Left Atrial Low-Voltage Areas Predict the Risk of Atrial Fibrillation Recurrence after Radiofrequency Ablation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Electroanatomic Mapping and Ablation
2.3. Fibrosis Assessment
- -
- <0.5 mV for patients in sinus rhythm at the time of ablation;
- -
- <0.25 mV for patients in AF at the time of ablation.
2.4. Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Clinical and Paraclinical Characteristics
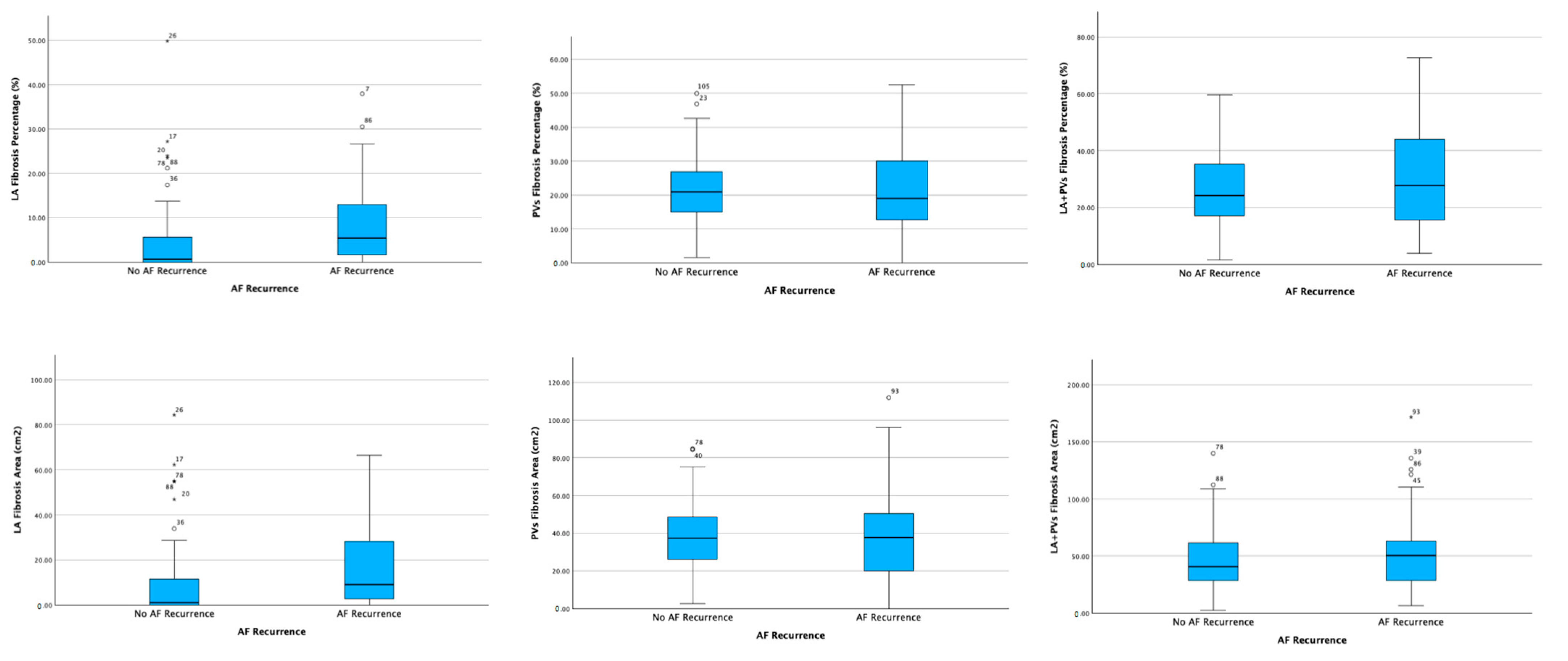
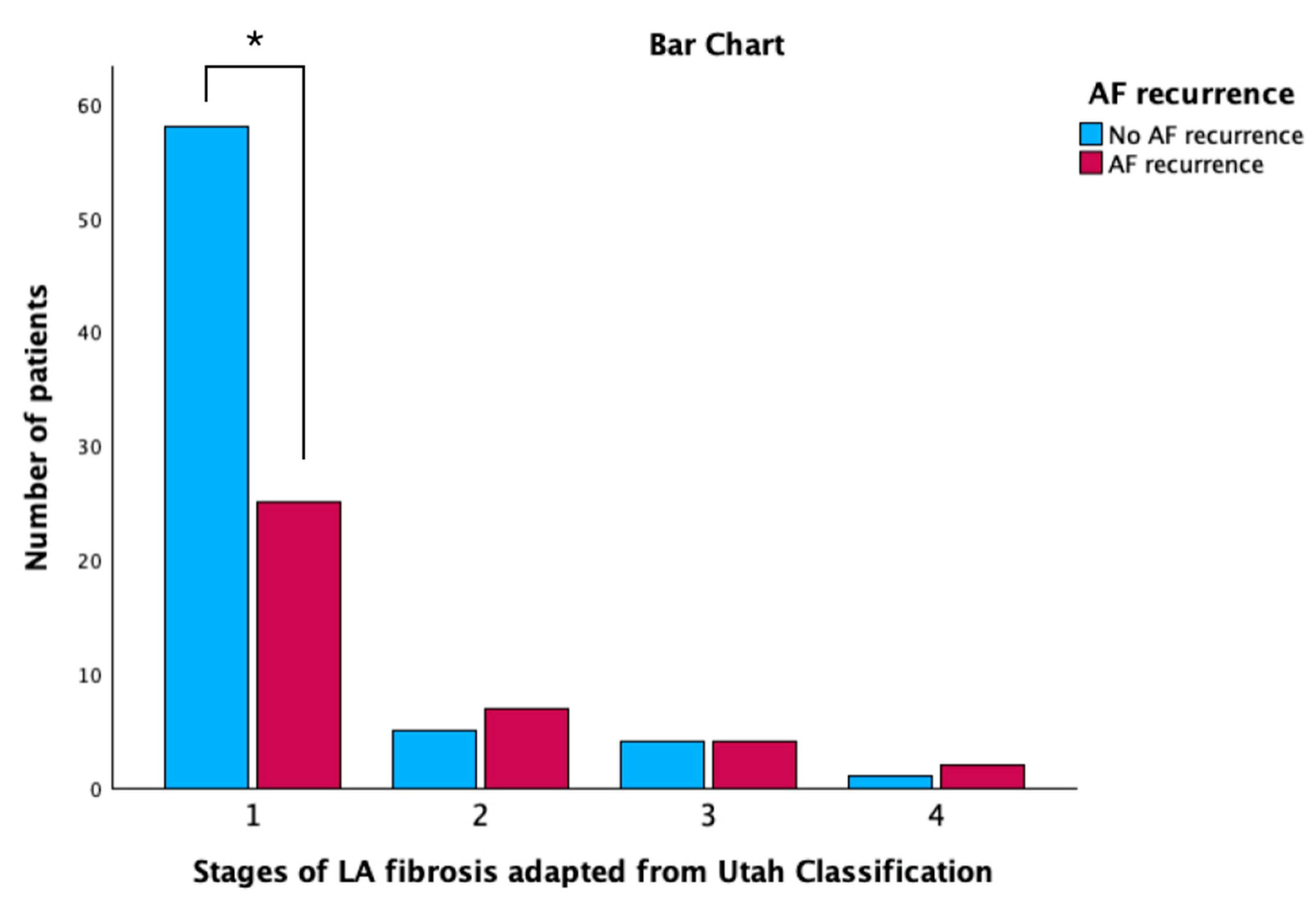
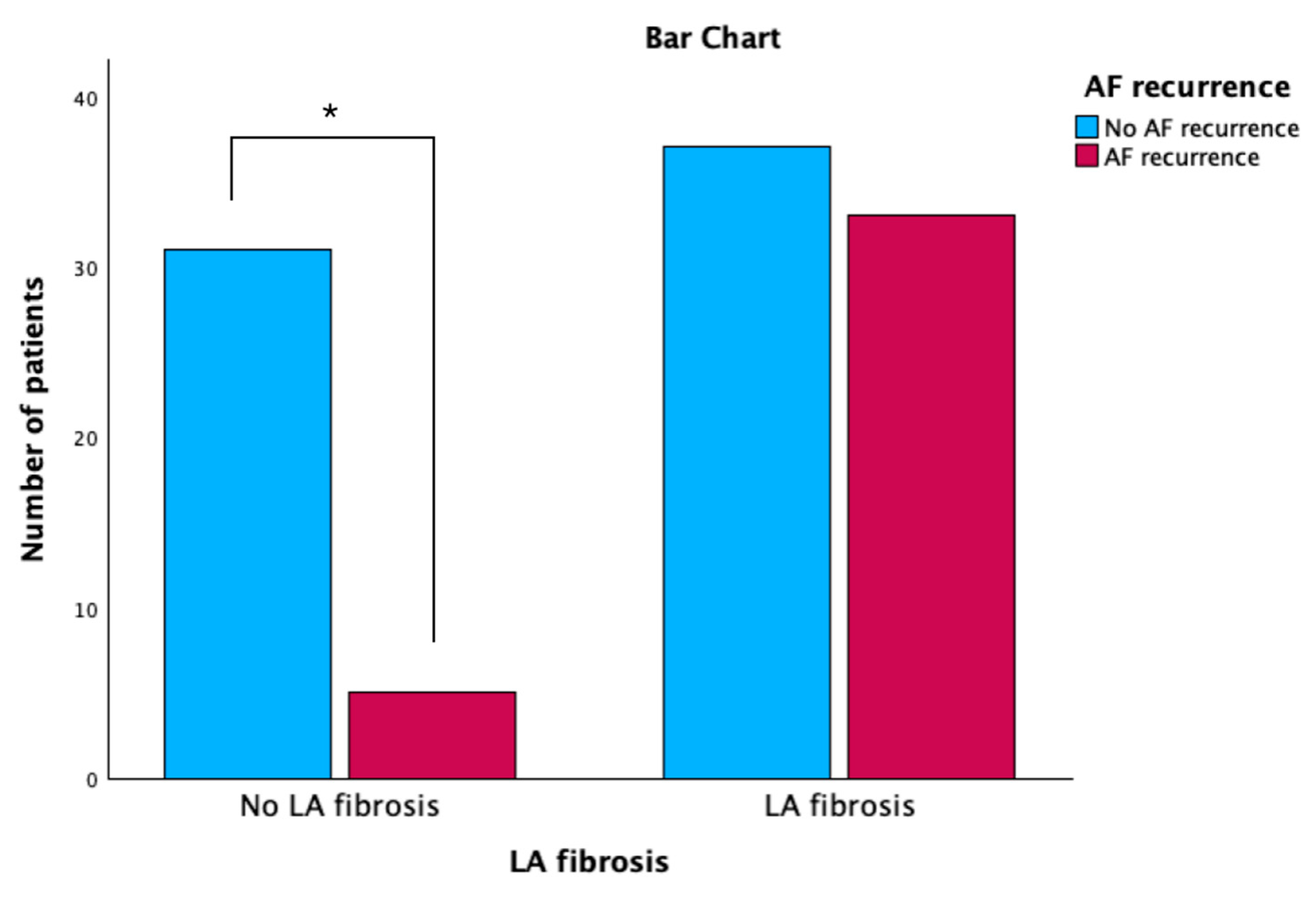
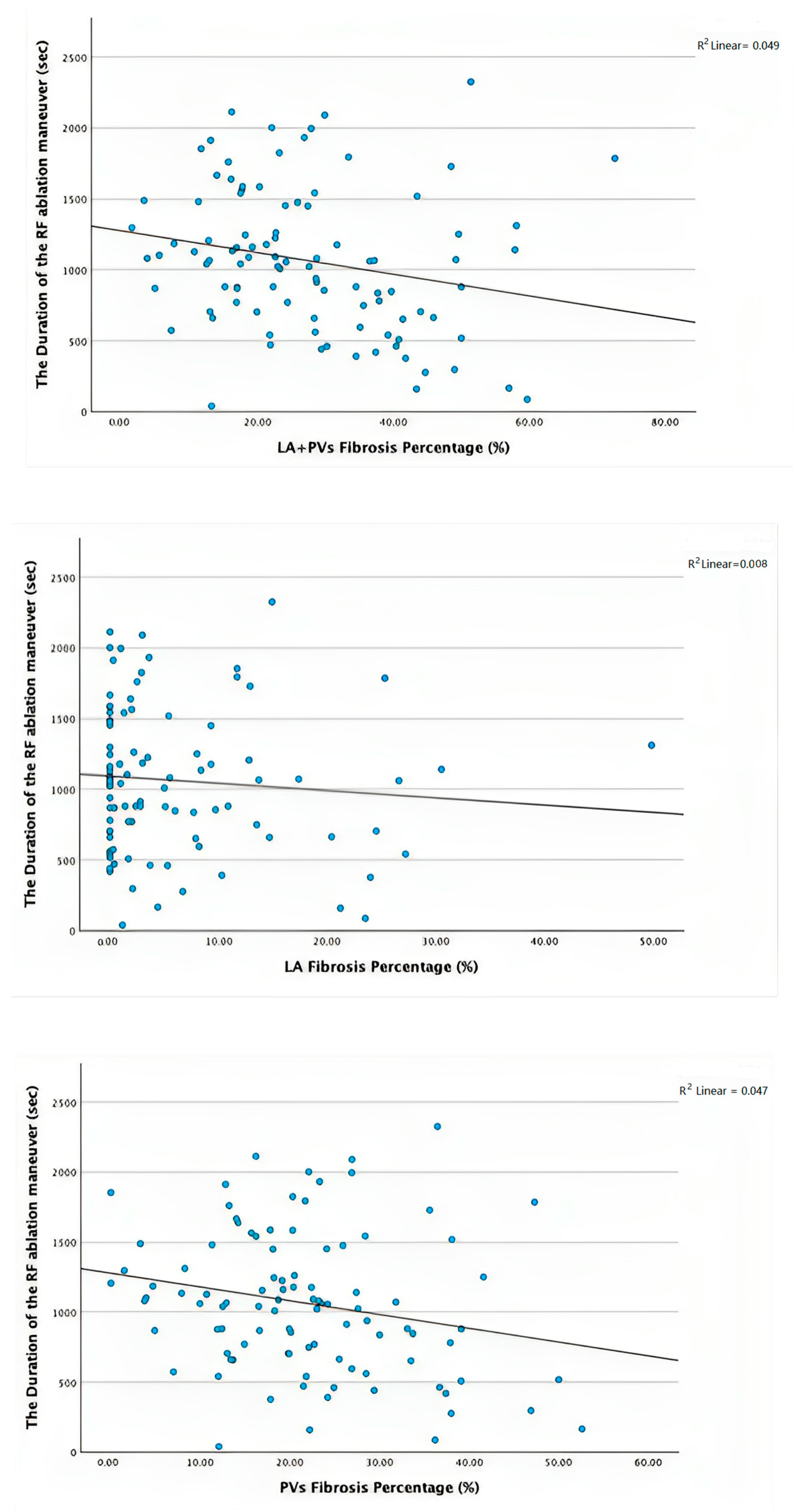
3.2. Electroanatomic Mapping and Low-Voltage Area Study
3.3. Reproducibility Assessment
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Vizzardi, E.; Curnis, A.; Latini, M.G.; Salghetti, F.; Rocco, E.; Lupi, L.; Rovetta, R.; Quinzani, F.; Bonadei, I.; Bontempi, L.; et al. Risk factors for atrial fibrillation recurrence: A literature review. J. Cardiovasc. Med. 2014, 15, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Pacchia, C.F.; Dosdall, D.J.; Ranjan, R.; Dibella, E. Alterations in atrial perfusion during atrial fibrillation. Exp. Physiol. 2014, 99, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Marrouche, N.F.; Wilber, D.; Hindricks, G.; Jais, P.; Akoum, N.; Marchlinski, F.; Kholmovski, E.; Burgon, N.; Hu, N.; Mont, L.; et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: The DECAAF study. JAMA 2014, 311, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Okamatsu, H.; Koyama, J.; Sakai, Y.; Negishi, K.; Hayashi, K.; Tsurugi, T.; Tanaka, Y.; Nakao, K.; Sakamoto, T.; Okumura, K. High-power application is associated with shorter procedure time and higher rate of first-pass pulmonary vein isolation in ablation index-guided atrial fibrillation ablation. J. Cardiovasc. Electrophysiol. 2019, 30, 2751–2758. [Google Scholar] [CrossRef] [PubMed]
- Phlips, T.; Taghji, P.; El Haddad, M.; Wolf, M.; Knecht, S.; Vandekerckhove, Y.; Tavernier, R.; Duytschaever, M. Improving procedural and one-year outcome after contact force-guided pulmonary vein isolation: The role of interlesion distance, ablation index, and contact force variability in the ‘CLOSE’-protocol. Europace 2018, 20, f419–f427. [Google Scholar] [CrossRef] [PubMed]
- Parwani, A.S.; Morris, D.A.; Blaschke, F.; Huemer, M.; Pieske, B.; Haverkamp, W.; Boldt, L.H. Left atrial strain predicts recurrence of atrial arrhythmias after catheter ablation of persistent atrial fibrillation. Open Heart 2017, 4, e000572. [Google Scholar] [CrossRef] [PubMed]
- Sarvari, S.I.; Haugaa, K.H.; Stokke, T.M.; Ansari, H.Z.; Leren, I.S.; Hegbom, F.; Smiseth, O.A.; Edvardsen, T. Strain echocardiographic assessment of left atrial function predicts recurrence of atrial fibrillation. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, R.; Murata, M.; Roberts, R.; Tokuda, H.; Minakata, Y.; Suzuki, K.; Tsuruta, H.; Kimura, T.; Nishiyama, N.; Fukumoto, K.; et al. Left atrial strain is a powerful predictor of atrial fibrillation recurrence after catheter ablation: Study of a heterogeneous population with sinus rhythm or atrial fibrillation. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.; Caracciolo, G.; Khan, U.; Mori, N.; Saha, S.K.; Srivathsan, K.; Altemose, G.; Scott, L.; Sengupta, P.; Jahangir, A. Left atrial reservoir function predicts atrial fibrillation recurrence after catheter ablation: A two-dimensional speckle strain study. J. Interv. Card. Electrophysiol. 2011, 31, 197. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.; Karim, S.; Kahaly, O.; Elzanaty, A.; Meenakshisundaram, C.; Abi-Saleh, B.; Eltahawy, E.; Chacko, P. Low voltage area guided substrate modification in nonparoxysmal atrial fibrillation: A systematic review and meta-analysis. J. Cardiovasc. Electrophysiol. 2023, 34, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Bijvoet, G.P.; Nies, H.M.J.M.; Holtackers, R.J.; Linz, D.; Adriaans, B.P.; Nijveldt, R.; Wildberger, J.E.; Vernooy, K.; Chaldoupi, S.M.; Mihl, C. Correlation between Cardiac MRI and Voltage Mapping in Evaluating Atrial Fibrosis: A Systematic Review. Radiol. Cardiothorac. Imaging 2022, 4, e220061. [Google Scholar] [CrossRef] [PubMed]
- Borne, R.T.; Sauer, W.H.; Zipse, M.M.; Zheng, L.; Tzou, W.; Nguyen, D.T. Longer Duration Versus Increasing Power During Radiofrequency Ablation Yields Different Ablation Lesion Characteristics. JACC Clin. Electrophysiol. 2018, 4, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Barkagan, M.; Leshem, E.; Shapira-Daniels, A.; Sroubek, J.; Buxton, A.E.; Saffitz, J.E.; Anter, E. Histopathological Characterization of Radiofrequency Ablation in Ventricular Scar Tissue. JACC Clin. Electrophysiol. 2019, 5, 920–931. [Google Scholar] [CrossRef] [PubMed]
- McDowell, K.S.; Vadakkumpadan, F.; Blake, R.; Blauer, J.; Plank, G.; Macleod, R.S.; Trayanova, N.A. Mechanistic Inquiry into the Role of Tissue Remodeling in Fibrotic Lesions in Human Atrial Fibrillation. Biophys. J. 2013, 104, 2764–2773. [Google Scholar] [CrossRef] [PubMed]
- Romano, L.R.; Scalzi, G.; Malizia, B.; Aquila, I.; Polimeni, A.; Indolfi, C.; Curcio, A. Impact of Percutaneous Mitral Valve Repair on Left Atrial Strain and Atrial Fibrillation Progression. J. Cardiovasc. Dev. Dis. 2023, 10, 320. [Google Scholar] [CrossRef] [PubMed]
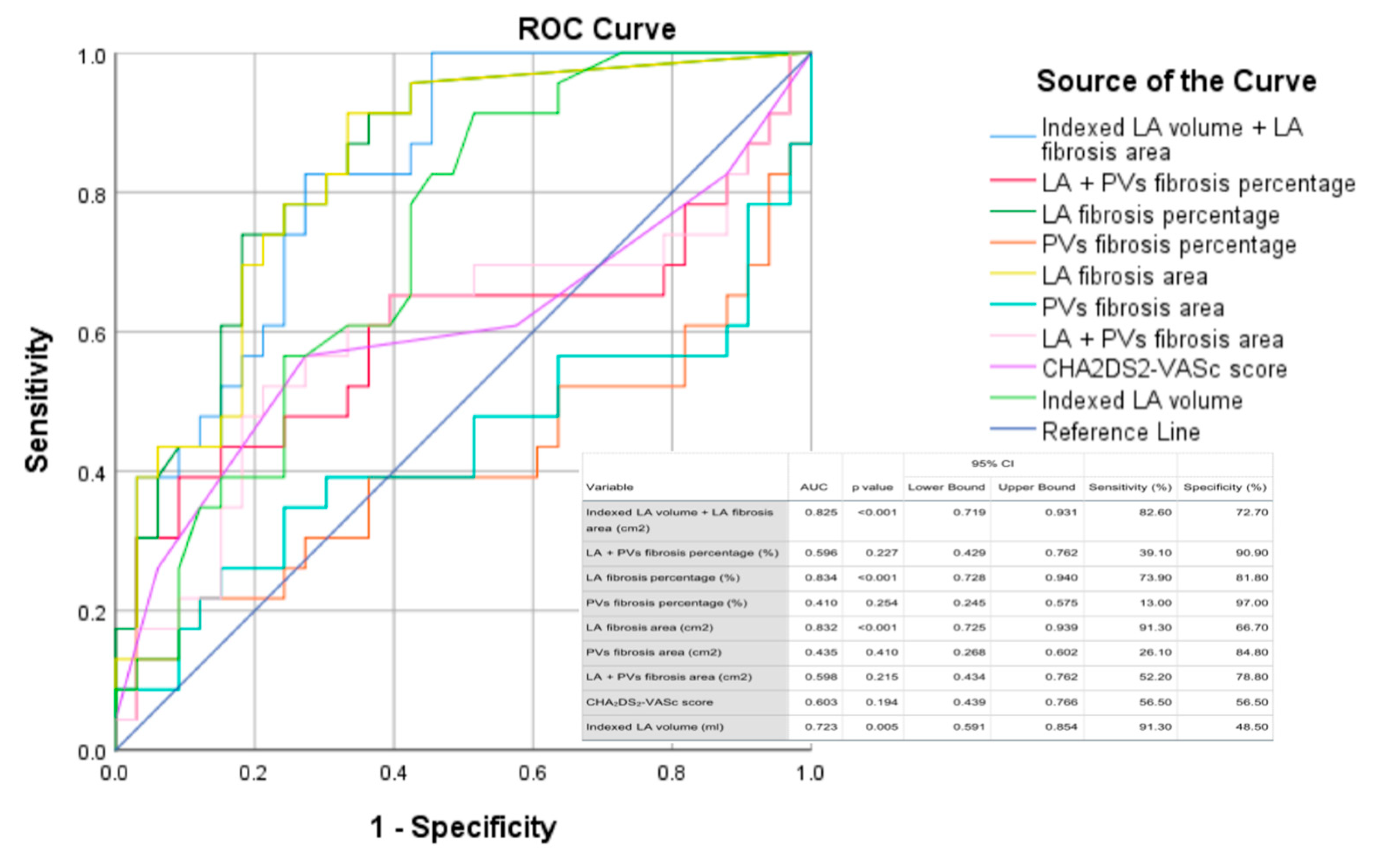
| Variable | Value |
|---|---|
| Age (years) | 58.89 ± 10.79 |
| Sex (male), n (%) | 61 (57.54%) |
| Smoking, n (%) | 16 (15.1%) |
| Hypertension, n (%) | 66 (62.3%) |
| Obesity, n (%) | 32 (30.2%) |
| Diabetes mellitus, n (%) | 16 (15.09%) |
| Obstructive sleep apnea syndrome, n (%) | 41 (38.68%) |
| Dyslipidemia, n (%) | 77 (72.64%) |
| Coronary Artery Disease, n (%) | 10 (9.4%) |
| Congestive Heart Failure, n (%) | 45 (42.45%) |
| CHA2DS2-VASc score at inclusion, n (%) | |
| 0 | 20 (18.9%) |
| 1 | 26 (24.5%) |
| 2 | 22 (20.8%) |
| 3 | 24 (22.6%) |
| 4 | 11 (10.4%) |
| 5 | 3 (2.8%) |
| Bipolar Voltage Mapping Parameter | Patients without AF Recurrence (n = 68) | Patients with AF Recurrence (n = 38) | p-Value |
|---|---|---|---|
| Area of LA fibrosis (cm2) | 9.06 ± 16.95 | 17.82 ± 19.9 | 0.018 |
| Percentage of LA fibrosis (%) | 4.62 ± 8.55 | 8.94 ± 9.72 | 0.019 |
| Area of PVs fibrosis (cm2) | 38.72 ± 18.75 | 39.29 ± 25.63 | 0.896 |
| Percentage of PVs fibrosis (%) | 21.58 ± 10.29 | 21.26 ± 12.95 | 0.888 |
| Total area (LA + PVs) of fibrosis (cm2) | 47.78 ± 27.71 | 57.11 ± 37.57 | 0.148 |
| Total percentage (LV + PVs) of fibrosis (%) | 26.20 ± 13.20 | 30.20 ± 16.47 | 0.175 |
| Total RF application time (s) | 1047.42 ± 479.55 | 1093.88 ± 554.26 | 0.667 |
| Total Ablation Time (s) | 3076.30 ± 1608.40 | 2936.97 ± 1592.42 | 0.684 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitran, R.-E.; Popa-Fotea, N.-M.; Iorgulescu, C.; Nastasa, A.; Pupaza, A.; Gondos, V.; Petre, I.-G.; Paja, S.-C.; Vatasescu, R.-G. Left Atrial Low-Voltage Areas Predict the Risk of Atrial Fibrillation Recurrence after Radiofrequency Ablation. Biomedicines 2023, 11, 3261. https://doi.org/10.3390/biomedicines11123261
Mitran R-E, Popa-Fotea N-M, Iorgulescu C, Nastasa A, Pupaza A, Gondos V, Petre I-G, Paja S-C, Vatasescu R-G. Left Atrial Low-Voltage Areas Predict the Risk of Atrial Fibrillation Recurrence after Radiofrequency Ablation. Biomedicines. 2023; 11(12):3261. https://doi.org/10.3390/biomedicines11123261
Chicago/Turabian StyleMitran, Raluca-Elena, Nicoleta-Monica Popa-Fotea, Corneliu Iorgulescu, Alexandrina Nastasa, Adelina Pupaza, Viviana Gondos, Ioana-Gabriela Petre, Steliana-Cosmina Paja, and Radu-Gabriel Vatasescu. 2023. "Left Atrial Low-Voltage Areas Predict the Risk of Atrial Fibrillation Recurrence after Radiofrequency Ablation" Biomedicines 11, no. 12: 3261. https://doi.org/10.3390/biomedicines11123261
APA StyleMitran, R.-E., Popa-Fotea, N.-M., Iorgulescu, C., Nastasa, A., Pupaza, A., Gondos, V., Petre, I.-G., Paja, S.-C., & Vatasescu, R.-G. (2023). Left Atrial Low-Voltage Areas Predict the Risk of Atrial Fibrillation Recurrence after Radiofrequency Ablation. Biomedicines, 11(12), 3261. https://doi.org/10.3390/biomedicines11123261






