Leukocyte- and Platelet-Rich Fibrin (L-PRF) Obtained from Smokers and Nonsmokers Shows a Similar Uniaxial Tensile Response In Vitro
Abstract
:1. Introduction
1.1. Summary Box
1.1.1. What Is Known?
1.1.2. What This Study Adds
2. Materials and Methods
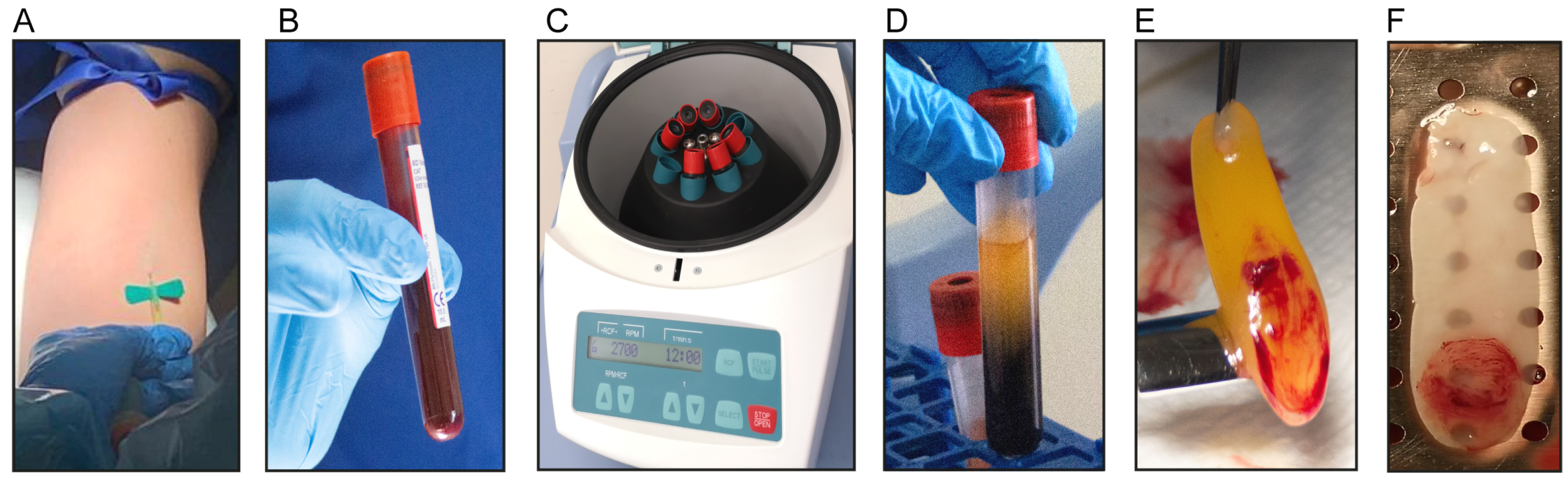
2.1. Isolation of L-PRF Membranes and Clots
2.2. Scanning Electron Microscopy (SEM)
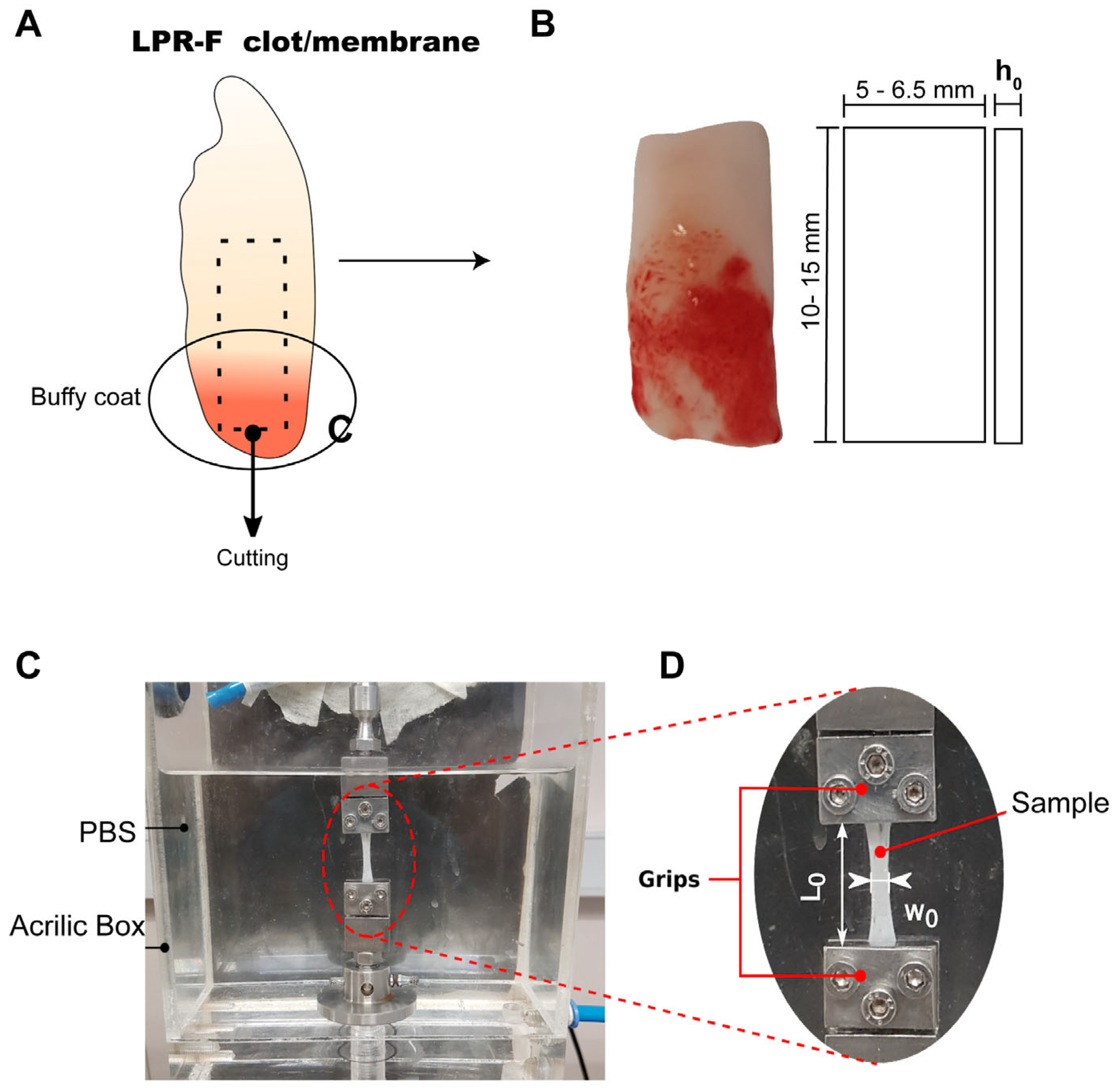
2.3. Preparation of L-PRF Samples for Uniaxial Tensile Testing
2.4. Uniaxial Tensile Tests
2.5. Statistical Analysis
3. Results
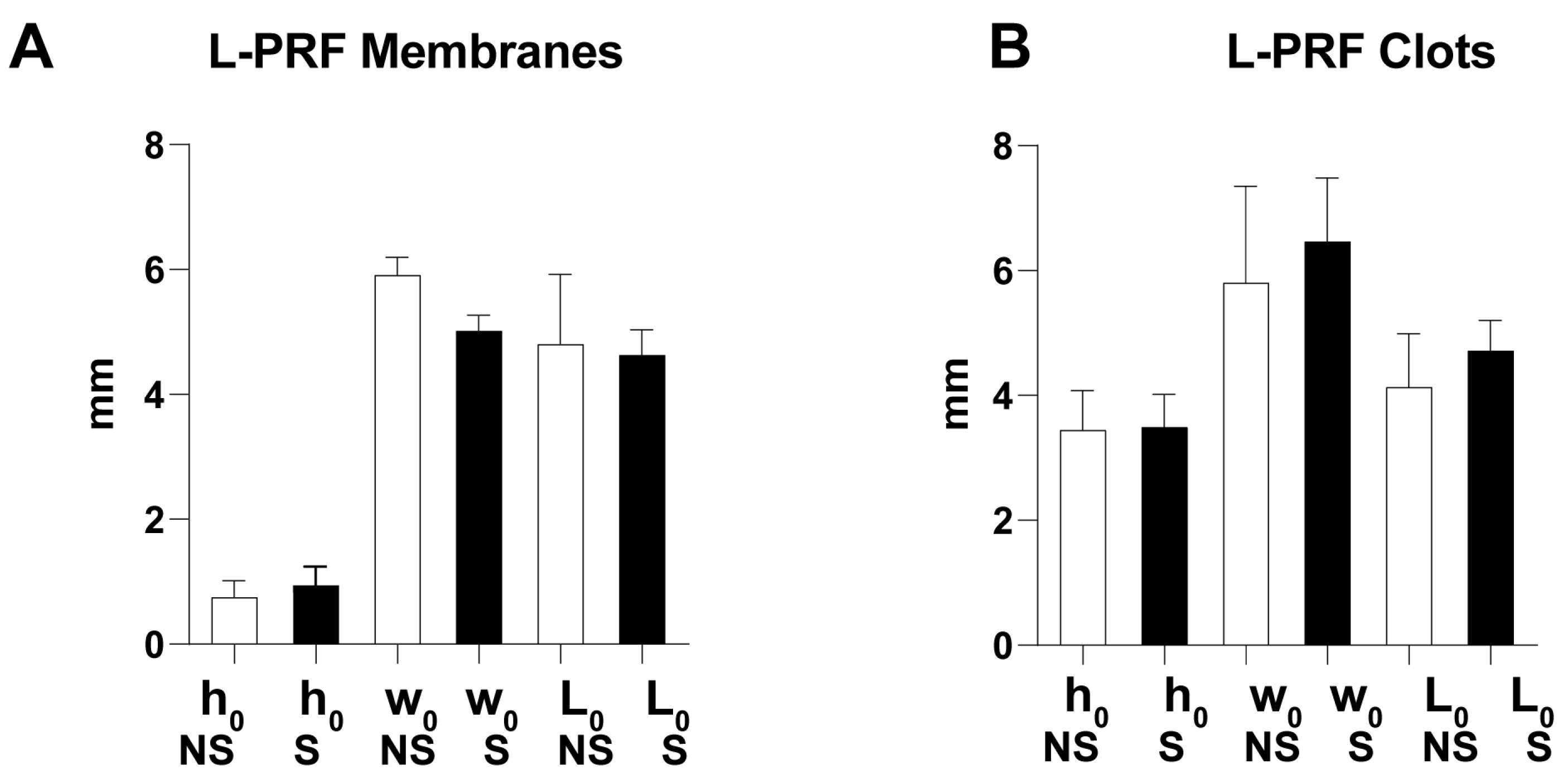
3.1. Morphological Characterization
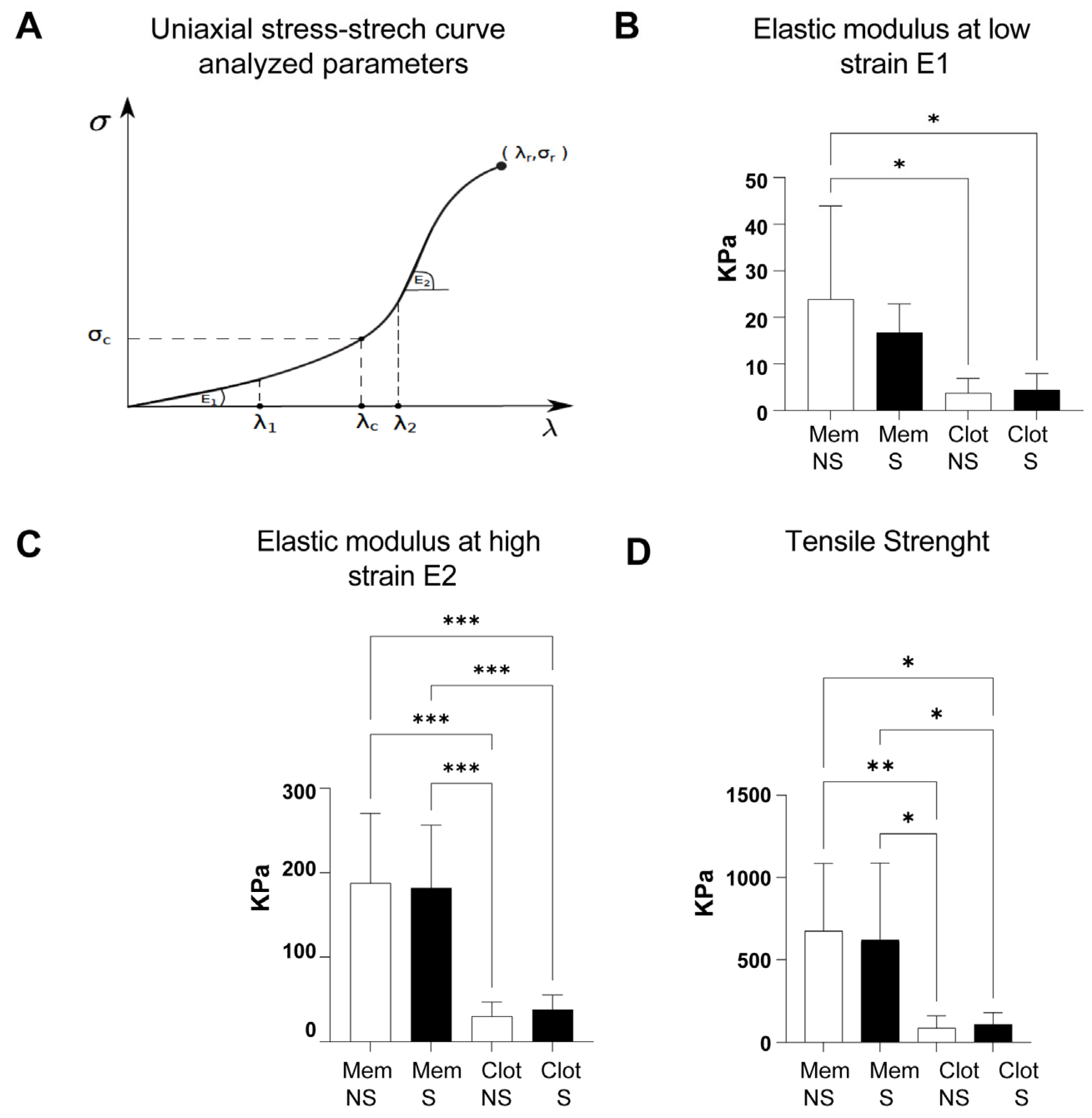
3.2. Uniaxial Stress–Stretch Curves and Mechanical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez, C.E.; Smith, P.C.; Palma Alvarado, V. The influence of platelet-derived products on angiogenesis and tissue repair: A concise update. Front. Physiol. 2015, 6, 290. [Google Scholar] [CrossRef]
- Avila-Ortiz, G.; Ambruster, J.; Barootchi, S.; Chambrone, L.; Chen, C.; Dixon, D.R.; Geisinger, M.L.; Giannobile, W.V.; Goss, K.; Gunsolley, J.C.; et al. American Academy of Periodontology best evidence consensus statement on the use of biologics in clinical practice. J. Periodontol. 2022, 93, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Andia, I.; Zumstein, M.A.; Zhang, C.-Q.; Pinto, N.R.; Bielecki, T. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: Current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014, 4, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Fujioka-Kobayashi, M.; Sculean, A.; Zhang, Y. Optimization of platelet-rich fibrin. Periodontology 2000 2023. early view. [Google Scholar] [CrossRef]
- Sato, A.; Kawabata, H.; Aizawa, H.; Tsujino, T.; Isobe, K.; Watanabe, T.; Kitamura, Y.; Miron, R.J.; Kawase, T. Distribution and quantification of activated platelets in platelet-rich fibrin matrices. Platelets 2020, 33, 110–115. [Google Scholar] [CrossRef]
- Castro, A.B.; Van Dessel, J.; Temmerman, A.; Jacobs, R.; Quirynen, M. Effect of different platelet-rich fibrin matrices for ridge preservation in multiple tooth extractions: A split-mouth randomized controlled clinical trial. J. Clin. Periodontol. 2021, 48, 984–995. [Google Scholar] [CrossRef]
- Castro, A.B.; Meschi, N.; Temmerman, A.; Pinto, N.; Lambrechts, P.; Teughels, W.; Quirynen, M. Regenerative potential of leucocyte- and platelet-rich fibrin. Part B: Sinus floor elevation, alveolar ridge preservation and implant therapy. A systematic review. J. Clin. Periodontol. 2017, 44, 225–234. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Eng. Part B Rev. 2017, 23, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Kao, R.T.; Nares, S.; Reynolds, M.A. Periodontal Regeneration—Intrabony Defects: A Systematic Review from the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S77–S104. [Google Scholar] [CrossRef]
- Strauss, F.-J.; Nasirzade, J.; Kargarpoor, Z.; Stähli, A.; Gruber, R. Effect of platelet-rich fibrin on cell proliferation, migration, differentiation, inflammation, and osteoclastogenesis: A systematic review of in vitro studies. Clin. Oral Investig. 2020, 24, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.B.; Herrero, E.R.; Slomka, V.; Pinto, N.; Teughels, W.; Quirynen, M. Antimicrobial capacity of Leucocyte-and Platelet Rich Fibrin against periodontal pathogens. Sci. Rep. 2019, 9, 8188. [Google Scholar] [CrossRef]
- Pinto, N.R.; Ubilla, M.; Zamora, Y.; Del Rio, V.; Ehrenfest, D.M.D.; Quirynen, M. Leucocyte- and platelet-rich fibrin (L-PRF) as a regenerative medicine strategy for the treatment of refractory leg ulcers: A prospective cohort study. Platelets 2018, 29, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, S.; Castro, A.B.; Temmerman, A.; Van Dessel, J.; Pinto, N.; Jacobs, R.; Quirynen, M. Leucocyte- and platelet-rich fibrin block for bone augmentation procedure: A proof-of-concept study. J. Clin. Periodontol. 2018, 45, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Siawasch, S.A.M.; Temmerman, A.; Cortellini, S.; Dhondt, R.; Teughels, W.; Castro, A.B. Do autologous platelet concentrates (APCs) have a role in intra-oral bone regeneration? A critical review of clinical guidelines on decision-making process. Periodontology 2000 2023. early view. [Google Scholar] [CrossRef] [PubMed]
- Lyris, V.; Millen, C.; Besi, E.; Pace-Balzan, A. Effect of leukocyte and platelet rich fibrin (L-PRF) on stability of dental implants. A systematic review and meta-analysis. Br. J. Oral Maxillofac. Surg. 2021, 59, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Jalkh, E.B.B.; Tovar, N.; Arbex, L.; Kurgansky, G.; Torroni, A.; Gil, L.F.; Wall, B.; Kohanbash, K.; Bonfante, E.A.; Coelho, P.G.; et al. Effect of leukocyte-platelet-rich fibrin in bone healing around dental implants placed in conventional and wide osteotomy sites: A pre-clinical study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Madurantakam, P.; Yoganarasimha, S.; Hasan, F.K. Characterization of Leukocyte-platelet Rich Fibrin, A Novel Biomaterial. J. Vis. Exp. 2015, e53221. [Google Scholar] [CrossRef]
- Khorshidi, H.; Raoofi, S.; Bagheri, R.; Banihashemi, H. Comparison of the Mechanical Properties of Early Leukocyte- and Platelet-Rich Fibrin versus PRGF/Endoret Membranes. Int. J. Dent. 2016, 2016, 1849207. [Google Scholar] [CrossRef]
- Ockerman, A.; Braem, A.; EzEldeen, M.; Castro, A.; Coucke, B.; Politis, C.; Verhamme, P.; Jacobs, R.; Quirynen, M. Mechanical and structural properties of leukocyte- and platelet-rich fibrin membranes: An in vitro study on the impact of anticoagulant therapy. J. Periodontal Res. 2020, 55, 686–693. [Google Scholar] [CrossRef]
- Simões-Pedro, M.; Tróia, P.M.B.P.S.; dos Santos, N.B.M.; Completo, A.M.G.; Castilho, R.M.; Fernandes, G.V.d.O. Tensile Strength Essay Comparing Three Different Platelet-Rich Fibrin Membranes (L-PRF, A-PRF, and A-PRF+): A Mechanical and Structural In Vitro Evaluation. Polymers 2022, 14, 1392. [Google Scholar] [CrossRef]
- Pascoal, M.d.A.N.C.; dos Santos, N.B.M.; Completo, A.M.G.; Fernandes, G.V.d.O. Tensile strength assay comparing the resistance between two different autologous platelet concentrates (leucocyte-platelet rich fibrin versus advanced-platelet rich fibrin): A pilot study. Int. J. Implant. Dent. 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-M.; Shen, E.-C.; Shen, J.T.; Fu, E.; Chiu, H.-C.; Hsia, Y.-J. Tensile strength, growth factor content and proliferation activities for two platelet concentrates of platelet-rich fibrin and concentrated growth factor. J. Dent. Sci. 2020, 15, 141–146. [Google Scholar] [CrossRef]
- Ravi, S.; Santhanakrishnan, M. Mechanical, chemical, structural analysis and comparative release of PDGF-AA from L-PRF, A-PRF and T-PRF—An in vitro study. Biomater. Res. 2020, 24, 16. [Google Scholar] [CrossRef]
- Sam, G.; Vadakkekuttical, R.J.; Amol, N.V. In vitro evaluation of mechanical properties of platelet-rich fibrin membrane and scanning electron microscopic examination of its surface characteristics. J. Indian Soc. Periodontol. 2015, 19, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Apatzidou, D.A. The role of cigarette smoking in periodontal disease and treatment outcomes of dental implant therapy. Periodontol. 2000 2022, 90, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhu, L.; Yang, C. The effect of preoperative smoking and smoke cessation on wound healing and infection in post-surgery subjects: A meta-analysis. Int. Wound J. 2022, 19, 2101–2106. [Google Scholar] [CrossRef]
- Ríos, S.; Álvarez, S.; Smith, P.C.; Sáez, C.G.; Andrade, C.; Pinto, N.; Martínez, C.E. Smoking habits do not affect biological responses induced by leucocyte and platelet-rich fibrin in periodontal ligament cells. J. Oral Pathol. Med. 2023, 52, 169–180. [Google Scholar] [CrossRef]
- Srirangarajan, S.; Sindhu, V.; Prabhu, S.; Rao, R.; Rudresh, V. Does Cigarette Smoking Induce Changes in Biologic and Mechanical Properties of Platelet-Rich Fibrin Membranes? Int. J. Periodontics Restor. Dent. 2021, 41, e213–e221. [Google Scholar] [CrossRef]
- Srirangarajan, S.; Sindhu, V.; Rao, R.; Prabhu, S.; Rudresh, V. Effect of Cigarette Smoking on Morphologic Characteristics of Two Different Platelet-Rich Fibrin Membranes: A Scanning Electron Microscopic Study. Int. J. Oral Maxillofac. Implant. 2020, 35, 275–280. [Google Scholar] [CrossRef]
- Miron, R.J.; Moraschini, V.; Fujioka-Kobayashi, M.; Zhang, Y.; Kawase, T.; Cosgarea, R.; Jepsen, S.; Bishara, M.; Canullo, L.; Shirakata, Y.; et al. Use of platelet-rich fibrin for the treatment of periodontal intrabony defects: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 2461–2478. [Google Scholar] [CrossRef]
- Miron, R.J.; Moraschini, V.; Del Fabbro, M.; Piattelli, A.; Fujioka-Kobayashi, M.; Zhang, Y.; Saulacic, N.; Schaller, B.; Kawase, T.; Cosgarea, R.; et al. Use of platelet-rich fibrin for the treatment of gingival recessions: A systematic review and meta-analysis. Clin. Oral Investig. 2020, 24, 2543–2557. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.-L.; et al. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin. Oral Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Perez, K.; Dym, H. Clinical Uses of Platelet-Rich Fibrin in Oral and Maxillofacial Surgery. Dent. Clin. N. Am. 2020, 64, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Neiva, R.F.; Gil, L.F.; Tovar, N.; Janal, M.N.; Marao, H.F.; Bonfante, E.A.; Pinto, N.; Coelho, P.G. The Synergistic Effect of Leukocyte Platelet-Rich Fibrin and Micrometer/Nanometer Surface Texturing on Bone Healing around Immediately Placed Implants: An Experimental Study in Dogs. BioMed. Res. Int. 2016, 2016, 9507342. [Google Scholar] [CrossRef] [PubMed]
- Lohse, T.; Rohrmann, S.; Bopp, M.; Faeh, D. Heavy Smoking Is More Strongly Associated with General Unhealthy Lifestyle than Obesity and Underweight. PLoS ONE 2016, 11, e0148563. [Google Scholar] [CrossRef]
- Barua, R.S.; Sy, F.; Srikanth, S.; Huang, G.; Javed, U.; Buhari, C.; Margosan, D.; Aftab, W.; Ambrose, J.A. Acute Cigarette Smoke Exposure Reduces Clot Lysis—Association between Altered Fibrin Architecture and the Re-sponse to t-PA. Thromb. Res. 2010, 126, 426–430. [Google Scholar] [CrossRef]
- Stępień, E.; Miszalski-Jamka, T.; Kapusta, P.; Tylko, G.; Pasowicz, M. Beneficial Effect of Cigarette Smoking Ces-sation on Fibrin Clot Properties. J. Thromb. Thrombolysis 2011, 32, 177–182. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e45–e50. [Google Scholar] [CrossRef]
- Choukroun, J.; Aalam, A. Platelet-Rich Fibrin: Natural and Biologic Blood Concentrate. In Horizontal Alveolar Ridge Augmentation in Implant Dentistry: A Surgical Manual; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 314–318. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara, C.; Bezmalinovic, A.; García-Herrera, C.; Ríos, S.; Valenzuela, L.M.; Martínez, C.E. Leukocyte- and Platelet-Rich Fibrin (L-PRF) Obtained from Smokers and Nonsmokers Shows a Similar Uniaxial Tensile Response In Vitro. Biomedicines 2023, 11, 3286. https://doi.org/10.3390/biomedicines11123286
Lara C, Bezmalinovic A, García-Herrera C, Ríos S, Valenzuela LM, Martínez CE. Leukocyte- and Platelet-Rich Fibrin (L-PRF) Obtained from Smokers and Nonsmokers Shows a Similar Uniaxial Tensile Response In Vitro. Biomedicines. 2023; 11(12):3286. https://doi.org/10.3390/biomedicines11123286
Chicago/Turabian StyleLara, Cesar, Alejandro Bezmalinovic, Claudio García-Herrera, Susana Ríos, Loreto M. Valenzuela, and Constanza E. Martínez. 2023. "Leukocyte- and Platelet-Rich Fibrin (L-PRF) Obtained from Smokers and Nonsmokers Shows a Similar Uniaxial Tensile Response In Vitro" Biomedicines 11, no. 12: 3286. https://doi.org/10.3390/biomedicines11123286
APA StyleLara, C., Bezmalinovic, A., García-Herrera, C., Ríos, S., Valenzuela, L. M., & Martínez, C. E. (2023). Leukocyte- and Platelet-Rich Fibrin (L-PRF) Obtained from Smokers and Nonsmokers Shows a Similar Uniaxial Tensile Response In Vitro. Biomedicines, 11(12), 3286. https://doi.org/10.3390/biomedicines11123286





