Shahzad, H.F.; Mehmood, A.; Choi, G.S. Diagnosis of vertebral column pathologies using concatenated resampling with machine learning algorithms. PeerJ Comput. Sci. 2021, 7, e547. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Zhou, Y.; Shi, S.; Zhang, Y.; Yin, S.; Liu, X.; Peng, Q.; Huang, S.; Jiang, Y.; Cui, C.; et al. How much can AI see in early pregnancy: A multi-center study of fetus head characterization in week 10–14 in ultrasound using deep learning. Comput. Methods Programs Biomed. 2022, 226, 107170. [Google Scholar] [CrossRef] [PubMed]
- Rustam, F.; Reshi, A.A.; Aljedaani, W.; Alhossan, A.; Ishaq, A.; Shafi, S.; Lee, E.; Alrabiah, Z.; Alsuwailem, H.; Ahmad, A.; et al. Vector mosquito image classification using novel RIFS feature selection and machine learning models for disease epidemiology. Saudi J. Biol. Sci. 2022, 29, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Celik, E.; Ilhan, H.O.; Elbir, A. Detection and estimation of down syndrome genes by machine learning techniques. In Proceedings of the 2017 25th Signal Processing and Communications Applications Conference (SIU), Antalya, Turkey, 15–18 May 2017. [Google Scholar]
- Hallowell, N.; Badger, S.; McKay, F.; Kerasidou, A.; Nellåker, C. Democratising or disrupting diagnosis? Ethical issues raised by the use of AI tools for rare disease diagnosis. SSM Qual. Res. Health 2023, 3, 100240. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.J. Health Supervision for Children with Down Syndrome. Pediatrics 2011, 128, 393–406. [Google Scholar] [CrossRef]
- Eadie, P.A.; Fey, M.E.; Douglas, J.M.; Parsons, C.L. Profiles of Grammatical Morphology and Sentence Imitation in Children with Specific Language Impairment and Down Syndrome. J. Speech Lang. Hear. Res. 2002, 45, 720–732. [Google Scholar] [CrossRef]
- Brunamonti, E.; Pani, P.; Papazachariadis, O.; Onorati, P.; Albertini, G.; Ferraina, S. Cognitive control of movement in down syndrome. Res. Dev. Disabil. 2011, 32, 1792–1797. [Google Scholar] [CrossRef]
- Ellis, N.R.; Woodley-Zanthos, P.; Dulaney, C.L. Memory for spatial location in children, adults, and mentally retarded persons. Am. J. Ment. Retard. 1989, 93, 521–526. [Google Scholar] [PubMed]
- Ulrich, D.A.; Burghardt, A.R.; Lloyd, M.; Tiernan, C.; Hornyak, J.E. Physical Activity Benefits of Learning to Ride a Two-Wheel Bicycle for Children with Down Syndrome: A Randomized Trial. Phys. Ther. 2011, 91, 1463–1477. [Google Scholar] [CrossRef] [PubMed]
- Lott, I.T.; Dierssen, M. Cognitive deficits and associated neurological complications in individuals with Down’s syndrome. Lancet Neurol. 2010, 9, 623–633. [Google Scholar] [CrossRef]
- Vicari, S. Motor Development and Neuropsychological Patterns in Persons with Down Syndrome. Behav. Genet. 2006, 36, 355–364. [Google Scholar] [CrossRef]
- Vicari, S.; Pontillo, M.; Armando, M. Neurodevelopmental and psychiatric issues in Down’s syndrome. Psychiatr. Genet. 2013, 23, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.; Hesketh, L. Language, cognition, and short-term memory in individuals with Down syndrome. Down Syndr. Res. Pract. 2001, 7, 1–7. [Google Scholar] [CrossRef]
- Shanahan, M.A.; Pennington, B.F.; Yerys, B.E.; Scott, A.; Boada, R.; Willcutt, E.G.; Olson, R.K.; DeFries, J.C. Processing Speed Deficits in Attention Deficit/Hyperactivity Disorder and Reading Disability. J. Abnorm. Child Psychol. 2006, 34, 584–601. [Google Scholar] [CrossRef]
- Borella, E.; Carretti, B.; Lanfranchi, S. Inhibitory mechanisms in Down syndrome: Is there a specific or general deficit? Res. Dev. Disabil. 2013, 34, 65–71. [Google Scholar] [CrossRef]
- Chen, C.C.; Spanò, G.; Edgin, J.O. The impact of sleep disruption on executive function in Down syndrome. Res. Dev. Disabil. 2013, 34, 2033–2039. [Google Scholar] [CrossRef]
- Marcell, M.M.; Weeks, S.L. Short-term memory difficulties and Down’s syndrome. J. Intellect. Disabil. Res. 2008, 32, 153–162. [Google Scholar] [CrossRef]
- Jarrold, C.; Baddeley, A.D.; Phillips, C.E. Verbal Short-Term Memory in Down Syndrome. J. Speech Lang. Hear. Res. 2002, 45, 531–544. [Google Scholar] [CrossRef]
- Karagianni, E.; Drigas, A. Language Development and Mobile Apps for Down Syndrome Children. Tech. Soc. Sci. J. 2022, 34, 193–213. [Google Scholar] [CrossRef]
- Kent, R.D.; Eichhorn, J.; Wilson, E.M.; Suk, Y.; Bolt, D.M.; Vorperian, H.K. Auditory-Perceptual Features of Speech in Children and Adults with Down Syndrome: A Speech Profile Analysis. J. Speech Lang. Hear. Res. 2021, 64, 1157–1175. [Google Scholar] [CrossRef] [PubMed]
- Robles-Bello, M.A.; Sánchez-Teruel, D.; Camacho-Conde, J.A. Variables that Predict the Potential Efficacy of Early Intervention in Reading in Down Syndrome. Psicol. Educ. 2020, 26, 95–100. [Google Scholar] [CrossRef]
- Schworer, E.K.; Voth, K.; Hoffman, E.K.; Esbensen, A.J. Short-term memory outcome measures: Psychometric evaluation and performance in youth with Down syndrome. Res. Dev. Disabil. 2022, 120, 104147. [Google Scholar] [CrossRef] [PubMed]
- Miles, S.; Chapman, R.; Sindberg, H. Sampling Context Affects MLU in the Language of Adolescents with Down Syndrome. J. Speech Lang. Hear. Res. 2006, 49, 325–337. [Google Scholar] [CrossRef]
- Mandal, A.S.; Fama, M.E.; Skipper-Kallal, L.M.; DeMarco, A.T.; Lacey, E.H.; Turkeltaub, P.E. Brain structures and cognitive abilities important for the self-monitoring of speech errors. Neurobiol. Lang. 2020, 1, 319–338. [Google Scholar] [CrossRef]
- Maessen, B.; Zink, I.; Maes, B.; Rombouts, E. The effect of manual movements on stuttering in individuals with down syndrome. J. Fluen. Disord. 2023, 75, 105958. [Google Scholar] [CrossRef]
- Parthimos, T.P.; Karavasilis, E.; Rankin, K.P.; Seimenis, I.; Leftherioti, K.; Papanicolaou, A.C.; Miller, B.; Papageorgiou, S.G.; Papatriantafyllou, J.D. The Neural Correlates of Impaired Self-Monitoring Among Individuals with Neurodegenerative Dementias. J. Neuropsychiatry Clin. Neurosci. 2019, 31, 201–209. [Google Scholar] [CrossRef]
- Traverso, L.; Fontana, M.; Usai, M.C.; Passolunghi, M.C. Response Inhibition and Interference Suppression in Individuals with Down Syndrome Compared to Typically Developing Children. Front. Psychol. 2018, 9, 660. [Google Scholar] [CrossRef]
- Martin, G.E.; Klusek, J.; Estigarribia, B.; Roberts, J.E. Language Characteristics of Individuals with Down Syndrome. Top. Lang. Disord. 2009, 29, 112–132. [Google Scholar] [CrossRef]
- Zhu, P.J.; Khatiwada, S.; Cui, Y.; Reineke, L.C.; Dooling, S.W.; Kim, J.J.; Li, W.; Walter, P.; Costa-Mattioli, M. Activation of the ISR mediates the behavioral and neurophysiological abnormalities in Down syndrome. Science 2019, 366, 843–849. [Google Scholar] [CrossRef]
- Vacca, R.A.; Bawari, S.; Valenti, D.; Tewari, D.; Nabavi, S.F.; Shirooie, S.; Sah, A.N.; Volpicella, M.; Braidy, N.; Nabavi, S.M. Down syndrome: Neurobiological alterations and therapeutic targets. Neurosci. Biobehav. Rev. 2019, 98, 234–255. [Google Scholar] [CrossRef]
- Lukowski, A.F.; Milojevich, H.M.; Eales, L. Cognitive Functioning in Children with Down Syndrome: Current Knowledge and Future Directions. In Advances in Child Development and Behavior; Elsevier: Amsterdam, The Netherlands, 2019; pp. 257–289. [Google Scholar] [CrossRef]
- Caloway, C.L.; Diercks, G.R.; Keamy, D.; de Guzman, V.; Soose, R.; Raol, N.; Shott, S.R.; Ishman, S.L.; Hartnick, C.J. Update on hypoglossal nerve stimulation in children with down syndrome and obstructive sleep apnea. Laryngoscope 2019, 130, E263–E267. [Google Scholar] [CrossRef]
- Santoro, S.L.; Steffensen, E.H. Congenital heart disease in Down syndrome—A review of temporal changes. J. Congenit. Cardiol. 2021, 5, 1. [Google Scholar] [CrossRef]
- Reller, M.D.; Strickland, M.J.; Riehle-Colarusso, T.; Mahle, W.T.; Correa, A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J. Pediatr. 2008, 153, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Roizen, N.J.; Patterson, D. Down’s syndrome. Lancet 2003, 361, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Morris-Rosendahl, D.J.; Crocq, M.-A. Neurodevelopmental disorders-the history and future of a diagnostic concept . Dialogues Clin. Neurosci. 2020, 22, 65–72. [Google Scholar] [CrossRef]
- Metwalley, K.A.; Farghaly, H.S. Endocrinal dysfunction in children with Down syndrome. Ann. Pediatr. Endocrinol. Metab. 2022, 27, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Martin-Perez, Y.; Gonzalez-Montero, G.; Gutierrez-Hernandez, A.L.; Blázquez-Sánchez, V.; Sánchez-Ramos, C. Vision Impairments in Young Adults with Down Syndrome. Vision 2023, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Patel, L.; Wolter-Warmerdam, K.; Hickey, F. Patterns of Behavior and Medical Comorbidities in Down syndrome. J. Ment. Health Res. Intellect. Disabil. 2020, 13, 267–280. [Google Scholar] [CrossRef]
- Horne, R.S.C.; Wijayaratne, P.; Nixon, G.M.; Walter, L.M. Sleep and sleep disordered breathing in children with down syndrome: Effects on behaviour, neurocognition and the cardiovascular system. Sleep Med. Rev. 2019, 44, 1–11. [Google Scholar] [CrossRef]
- Lal, C.; White, D.R.; Joseph, J.E.; van Bakergem, K.; LaRosa, A. Sleep-Disordered Breathing in Down Syndrome. Chest 2015, 147, 570–579. [Google Scholar] [CrossRef]
- Sibarani, C.R.; Walter, L.M.; Davey, M.J.; Nixon, G.M.; Horne, R.S.C. Sleep-disordered breathing and sleep macro- and micro-architecture in children with Down syndrome. Pediatr. Res. 2021, 91, 1248–1256. [Google Scholar] [CrossRef]
- Subramaniam, D.R.; Mylavarapu, G.; McConnell, K.; Fleck, R.J.; Shott, S.R.; Amin, R.S.; Gutmark, E.J. Compliance Measurements of the Upper Airway in Pediatric Down Syndrome Sleep Apnea Patients. Ann. Biomed. Eng. 2016, 44, 873–885. [Google Scholar] [CrossRef] [PubMed]
- de Miguel-Díez, J.; Villa-Asensi, J.R.; Álvarez-Sala, J.L. Prevalence of Sleep-Disordered Breathing in Children with Down Syndrome: Polygraphic Findings in 108 Children. Sleep 2003, 26, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Maris, M.; Verhulst, S.; Wojciechowski, M.; Van de Heyning, P.; Boudewyns, A. Prevalence of Obstructive Sleep Apnea in Children with Down Syndrome. Sleep 2016, 39, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Chawla, J.K.; Cooke, E.; Miguel, M.C.; Burgess, S.; Staton, S. Parents’ Experiences of Having a Child with Down Syndrome and Sleep Difficulties. Behav. Sleep Med. 2022, 21, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Bush, D.; Galambos, C.; Ivy, D.D.; Abman, S.H.; Wolter-Warmerdam, K.; Hickey, F. Clinical Characteristics and Risk Factors for Developing Pulmonary Hypertension in Children with Down Syndrome. J. Pediatr. 2018, 202, 212–219.e2. [Google Scholar] [CrossRef] [PubMed]
- Altuna, M.; Giménez, S.; Fortea, J. Epilepsy in Down Syndrome: A Highly Prevalent Comorbidity. J. Clin. Med. 2021, 10, 2776. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.M.; Boison, D. The metabolic basis of epilepsy. Nat. Rev. Neurol. 2022, 18, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Casanova, M.F.; Fatemi, S.H.; Folsom, T.D.; Reutiman, T.J.; Brown, G.L.; Edelson, S.M.; Slattery, J.C.; Adams, J.B. Neuropathological Mechanisms of Seizures in Autism Spectrum Disorder. Front. Neurosci. 2016, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Hithersay, R.; Startin, C.M.; Hamburg, S.; Mok, K.Y.; Hardy, J.; Fisher, E.M.C.; Tybulewicz, V.L.J.; Nizetic, D.; Strydom, A. Association of Dementia with Mortality Among Adults with Down Syndrome Older Than 35 Years. JAMA Neurol. 2019, 76, 152–160. [Google Scholar] [CrossRef]
- Bayen, E.; Possin, K.L.; Chen, Y.; Cleret de Langavant, L.; Yaffe, K. Prevalence of Aging, Dementia, and Multimorbidity in Older Adults with Down Syndrome. JAMA Neurol. 2018, 75, 1399–1406. [Google Scholar] [CrossRef]
- Pujol, J.; Fenoll, R.; Ribas-Vidal, N.; Martínez-Vilavella, G.; Blanco-Hinojo, L.; García-Alba, J.; Deus, J.; Novell, R.; Esteba-Castillo, S. A longitudinal study of brain anatomy changes preceding dementia in Down syndrome. Neuroimage Clin. 2018, 18, 160–166. [Google Scholar] [CrossRef]
- Dekker, A.D.; Sacco, S.; Carfi, A.; Benejam, B.; Vermeiren, Y.; Beugelsdijk, G.; Schippers, M.; Hassefras, L.; Eleveld, J.; Grefelman, S.; et al. The Behavioral and Psychological Symptoms of Dementia in Down Syndrome (BPSD-DS) Scale: Comprehensive Assessment of Psychopathology in Down Syndrome. J. Alzheimer–s Dis. 2018, 63, 797–819. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.L.; Holland, A.J.; Hon, J.; Huppert, F.A.; Treppner, P.; Watson, P.C. Personality and behaviour changes mark the early stages of Alzheimer’s disease in adults with Down’s syndrome: Findings from a prospective population-based study. Int. J. Geriatr. Psychiatry 2006, 21, 661–673. [Google Scholar] [CrossRef]
- Ball, S.L.; Holland, A.J.; Treppner, P.; Watson, P.C.; Huppert, F.A. Executive dysfunction and its association with personality and behaviour changes in the development of Alzheimer’s disease in adults with Down syndrome and mild to moderate learning disabilities. Br. J. Clin. Psychol. 2008, 47, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Pentkowski, N.S.; Rogge-Obando, K.K.; Donaldson, T.N.; Bouquin, S.J.; Clark, B.J. Anxiety and Alzheimer’s disease: Behavioral analysis and neural basis in rodent models of Alzheimer’s-related neuropathology. Neurosci. Biobehav. Rev. 2021, 127, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Oxelgren, U.W.; Myrelid, Å.; Annerén, G.; Ekstam, B.; Göransson, C.; Holmbom, A.; Isaksson, A.; Åberg, M.; Gustafsson, J.; Fernell, E. Prevalence of autism and attention-deficit–hyperactivity disorder in Down syndrome: A population-based study. Dev. Med. Child Neurol. 2016, 59, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Weijerman, M.E.; van Furth, A.M.; van der Mooren, M.D.; van Weissenbruch, M.M.; Rammeloo, L.; Broers, C.J.M.; Gemke, R.J.B.J. Prevalence of congenital heart defects and persistent pulmonary hypertension of the neonate with Down syndrome. Eur. J. Pediatr. 2010, 169, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Paladini, D.; Tartaglione, A.; Agangi, A.; Teodoro, A.; Forleo, F.; Borghese, A.; Martinelli, P. The association between congenital heart disease and Down syndrome in prenatal life. Ultrasound Obstet. Gynecol. 2000, 15, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, B.E.B.V.; Medeiros, S.L.; Bermudez, M.B.; Novadzki, I.M.; Magdalena, N.I.R. Down syndrome: Prevalence and distribution of congenital heart disease in Brazil. Sao Paulo Med. J. 2015, 133, 521–524. [Google Scholar] [CrossRef]
- Kim, M.-A.; Lee, Y.S.; Yee, N.H.; Choi, J.S.; Choi, J.Y.; Seo, K. Prevalence of congenital heart defects associated with Down syndrome in Korea. J. Korean Med. Sci. 2014, 29, 1544–1549. [Google Scholar] [CrossRef] [PubMed]
- Al-Aama, J.Y.; Bondagji, N.S.; El-Harouni, A.A. Congenital heart defects in Down syndrome patients from western Saudi Arabia. Saudi Med. J. 2012, 33, 1211–1215. [Google Scholar]
- Baban, A.; Olivini, N.; Cantarutti, N.; Calì, F.; Vitello, C.; Valentini, D.; Adorisio, R.; Calcagni, G.; Alesi, V.; Di Mambro, C.; et al. Differences in morbidity and mortality in Down syndrome are related to the type of congenital heart defect. Am. J. Med. Genet. Part A 2020, 182, 1342–1350. [Google Scholar] [CrossRef]
- Bergström, S.; Carr, H.; Petersson, G.; Stephansson, O.; Bonamy, A.-K.E.; Dahlström, A.; Halvorsen, C.P.; Johansson, S. Trends in Congenital Heart Defects in Infants with Down Syndrome. Pediatrics 2016, 138, e20160123. [Google Scholar] [CrossRef]
- Santos, F.C.G.B.; Croti, U.A.; Marchi, C.H.D.; Murakami, A.N.; Brachine, J.D.P.; Borim, B.C.; Finoti, R.G.; Godoy, M.F.d. Surgical Treatment for Congenital Heart Defects in Down Syndrome Patients. Braz. J. Cardiovasc. Surg. 2019, 34, 1–7. [Google Scholar] [CrossRef]
- Lu, E.; Pyatka, N.; Burant, C.J.; Sajatovic, M. Systematic Literature Review of Psychiatric Comorbidities in Adults with Epilepsy. J. Clin. Neurol. 2021, 17, 176–186. [Google Scholar] [CrossRef]
- Startin, C.M.; D’Souza, H.; Ball, G.; Hamburg, S.; Hithersay, R.; Hughes, K.M.O.; Massand, E.; Karmiloff-Smith, A.; Thomas, M.S.C.; LonDown, S.C.; et al. Health comorbidities and cognitive abilities across the lifespan in Down syndrome. J. Neurodev. Disord. 2020, 12, 4. [Google Scholar] [CrossRef]
- Jojoa-Acosta, M.F.; Signo-Miguel, S.; Garcia-Zapirain, M.B.; Gimeno-Santos, M.; Mendez-Zorrilla, A.; Vaidya, C.J.; Molins-Sauri, M.; Guerra-Balic, M.; Bruna-Rabassa, O. Executive Functioning in Adults with Down Syndrome: Machine-Learning-Based Prediction of Inhibitory Capacity. Int. J. Environ. Res. Public Health 2021, 18, 785. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.H.; Annus, T.; Wilson, L.R.; Remtulla, R.; Hong, Y.T.; Fryer, T.D.; Acosta-Cabronero, J.; Cardenas-Blanco, A.; Smith, R.; Menon, D.K.; et al. Brain-predicted age in Down syndrome is associated with beta amyloid deposition and cognitive decline. Neurobiol. Aging 2017, 56, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Aldoseri, A.; Al-Khalifa, K.N.; Hamouda, A.M. Re-Thinking Data Strategy and Integration for Artificial Intelligence: Concepts, Opportunities, and Challenges. Appl. Sci. 2023, 13, 7082. [Google Scholar] [CrossRef]
| S. No. | Down Syndrome Associated Complications | Occurrence |
|---|---|---|
| 1. | Cataracts | 15% |
| 2. | Congenital heart ailments | 40–50% |
| 3. | Dental eruption (Delayed) | 23% |
| 4. | Gastrointestinal atresias | 12% |
| 5. | Hearing issues | 75% |
| 6. | Hip dislocation | 6% |
| 7. | Neurological Impairment | 1–13% |
| 8. | Otitis media | 50–70% |
| 9. | Refractive errors | 50% |
| 10. | Sleep apnea (Obstructive) | 50–75% |
| 11. | Thyroid disorders | 4–18% |
| 12. | Vision impairments | 60% |
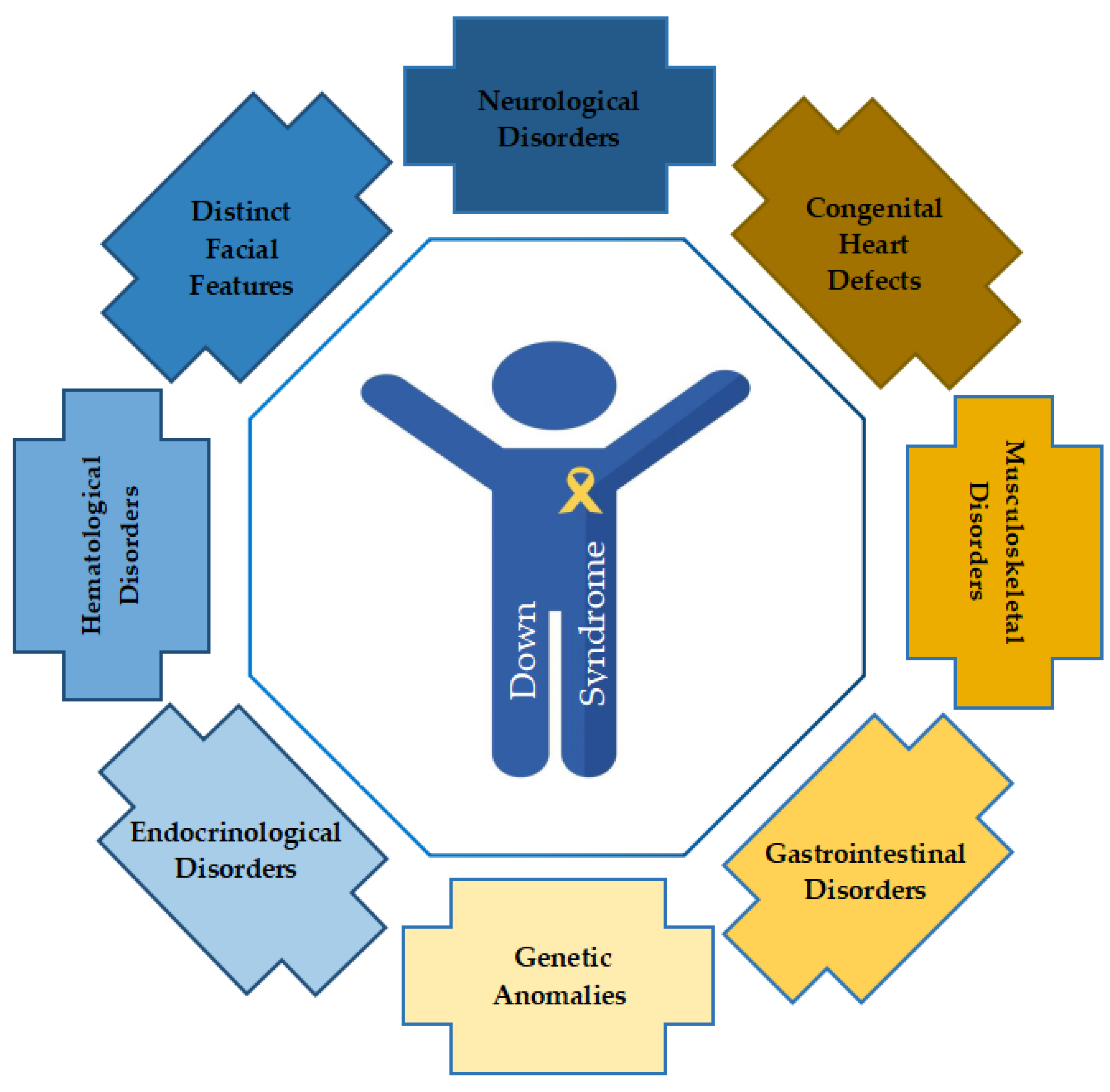
| Co-Morbidity | Disorder/Disease |
|---|---|
| Neurological Disorders |
|
| Congenital Heart Defects |
|
| Musculoskeletal Disorders |
|
| Gastrointestinal Disorders |
|
| Possible Genetic Anomalies |
|
| Endocrinological Disorders |
|
| Hematological Disorders |
|
| Distinct Facial Features |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koul, A.M.; Ahmad, F.; Bhat, A.; Aein, Q.-u.; Ahmad, A.; Reshi, A.A.; Kaul, R.-u.-R. Unraveling Down Syndrome: From Genetic Anomaly to Artificial Intelligence-Enhanced Diagnosis. Biomedicines 2023, 11, 3284. https://doi.org/10.3390/biomedicines11123284
Koul AM, Ahmad F, Bhat A, Aein Q-u, Ahmad A, Reshi AA, Kaul R-u-R. Unraveling Down Syndrome: From Genetic Anomaly to Artificial Intelligence-Enhanced Diagnosis. Biomedicines. 2023; 11(12):3284. https://doi.org/10.3390/biomedicines11123284
Chicago/Turabian StyleKoul, Aabid Mustafa, Faisel Ahmad, Abida Bhat, Qurat-ul Aein, Ajaz Ahmad, Aijaz Ahmad Reshi, and Rauf-ur-Rashid Kaul. 2023. "Unraveling Down Syndrome: From Genetic Anomaly to Artificial Intelligence-Enhanced Diagnosis" Biomedicines 11, no. 12: 3284. https://doi.org/10.3390/biomedicines11123284
APA StyleKoul, A. M., Ahmad, F., Bhat, A., Aein, Q.-u., Ahmad, A., Reshi, A. A., & Kaul, R.-u.-R. (2023). Unraveling Down Syndrome: From Genetic Anomaly to Artificial Intelligence-Enhanced Diagnosis. Biomedicines, 11(12), 3284. https://doi.org/10.3390/biomedicines11123284







