The Role of Irisin throughout Women’s Life Span
Abstract
1. Introduction
2. Irisin and Sex Differences: Towards Gender Medicine
3. Irisin and Pubertal Development
4. Irisin and Polycystic Ovarian Syndrome (PCOS)
5. Irisin and Functional Hypothalamic Amenorrhea
6. Irisin and Endometriosis
7. Irisin and Gestational Diabetes Mellitus
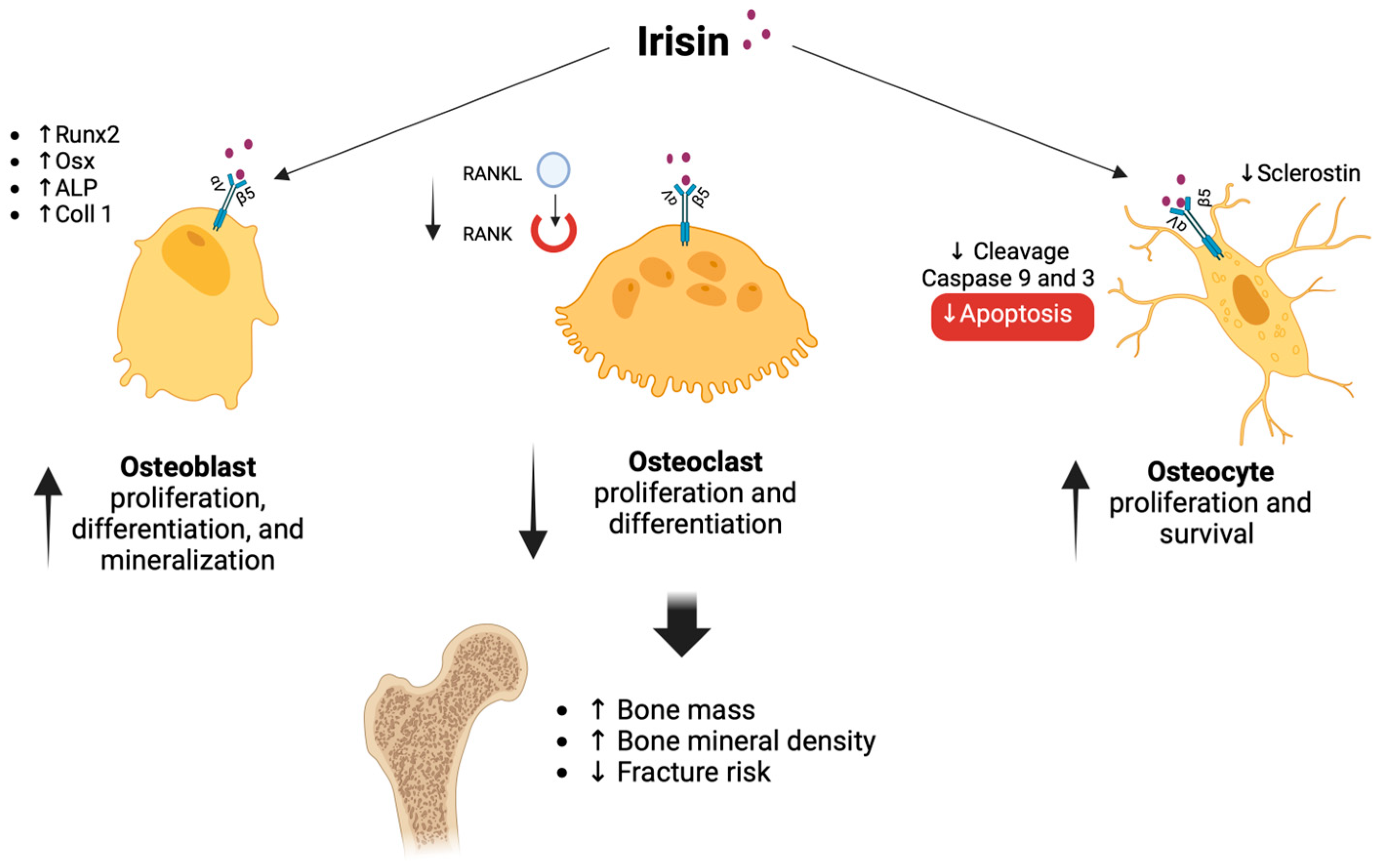
8. Irisin and Menopause
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Pukajło, K.; Kolackov, K.; Łaczmański, Ł.; Daroszewski, J. Irisin, a new mediator of energy homeostasis. Adv. Hyg. Exp. Med. 2015, 69, 233–242. [Google Scholar] [CrossRef]
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Natalicchio, A.; Marrano, N.; Giorgino, F. Irisina: Ruolo nell’omeostasi del glucosio. L’Endocrinologo 2018, 19, 292. [Google Scholar] [CrossRef]
- Norman, D.; Drott, C.J.; Carlsson, P.O.; Espes, D. Irisin—A Pancreatic Islet Hormone. Biomedicines 2022, 10, 258. [Google Scholar] [CrossRef]
- Song, R.; Zhao, X.; Zhang, D.Q.; Wang, R.; Feng, Y. Lower levels of irisin in patients with type 2 diabetes mellitus: A meta-analysis. Diabetes Res. Clin. Pract. 2021, 175, 108788. [Google Scholar] [CrossRef] [PubMed]
- Marrano, N.; Biondi, G.; Borrelli, A.; Cignarelli, A.; Perrini, S.; Laviola, L.; Giorgino, F.; Natalicchio, A. Irisin and Incretin Hormones: Similarities, Differences, and Implications in Type 2 Diabetes and Obesity. Biomolecules 2021, 11, 286. [Google Scholar] [CrossRef]
- Martinez Munoz, I.Y.; Camarillo Romero, E.D.S.; Garduno Garcia, J.J. Irisin a Novel Metabolic Biomarker: Present Knowledge and Future Directions. Int. J. Endocrinol. 2018, 2018, 7816806. [Google Scholar] [CrossRef]
- Korta, P.; Pocheć, E.; Mazur-Biały, A. Irisin as a Multifunctional Protein: Implications for Health and Certain Diseases. Medicina 2019, 55, 485. [Google Scholar] [CrossRef]
- Ma, C.; Ding, H.; Deng, Y.; Liu, H.; Xiong, X.; Yang, Y. Irisin: A New Code Uncover the Relationship of Skeletal Muscle and Cardiovascular Health During Exercise. Front. Physiol. 2021, 2, 620608. [Google Scholar] [CrossRef]
- Yi, P.; Park, J.S.; Melton, D.A. Betatrophin: A hormone that controls pancreatic β cell proliferation. Cell 2013, 53, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Kim, D.I.; Choi, J.H.; Heo, Y.R.; Park, S.H. New role of irisin in hepatocytes: The protective effect of hepatic steatosis in vitro. Cell Signal 2015, 27, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Oranger, A.; Mori, G.; Brunetti, G.; Colucci, S.; Cinti, S.; Grano, M. Irisin enhances osteoblast differentiation in vitro. Int. J. Endocrinol. 2014, 2014, 902186. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Bialy, A.I.; Pocheć, E.; Zarawski, M. Anti-Inflammatory Properties of Irisin, Mediator of Physical Activity, Are Connected with TLR4/MyD88 Signaling Pathway Activation. Int. J. Mol. Sci. 2017, 18, 701. [Google Scholar] [CrossRef]
- Al-Ghazali, M.J.; Ali, H.A.; Al-Rufaie, M.M. Serum irisin levels as a potential marker for diagnosis of gestational diabetes mellitus. Acta Biomed. 2020, 91, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Qiao, X.; Cai, Y.; Li, A.; Shan, D. Lower circulating irisin in middle-aged and older adults with osteoporosis: A systematic review and meta-analysis. Menopause 2019, 26, 1302–1310. [Google Scholar] [CrossRef]
- Luo, Y.; Qiao, X.; Xu, L.; Huang, G. Irisin: Circulating levels in serum and its relation to gonadal axis. Endocrine 2022, 75, 663–671. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Alkharfy, K.M.; Rahman, S.; Amer, O.E.; Vinodson, B.; Sabico, S.; Piya, M.K.; Harte, A.L.; McTernan, P.G.; Alokail, M.S.; et al. Irisin as a predictor of glucose metabolism in children: Sexually dimorphic effects. Eur. J. Clin. Investig. 2014, 44, 119–124. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Polyzos, S.A.; Saridakis, Z.G.; Kynigopoulos, G.; Skouvaklidou, E.C.; Molyvas, D.; Vasiloglou, M.F.; Apostolou, A.; Karagiozoglou-Lampoudi, T.; Siopi, A.; et al. Circulating irisin in healthy, young individuals: Day-night rhythm, effects of food intake and exercise, and associations with gender, physical activity, diet, and body composition. J. Clin. Endocrinol. Metab. 2014, 99, 3247–3255. [Google Scholar] [CrossRef]
- Zügel, M.; Qiu, S.; Laszlo, R.; Bosnyák, E.; Weigt, C.; Müller, D.; Diel, P.; Steinacker, J.M.; Schumann, U. The role of sex, adiposity, and gonadectomy in the regulation of irisin secretion. Endocrine 2016, 54, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernández-Real, J.M. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef] [PubMed]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Crujeiras, A.B.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/Irisin Is Not Only a Myokine but Also an Adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef]
- Wahab, F.; Khan, I.U.; Polo, I.R.; Zubair, H.; Drummer, C.; Shahab, M.; Behr, R. Irisin in the primate hypothalamus and its effect on GnRH in vitro. J. Endocrinol. 2019, 241, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Wahab, F.; Shahab, M.; Behr, R. Hypothesis: Irisin is a metabolic trigger for the activation of the neurohormonal axis governing puberty onset. Med. Hypotheses 2016, 95, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ulker, N.; Yardimci, A.; Kaya Tektemur, N.; Bulmus, O.; Ozer Kaya, S.; Gulcu Bulmus, F.; Turk, G.; Ozcan, M.; Canpolat, S. Irisin may have a role in pubertal development and regulation of reproductive function in rats. Reproduction 2020, 160, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, Q.; Lian, A.; Xu, Y. Irisin stimulates gonadotropins gene expression in tilapia (Oreochromis niloticus) pituitary cells. Anim. Reprod. Sci. 2017, 85, 140–147. [Google Scholar] [CrossRef]
- Poretsky, L.; Islam, J.; Avtanski, D.; Lin, Y.K.; Shen, Y.L.; Hirth, Y.; Lesser, M.; Rosenwaks, Z.; Seto-Young, D. Reproductive effects of irisin: Initial in vitro studies. Reprod. Biol. 2017, 17, 285–288. [Google Scholar] [CrossRef]
- Bastu, E.; Zeybek, U.; Gurel Gurevin, E.; Yüksel Ozgor, B.; Celik, F.; Okumus, N.; Demiral, I.; Dural, O.; Celik, C.; Bulut, H.; et al. Effects of Irisin and Exercise on Metabolic Parameters and Reproductive Hormone Levels in High-Fat Diet-Induced Obese Female Mice. Reprod. Sci. 2018, 25, 281–291. [Google Scholar] [CrossRef]
- Tekin, S.; Beytur, A.; Erden, Y.; Beytur, A.; Cigremis, Y.; Vardi, N.; Turkoz, Y.; Tekedereli, I.; Sandal, S. Effects of intracerebroventricular administration of irisin on the hypothalamus-pituitary-gonadal axis in male rats. J. Cell Physiol. 2019, 234, 8815–8824. [Google Scholar] [CrossRef]
- Klenke, U.; Taylor-Burds, C.; Wray, S. Metabolic influences on reproduction: Adiponectin attenuates GnRH neuronal activity in female mice. Endocrinology 2014, 155, 1851–1863. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Tena-Sempere, M. Metabolic control of female puberty: Potential therapeutic targets. Expert. Opin. Ther. Targets 2016, 20, 1181–1193. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garrido, M.A.; Tena-Sempere, M. Metabolic control of puberty: Roles of leptin and kisspeptins. Horm. Behav. 2013, 64, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Wahab, F.; Shahab, M.; Behr, R. The involvement of gonadotropin inhibitory hormone and kisspeptin in the metabolic regulation of reproduction. J. Endocrinol. 2015, 225, R49–R66. [Google Scholar] [CrossRef] [PubMed]
- De Leonibus, C.; Marcovecchio, M.L.; Chiavaroli, V.; De Giorgis, T.; Chiarelli, F.; Mohn, A. Timing of puberty and physical growth in obese children: A longitudinal study in boys and girls. Pediatr. Obes. 2014, 9, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Atay, Z.; Turan, S.; Guran, T.; Furman, A.; Bereket, A. The prevalence and risk factors of premature thelarche and pubarche in 4- to 8-year-old girls. Acta Paediatr. Int. J. Paediatr. 2012, 101, 71–75. [Google Scholar] [CrossRef]
- Hannon, T.S.; Janosky, J.; Arslanian, S.A. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr. Res. 2006, 60, 759–763. [Google Scholar] [CrossRef]
- Reinehr, T.; Elfers, C.; Lass, N.; Roth, C.L. Irisin and its relation to insulin resistance and puberty in obese children: A longitudinal analysis. J. Clin. Endocrinol. Metab. 2015, 100, 2123–2130. [Google Scholar] [CrossRef]
- Kutlu, E.; Özgen, I.T.; Bulut, H.; Koçyiǧit, A.; Otçu, H.; Cesur, Y. Serum Irisin Levels in Central Precocious Puberty and Its Variants. J. Clin. Endocrinol. Metab. 2021, 106, E247–E254. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic ovary syndrome. Nat. Rev. Dis. Primers 2016, 2, 16057. [Google Scholar] [CrossRef]
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef]
- Lim, S.S.; Davies, M.J.; Norman, R.J.; Moran, L.J. Overweight, obesity and central obesity in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2012, 18, 618–637. [Google Scholar] [CrossRef] [PubMed]
- Amisi, C.A. Markers of insulin resistance in Polycystic ovary syndrome women: An update. World J. Diabetes 2022, 13, 129–149. [Google Scholar] [CrossRef] [PubMed]
- Pant, P.; Chitme, H.; Sircar, R.; Prasad, R.; Prasad, H.O. Genome-wide association study for single nucleotide polymorphism associated with mural and cumulus granulosa cells of PCOS (polycystic ovary syndrome) and non-PCOS patients. Future J. Pharm. Sci. 2023, 27. [Google Scholar] [CrossRef]
- Gizaw, M.; Anandakumar, P.; Debela, T. A Review on the Role of Irisin in Insulin Resistance and Type 2 Diabetes Mellitus. J. Pharmacopunct. 2017, 20, 235–242. [Google Scholar] [CrossRef]
- Park, K.H.; Zaichenko, L.; Brinkoetter, M.; Thakkar, B.; Sahin-Efe, A.; Joung, K.E.; Tsoukas, M.A.; Geladari, E.V.; Huh, J.Y.; Dincer, F.; et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 4899–4907. [Google Scholar] [CrossRef]
- Chang, C.L.; Huang, S.Y.; Soong, Y.K.; Cheng, P.J.; Wang, C.J.; Liang, I.T. Circulating irisin and glucose-dependent insulinotropic peptide are associated with the development of polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2014, 99, E2539–E2548. [Google Scholar] [CrossRef] [PubMed]
- Pukajło, K.; Łaczmański, Ł.; Kolackov, K.; Kuliczkowska-Płaksej, J.; Bolanowski, M.; Milewicz, A.; Daroszewski, J. Irisin plasma concentration in PCOS and healthy subjects is related to body fat content and android fat distribution. Gynecol. Endocrinol. 2015, 31, 907–911. [Google Scholar] [CrossRef]
- Abali, R.; Temel Yuksel, I.; Yuksel, M.A.; Bulut, B.; Imamoglu, M.; Emirdar, V.; Unal, F.; Guzel, S.; Celik, C. Implications of circulating irisin and Fabp4 levels in patients with polycystic ovary syndrome. J. Obs. Gynaecol. 2016, 36, 897–901. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.Y.; Sun, Y.; Hou, X.G.; Chen, L. Higher circulating irisin levels in patients with polycystic ovary syndrome: A meta-analysis. Gynecol. Endocrinol. 2018, 34, 290–293. [Google Scholar] [CrossRef]
- Cai, X.; Qiu, S.; Li, L.; Zügel, M.; Steinacker, J.M.; Schumann, U. Circulating irisin in patients with polycystic ovary syndrome: A meta-analysis. Reprod. Biomed. Online 2018, 36, 172–180. [Google Scholar] [CrossRef]
- Li, Q.; Jia, S.; Xu, L.; Li, B.; Chen, N. Metformin-induced autophagy and irisin improves INS-1 cell function and survival in high-glucose environment via AMPK/SIRT1/PGC-1α signal pathway. Food Sci. Nutr. 2019, 7, 1695–1703. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Pardo, M.; Arturo, R.R.; Navas-Carretero, S.; Zulet, M.A.; Martínez, J.A.; Casanueva, F.F. Longitudinal variation of circulating irisin after an energy restriction-induced weight loss and following weight regain in obese men and women. Am J. Hum. Biol. 2014, 26, 198–207. [Google Scholar] [CrossRef]
- Bostancı, M.S.; Akdemir, N.; Cinemre, B.; Cevrioglu, A.S.; Özden, S.; Ünal, O. Serum irisin levels in patients with polycystic ovary syndrome. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4462–4468. [Google Scholar] [PubMed]
- Zhang, L.; Fang, X.; Li, L.; Liu, R.; Zhang, C.; Liu, H.; Tan, M.; Yang, G. The association between circulating irisin levels and different phenotypes of polycystic ovary syndrome. J. Endocrinol. Investig. 2018, 41, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, X.; Wang, X.; Liao, X.; Li, L.; Yang, G.; Gao, L. Free androgen index and Irisin in polycystic ovary syndrome. J. Endocrinol. Investig. 2016, 39, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.R.; Mamede, M.; Bizzi, M.F.; Rocha, A.L.L.; Ferreira, C.N.; Gomes, K.B.; Cândido, A.L.; Reis, F.M. Brown adipose tissue activity is reduced in women with polycystic ovary syndrome. Eur. J. Endocrinol. 2019, 181, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Shorakae, S.; Jona, E.; de Courten, B.; Lambert, G.W.; Lambert, E.A.; Phillips, S.E.; Clarke, I.J.; Teede, H.J.; Henry, B.A. Brown adipose tissue thermogenesis in polycystic ovary syndrome. Clin. Endocrinol. 2019, 90, 425–432. [Google Scholar] [CrossRef]
- Zheng, S.; Guo, W.; Wang, X. Study on the relationship between the levels of irisin in umbilical cord blood and neonatal growth in China. J. Matern.-Fetal Neonatal Med. 2020, 33, 4133–4138. [Google Scholar] [CrossRef]
- Varikasuvu, S.R.; Pérez-López, F.R.; Thangappazham, B.; Raj, H.; Vykunta, A. Effect of metformin intervention on circulating irisin levels in polycystic ovary syndrome: A systematic review and collaborative meta-analysis. Gynecol. Endocrinol. 2021, 38, 207–212. [Google Scholar] [CrossRef]
- Gordon, C.M.; Ackerman, K.E.; Berga, S.L.; Kaplan, J.R.; Mastorakos, G.; Misra, M.; Murad, M.H.; Santoro, N.F.; Warren, M.P. Functional Hypothalamic Amenorrhea: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 1413–1439. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Current evaluation of amenorrhea. Fertil. Steril. 2006, 86, S148–S155. [Google Scholar] [CrossRef] [PubMed]
- Indirli, R.; Lanzi, V.; Mantovani, G.; Arosio, M.; Ferrante, E. Bone health in functional hypothalamic amenorrhea: What the endocrinologist needs to know. Front. Endocrinol. 2022, 13, 946695. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Qiao, X.; Zeng, R.; Cheng, R.; Zhang, J.; Luo, Y.; Nie, Y.; Hu, Y.; Yang, Z.; Zhang, J.; et al. Irisin promotes proliferation but inhibits differentiation in osteoclast precursor cells. FASEB J. 2018, 32, 5813–5823. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Brychta, R.J.; Collins, M.T.; Linderman, J.; Smith, S.; Herscovitch, P.; Millo, C.; Chen, K.Y.; Celi, F.S. Cold-activated brown adipose tissue is an independent predictor of higher bone mineral density in women. Osteoporos. Int. 2013, 4, 1513–1518. [Google Scholar] [CrossRef]
- Klangjareonchai, T.; Nimitphong, H.; Saetung, S.; Bhirommuang, N.; Samittarucksa, R.; Chanprasertyothin, S.; Sudatip, R.; Ongphiphadhanakul, B. Circulating sclerostin and irisin are related and interact with gender to influence adiposity in adults with prediabetes. Int. J. Endocrinol. 2014, 2014, 261545. [Google Scholar] [CrossRef]
- Singhal, V.; Lawson, E.A.; Ackerman, K.E.; Fazeli, P.K.; Clarke, H.; Lee, H.; Eddy, K.; Marengi, D.A.; Derrico, N.P.; Bouxsein, M.L.; et al. Irisin levels are lower in young amenorrheic athletes compared with eumenorrheic athletes and non-athletes and are associated with bone density and strength estimates. PLoS ONE 2014, 9, e100218. [Google Scholar] [CrossRef]
- Stengel, A.; Hofmann, T.; Goebel-Stengel, M.; Elbelt, U.; Kobelt, P.; Klapp, B.F. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity--correlation with body mass index. Peptides 2013, 39, 125–130. [Google Scholar] [CrossRef]
- Bredella, M.A.; Fazeli, P.K.; Freedman, L.M.; Calder, G.; Lee, H.; Rosen, C.J.; Klibanski, A. Young women with cold-activated brown adipose tissue have higher bone mineral density and lower Pref-1 than women without brown adipose tissue: A study in women with anorexia nervosa, women recovered from anorexia nervosa, and normal-weight women. J. Clin. Endocrinol. Metab. 2012, 97, E584–E590. [Google Scholar] [CrossRef]
- Notaristefano, G.; Merola, A.; Scarinci, E.; Ubaldi, N.; Ranalli, M.; Tropea, A.; Diterlizzi, A.; Fabozzi, S.M.; Alesiani, O.; Silvestrini, A.; et al. Circulating irisin levels in functional hypothalamic amenorrhea: A new bone damage index? A pilot study. Endocrine 2022, 77, 168–176. [Google Scholar] [CrossRef]
- Gamal, R.M.; Mohamed, M.E.; Hammam, N.; El Fetoh, N.A.; Rashed, A.M.; Furst, D.E. Preliminary study of the association of serum irisin levels with poor sleep quality in rheumatoid arthritis patients. Sleep Med. 2020, 67, 71–76. [Google Scholar] [CrossRef]
- Slate-Romano, J.J.; Yano, N.; Zhao, T.C. Irisin reduces inflammatory signaling pathways in inflammation-mediated metabolic syndrome. Mol. Cell Endocrinol. 2022, 552, 111676. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Primers 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Kaya Sezginer, E.; Kirlangiç, Ö.F.; Eşkin Tanriverdi, M.D.; Topçu, H.O.; Gür, S. Analysis of Changes in Serum Levels and Gene Expression Profiles of Novel Adipocytokines (Omentin, Vaspin, Irisin and Visfatin) and Their Correlation with Serum C-reactive Protein Levels in Women Diagnosed with Endometriosis. Turk. J. Pharm. Sci. 2022, 19, 48–53. [Google Scholar] [CrossRef]
- Ali, E.Y.; Hegazy, G.A.; Hashem, E.M. Evaluation of irisin, retinol-binding protein 4, and leptin serum levels as biomarkers of macrovascular complications involvement in Saudi type 2 diabetes mellitus. A case-control study. Saudi Med. J. 2020, 41, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Kuloglu, T.; Celik, O.; Aydin, S.; Hanifi Ozercan, I.; Acet, M.; Aydin, Y.; Artas, G.; Turk, A.; Yardim, M.; Ozan, G.; et al. Irisin immunostaining characteristics of breast and ovarian cancer cells. Cell Mol. Biol. 2016, 62, 40–44. [Google Scholar]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Wang, H.; Li, N.; Chivese, T.; Werfalli, M.; Sun, H.; Yuen, L.; Hoegfeldt, C.A.; Elise Powe, C.; Immanuel, J.; Karuranga, S.; et al. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res. Clin. Pract. 2022, 183, 109050. [Google Scholar] [CrossRef]
- Catalano, P.M.; Tyzbir, E.D.; Roman, N.M.; Amini, S.B.; Sims, E.A.H. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am. J. Obstet. Gynecol. 1991, 165, 1667–1672. [Google Scholar] [CrossRef]
- Vohr, B.R.; Boney, C.M. Gestational diabetes: The forerunner for the development of maternal and childhood obesity and metabolic syndrome? J. Matern.-Fetal Neonatal Med. 2008, 21, 149–157. [Google Scholar] [CrossRef]
- Lain, K.Y.; Catalano, P.M. Factors That Affect Maternal Insulin Resistance and Modify Fetal Growth and Body Composition. Metab. Syndr. Relat. Disord. 2006, 4, 91–100. [Google Scholar] [CrossRef]
- Briana, D.D.; Malamitsi-Puchner, A. Adipocytokines in Normal and Complicated Pregnancies. Reprod. Sci. 2009, 16, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Lowe, W.L.; Karban, J. Genetics, genomics and metabolomics: New insights into maternal metabolism during pregnancy. Diabet. Med. 2014, 31, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Garcés, M.F.; Peralta, J.J.; Ruiz-Linares, C.E.; Lozano, A.R.; Poveda, N.E.; Torres-Sierra, A.L.; Eslava-Schmalbach, J.H.; Alzate, J.P.; Sánchez, Á.Y.; Sanchez, E.; et al. Irisin Levels During Pregnancy and Changes Associated With the Development of Preeclampsia. J. Clin. Endocrinol. Metab. 2014, 99, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- Kuzmicki, M.; Telejko, B.; Lipinska, D.; Pliszka, J.; Szamatowicz, M.; Wilk, J.; Zbucka-Kretowska, M.; Laudanski, P.; Kretowski, A.; Gorska, M.; et al. Serum irisin concentration in women with gestational diabetes. Gynecol. Endocrinol. 2014, 30, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, M.A.; Oncul, M.; Tuten, A.; Imamoglu, M.; Acikgoz, A.S.; Kucur, M.; Madazli, R. Maternal serum and fetal cord blood irisin levels in gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2014, 104, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Erol, O.; Erkal, N.; Ellidağ, H.Y.; İsenlik, B.S.; Aydın, Ö.; Derbent, A.U.; Yılmaz, N. Irisin as an early marker for predicting gestational diabetes mellitus: A prospective study. J. Matern.-Fetal Neonatal Med. 2016, 29, 3590–3595. [Google Scholar] [CrossRef] [PubMed]
- Ebert, T.; Stepan, H.; Schrey, S.; Kralisch, S.; Hindricks, J.; Hopf, L.; Platz, M.; Lossner, U.; Jessnitzer, B.; Drewlo, S.; et al. Serum levels of irisin in gestational diabetes mellitus during pregnancy and after delivery. Cytokine 2014, 65, 153–158. [Google Scholar] [CrossRef]
- Piya, M.K.; Harte, A.L.; Sivakumar, K.; Tripathi, G.; Voyias, P.D.; James, S.; Sabico, S.; Al-Daghri, N.M.; Saravanan, P.; Barber, T.M.; et al. The identification of irisin in human cerebrospinal fluid: Influence of adiposity, metabolic markers, and gestational diabetes. Am. J. Physiol. Endocrinol. Metab. 2014, 306, 512–518. [Google Scholar] [CrossRef]
- Cai, L.; Wu, W.; Lin, L.; Chen, Y.; Gao, R.; Shi, B.; Ma, B.; Chen, Y.; Jing, J. Association between plasma irisin and glucose metabolism in pregnant women is modified by dietary n-3 polyunsaturated fatty acid intake. J. Diabetes Investig. 2020, 11, 1326–1335. [Google Scholar] [CrossRef]
- Vaughan, R.A.; Gannon, N.P.; Barberena, M.A.; Garcia-Smith, R.; Bisoffi, M.; Mermier, C.M.; Conn, C.A.; Trujillo, K.A. Characterization of the metabolic effects of irisin on skeletal muscle in vitro. Diabetes Obes. Metab. 2014, 16, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Shi, C.X.; Gao, R.; Sun, H.J.; Xiong, X.Q.; Ding, L.; Chen, Q.; Li, Y.H.; Wang, J.J.; Kang, Y.M.; et al. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin. Sci. 2015, 129, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Qiao, T.; Xu, F.; Li, Z.; Chen, T.; Su, H.; Chen, G.; Zhang, L.; Xu, D.; Zhang, X. Circulating irisin levels of prenatal and postnatal patients with gestational diabetes mellitus: A systematic review and meta-analysis. Cytokine 2020, 126, 154924. [Google Scholar] [CrossRef] [PubMed]
- Du, X.L.; Jiang, W.X.; Lv, Z.T. Lower Circulating Irisin Level in Patients with Diabetes Mellitus: A Systematic Review and Meta-Analysis. Horm. Metab. Res. 2016, 48, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, J.; Li, Z.L.; Yang, J.; Li, M.L.; Wang, G.L. Circulating irisin is lower in gestational diabetes mellitus. Endocr. J. 2015, 62, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Onat, T.; Inandiklioglu, N. Circulating Myonectin and Irisin Levels in Gestational Diabetes Mellitus—A Case-control Study. Z. Für Geburtshilfe Neonatol. 2021, 225, 320–326. [Google Scholar] [CrossRef]
- Kulhan, N.G.; Kulhan, M.; Turkler, C.; Ata, N.; Kiremitli, T.; Kiremitli, S. Could serum levels of irisin be used in gestational diabetes predicting? Taiwan J. Obstet. Gynecol. 2019, 58, 434–437. [Google Scholar] [CrossRef]
- Wang, P.; Ma, H.H.; Hou, X.Z.; Song, L.L.; Song, X.L.; Zhang, J.F. Reduced plasma level of irisin in first trimester as a risk factor for the development of gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2018, 142, 130–138. [Google Scholar] [CrossRef]
- Zbucka-Kretowska, M.; Kuzmicki, M.; Telejko, B.; Goscik, J.; Ciborowski, M.; Lipinska, D.; Hryniewicka, J.; Citko, A.; Lawicki, S.; Wolczynski, S. First-trimester irisin and fetuin-A concentration in predicting macrosomia. J. Matern.-Fetal Neonatal Med. 2019, 32, 2868–2873. [Google Scholar] [CrossRef]
- Ersahin, S.S.; Yurci, A. Cord blood and maternal serum preptin and irisin concentrations are regulated independently in GDM. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1954–1958. [Google Scholar] [CrossRef]
- Fatima, S.S.; Khalid, E.; Ladak, A.A.; Ali, S.A. Colostrum and mature breast milk analysis of serum irisin and sterol regulatory element-binding proteins-1c in gestational diabetes mellitus. J. Matern.-Fetal Neonatal Med. 2019, 32, 2993–2999. [Google Scholar] [CrossRef] [PubMed]
- Drewlo, S.; Johnson, E.; Kilburn, B.A.; Kadam, L.; Armistead, B.; Kohan-Ghadr, H.R. Irisin induces trophoblast differentiation via AMPK activation in the human placenta. J. Cell. Physiol. 2020, 235, 7146–7158. [Google Scholar] [CrossRef] [PubMed]
- Yong, E.; Logan, S. Menopausal osteoporosis: Screening, prevention and treatment. Singap. Med. J. 2021, 62, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Akkawi, I.; Zmerly, H. Osteoporosis: Current Concepts. Joints 2018, 6, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Kornel, A.; Den Hartogh, D.J.; Klentrou, P.; Tsiani, E. Role of the myokine irisin on bone homeostasis: Review of the current evidence. Int. J. Mol. Sci. 2021, 22, 9136. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Nie, Y.; Ma, Y.; Chen, Y.; Cheng, R.; Yin, W.; Hu, Y.; Xu, W.; Xu, L. Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci. Rep. 2016, 6, 18732. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, K.; Zhao, S.; Geng, T.; Fan, X.; Sun, S.; Zheng, M.; Jin, Q. Irisin promotes osteogenic differentiation of bone marrow mesenchymal stem cells by activating autophagy via the Wnt//β-catenin signal pathway. Cytokine 2020, 136, 155292. [Google Scholar] [CrossRef]
- Zhang, J.; Valverde, P.; Zhu, X.; Murray, D.; Wu, Y.; Yu, L.; Jiang, H.; Dard, M.M.; Huang, J.; Xu, Z.; et al. Exercise-induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. Bone Res. 2017, 5, 16056. [Google Scholar] [CrossRef]
- He, Z.; Li, H.; Han, X.; Zhou, F.; Du, J.; Yang, Y.; Xu, Q.; Zhang, S.; Zhang, S.; Zhao, N.; et al. Irisin inhibits osteocyte apoptosis by activating the Erk signaling pathway in vitro and attenuates ALCT-induced osteoarthritis in mice. Bone 2020, 141, 115573. [Google Scholar] [CrossRef]
- Storlino, G.; Colaianni, G.; Sanesi, L.; Lippo, L.; Brunetti, G.; Errede, M.; Colucci, S.; Passeri, G.; Grano, M. Irisin Prevents Disuse-Induced Osteocyte Apoptosis. J. Bone Miner. Res. 2020, 35, 766–775. [Google Scholar] [CrossRef]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell 2018, 175, 1756–1768.e17. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.C.; Costa, A.G.; Cusano, N.E.; Kousteni, S.; Bilezikian, J.P. Catabolic and anabolic actions of parathyroid hormone on the skeleton. J. Endocrinol. Investig. 2011, 34, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Sanesi, L.; Storlino, G.; Brunetti, G.; Colucci, S.; Grano, M. Irisin and bone: From preclinical studies to the evaluation of its circulating levels in different populations of human subjects. Cells 2019, 8, 451. [Google Scholar] [CrossRef]
- Morgan, E.N.; Alsharidah, A.S.; Mousa, A.M.; Edrees, H.M. Irisin Has a Protective Role against Osteoporosis in Ovariectomized Rats. BioMed Res. Int. 2021, 2021, 5570229. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Animal models for osteoporosis. Eur. J. Pharmacol. 2015, 759, 287–294. [Google Scholar] [CrossRef]
- Engin-Üstün, Y.; Çağlayan, E.K.; Göçmen, A.Y.; Polat, M.F. Postmenopausal Osteoporosis Is Associated with Serum Chemerin and Irisin but Not with Apolipoprotein M Levels. J. Menopausal Med. 2016, 22, 76. [Google Scholar] [CrossRef][Green Version]
- Palermo, A.; Strollo, R.; Maddaloni, E.; Tuccinardi, D.; D’Onofrio, L.; Briganti, S.I.; Defeudis, G.; De Pascalis, M.; Lazzaro, M.C.; Colleluori, G.; et al. Irisin is associated with osteoporotic fractures independently of bone mineral density, body composition or daily physical activity. Clin. Endocrinol. 2015, 82, 615–619. [Google Scholar] [CrossRef]
- Liu, K.; Jing, P.; Liu, Z.; Wang, Y.; Han, Z.; Wang, Y.; Zheng, Z.; Wu, Y.; Wang, T.; Li, Y.; et al. Serum levels of irisin in postmenopausal women with osteoporotic hip fractures. Cytokine 2021, 148, 155708. [Google Scholar] [CrossRef]
- Yan, J.; Liu, H.J.; Guo, W.C.; Yang, J. Low serum concentrations of Irisin are associated with increased risk of hip fracture in Chinese older women. Jt. Bone Spine 2018, 85, 353–358. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, H.C.; Zhang, D.; Yeom, H.; Lim, S.K. The novel myokine irisin: Clinical implications and potential role as a biomarker for sarcopenia in postmenopausal women. Endocrine 2019, 64, 341–348. [Google Scholar] [CrossRef]
- Palermo, A.; Sanesi, L.; Colaianni, G.; Tabacco, G.; Naciu, A.M.; Cesareo, R.; Pedone, C.; Lelli, D.; Brunetti, G.; Mori, G.; et al. A Novel Interplay Between Irisin and PTH: From Basic Studies to Clinical Evidence in Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2019, 104, 3088–3096. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Polyzos, S.A.; Makras, P.; Gkiomisi, A.; Bisbinas, I.; Katsarou, A.; Filippaios, A.; Mantzoros, C.S. Circulating irisin is associated with osteoporotic fractures in postmenopausal women with low bone mass but is not affected by either teriparatide or denosumab treatment for 3 months. Osteoporos. Int. 2014, 25, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Welten, S.J.G.C.; Onland-Moret, N.C.; Boer, J.M.A.; Verschuren, W.M.M.; van der Schouw, Y.T. Age at Menopause and Risk of Ischemic and Hemorrhagic Stroke. Stroke 2021, 52, 2583–2591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, W. Can irisin be a linker between physical activity and brain function? Biomol. Concepts 2016, 7, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.S.; Dincer, F.; Mantzoros, C.S. Pharmacological concentrations of irisin increase cell proliferation without influencing markers of neurite outgrowth and synaptogenesis in mouse H19-7 hippocampal cell lines. Metabolism 2013, 62, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Deng, X.; Huang, W.; Yu, J.; Wang, J.; Wang, J.; Yang, S.; Liu, X.; Wang, L.; Zhang, Y.; et al. Irisin protects against neuronal injury induced by oxygen-glucose deprivation in part depends on the inhibition of ROS-NLRP3 inflammatory signaling pathway. Mol. Immunol. 2017, 91, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Huberman, M.A.; d’Adesky, N.D.; Niazi, Q.B.; Perez-Pinzon, M.A.; Bramlett, H.M.; Raval, A.P. Irisin-Associated Neuroprotective and Rehabilitative Strategies for Stroke. NeuroMolecular Med. 2022, 24, 62–73. [Google Scholar] [CrossRef]
- Kim, S.J.; Yoon, E.S.; Jung, S.Y.; Kim, D.Y. Effect of uphill walking on browning factor and high molecular weight-adiponectin in postmenopausal women. J. Exerc. Rehabil. 2020, 16, 265–271. [Google Scholar] [CrossRef]
- Rashti, B.A.; Mehrabani, J.; Damirchi, A.; Babaei, P. The influence of concurrent training intensity on serum irisin and abdominal fat in postmenopausal women. Prz. Menopauzalny 2019, 18, 166–173. [Google Scholar] [CrossRef]
- Wiecek, M.; Szymura, J.; Sproull, J.; Szygula, Z. Whole-body cryotherapy is an effective method of reducing abdominal obesity in menopausal women with metabolic syndrome. J. Clin. Med. 2020, 9, 2797. [Google Scholar] [CrossRef]
- Shen, H.H.; Huang, S.Y.; Kung, C.W.; Chen, S.Y.; Chen, Y.F.; Cheng, P.Y.; Lam, K.K.; Lee, Y.M. Genistein ameliorated obesity accompanied with adipose tissue browning and attenuation of hepatic lipogenesis in ovariectomized rats with high-fat diet. J. Nutr. Biochem. 2019, 67, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Assyov, Y.; Gateva, A.; Karamfilova, V.; Gatev, T.; Nedeva, I.; Velikova, T.; Kamenov, Z.A. Impact of testosterone treatment on circulating irisin in men with late-onset hypogonadism and metabolic syndrome. Aging Male 2021, 23, 1381–1387. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbagallo, F.; Cannarella, R.; Garofalo, V.; Marino, M.; La Vignera, S.; Condorelli, R.A.; Tiranini, L.; Nappi, R.E.; Calogero, A.E. The Role of Irisin throughout Women’s Life Span. Biomedicines 2023, 11, 3260. https://doi.org/10.3390/biomedicines11123260
Barbagallo F, Cannarella R, Garofalo V, Marino M, La Vignera S, Condorelli RA, Tiranini L, Nappi RE, Calogero AE. The Role of Irisin throughout Women’s Life Span. Biomedicines. 2023; 11(12):3260. https://doi.org/10.3390/biomedicines11123260
Chicago/Turabian StyleBarbagallo, Federica, Rossella Cannarella, Vincenzo Garofalo, Marta Marino, Sandro La Vignera, Rosita A. Condorelli, Lara Tiranini, Rossella E. Nappi, and Aldo E. Calogero. 2023. "The Role of Irisin throughout Women’s Life Span" Biomedicines 11, no. 12: 3260. https://doi.org/10.3390/biomedicines11123260
APA StyleBarbagallo, F., Cannarella, R., Garofalo, V., Marino, M., La Vignera, S., Condorelli, R. A., Tiranini, L., Nappi, R. E., & Calogero, A. E. (2023). The Role of Irisin throughout Women’s Life Span. Biomedicines, 11(12), 3260. https://doi.org/10.3390/biomedicines11123260







