On the Role of Iron in Idiopathic Parkinson’s Disease
Abstract
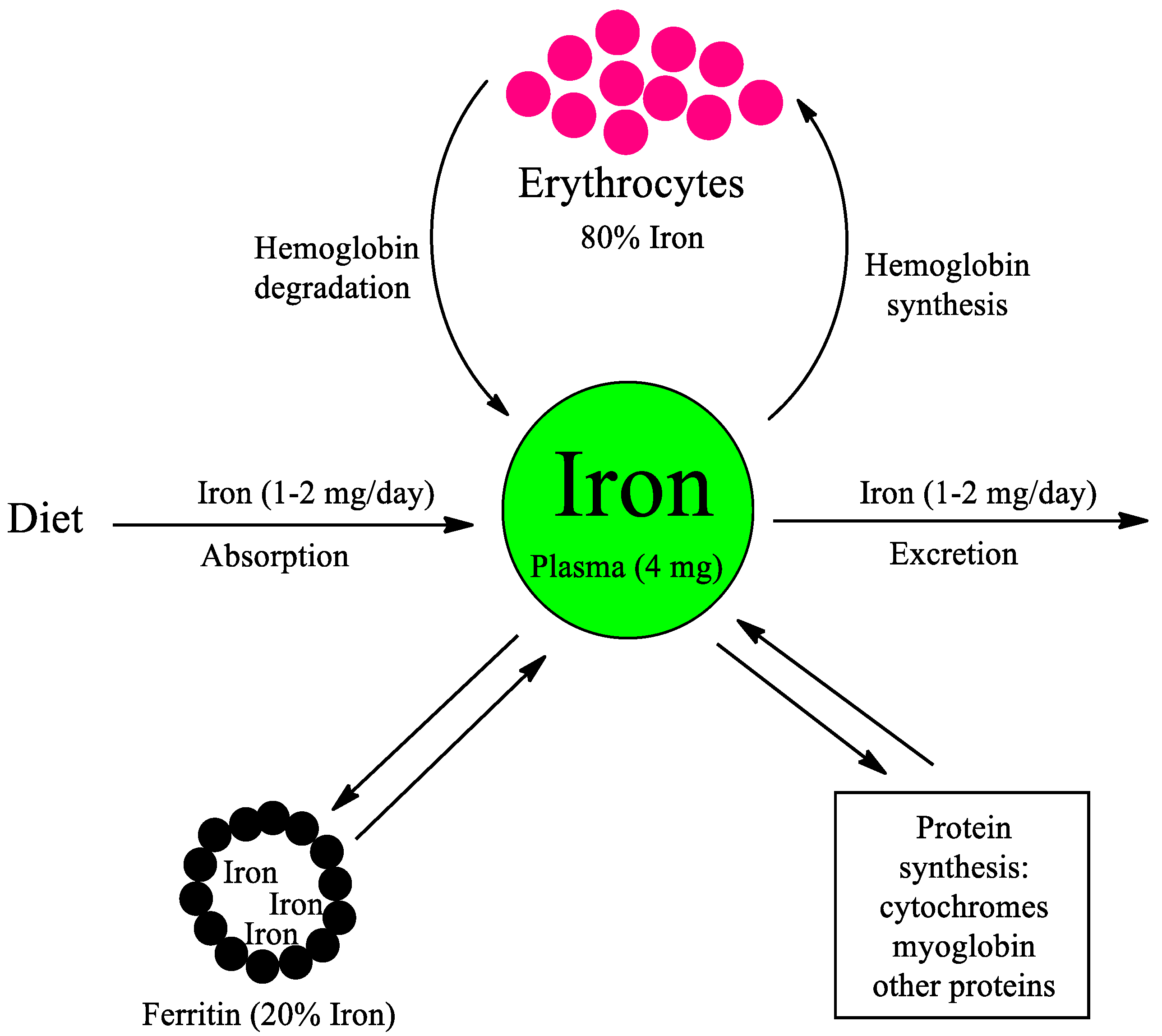
1. Iron
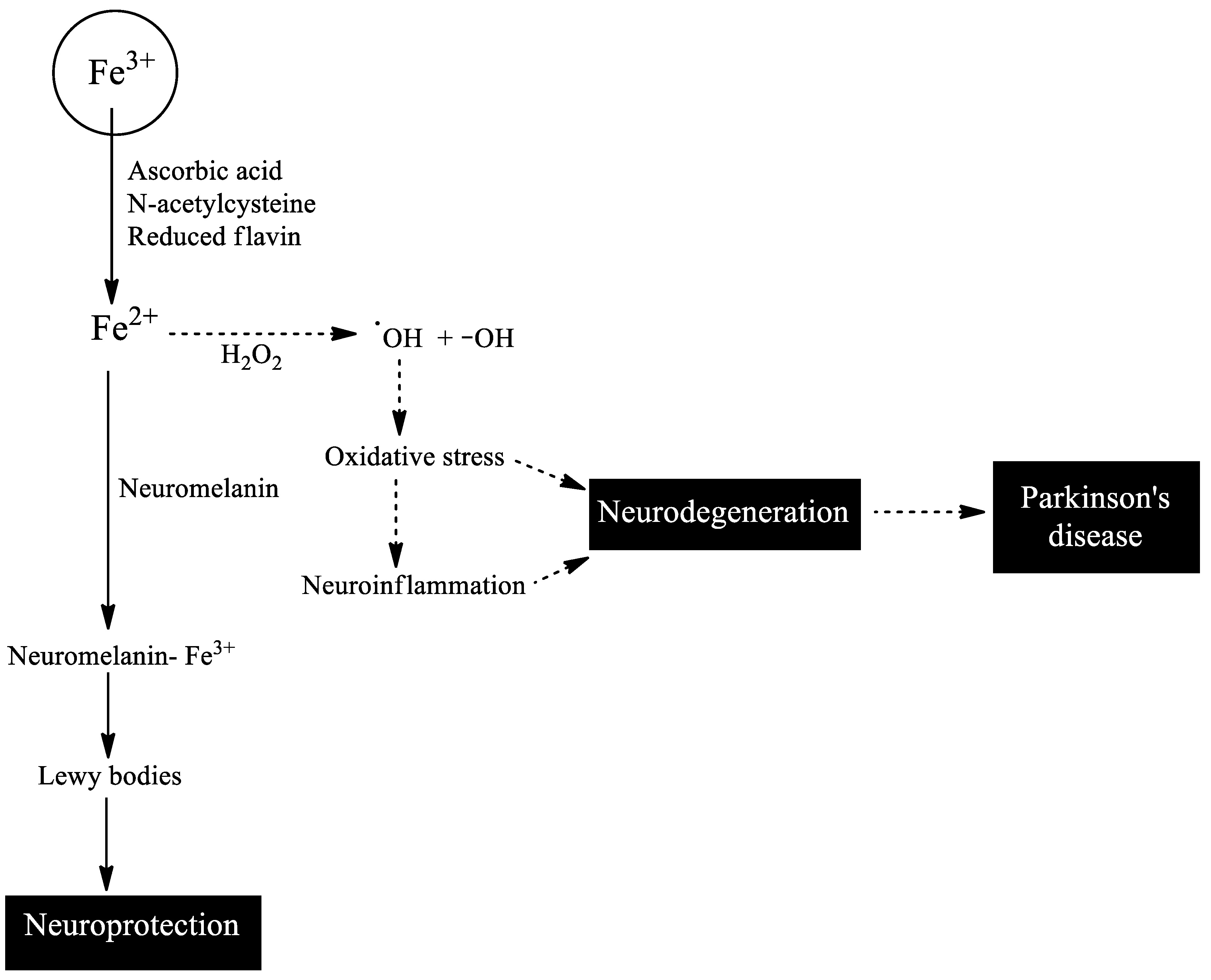
2. Iron Toxicity
3. Iron and Parkinson’s Disease
4. Clinical Study with the Iron Chelator Deferiprone
| Disease | Aim of Treatment | Result | Reference |
|---|---|---|---|
| Transfusion-dependent thalassemia | Prevent the toxic effects of iron overload | Positive | Elalfy et al., 2023 [51] |
| Pantothenate kinase-associated neurodegeneration | Prevent iron accumulation | Positive | Klopstock et al., 2019 [52] |
| Transfusional iron overload in sickle cell disease | Prevent iron accumulation | Positive | Kwiatkowski et al., 2022 [62] |
| Friedreich’s ataxia | Prevent iron accumulation | Positive | Pandolfo M, Hausmann, 2013 [63] |
| Transfusion-dependent haemoglobinopathies-2 | Prevent iron accumulation | Positive | Maggio et al., 2020 [64] |
| Parkinson’s disease | Prevent iron-dependent disease progression | Negative | Devos et al., 2022 [55] |
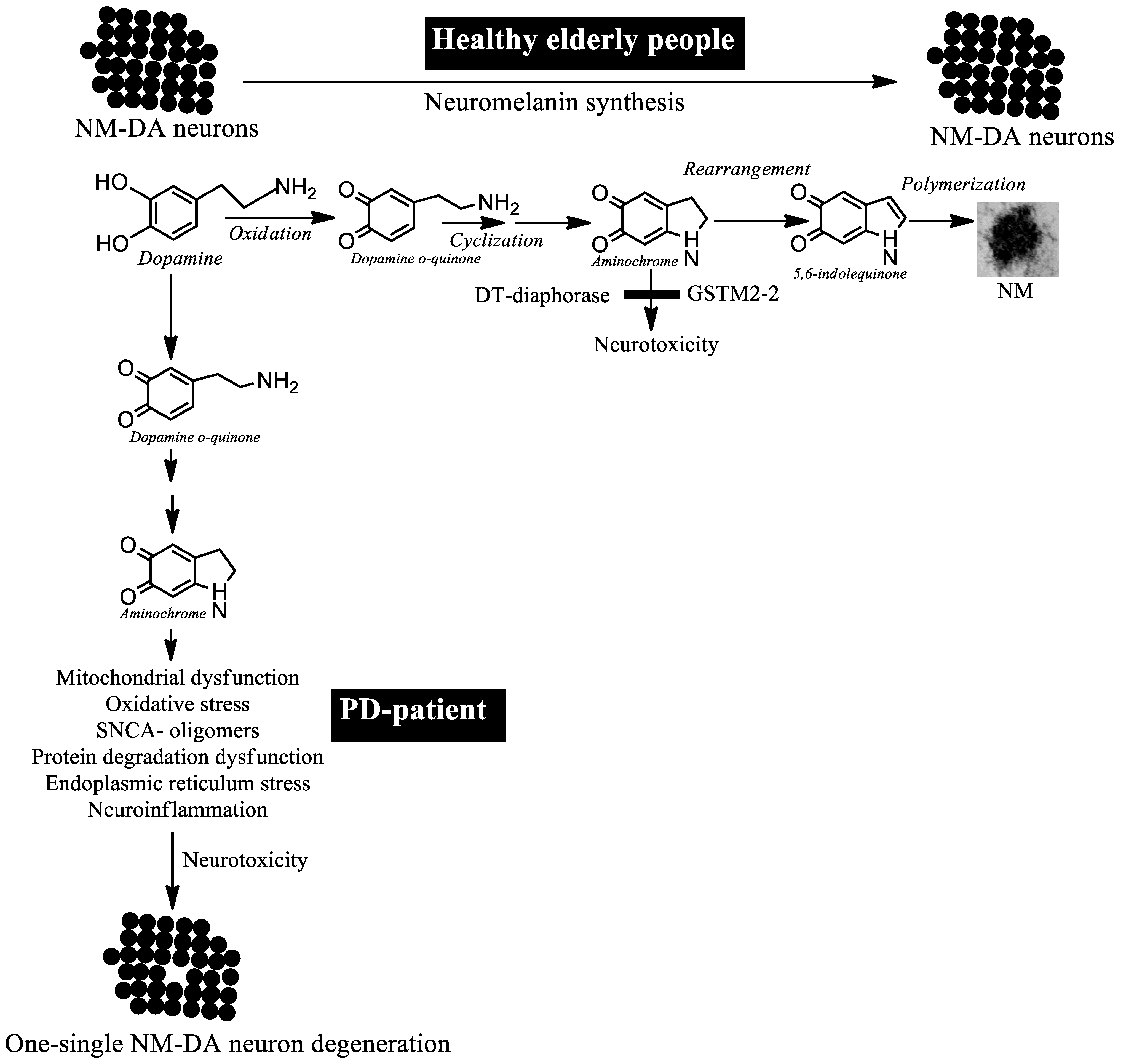
5. The Degenerative Process in Parkinson’s Disease
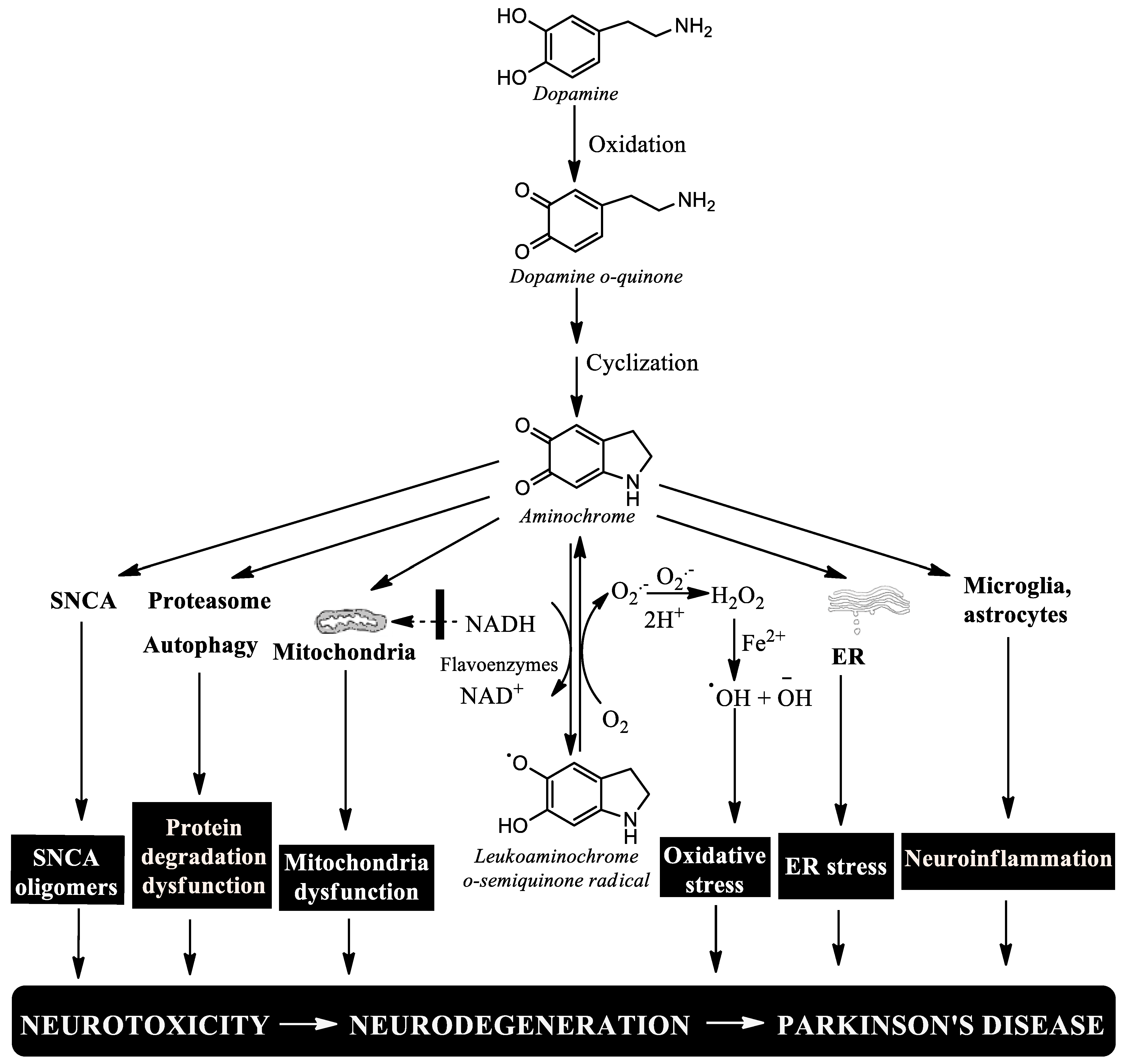
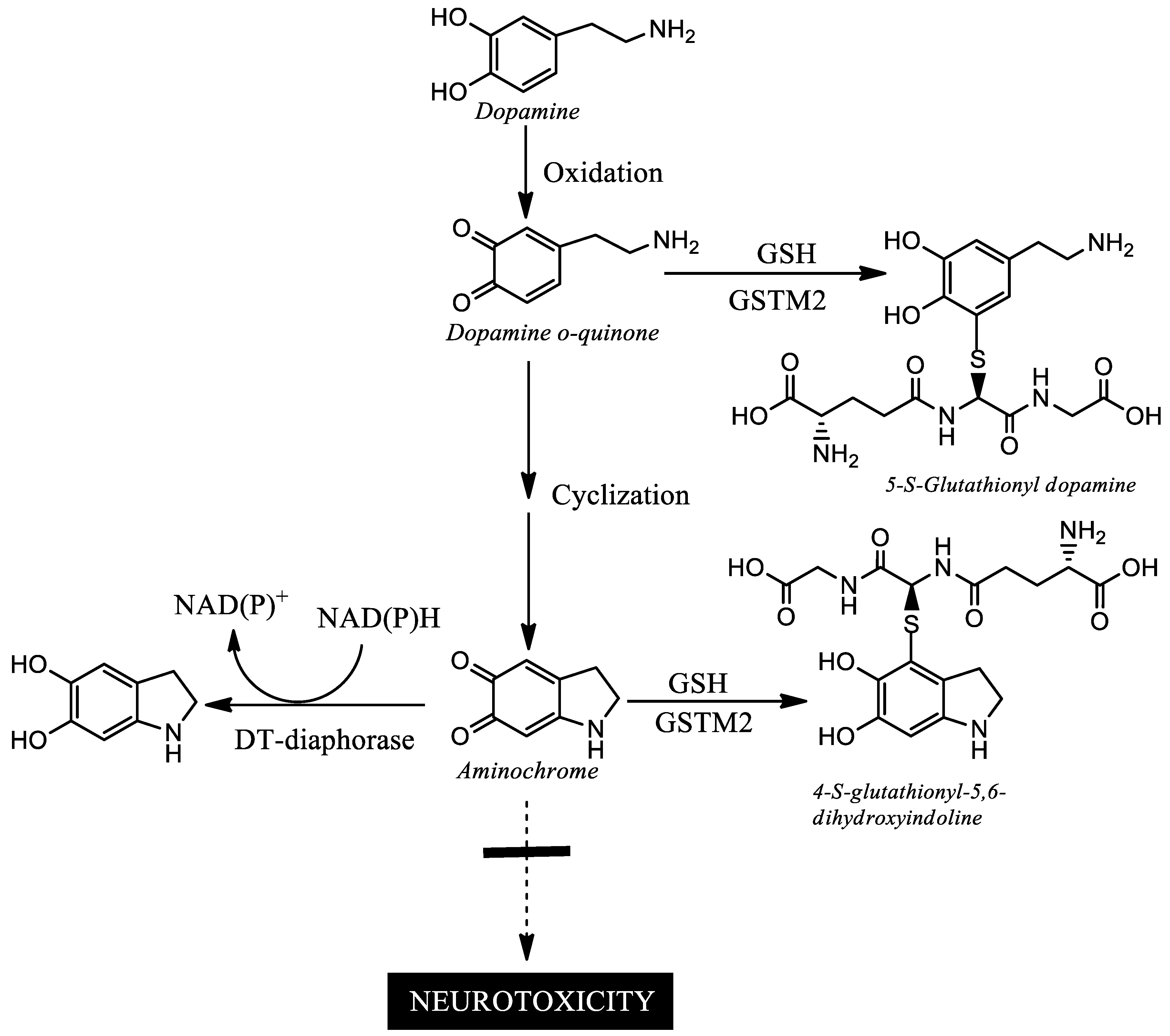
6. Aminochrome
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dutt, S.; Hamza, I.; Bartnikas, T.B. Molecular Mechanisms of Iron and Heme Metabolism. Annu. Rev. Nutr. 2022, 42, 311–335. [Google Scholar] [CrossRef]
- Vogt, A.-C.S.; Arsiwala, T.; Mohsen, M.; Vogel, M.; Manolova, V.; Bachmann, M.F. On Iron Metabolism and Its Regulation. Int. J. Mol. Sci. 2021, 22, 4591. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.; Hider, R. Iron and redox cycling. Do’s and don’ts. Free. Radic. Biol. Med. 2019, 133, 3–10. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J.; Frazer, D.M. Current understanding of iron homeostasis. Am. J. Clin. Nutr. 2017, 106, 1559S–1566S. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, N.; Pantopoulos, K. The IRP/IRE system in vivo: Insights from mouse models. Front. Pharmacol. 2014, 5, 176. [Google Scholar] [CrossRef]
- Golfeyz, S.; Lewis, S.; Weisberg, I.S. Hemochromatosis: Pathophysiology, evaluation, and management of hepatic iron overload with a focus on MRI. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 767–778. [Google Scholar] [CrossRef]
- Mehta, K.J.; Farnaud, S.J.; Sharp, P.A. Iron and liver fibrosis: Mechanistic and clinical aspects. World J. Gastroenterol. 2019, 25, 521–538. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, E.; Marley, A.; Samaan, M.A.; Brookes, M.J. Iron deficiency anaemia: Pathophysiology, assessment, practical management. BMJ Open Gastroenterol. 2022, 9, e000759. [Google Scholar] [CrossRef]
- Gattermann, N.; Muckenthaler, M.U.; Kulozik, A.E.; Metzgeroth, G.; Hastka, J. The evaluation of iron deficiency and iron overload. Dtsch. Aerzteblatt Online 2021, 118, 847–856. [Google Scholar] [CrossRef]
- Petraglia, F.; Dolmans, M.M. Iron deficiency anemia: Impact on women’s reproductive health. Fertil. Steril. 2022, 118, 605–606. [Google Scholar] [CrossRef] [PubMed]
- Zucca, F.A.; Segura-Aguilar, J.; Ferrari, E.; Muñoz, P.; Paris, I.; Sulzer, D.; Sarna, T.; Casella, L.; Zecca, L. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog. Neurobiol. 2017, 155, 96–119. [Google Scholar] [CrossRef]
- Zucca, F.A.; Capucciati, A.; Bellei, C.; Sarna, M.; Sarna, T.; Monzani, E.; Casella, L.; Zecca, L. Neuromelanins in brain aging and Parkinson’s disease: Synthesis, structure, neuroinflammatory, and neurodegenerative role. IUBMB Life 2023, 75, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Sulzer, D.; Cassidy, C.; Horga, G.; Kang, U.J.; Fahn, S.; Casella, L.; Pezzoli, G.; Langley, J.; Hu, X.P.; Zucca, F.A.; et al. Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson’s disease. Npj Park. Dis. 2018, 4, 11. [Google Scholar] [CrossRef]
- Zecca, L.; Casella, L.; Albertini, A.; Bellei, C.; Zucca, F.A.; Engelen, M.; Zadlo, A.; Szewczyk, G.; Zareba, M.; Sarna, T. Neuromelanin can protect against iron-mediated oxidative damage in system modeling iron overload of brain aging and Parkinson’s disease. J. Neurochem. 2008, 106, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Sulzer, D.; Bogulavsky, J.; Larsen, K.E.; Behr, G.; Karatekin, E.; Kleinman, M.H.; Turro, N.; Krantz, D.; Edwards, R.H.; Greene, L.A.; et al. Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc. Natl. Acad. Sci. USA 2000, 97, 11869–11874. [Google Scholar] [CrossRef]
- Segura-Aguilar, J.; Sulzer, D.; Zucca, F.A.; Zecca, L. Overexpression of Vesicular Monoamine Transporter-2 may Block Neurotoxic Metabolites from Cytosolic Dopamine: A Potential Neuroprotective Therapy for Parkinson’s Disease. Clin. Pharmacol. Transl. Med. 2019, 3, 143–148. [Google Scholar]
- Liang, C.-L.; Nelson, O.; Yazdani, U.; Pasbakhsh, P.; German, D.C. Inverse relationship between the contents of neuromelanin pigment and the vesicular monoamine transporter-2: Human midbrain dopamine neurons. J. Comp. Neurol. 2004, 473, 97–106. [Google Scholar] [CrossRef]
- Segura-Aguilar, J. (Ed.) Dopamine oxidation to neuromelanin and neurotoxic metabolites. In Clinical Studies and Therapies in Parkinson’s Disease: Translations from Preclinical Models; Elsevier: Cambridge, MA, USA, 2021; pp. 213–223. [Google Scholar]
- Zhang, W.; Phillips, K.; Wielgus, A.R.; Liu, J.; Albertini, A.; Zucca, F.A.; Faust, R.; Qian, S.Y.; Miller, D.S.; Chignell, C.F.; et al. Neuromelanin Activates Microglia and Induces Degeneration of Dopaminergic Neurons: Implications for Progression of Parkinson’s Disease. Neurotox. Res. 2011, 19, 63–72. [Google Scholar] [CrossRef]
- Wilms, H.; Rosenstiel, P.; Sievers, J.; Deuschl, G.; Zecca, L.; Lucius, R.; Zhang, W.; Wang, T.; Pei, Z.; Miller, D.S.; et al. Activation of microglia by human neuromelanin is NF-κB-dependent and involves p38 mitogen-activated protein kinase: Implications for Parkinson’s disease. FASEB J. 2003, 17, 1–20. [Google Scholar] [CrossRef]
- Segura-Aguilar, J.; Paris, I.; Muñoz, P.; Ferrari, E.; Zecca, L.; Zucca, F.A. Protective and toxic roles of dopamine in Parkinson’s disease. J. Neurochem. 2014, 129, 898–915. [Google Scholar] [CrossRef]
- Segura-Aguilar, J.; Muñoz, P.; Inzunza, J.; Varshney, M.; Nalvarte, I.; Mannervik, B. Neuroprotection against Aminochrome Neurotoxicity: Glutathione Transferase M2-2 and DT-Diaphorase. Antioxidants 2022, 11, 296. [Google Scholar] [CrossRef]
- Zecca, L.; Fariello, R.; Riederer, P.; Sulzer, D.; Gatti, A.; Tampellini, D. The absolute concentration of nigral neuromelanin, assayed by a new sensitive method, increases throughout the life and is dramatically decreased in Parkinson’s disease. FEBS Lett. 2002, 510, 216–220. [Google Scholar] [CrossRef]
- Scarcello, E.; Herpain, A.; Tomatis, M.; Turci, F.; Jacques, P.; Lison, D. Hydroxyl radicals and oxidative stress: The dark side of Fe corrosion. Colloids Surf. B Biointerfaces 2020, 185, 110542. [Google Scholar] [CrossRef]
- Pandrangi, S.L.; Chittineedi, P.; Chikati, R.; Lingareddy, J.R.; Nagoor, M.; Ponnada, S.K. Role of dietary iron revisited: In metabolism, ferroptosis and pathophysiology of cancer. Am. J. Cancer Res. 2022, 12, 974–985. [Google Scholar] [PubMed]
- Sinha, S.; Pereira-Reis, J.; Guerra, A.; Rivella, S.; Duarte, D. The Role of Iron in Benign and Malignant Hematopoiesis. Antioxid. Redox Signal. 2021, 35, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ding, Y.; Zhang, J.; Zhang, Y.; Cui, Y.; Zhang, Y.; Chang, S.; Chang, Y.; Gao, G. Iron overload suppresses hippocampal neurogenesis in adult mice: Implication for iron dysregulation-linked neurological diseases. CNS Neurosci. Ther. 2023. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-Y.; Gan, C.-L.; Peng, J.-C.; Xie, Y.-H.; Song, H.-X.; Mo, Y.-Q.; Ou, S.-Y.; Aschner, M.; Jiang, Y.-M. Effects of Manganese and Iron, Alone or in Combination, on Apoptosis in BV2 Cells. Biol. Trace Element Res. 2023. [Google Scholar] [CrossRef]
- Sripetchwandee, J.; Kongkaew, A.; Kumfu, S.; Chunchai, T.; Chattipakorn, N.; Chattipakorn, S.C. Ferrostatin-1 and Z-VAD-FMK potentially attenuated Iron-mediated neurotoxicity and rescued cognitive function in Iron-overloaded rats. Life Sci. 2023, 313, 121269. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Wei, R.; Khan, S.; Zhang, R.; Zhang, Y.; Yong, V.W.; Xue, M. Iron Neurotoxicity and Protection by Deferoxamine in Intracerebral Hemorrhage. Front. Mol. Neurosci. 2022, 15, 927334. [Google Scholar] [CrossRef]
- Xie, B.; Wang, Y.; Lin, Y.; Mao, Q.; Feng, J.; Gao, G.; Jiang, J. Inhibition of ferroptosis attenuates tissue damage and improves long-term outcomes after traumatic brain injury in mice. CNS Neurosci. Ther. 2019, 25, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhang, T.; Ding, X.; Zhao, X.; Liu, Q.; Xia, Z.; Cao, Q.; Yan, F.; Chen, L.; Zhu, M.; et al. Iron overload facilitates neonatal hypoxic–ischemic brain damage via SLC7A11-mediated ferroptosis. J. Neurosci. Res. 2023, 101, 1107–1124. [Google Scholar] [CrossRef]
- Gleitze, S.; Paula-Lima, A.; Núñez, M.T.; Hidalgo, C. The calcium–iron connection in ferroptosis-mediated neuronal death. Free. Radic. Biol. Med. 2021, 175, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Thapa, K.; Khan, H.; Kanojia, N.; Singh, T.G.; Kaur, A.; Kaur, G. Therapeutic Insights on Ferroptosis in Parkinson’s disease. Eur. J. Pharmacol. 2022, 930, 175133. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.K.; Zelic, M.; Han, Y.; Teeple, E.; Chen, L.; Sadeghi, M.; Shankara, S.; Guo, L.; Li, C.; Pontarelli, F.; et al. Microglia ferroptosis is regulated by SEC24B and contributes to neurodegeneration. Nat. Neurosci. 2023, 26, 12–26. [Google Scholar] [CrossRef]
- Qu, W.; Cheng, Y.; Peng, W.; Wu, Y.; Rui, T.; Luo, C.; Zhang, J. Targeting iNOS Alleviates Early Brain Injury After Experimental Subarachnoid Hemorrhage via Promoting Ferroptosis of M1 Microglia and Reducing Neuroinflammation. Mol. Neurobiol. 2022, 59, 3124–3139. [Google Scholar] [CrossRef]
- Pap, R.; Pandur, E.; Jánosa, G.; Sipos, K.; Nagy, T.; Agócs, A.; Deli, J. Lutein Decreases Inflammation and Oxidative Stress and Prevents Iron Accumulation and Lipid Peroxidation at Glutamate-Induced Neurotoxicity. Antioxidants 2022, 11, 2269. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Q.; Shan, X.; Zhou, H.; Wang, J.; Hu, Y.; Chen, J.; Lv, Z. Ginkgolide B attenuates cerebral ischemia-reperfusion injury via inhibition of ferroptosis through disrupting NCOA4-FTH1 interaction. J. Ethnopharmacol. 2023, 318 Pt B, 116982. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Feng, W.; Wang, C.; Chen, M.; Li, Y.; Chen, J.; Liu, X.; Liu, Q.; Tian, J. Berberine ameliorates iron levels and ferroptosis in the brain of 3 × Tg-AD mice. Phytomedicine 2023, 118, 154962. [Google Scholar] [CrossRef]
- Zhang, W.; Qian, S.; Tang, B.; Kang, P.; Zhang, H.; Shi, C. Resveratrol inhibits ferroptosis and decelerates heart failure progression via Sirt1/p53 pathway activation. J. Cell. Mol. Med. 2023, 27, 3075–3089. [Google Scholar] [CrossRef]
- Jiang, P.; Zhou, L.; Zhao, L.; Fei, X.; Wang, Z.; Liu, T.; Tang, Y.; Li, D.; Gong, H.; Luo, Y.; et al. Puerarin attenuates valproate-induced features of ASD in male mice via regulating Slc7a11-dependent ferroptosis. Neuropsychopharmacology 2023. [Google Scholar] [CrossRef]
- Zhang, D.; Yao, J.; Sun, J.; Wang, J.; Chen, L.; He, H.; Wu, T. Iron accumulation in the ventral tegmental area in Parkinson’s disease. Front. Aging Neurosci. 2023, 15, 1187684. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Ma, P.; Yang, P.; Zhai, S.; He, M.; Zhang, X.; Tu, Q.; Jiao, L.; Ye, L.; Feng, Z.; et al. Alpha lipoic acid ameliorates motor deficits by inhibiting ferroptosis in Parkinson’s disease. Neurosci. Lett. 2023, 810, 137346. [Google Scholar] [CrossRef]
- Li, Y.; Shi, C.; Liu, R.; Yang, J.; Xie, J.; Wang, J. Alpha-synuclein Affects Certain Iron Transporters of BV2 Microglia Cell through its ferric reductase activity. J. Neurophysiol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chang, Q.; Sun, T.; He, X.; Wen, L.; An, J.; Feng, J.; Zhao, Y. Metabolic reprogramming and polarization of microglia in Parkinson’s disease: Role of inflammasome and iron. Ageing Res. Rev. 2023, 90, 102032. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, X.; Geng, X.; Wang, F. Meta-analysis of iron metabolism markers levels of Parkinson’s disease patients determined by fluid and MRI measurements. J. Trace Elements Med. Biol. 2023, 78, 127190. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Rutledge, J.; Schallert, T. MRI, brain iron and experimental Parkinson’s disease. J. Neurol. Sci. 1992, 113, 198–208. [Google Scholar] [CrossRef]
- Lee, H.; Baek, S.-Y.; Chun, S.Y.; Lee, J.-H.; Cho, H. Specific visualization of neuromelanin-iron complex and ferric iron in the human post-mortem substantia nigra using MR relaxometry at 7T. NeuroImage 2018, 172, 874–885. [Google Scholar] [CrossRef]
- Segura-Aguilar, J.; Mannervik, B. A Preclinical Model for Parkinson’s Disease Based on Transcriptional Gene Activation via KEAP1/NRF2 to Develop New Antioxidant Therapies. Antioxidants 2023, 12, 673. [Google Scholar] [CrossRef]
- Elalfy, M.S.; Hamdy, M.; Adly, A.; Ebeid, F.S.E.; Temin, N.T.; Rozova, A.; Lee, D.; Fradette, C.; Tricta, F. Efficacy and safety of early-start deferiprone in infants and young children with transfusion-dependent beta thalassemia: Evidence for iron shuttling to transferrin in a randomized, double-blind, placebo-controlled, clinical trial (START). Am. J. Hematol. 2023, 98, 1415–1424. [Google Scholar] [CrossRef]
- Klopstock, T.; Tricta, F.; Neumayr, L.; Karin, I.; Zorzi, G.; Fradette, C.; Kmieć, T.; Büchner, B.; Steele, H.E.; Horvath, R.; et al. Safety and efficacy of deferiprone for pantothenate kinase-associated neurodegeneration: A randomised, double-blind, controlled trial and an open-label extension study. Lancet Neurol. 2019, 18, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.M.; Wagener, A.; Fietzek, U.M.; Klopstock, T.; Mosharov, E.V.; Zucca, F.A.; Sulzer, D.; Zecca, L.; Burbulla, L.F. Interactions of dopamine, iron, and alpha-synuclein linked to dopaminergic neuron vulnerability in Parkinson’s disease and Neurodegeneration with Brain Iron Accumulation disorders. Neurobiol. Dis. 2022, 175, 105920. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, P.; Mena, N.P.; Carrasco, C.M.; Muñoz, Y.; Pérez-Henríquez, P.; Morales, R.A.; Cassels, B.K.; Méndez-Gálvez, C.; García-Beltrán, O.; González-Billault, C.; et al. Iron Chelators and Antioxidants Regenerate Neuritic Tree and Nigrostriatal Fibers of MPP+/MPTP-Lesioned Dopaminergic Neurons. PLoS ONE 2015, 10, e0144848. [Google Scholar] [CrossRef]
- Devos, D.; Labreuche, J.; Rascol, O.; Corvol, J.-C.; Duhamel, A.; Delannoy, P.G.; Poewe, W.; Compta, Y.; Pavese, N.; Růžička, E.; et al. Trial of Deferiprone in Parkinson’s Disease. New Engl. J. Med. 2022, 387, 2045–2055. [Google Scholar] [CrossRef] [PubMed]
- Williams, A. MPTP parkinsonism. BMJ 1984, 289, 1401–1402. [Google Scholar] [CrossRef][Green Version]
- Athauda, D.; Foltynie, T. The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat. Rev. Neurol. 2015, 11, 25–40. [Google Scholar] [CrossRef]
- Athauda, D.; Foltynie, T. Challenges in detecting disease modification in Parkinson’s disease clinical trials. Park. Relat. Disord. 2016, 32, 1–11. [Google Scholar] [CrossRef]
- Olanow, C.; Kieburtz, K.; Katz, R. Clinical approaches to the development of a neuroprotective therapy for PD. Exp. Neurol. 2017, 298, 246–251. [Google Scholar] [CrossRef]
- Schneider, J.S. GM1 Ganglioside as a Disease-Modifying Therapeutic for Parkinson’s Disease: A Multi-Functional Glycosphingolipid That Targets Multiple Parkinson’s Disease-Relevant Pathogenic Mechanisms. Int. J. Mol. Sci. 2023, 24, 9183. [Google Scholar] [CrossRef]
- Mari, Z.; Mestre, T.A. The Disease Modification Conundrum in Parkinson’s Disease: Failures and Hopes. Front. Aging Neurosci. 2022, 14, 810860. [Google Scholar] [CrossRef]
- Kwiatkowski, J.L.; Hamdy, M.; El-Beshlawy, A.; Ebeid, F.S.E.; Badr, M.; Alshehri, A.; Kanter, J.; Inusa, B.; Adly, A.A.M.; Williams, S.; et al. Deferiprone vs deferoxamine for transfusional iron overload in SCD and other anemias: A randomized, open-label noninferiority study. Blood Adv. 2022, 6, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, M.; Hausmann, L. Deferiprone for the treatment of Friedreich’s ataxia. J. Neurochem. 2013, 126 (Suppl. 1), 142–146. [Google Scholar] [CrossRef] [PubMed]
- Maggio, A.; Kattamis, A.; Felisi, M.; Reggiardo, G.; El-Beshlawy, A.; Bejaoui, M.; Sherief, L.; Christou, S.; Cosmi, C.; Della Pasqua, O.; et al. Evaluation of the efficacy and safety of deferiprone compared with deferasirox in paediatric patients with transfusion-dependent haemoglobinopathies (DEEP-2): A multicentre, randomised, open-label, non-inferiority, phase 3 trial. Lancet Haematol. 2020, 7, e469–e478. [Google Scholar] [CrossRef] [PubMed]
- Ni, A.; Ernst, C. Evidence That Substantia Nigra Pars Compacta Dopaminergic Neurons Are Selectively Vulnerable to Oxidative Stress Because They Are Highly Metabolically Active. Front. Cell. Neurosci. 2022, 16, 826193. [Google Scholar] [CrossRef] [PubMed]
- Grünblatt, E.; Ruder, J.; Monoranu, C.M.; Riederer, P.; Youdim, M.B.; Mandel, S.A. Differential Alterations in Metabolism and Proteolysis-Related Proteins in Human Parkinson’s Disease Substantia Nigra. Neurotox. Res. 2018, 33, 560–568. [Google Scholar] [CrossRef]
- Bisaglia, M.; Mammi, S.; Bubacco, L. Kinetic and structural analysis of the early oxidation products of dopamine: Analysis of the interactions with alpha-synuclein. J. Biol. Chem. 2007, 282, 15597–15605. [Google Scholar] [CrossRef]
- Zafar, K.S.; Siegel, D.; Ross, D. A Potential Role for Cyclized Quinones Derived from Dopamine, DOPA, and 3,4-Dihydroxyphenylacetic Acid in Proteasomal Inhibition. Mol. Pharmacol. 2006, 70, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Siegel, D.; Ross, D. Quinone-induced protein handling changes: Implications for major protein handling systems in quinone-mediated toxicity. Toxicol. Appl. Pharmacol. 2014, 280, 285–295. [Google Scholar] [CrossRef]
- Herrera, A.; Muñoz, P.; Steinbusch, H.W.M.; Segura-Aguilar, J. Are Dopamine Oxidation Metabolites Involved in the Loss of Dopaminergic Neurons in the Nigrostriatal System in Parkinson’s Disease? ACS Chem. Neurosci. 2017, 8, 702–711. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huenchuguala, S.; Segura-Aguilar, J. On the Role of Iron in Idiopathic Parkinson’s Disease. Biomedicines 2023, 11, 3094. https://doi.org/10.3390/biomedicines11113094
Huenchuguala S, Segura-Aguilar J. On the Role of Iron in Idiopathic Parkinson’s Disease. Biomedicines. 2023; 11(11):3094. https://doi.org/10.3390/biomedicines11113094
Chicago/Turabian StyleHuenchuguala, Sandro, and Juan Segura-Aguilar. 2023. "On the Role of Iron in Idiopathic Parkinson’s Disease" Biomedicines 11, no. 11: 3094. https://doi.org/10.3390/biomedicines11113094
APA StyleHuenchuguala, S., & Segura-Aguilar, J. (2023). On the Role of Iron in Idiopathic Parkinson’s Disease. Biomedicines, 11(11), 3094. https://doi.org/10.3390/biomedicines11113094







