Erucin Exerts Cardioprotective Effects on Ischemia/Reperfusion Injury through the Modulation of mitoKATP Channels
Abstract
1. Introduction
2. Materials and Methods
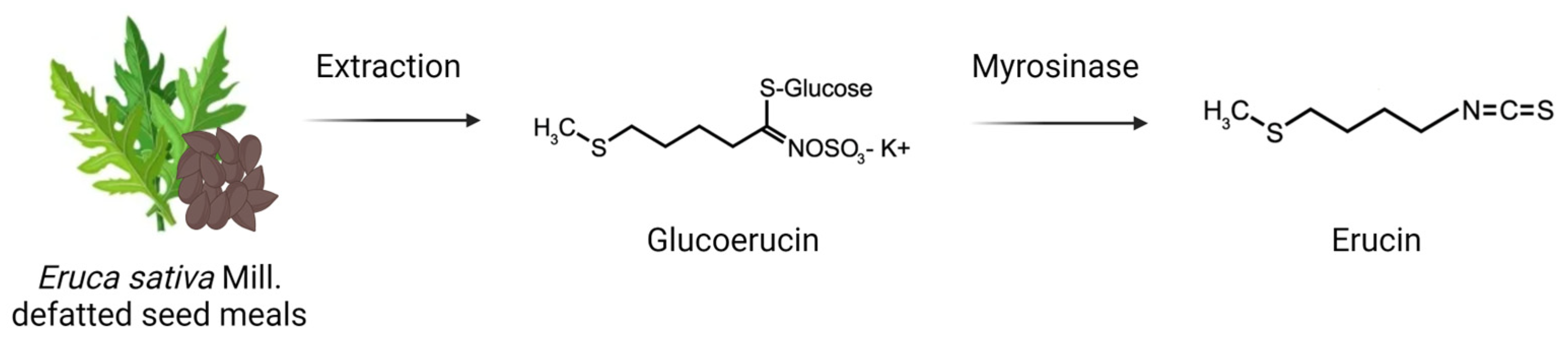
2.1. Erucin Production
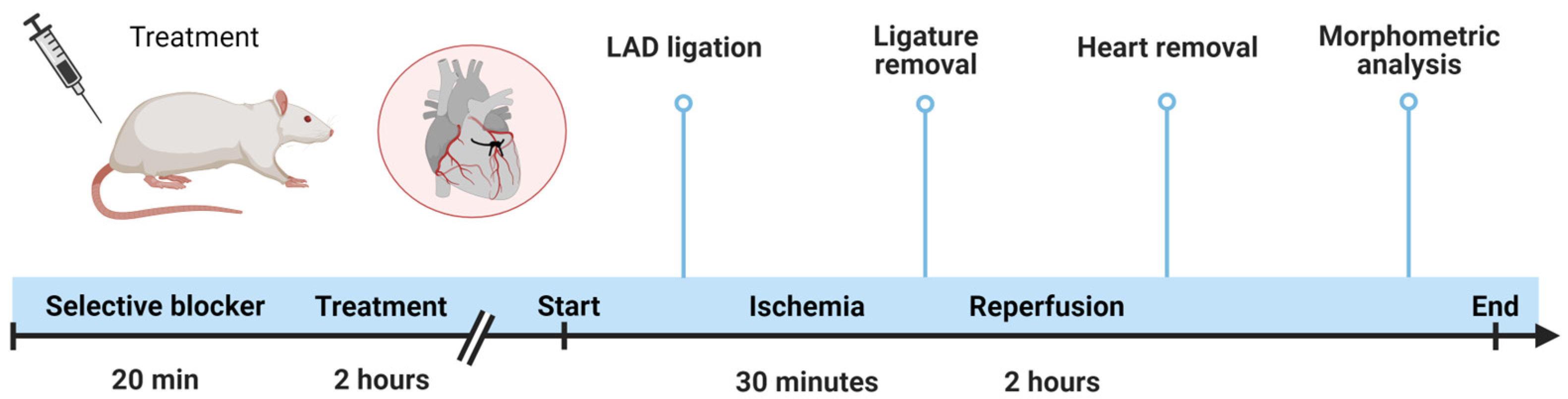
2.2. Animal Procedures
2.2.1. In Vivo Acute Myocardial Infarction
2.2.2. Evaluation of Systemic Troponin I Levels
2.3. Ex Vivo Procedure
2.3.1. Evaluation of Mitochondrial Membrane Potential
2.3.2. Modulation of K+ Flow through the Involvement of mitoK Channels
2.4. Data Analysis
3. Results
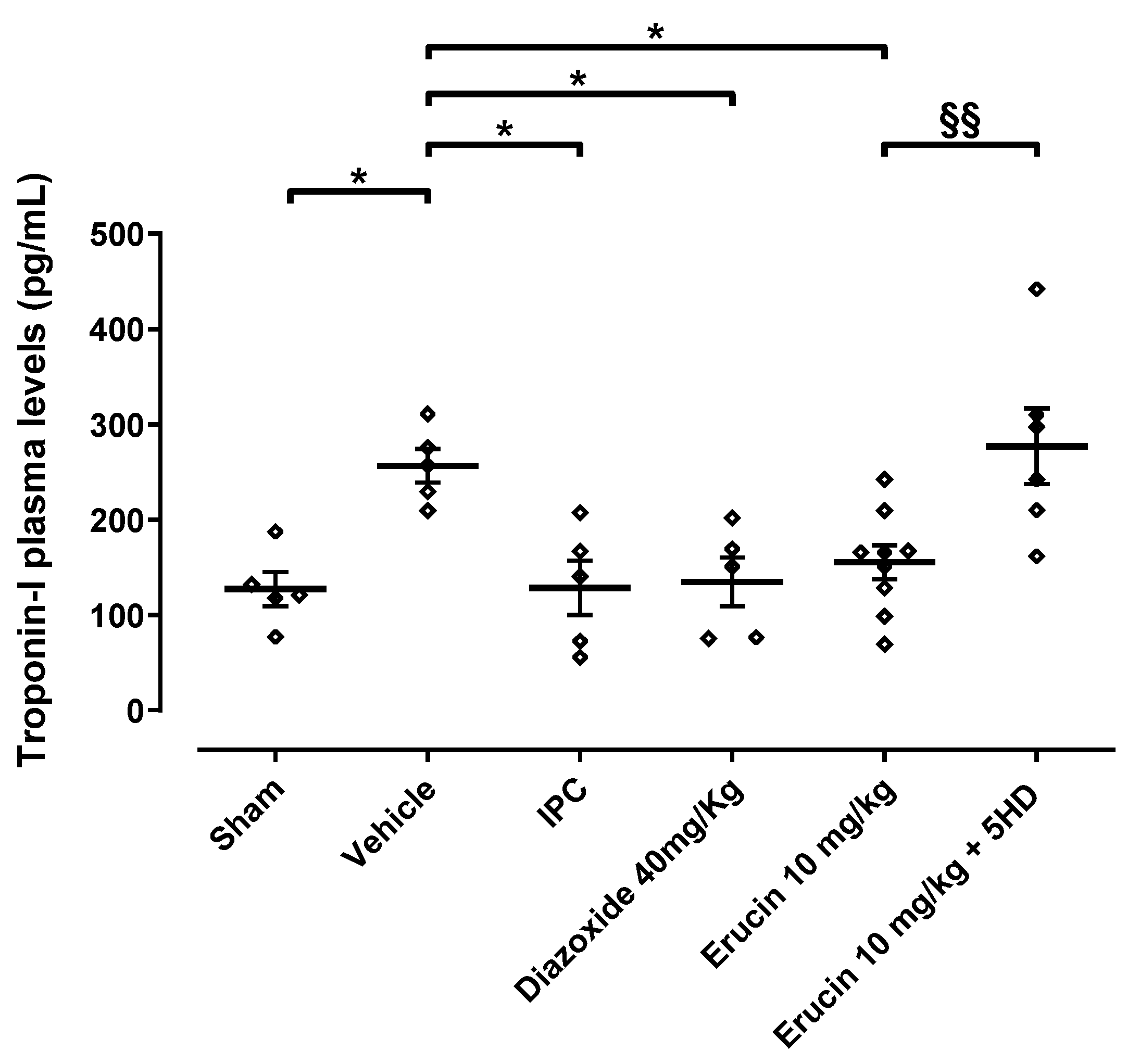
3.1. Involvement of mitoKATP Channels in the Cardioprotective Effect of ERU—Morphometric Analysis
3.2. Involvement of mitoKATP Channels in the Cardioprotective Effect of ERU—Troponin I Release
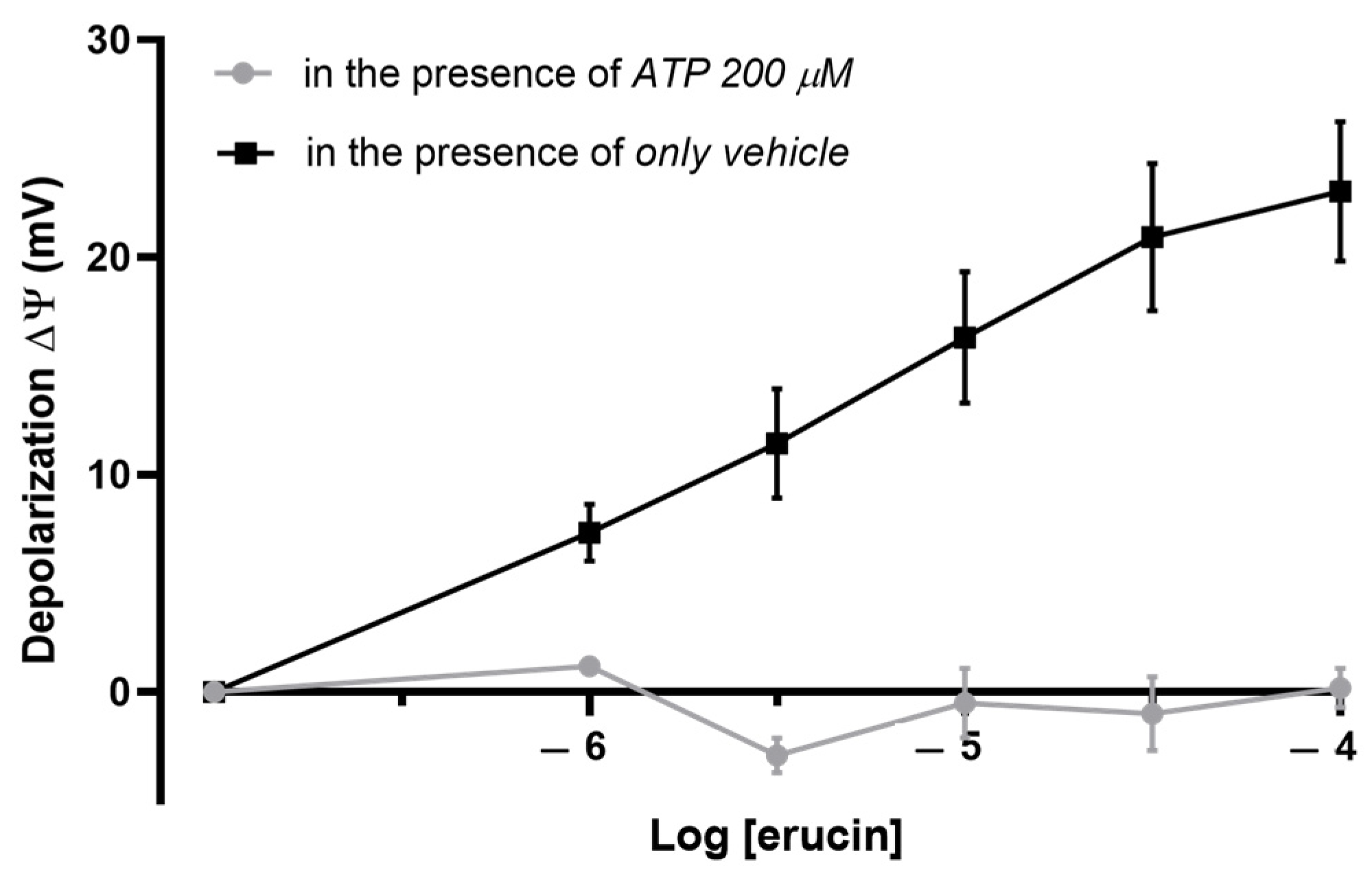
3.3. Involvement of mitoKATP Channels in the Effect of ERU: Evaluation of the Mitochondrial Membrane Potential
3.4. Involvement of mitoKATP Channels in the Effect of ERU: Evaluation of the Mitochondrial Potassium Flows
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Jeevaratnam, K.; Chadda, K.R.; Huang, C.L.-H.; Camm, A.J. Cardiac potassium channels: Physiological insights for targeted therapy. J. Cardiovasc. Pharmacol. Ther. 2018, 23, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.J.; Fryer, R.M. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ. Res. 1999, 84, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Rapposelli, S.; Martelli, A.; Breschi, M.C.; Calderone, V. Mitochondrial potassium channels as pharmacological target for cardioprotective drugs. Med. Res. Rev. 2015, 35, 520–553. [Google Scholar] [CrossRef] [PubMed]
- Garlid, K.D. Opening mitochondrial K ATP in the heart–what happens, and what does not happen. Basic Res. Cardiol. 2000, 95, 275–279. [Google Scholar] [CrossRef]
- Holmuhamedov, E.L.; Jovanović, S.; Dzeja, P.P.; Jovanović, A.; Terzic, A. Mitochondrial ATP-sensitive K+ channels modulate cardiac mitochondrial function. Am. J. Physiol. Heart Circ. Physiol. 1998, 275, H1567–H1576. [Google Scholar] [CrossRef] [PubMed]
- Maslov, L.N.; Popov, S.V.; Naryzhnaya, N.V.; Mukhomedzyanov, A.V.; Kurbatov, B.K.; Derkachev, I.A.; Boshchenko, A.A.; Prasad, N.R.; Ma, H.; Zhang, Y. KATP channels are regulators of programmed cell death and targets for creation of novel drugs against ischemia/reperfusion cardiac injury. Fundam. Clin. Pharmacol. 2023, 37, 1020–1049. [Google Scholar] [CrossRef]
- Chapa-Dubocq, X.R.; Rodríguez-Graciani, K.M.; Escobales, N.; Javadov, S. Mitochondrial Volume Regulation and Swelling Mechanisms in Cardiomyocytes. Antioxidants 2023, 12, 1517. [Google Scholar] [CrossRef]
- Makarov, V.I.; Khmelinskii, I.; Khuchua, Z.; Javadov, S. In silico simulation of reversible and irreversible swelling of mitochondria: The role of membrane rigidity. Mitochondrion 2020, 50, 71–81. [Google Scholar] [CrossRef]
- Holmuhamedov, E.L.; Wang, L.; Terzic, A. ATP-sensitive K+ channel openers prevent Ca2+ overload in rat cardiac mitochondria. J. Physiol. 1999, 519, 347. [Google Scholar] [CrossRef]
- Yellon, D.M.; Beikoghli Kalkhoran, S.; Davidson, S.M. The RISK pathway leading to mitochondria and cardioprotection: How everything started. Basic Res. Cardiol. 2023, 118, 22. [Google Scholar] [CrossRef] [PubMed]
- Behera, R.; Sharma, V.; Grewal, A.K.; Kumar, A.; Arora, B.; Najda, A.; Albadrani, G.M.; Altyar, A.E.; Abdel-Daim, M.M.; Singh, T.G. Mechanistic correlation between mitochondrial permeability transition pores and mitochondrial ATP dependent potassium channels in ischemia reperfusion. Biomed. Pharmacother. 2023, 162, 114599. [Google Scholar] [CrossRef]
- Robichaux, D.J.; Harata, M.; Murphy, E.; Karch, J. Mitochondrial permeability transition pore-dependent necrosis. J. Mol. Cell. Cardiol. 2023, 174, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, O.; Cohen, M.V.; Yellon, D.M.; Downey, J.M. Mitochondrial KATP channels: Role in cardioprotection. Cardiovasc. Res. 2002, 55, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Nichols, C.G. Adenosine triphosphate-sensitive potassium currents in heart disease and cardioprotection. Card. Electrophysiol. Clin. 2016, 8, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Barrese, V.; Soldovieri, M.V.; Ambrosino, P.; Martelli, A.; Vinciguerra, I.; Miceli, F.; Greenwood, I.A.; Curtis, M.J.; Breschi, M.C. Expression and function of Kv7.4 channels in rat cardiac mitochondria: Possible targets for cardioprotection. Cardiovasc. Res. 2016, 110, 40–50. [Google Scholar] [CrossRef]
- Inoue, I.; Nagase, H.; Kishi, K.; Higuti, T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature 1991, 352, 244–247. [Google Scholar] [CrossRef]
- Siemen, D.; Loupatatzis, C.; Borecky, J.; Gulbins, E.; Lang, F. Ca2+-activated K channel of the BK-type in the inner mitochondrial membrane of a human glioma cell line. Biochem. Biophys. Res. Commun. 1999, 257, 549–554. [Google Scholar] [CrossRef]
- Cirino, G.; Szabo, C.; Papapetropoulos, A. Physiological roles of hydrogen sulfide in mammalian cells, tissues, and organs. Physiol. Rev. 2023, 103, 31–276. [Google Scholar] [CrossRef]
- Ansari, M.; Prem, P.N.; Kurian, G.A. Hydrogen sulfide postconditioning rendered cardioprotection against myocardial ischemia-reperfusion injury is compromised in rats with diabetic cardiomyopathy. Microvasc. Res. 2022, 141, 104322. [Google Scholar] [CrossRef]
- Sun, H.-J.; Wu, Z.-Y.; Nie, X.-W.; Wang, X.-Y.; Bian, J.-S. An updated insight into molecular mechanism of hydrogen sulfide in cardiomyopathy and myocardial ischemia/reperfusion injury under diabetes. Front. Pharmacol. 2021, 12, 651884. [Google Scholar] [CrossRef]
- King, A.L.; Polhemus, D.J.; Bhushan, S.; Otsuka, H.; Kondo, K.; Nicholson, C.K.; Bradley, J.M.; Islam, K.N.; Calvert, J.W.; Tao, Y.-X. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc. Natl. Acad. Sci. USA 2014, 111, 3182–3187. [Google Scholar] [CrossRef]
- Wu, D.; Gu, Y.; Zhu, D. Cardioprotective effects of hydrogen sulfide in attenuating myocardial ischemia-reperfusion injury. Mol. Med. Rep. 2021, 24, 1–11. [Google Scholar] [CrossRef]
- Wu, S.Y.; Pan, C.S.; Geng, B.; Zhao, J.; Yu, F.; Pang, Y.Z.; Tang, C.S.; Qi, Y.F. Hydrogen sulfide ameliorates vascular calcification induced by vitamin D3 plus nicotine in rats 1. Acta Pharmacol. Sin. 2006, 27, 299–306. [Google Scholar] [CrossRef]
- Andreadou, I.; Schulz, R.; Papapetropoulos, A.; Turan, B.; Ytrehus, K.; Ferdinandy, P.; Daiber, A.; Di Lisa, F. The role of mitochondrial reactive oxygen species, NO and H2S in ischaemia/reperfusion injury and cardioprotection. J. Cell. Mol. Med. 2020, 24, 6510–6522. [Google Scholar] [CrossRef]
- Walewska, A.; Szewczyk, A.; Krajewska, M.; Koprowski, P. Targeting mitochondrial large-conductance calcium-activated potassium channel by hydrogen sulfide via heme-binding site. J. Pharmacol. Exp. Ther. 2022, 381, 137–150. [Google Scholar] [CrossRef]
- Donnarumma, E.; Trivedi, R.K.; Lefer, D.J. Protective actions of H2S in acute myocardial infarction and heart failure. Compr. Physiol. 2011, 7, 583–602. [Google Scholar]
- Testai, L.; Marino, A.; Piano, I.; Brancaleone, V.; Tomita, K.; Mannelli, L.D.C.; Martelli, A.; Citi, V.; Breschi, M.C.; Levi, R. The novel H2S-donor 4-carboxyphenyl isothiocyanate promotes cardioprotective effects against ischemia/reperfusion injury through activation of mitoKATP channels and reduction of oxidative stress. Pharmacol. Res. 2016, 113, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Chen, J.; Mo, L.; Ke, X.; Zhang, W.; Zheng, D.; Pan, W.; Wu, S.; Feng, J.; Song, M. ATP-sensitive K+ channels contribute to the protective effects of exogenous hydrogen sulfide against high glucose-induced injury in H9c2 cardiac cells. Int. J. Mol. Med. 2016, 37, 763–772. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barodka, V.M.; Bhunia, A.K.; Gazi, S.K.; Mustafa, A.K.; Sikka, G.; Snyder, S.H.; Gazi, F.K.; Steppan, J.; Barrow, R.K. Hydrogen Sulfide as Endothelium-Derived Hyperpolarizing Factor Sulfhydrates Potassium Channels. Circ. Res. 2011, 109, 1259–1268. [Google Scholar]
- Citi, V.; Martelli, A.; Testai, L.; Marino, A.; Breschi, M.C.; Calderone, V. Hydrogen sulfide releasing capacity of natural isothiocyanates: Is it a reliable explanation for the multiple biological effects of Brassicaceae? Planta Med. 2014, 80, 610–613. [Google Scholar] [CrossRef]
- Karwi, Q.G.; Whiteman, M.; Wood, M.E.; Torregrossa, R.; Baxter, G.F. Pharmacological postconditioning against myocardial infarction with a slow-releasing hydrogen sulfide donor, GYY4137. Pharmacol. Res. 2016, 111, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Citi, V.; Corvino, A.; Fiorino, F.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Brogi, S.; Flori, L.; Gorica, E. Structure-activity relationships study of isothiocyanates for H2S releasing properties: 3-Pyridyl-isothiocyanate as a new promising cardioprotective agent. J. Adv. Res. 2021, 27, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Montanaro, R.; Flori, L.; Pagnotta, E.; Vellecco, V.; Gorica, E.; Ugolini, L.; Righetti, L.; Brancaleone, V.; Bucci, M. Persulfidation of mitoKv7.4 channels contributes to the cardioprotective effects of the H2S-donor Erucin against ischemia/reperfusion injury. Biochem. Pharmacol. 2023, 215, 115728. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Pagnotta, E.; Piragine, E.; Flori, L.; Citi, V.; Martelli, A.; Mannelli, L.D.C.; Ghelardini, C.; Matteo, R.; Suriano, S. Cardiovascular benefits of Eruca sativa mill. Defatted seed meal extract: Potential role of hydrogen sulfide. Phytother. Res. 2022, 36, 2616–2627. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, V.; Piragine, E.; Gorica, E.; Citi, V.; Testai, L.; Pagnotta, E.; Matteo, R.; Pecchioni, N.; Montanaro, R.; Di Cesare Mannelli, L. Anti-inflammatory effect of the natural H2S-donor erucin in vascular endothelium. Int. J. Mol. Sci. 2022, 23, 5593. [Google Scholar] [CrossRef]
- Martelli, A.; Piragine, E.; Gorica, E.; Citi, V.; Testai, L.; Pagnotta, E.; Lazzeri, L.; Pecchioni, N.; Ciccone, V.; Montanaro, R. The H2S-donor erucin exhibits protective effects against vascular inflammation in human endothelial and smooth muscle cells. Antioxidants 2021, 10, 961. [Google Scholar] [CrossRef]
- Martelli, A.; Piragine, E.; Citi, V.; Testai, L.; Pagnotta, E.; Ugolini, L.; Lazzeri, L.; Di Cesare Mannelli, L.; Manzo, O.L.; Bucci, M. Erucin exhibits vasorelaxing effects and antihypertensive activity by H2S-releasing properties. Br. J. Pharmacol. 2020, 177, 824–835. [Google Scholar] [CrossRef]
- ISO 9167:2019; Rapeseed and rapeseed meals—Determination of Glucosinolates Content—Method using High performance Liquid Chromatography. ISO: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/72207.html (accessed on 1 December 2023).
- Pessina, A.; Thomas, R.M.; Palmieri, S.; Luisi, P.L. An improved method for the purification of myrosinase and its physicochemical characterization. Arch. Biochem. Biophys. 1990, 280, 383–389. [Google Scholar] [CrossRef]
- Ugolini, L.; Scarafile, D.; Matteo, R.; Pagnotta, E.; Malaguti, L.; Lazzeri, L.; Modesto, M.; Checcucci, A.; Mattarelli, P.; Braschi, I. Effect of bioactive compounds released from Brassicaceae defatted seed meals on bacterial load in pig manure. Environ. Sci. Pollut. Res. 2021, 28, 62353–62367. [Google Scholar] [CrossRef]
- Labajova, A.; Vojtiskova, A.; Krivakova, P.; Kofranek, J.; Drahota, Z.; Houstek, J. Evaluation of mitochondrial membrane potential using a computerized device with a tetraphenylphosphonium-selective electrode. Anal. Biochem. 2006, 353, 37–42. [Google Scholar] [CrossRef]
- Wojtovich, A.P.; Williams, D.M.; Karcz, M.K.; Lopes, C.M.; Gray, D.A.; Nehrke, K.W.; Brookes, P.S. A novel mitochondrial KATP channel assay. Circ. Res. 2010, 106, 1190–1196. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y. Heart disease and stroke statistics—2022 update: A report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- He, J.; Liu, D.; Zhao, L.; Zhou, D.; Rong, J.; Zhang, L.; Xia, Z. Myocardial ischemia/reperfusion injury: Mechanisms of injury and implications for management. Exp. Ther. Med. 2022, 23, 1–11. [Google Scholar] [CrossRef]
- Salloum, F.N. Hydrogen sulfide and cardioprotection—Mechanistic insights and clinical translatability. Pharmacol. Ther. 2015, 152, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, X.; Cheng, L.; Han, J.; Mu, J. Hydrogen sulfide restores cardioprotective effects of remote ischemic preconditioning in aged rats via HIF-1α/Nrf2 signaling pathway. Korean J. Physiol. Pharmacol. 2021, 25, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cai, X.; Zhang, Q.; Li, X.; Li, S.; Ma, J.; Zhu, W.; Liu, X.; Wei, M.; Tu, W. Hydrogen sulfide restores sevoflurane postconditioning mediated cardioprotection in diabetic rats: Role of SIRT1/Nrf2 signaling-modulated mitochondrial dysfunction and oxidative stress. J. Cell. Physiol. 2021, 236, 5052–5068. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gao, J.; Sun, W.; Li, L.; Wang, Y.; Bai, S.; Li, X.; Wang, R.; Wu, L.; Li, H. Involvement of exogenous H2S in recovery of cardioprotection from ischemic post-conditioning via increase of autophagy in the aged hearts. Int. J. Cardiol. 2016, 220, 681–692. [Google Scholar] [CrossRef]
- Wei, C.; Zhao, Y.; Wang, L.; Peng, X.; Li, H.; Zhao, Y.; He, Y.; Shao, H.; Zhong, X.; Li, H. H2S restores the cardioprotection from ischemic post-conditioning in isolated aged rat hearts. Cell Biol. Int. 2015, 39, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Predmore, B.L.; Kondo, K.; Bhushan, S.; Zlatopolsky, M.A.; King, A.L.; Aragon, J.P.; Grinsfelder, D.B.; Condit, M.E.; Lefer, D.J. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H2410–H2418. [Google Scholar] [CrossRef]
- Calvert, J.W.; Elston, M.; Nicholson, C.K.; Gundewar, S.; Jha, S.; Elrod, J.W.; Ramachandran, A.; Lefer, D.J. Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice. Circulation 2010, 122, 11–19. [Google Scholar] [CrossRef]
- Bello, I.; Smimmo, M.; d’Emmanuele di Villa Bianca, R.; Bucci, M.; Cirino, G.; Panza, E.; Brancaleone, V. Erucin, an H2S-Releasing Isothiocyanate, Exerts Anticancer Effects in Human Triple-Negative Breast Cancer Cells Triggering Autophagy-Dependent Apoptotic Cell Death. Int. J. Mol. Sci. 2023, 24, 6764. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhou, J.; Budhraja, A.; Hu, X.; Chen, Y.; Cheng, Q.; Liu, L.; Zhou, T.; Li, P.; Liu, E. Mitochondrial translocation and interaction of cofilin and Drp1 are required for erucin-induced mitochondrial fission and apoptosis. Oncotarget 2015, 6, 1834. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, J.; Du, J.; Xu, G.; Tang, C.; Geng, B. Hydrogen sulfide regulates cardiac sarcoplasmic reticulum Ca2+ uptake via KATP channel and PI3K/Akt pathway. Life Sci. 2012, 91, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Andrukhiv, A.; Costa, A.D.; West, I.C.; Garlid, K.D. Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2067–H2074. [Google Scholar] [CrossRef]
- Bao, L.; Hadjiolova, K.; Coetzee, W.A.; Rindler, M.J. Endosomal KATP channels as a reservoir after myocardial ischemia: A role for SUR2 subunits. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H262–H270. [Google Scholar] [CrossRef]
- Leanza, L.; Biasutto, L.; Managò, A.; Gulbins, E.; Zoratti, M.; Szabò, I. Intracellular ion channels and cancer. Front. Physiol. 2013, 4, 227. [Google Scholar] [CrossRef]
- Palácio, P.B.; de Freitas Soares, G.C.; Lima, G.M.B.; Cunha, P.L.O.; Varela, A.L.N.; Facundo, H.T. Competitive interaction between ATP and GTP regulates mitochondrial ATP-sensitive potassium channels. Chem. Biol. Interact. 2023, 381, 110560. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flori, L.; Montanaro, R.; Pagnotta, E.; Ugolini, L.; Righetti, L.; Martelli, A.; Di Cesare Mannelli, L.; Ghelardini, C.; Brancaleone, V.; Testai, L.; et al. Erucin Exerts Cardioprotective Effects on Ischemia/Reperfusion Injury through the Modulation of mitoKATP Channels. Biomedicines 2023, 11, 3281. https://doi.org/10.3390/biomedicines11123281
Flori L, Montanaro R, Pagnotta E, Ugolini L, Righetti L, Martelli A, Di Cesare Mannelli L, Ghelardini C, Brancaleone V, Testai L, et al. Erucin Exerts Cardioprotective Effects on Ischemia/Reperfusion Injury through the Modulation of mitoKATP Channels. Biomedicines. 2023; 11(12):3281. https://doi.org/10.3390/biomedicines11123281
Chicago/Turabian StyleFlori, Lorenzo, Rosangela Montanaro, Eleonora Pagnotta, Luisa Ugolini, Laura Righetti, Alma Martelli, Lorenzo Di Cesare Mannelli, Carla Ghelardini, Vincenzo Brancaleone, Lara Testai, and et al. 2023. "Erucin Exerts Cardioprotective Effects on Ischemia/Reperfusion Injury through the Modulation of mitoKATP Channels" Biomedicines 11, no. 12: 3281. https://doi.org/10.3390/biomedicines11123281
APA StyleFlori, L., Montanaro, R., Pagnotta, E., Ugolini, L., Righetti, L., Martelli, A., Di Cesare Mannelli, L., Ghelardini, C., Brancaleone, V., Testai, L., & Calderone, V. (2023). Erucin Exerts Cardioprotective Effects on Ischemia/Reperfusion Injury through the Modulation of mitoKATP Channels. Biomedicines, 11(12), 3281. https://doi.org/10.3390/biomedicines11123281










