Early Effects of Alpha-Synuclein Depletion by Pan-Neuronal Inactivation of Encoding Gene on Electroencephalogram Coherence between Different Brain Regions in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Genotyping
2.3. Alpha-Synuclein Depletion and Procedures
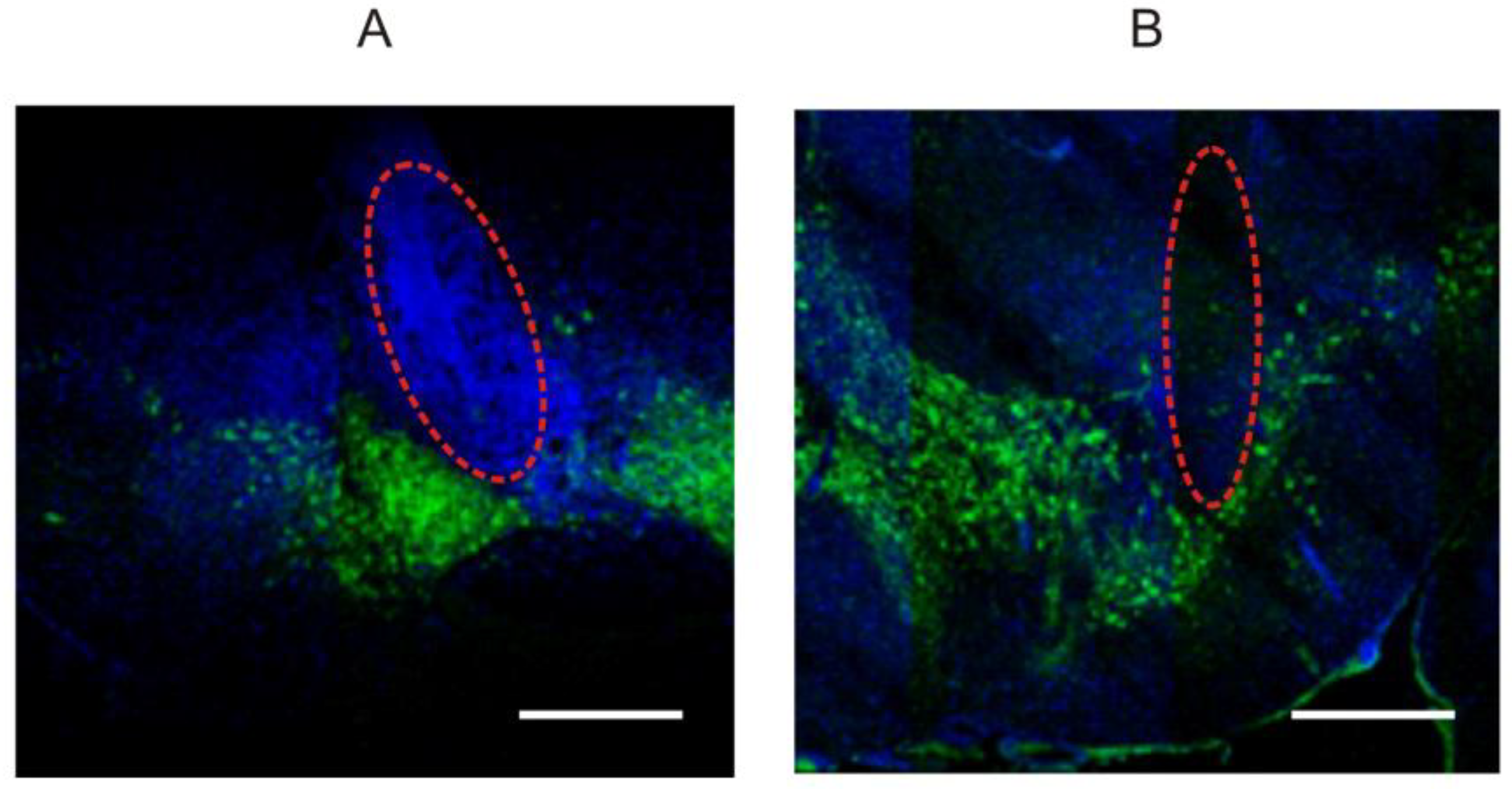
2.4. Preparation of Histological Sections and Immunohistochemistry
2.5. High-Pressure Liquid Chromatography (HPLC) with Electrochemical Detection
2.6. Analysis of mRNA Expression
2.7. Implantation of Electrodes
2.8. Baseline EEG Recording and Computation of the EEG Coherence
2.9. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Brain Areas | MCsin—Ptsin (A) | |||||||
| Drugs/Mice | Tamoxifen (TAM) vs. vehicle | Knockouts Versus Normal Mice | ||||||
| Months after TAM | 1 | 2 | 3 | |||||
| Bands/Coherence | F30 | p | F27 | p | F36 | p | F66 | p |
| delta1 | 5.8 | 0.022 | 10.6 | 0.003 | 18.7 | <0.001 | 4.8 | 0.032 |
| delta2 | 12.9 | 0.001 | 12.8 | 0.001 | 10.7 | 0.002 | 4.4 | 0.039 |
| theta | 12.5 | 0.001 | 19.1 | <0.001 | 9.9 | 0.002 | 13.1 | <0.001 |
| alpha | 11.8 | 0.002 | 19.8 | <0.001 | 8.6 | 0.005 | 12.0 | <0.001 |
| beta1 | 11.9 | 0.002 | 14.6 | <0.001 | 6.7 | 0.012 | 10.3 | 0.002 |
| beta2 | 14.1 | <0.001 | 8.6 | 0.007 | 6.4 | 0.014 | 5.5 | 0.022 |
| Brain Areas | MCsin—VTAsin (B) | |||||||
| Drugs/Mice | Tamoxifen (TAM) vs. vehicle | Knockouts Versus Normal Mice | ||||||
| Months after TAM | 1 | 2 | 3 | |||||
| Bands/Coherence | F30 | p | F27 | p | F36 | p | F66 | p |
| delta1 | 10.1 | 0.003 | 3.1 | 0.091 | 2.8 | 0.052 | 1.2 | 0.276 |
| delta2 | 9.1 | 0.005 | 4.1 | 0.053 | 1.9 | 0.168 | 0.3 | 0.616 |
| theta | 1.1 | 0.298 | 12.5 | 0.002 | 2.4 | 0.130 | 5.6 | 0.041 |
| alpha | 0.7 | 0.422 | 15.4 | <0.001 | 2.4 | 0.128 | 10.0 | 0.002 |
| beta1 | 0.4 | 0.525 | 9.5 | 0.005 | 1.9 | 0.173 | 9.5 | 0.005 |
| beta2 | 0.1 | 0.897 | 7.6 | 0.010 | 1.8 | 0.191 | 4.3 | 0.054 |
| Brain Areas | MCsin—SNdex (C) | |||||||
| Drugs/Mice | Tamoxifen (TAM) vs. vehicle | Knockouts Versus Normal Mice | ||||||
| Months after TAM | 1 | 2 | 3 | |||||
| Bands/Coherence | F30 | p | F27 | p | F36 | p | F66 | p |
| delta1 | 3.7 | 0.065 | 9.2 | 0.005 | 22.5 | <0.001 | 17.4 | <0.001 |
| delta2 | 11.5 | 0.002 | 7.8 | 0.009 | 15.4 | <0.001 | 15.8 | <0.001 |
| theta | 20.8 | <0.001 | 21.2 | <0.001 | 12.2 | <0.001 | 23.6 | <0.001 |
| alpha | 20.5 | <0.001 | 29.1 | <0.001 | 11.1 | 0.001 | 24.7 | <0.001 |
| beta1 | 18.9 | <0.001 | 16.4 | <0.001 | 8.5 | 0.005 | 26.6 | <0.001 |
| beta2 | 22.2 | <0.001 | 6.8 | 0.015 | 7.6 | 0.008 | 18.3 | <0.001 |
| Brain Areas | Ptsin—VTAsin (D) | |||||||
| Drugs/Mice | Tamoxifen (TAM) vs. vehicle | Knockouts Versus Normal Mice | ||||||
| Months after TAM | 1 | 2 | 3 | |||||
| Bands/Coherence | F30 | p | F27 | p | F36 | p | F66 | p |
| delta1 | 1.2 | 0.291 | 6.6 | 0.016 | 16.5 | <0.001 | 43.4 | <0.001 |
| delta2 | 3.7 | 0.636 | 7.1 | 0.013 | 12.6 | <0.001 | 25.3 | <0.001 |
| theta | 14.9 | <0.001 | 7.3 | 0.012 | 12.0 | <0.001 | 33.1 | <0.001 |
| alpha | 12.5 | 0.001 | 8.2 | 0.008 | 11.1 | 0.001 | 38.0 | <0.001 |
| beta1 | 12.0 | 0.002 | 8.7 | 0.007 | 11.4 | 0.001 | 37.2 | <0.001 |
| beta2 | 13.8 | <0.001 | 9.1 | 0.005 | 12.5 | <0.001 | 36.3 | <0.001 |
| Brain Areas | Ptsin—SNdex (E) | |||||||
| Drugs/Mice | Tamoxifen (TAM) vs. vehicle | Knockouts Versus Normal Mice | ||||||
| Months after TAM | 1 | 2 | 3 | |||||
| Bands/Coherence | F30 | p | F27 | p | F36 | p | F66 | p |
| delta1 | 6.6 | 0.015 | 7.4 | 0.011 | 46.2 | <0.001 | 25.2 | <0.001 |
| delta2 | 7.7 | 0.009 | 7.9 | 0.009 | 40.5 | <0.001 | 14.8 | <0.001 |
| theta | 5.8 | 0.022 | 10.7 | 0.003 | 35.4 | <0.001 | 34.2 | <0.001 |
| alpha | 6.4 | 0.017 | 13.1 | 0.001 | 31.4 | <0.001 | 37.0 | <0.001 |
| beta1 | 5.3 | 0.029 | 11.3 | 0.002 | 28.7 | <0.001 | 37.7 | <0.001 |
| beta2 | 5.2 | 0.029 | 10.1 | 0.004 | 32.1 | <0.001 | 41.9 | <0.001 |
| Brain Areas | VTAsin—SNdex (F) | |||||||
| Drugs/Mice | Tamoxifen (TAM) vs. vehicle | Alpha-Knockouts vs. Normal Mice | ||||||
| Months after TAM | 1 | 2 | 3 | |||||
| Bands/Coherence | F30 | p | F27 | p | F36 | p | F66 | p |
| delta1 | 0.0 | 0.916 | 0.5 | 0.501 | 34.6 | <0.001 | 24.7 | <0.001 |
| delta2 | 0.0 | 0.874 | 0.3 | 0.612 | 26.3 | <0.001 | 8.8 | 0.004 |
| theta | 1.3 | 0.257 | 0.1 | 0.760 | 27.2 | <0.001 | 18.9 | <0.001 |
| alpha | 1.5 | 0.236 | 0.0 | 0.869 | 28.7 | <0.001 | 18.2 | <0.001 |
| beta1 | 1.6 | 0.215 | 0.0 | 0.871 | 31.2 | <0.001 | 37.7 | <0.001 |
| beta2 | 2.45 | 0.128 | 0.0 | 0.833 | 30.3 | <0.001 | 22.6 | <0.001 |
Appendix B
| Drugs/Mice | Tamoxifen (TAM) vs. vehicle | Knockouts versus Normal Mice (D) | ||||||
|---|---|---|---|---|---|---|---|---|
| Months after TAM | 1 (A) | 2 (B) | 3 (C) | |||||
| Brain Areas | F180 | p | F162 | p | F216 | p | F396 | p |
| MCsin—Ptsin | 68.4 | <0.001 | 83.7 | <0.001 | 23.3 | <0.001 | 46.4 | <0.001 |
| MCsin—VTAsin | 19.8 | 0.001 | 48.7 | <0.001 | 3.7 | 0.055 | 8.8 | 0.032 |
| MCsin—SNdex | 90.9 | <0.001 | 82.6 | <0.001 | 15.5 | <0.001 | 125 | <0.001 |
| Ptsin—VTAsin | 51.7 | <0.001 | 46.9 | <0.001 | 24.4 | <0.001 | 210 | <0.001 |
| Ptsin—SNdex | 36.5 | <0.001 | 60.3 | <0.001 | 72.2 | <0.001 | 185 | <0.001 |
| VTAsin—SNdex | 5.2 | 0.024 | 0.1 | 0.864 | 90.8 | <0.001 | 107 | <0.001 |
References
- Osterberg, V.R.; Spinelli, K.J.; Weston, L.J.; Luk, K.C.; Woltjer, R.L.; Unni, V.K. Progressive aggregation of alpha-synuclein and selective degeneration of lewy inclusion-bearing neurons in a mouse model of parkinsonism. Cell Rep. 2015, 10, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Venda, L.L.; Cragg, S.J.; Buchman, V.L.; Wade-Martins, R. Alpha-synuclein and dopamine at the crossroads of Parkinson’s disease. Trends Neurosci. 2010, 33, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Benskey, M.J.; Perez, R.G.; Manfredsson, F.P. The contribution of alpha synuclein to neuronal survival and function—Implications for Parkinson’s disease. J. Neurochem. 2016, 137, 331–359. [Google Scholar] [CrossRef] [PubMed]
- Sulzer, D.; Edwards, R.H. The physiological role of alpha-synuclein and its relationship to Parkinson’s disease. J. Neurochem. 2019, 150, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Connor-Robson, N.; Peters, O.M.; Millership, S.; Ninkina, N.; Buchman, V.L. Combinational losses of synucleins reveal their differential requirements for compensating age-dependent alterations in motor behavior and dopamine metabolism. Neurobiol. Aging 2016, 46, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Yoo, G.; Yeou, S.; Son, J.B.; Shin, Y.K.; Lee, N.K. Cooperative inhibition of SNARE-mediated vesicle fusion by α-synuclein monomers and oligomers. Sci. Rep. 2021, 11, 10955. [Google Scholar] [CrossRef]
- Carnazza, K.E.; Komer, L.E.; Xie, Y.X.; Pineda, A.; Briano, J.A.; Gao, V.; Na, Y.; Ramlall, T.; Buchman, V.L.; Eliezer, D.; et al. Synaptic vesicle binding of α-synuclein is modulated by β- and γ-synucleins. Cell Rep. 2022, 39, 110675. [Google Scholar] [CrossRef]
- Ninkina, N.; Connor-Robson, N.; Ustyugov, A.A.; Tarasova, T.V.; Shelkovnikova, T.A.; Buchman, V.L. A novel resource for studying function and dysfunction of alpha-synuclein: Mouse lines for modulation of endogenous snca gene expression. Sci. Rep. 2015, 5, 16615. [Google Scholar] [CrossRef]
- Ninkina, N.; Tarasova, T.V.; Chaprov, K.D.; Roman, A.Y.; Kukharsky, M.S.; Kolik, L.G.; Ovchinnikov, R.; Ustyugov, A.A.; Durnev, A.D.; Buchman, V.L. Alterations in the nigrostriatal system following conditional inactivation of alpha-synuclein in neurons of adult and aging mice. Neurobiol. Aging 2020, 91, 76–87. [Google Scholar] [CrossRef]
- Chaprov, K.D.; Lysikova, E.A.; Teterina, E.V.; Buchman, V.L. Kinetics of alpha-synuclein depletion in three brain regions following conditional pan-neuronal inactivation of the encoding gene (Snca) by tamoxifen-induced Cre-recombination in adult mice. Transgenic Res. 2021, 30, 867–873. [Google Scholar] [CrossRef]
- Mikelman, S.R.; Guptaroy, B.; Gnegy, M.E. Tamoxifen and its active metabolites inhibit dopamine transporter function independently of the estrogen receptors. J. Neurochem. 2017, 141, 31–36. [Google Scholar] [CrossRef]
- Pandey, D.; Banerjee, S.; Basu, M.; Mishra, N. Memory enhancement by tamoxifen on amyloidosis mouse model. J. Horm. Behav. 2016, 79, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wu, C.F.; Shi, B.; Xu, Y.M. Tamoxifen and toremifene cause impairment of learning and memory function in mice. Pharmacol. Biochem. Behav. 2002, 71, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Nimmrich, V.; Draguhn, A.; Axmacher, N. Neuronal Network Oscillations in Neurodegenerative Diseases. Neuromol. Med. 2015, 17, 270–284. [Google Scholar] [CrossRef]
- Nunez, P.L.; Srinivasan, R. Electric Fields of the Brain: The Neurophysics of EEG; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Wang, R.; Wang, J.; Yu, H.; Wei, X.; Yang, C.; Deng, B. Power spectral density and coherence analysis of Alzheimer’s EEG. Cogn. Neurodyn. 2015, 9, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Vorobyov, V.; Deev, A.; Sukhanova, I.; Morozova, O.; Oganesyan, Z.; Chaprov, K.; Buchman, V.L. Loss of the Synuclein Family Members Differentially Affects Baseline- and Apomorphine- Associated EEG Determinants in Single-, Double- and Triple- Knockout Mice. Biomedicines 2022, 10, 3128. [Google Scholar] [CrossRef]
- Vorobyov, V.; Deev, A.; Chaprov, K.; Ustyugov, A.A.; Lysikova, E. Age-Related Modifications of Electroencephalogram Coherence in Mice Models of Alzheimer’s Disease and Amyotrophic Lateral Sclerosis. Biomedicines 2023, 11, 1151. [Google Scholar] [CrossRef]
- Chaprov, K.D.; Teterina, E.V.; Roman, A.Y.; Ivanova, T.A.; Goloborshcheva, V.V.; Kucheryanu, V.G.; Morozov, S.G.; Lysikova, E.A.; Lytkina, O.A.; Koroleva, I.V.; et al. Comparative Analysis of MPTP Neurotoxicity in Mice with a Constitutive Knockout of the α-Synuclein Gene. Mol. Biol. 2021, 55, 133–142. [Google Scholar] [CrossRef]
- Goloborshcheva, V.V.; Chaprov, K.D.; Teterina, E.V.; Ovchinnikov, R.; Buchman, V.L. Reduced complement of dopaminergic neurons in the substantia nigra pars compacta of mice with a constitutive “low footprint” genetic knockout of alpha-synuclein. Mol. Brain 2020, 13, 75. [Google Scholar] [CrossRef]
- Hökfelt, T.; Martensson, R.; Björklund, A.; Kheinau, S.; Goldstein, M. Distributional maps of tyrosinehydroxylaseimmunoreactive neurons in the rat brain. In Handbook of Chemical Neuroanatomy, Vol. 2: Classical Transmitters in the CNS, Part I; Björklund, A., Hökfelt, T., Eds.; Elsevier Science B.V: Amsterdam, The Netherlands, 1984; pp. 277–379. [Google Scholar]
- Franklin, K.B.J.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates, 3rd ed.; Academic Press: New York, NY, USA, 2007. [Google Scholar]
- Fell, J.; Axmacher, N. The role of phase synchronization in memory processes. Nat. Rev. Neurosci. 2011, 12, 105–118. [Google Scholar] [CrossRef]
- Eberling, J.L.; Wu, C.; Tong-Turnbeaugh, R.; Jagust, W.J. Estrogen-and tamoxifen-associated effects on brain structure and function. Neuroimage 2004, 21, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Denk, F.; Ramer, L.M.; Erskine, E.L.; Nassar, M.A.; Bogdanov, Y.; Signore, M.; Wood, J.N.; McMahon, S.B.; Ramer, M. Tamoxifen induces cellular stress in the nervous system by inhibiting cholesterol synthesis. Acta Neuropathol. Commun. 2015, 3, 74. [Google Scholar] [CrossRef] [PubMed]
- Novick, A.M.; Scott, A.T.; Epperson, C.N.; Schneck, C.D. Neuropsychiatric effects of tamoxifen: Challenges and opportunities. Front. Neuroendocrinol. 2020, 59, 100869. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.G.; Bourque, M.; Morissette, M.; Di Paolo, T. Steroids-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci. Ther. 2010, 16, e43–e71. [Google Scholar] [CrossRef]
- Mikelman, S.R.; Guptaroy, B.; Schmitt, K.C.; Jones, K.T.; Zhen, J.; Reith, M.E.A.; Gnegy, M.E. Tamoxifen Directly Interacts with the Dopamine Transporter. J. Pharmacol. Exp. Ther. 2018, 367, 119–128. [Google Scholar] [CrossRef]
- Hiemke, C.; Ghraf, R. Interaction of non-steroidal antiestrogens with dopamine receptor binding. J. Steroid Biochem. 1984, 21, 663–667. [Google Scholar] [CrossRef]
- McDermott, J.L.; Anderson, L.I.; Dluzen, D.E. Interactive effects of tamoxifen and estrogen upon the nigrostriatal dopaminergic system. Neuroendocrinology 1997, 66, 181–187. [Google Scholar] [CrossRef]
- Li, X.; Du, Z.-J.; Chen, M.-Q.; Chen, J.-J.; Liang, Z.-M.; Ding, X.-T.; Zhou, M.; Li, S.-J.; Li, X.-W.; Yang, J.-M.; et al. The effects of tamoxifen on mouse behavior. Genes Brain Behav. 2020, 19, e12620. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorobyov, V.; Deev, A.; Morozova, O.; Oganesyan, Z.; Krayushkina, A.M.; Ivanova, T.A.; Chaprov, K. Early Effects of Alpha-Synuclein Depletion by Pan-Neuronal Inactivation of Encoding Gene on Electroencephalogram Coherence between Different Brain Regions in Mice. Biomedicines 2023, 11, 3282. https://doi.org/10.3390/biomedicines11123282
Vorobyov V, Deev A, Morozova O, Oganesyan Z, Krayushkina AM, Ivanova TA, Chaprov K. Early Effects of Alpha-Synuclein Depletion by Pan-Neuronal Inactivation of Encoding Gene on Electroencephalogram Coherence between Different Brain Regions in Mice. Biomedicines. 2023; 11(12):3282. https://doi.org/10.3390/biomedicines11123282
Chicago/Turabian StyleVorobyov, Vasily, Alexander Deev, Olga Morozova, Zoya Oganesyan, Anastasia M. Krayushkina, Tamara A. Ivanova, and Kirill Chaprov. 2023. "Early Effects of Alpha-Synuclein Depletion by Pan-Neuronal Inactivation of Encoding Gene on Electroencephalogram Coherence between Different Brain Regions in Mice" Biomedicines 11, no. 12: 3282. https://doi.org/10.3390/biomedicines11123282
APA StyleVorobyov, V., Deev, A., Morozova, O., Oganesyan, Z., Krayushkina, A. M., Ivanova, T. A., & Chaprov, K. (2023). Early Effects of Alpha-Synuclein Depletion by Pan-Neuronal Inactivation of Encoding Gene on Electroencephalogram Coherence between Different Brain Regions in Mice. Biomedicines, 11(12), 3282. https://doi.org/10.3390/biomedicines11123282






