Diagnosis of Carcinogenic Pathologies through Breath Biomarkers: Present and Future Trends
Abstract
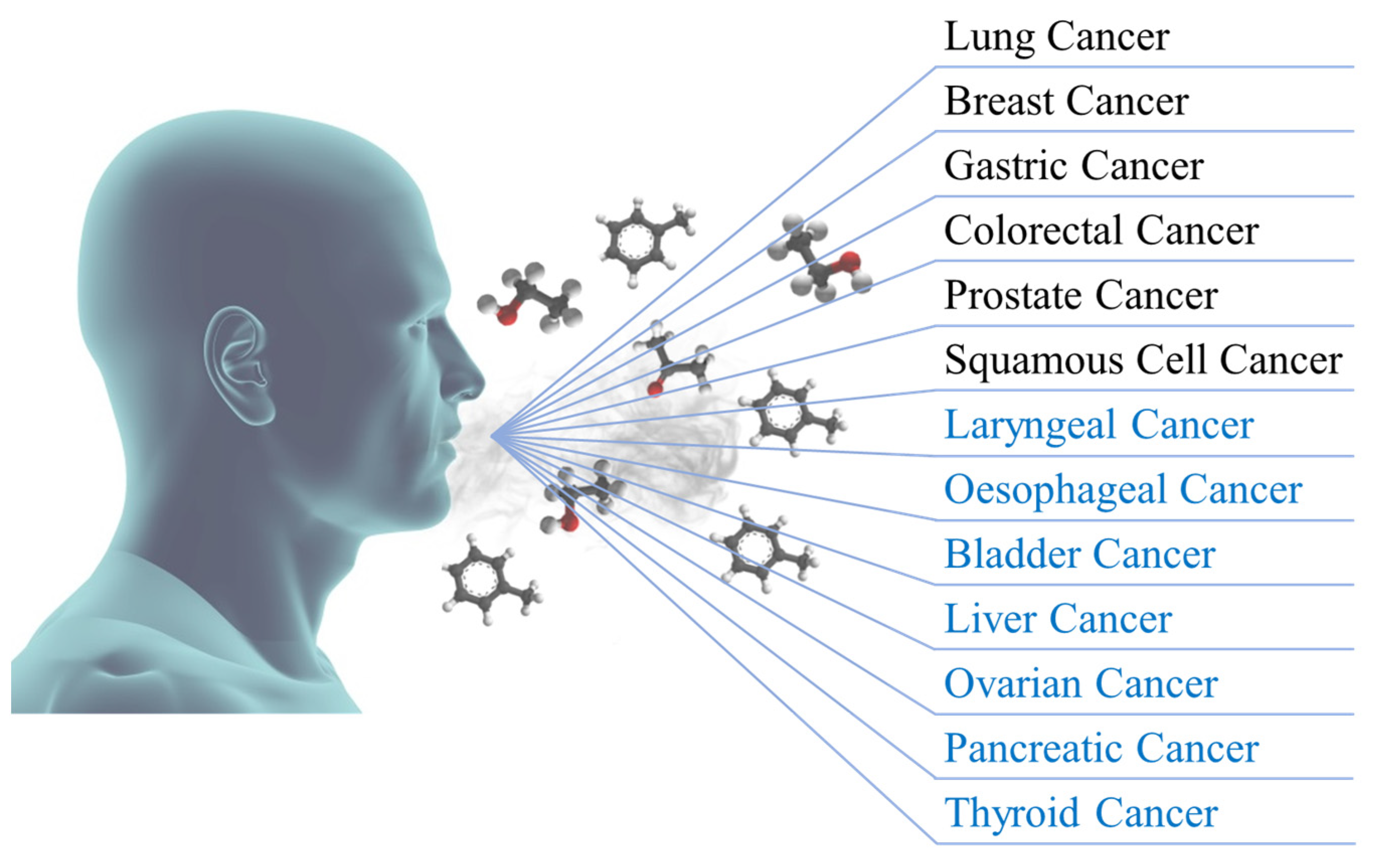
:1. Introduction
2. Carcinogenic Biomarkers from Breath
2.1. Lung Cancer
2.2. Breast Cancer
2.3. Gastric Cancer
2.4. Colorectal Cancer
2.5. Prostate Cancer
2.6. Squamous Cell Cancer
2.6.1. Laryngeal Cancer
2.6.2. Oesophageal Cancer
2.7. Pathologies with Research Potential
2.7.1. Bladder Cancer
2.7.2. Liver Cancer
2.7.3. Ovarian Cancer
2.7.4. Pancreatic Cancer
2.7.5. Thyroid Cancer
2.8. Current Limitations in Breath Biomarkers
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jernal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Cancer Facts and Statistics. 2023. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html (accessed on 26 June 2023).
- Cancer Facts and Statistics. 2013. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2013.html (accessed on 26 June 2023).
- Mendelsohn, J.; Ringborg, U.; Schilsky, R.L. Personalized cancer medicine—A strategy to counteract an increasing cancer challenge. Mol. Oncol. 2012, 6, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Daneshkhah, A.; Siegel, A.P.; Agarwal, M. Volatile organic compounds: Potential biomarkers for improved diagnosis and monitoring of diabetic wounds. In Wound Healing, Tissue Repair, and Regeneration Diabetes, 1st ed.; Bagchi, D., Das, A., Roy, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 491–512. [Google Scholar]
- Chatterjee, S.; Zetter, B.R. Cancer biomarkers: Knowing the present and predicting the future. Future Med. 2005, 1, 37–50. [Google Scholar] [CrossRef]
- Pauling, L.; Robinson, A.B.; Teranishi, R.; Cary, P. Quantitative analysis of urine vapor and breath by gas-liquid partition chromatography. Proc. Nat. Acad. Sci. USA 1971, 68, 2374–2376. [Google Scholar] [CrossRef]
- Hartwell, L.; Mankoff, D.; Paulovich, A.; Ramsey, S.; Swisher, E. Cancer biomarkers: A systems approach. Nat. Biotechnol. 2006, 24, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.; Peruzzi, P.P.; Lawler, S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol. Med. 2014, 20, 460–469. [Google Scholar] [CrossRef]
- Biomarker. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/biomarker (accessed on 26 June 2023).
- Henry, N.L.; Hayes, D.F. Cancer biomarkers. Mol. Oncol. 2012, 6, 140–146. [Google Scholar] [CrossRef]
- Bruice, P.Y. Organic Chemistry, 8th ed.; Pearson Education: New York, NY, USA, 2016. [Google Scholar]
- Solomons, T.W.G.; Fryhle, C.B. Organic Chemistry, 12th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2009. [Google Scholar]
- Ruzsanyi, V.; Sielemann, S.; Baumbach, J.I. Analysis of human breath using IMS. Int. J. Ion Mobil. Spectrom. 2005, 8, 5–7. [Google Scholar]
- Moura, P.C.; Vassilenko, V. Contemporary ion mobility spectrometry applications and future trends towards environmental, health and food research: A review. Int. J. Mass Spectrom. 2023, 486, 117012. [Google Scholar] [CrossRef]
- Turner, M.A.; Bandelow, S.; Edwards, L.; Patel, P.; Martin, H.J.; Wilson, I.D.; Thomas, C.L.P. The effect of a paced auditory serial addition test (PASAT) intervention on the profile of volatile organic compounds in human breath: A pilot study. J. Breath Res. 2013, 7, 017102. [Google Scholar] [CrossRef]
- Fiehn, O. Extending the breadth of metabolite profiling by gas chromatography coupled to mass spectrometry. Trends Anal. Chem. 2008, 27, 261–269. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, M.E.; Clutton-Brock, T.H.; Green, S.; Mayhew, C.A. Endogenous volatile organic compounds in breath and blood of healthy volunteers: Examining breath analysis as a surrogate for blood measurements. J. Breath Res. 2009, 3, 027005. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.; Spanel, P.; Smith, D. A longitudinal study of breath isoprene in healthy volunteers using selected ion flow tube mass spectrometry (SIFT-MS). Physiol. Meas. 2005, 27, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Natale, C.D.; Macagnano, A.; Martinelli, E.; Paolesse, R.; D’Arcangelo, G.; Rosciani, C.; Finazzi-Agrò, A.; D’Amico, A. Lung cancer identification by the analysis of breath by means of an array of non-selective gas sensors. Biosens. Bioelectron. 2003, 18, 1209–1218. [Google Scholar] [CrossRef]
- Amann, A.; Costello, B.L.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef] [PubMed]
- Moura, P.C.; Raposo, M.; Vassilenko, V. Breath Volatile Organic Compounds (VOCs) as Biomarkers for the Diagnosis of Pathological Conditions: A Review. Biomed. J. 2023, 46, 100623. [Google Scholar] [CrossRef]
- Amann, A.; Smith, D. Volatile Biomarkers: Non-Invasive Diagnosis in Physiology and Medicine, 1st ed.; Elsevier: Cambridge, MA, USA, 2013. [Google Scholar]
- Santos, P.; Moura, P.C.; Vassilenko, V. Suitability of Short- and Long-Term Storage of Volatile Organic Compounds Samples in Syringe-Based Containers: A Comparison Study. Metabolites 2023, 13, 903. [Google Scholar] [CrossRef]
- Risby, T.H.; Solga, S.F. Current status of clinical breath analysis. Appl. Phys. B 2006, 85, 421–426. [Google Scholar] [CrossRef]
- Miekisch, W.; Schubert, J.K.; Noeldge-Schomburg, F.E. Diagnostic potential of breath analysis--focus on volatile organic compounds. Clin. Chim. Acta 2004, 347, 25–39. [Google Scholar] [CrossRef]
- Focus Area: Biomarkers. Available online: https://www.fda.gov/science-research/focus-areas-regulatory-science-report/focus-area-biomarkers (accessed on 3 October 2023).
- Jones, A.W.; Andersson, L. Determination of ethanol in breath for legal purposes using a five-filter infrared analyzer: Studies on response to volatile interfering substances. J. Breath Res. 2008, 2, 026006. [Google Scholar] [CrossRef]
- Taylor, D.R. Advances in the clinical applications of exhaled nitric oxide measurements. J. Breath Res. 2012, 6, 047102. [Google Scholar] [CrossRef] [PubMed]
- Herman-Saffar, O.; Boger, Z.; Libson, S.; Lieberman, D.; Gonen, R.; Zeiri, Y. Early non-invasive detection of breast cancer using exhaled breath and urine analysis. Comput. Biol. Med. 2018, 96, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ke, C.; Wang, X.; Chi, C.; Guo, L.; Luo, S.; Guo, Z.; Xu, G.; Zhang, F.; Li, E. Noninvasive detection of colorectal cancer by analysis of exhaled breath. Anal. Bioanal. Chem. 2014, 406, 4757–4763. [Google Scholar] [CrossRef] [PubMed]
- Catalin, D.A.; Vasile, B.D.; Alina, D. Diagnostic Application of Volatile Organic Compounds as Potential Biomarkers for Detecting Digestive Neoplasia: A Systematic Review. Diagnostics 2021, 11, 2317. [Google Scholar]
- Vries, R.; Farzan, N.; Fabius, T.; Jongh, F.H.C.; Jack, P.M.C.; Haarman, R.G.; Snoey, E.; Veen, J.; Dagelet, Y.; Zee, A.; et al. Prospective Detection of Early Lung Cancer in Patients with COPD in Regular Care by Electronic Nose Analysis of Exhaled Breath. Chest 2023, 164, 1315–1324. [Google Scholar] [CrossRef]
- Peng, G.; Hakim, M.; Broza, Y.Y.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br. J. Cancer 2010, 103, 542–551. [Google Scholar] [CrossRef]
- Yazbeck, R.; Jaenisch, S.E.; Watson, D.I. From blood to breath: New horizons for esophageal cancer biomarkers. World J. Gastroenterol. 2016, 22, 10077–10083. [Google Scholar] [CrossRef]
- Cauchi, M.; Weber, C.M.; Bolt, B.J.; Spratt, P.B.; Bessant, C.; Turner, D.C.; Willis, C.M.; Britton, L.E.; Turner, C.; Morgan, G. Evaluation of gas chromatography mass spectrometry and pattern recognition for the identification of bladder cancer from urine headspace. Anal. Methods 2016, 8, 4037. [Google Scholar] [CrossRef]
- Stavropoulos, G.; Munster, K.; Ferrandino, G.; Sauca, M.; Ponsioen, C.; Schooten, F.J.; Smolinska, A. Liver Impairment—The Potential Application of Volatile Organic Compounds in Hepatology. Metabolites 2021, 11, 618. [Google Scholar] [CrossRef]
- Raspagliesi, F.; Bogani, G.; Benedetti, S.; Grassi, S.; Ferla, S.; Buratti, S. Detection of Ovarian Cancer through Exhaled Breath by Electronic Nose: A Prospective Study. Cancers 2020, 12, 2408. [Google Scholar] [CrossRef]
- Markar, S.R.; Brodie, B.; Chin, S.T.; Romano, A.; Spalding, D.; Hanna, G.B. Profile of exhaled-breath volatile organic compounds to diagnose pancreatic cancer. Br. J. Surg. 2018, 105, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, C.; Chi, C.; Wang, X.; Liu, S.; Zhao, W.; Ke, C.; Xu, G.; Li, E. Exhaled breath volatile biomarker analysis for thyroid cancer. Transl. Res. 2015, 166, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Chemical Abstract Service. Available online: https://www.cas.org/ (accessed on 3 October 2023).
- The Human Metabolome Database. Available online: https://hmdb.ca/ (accessed on 3 October 2023).
- Key Statistics for Lung Cancer. Available online: https://www.cancer.org/cancer/types/lung-cancer/about/key-statistics.html (accessed on 3 October 2023).
- Travis, W.D. Pathology of Lung Cancer. Clin. Chest Med. 2011, 32, 669–692. [Google Scholar] [CrossRef]
- Tanoue, L.T.; Tanner, N.T.; Gould, M.K.; Silvestri, G.A. Lung cancer screening. Am. J. Respir. Crit. Care Med. 2015, 191, 19–33. [Google Scholar] [CrossRef]
- Wakelee, H.; Chang, E.T.; Gomez, S.L.; Keegan, T.H.M.; Feskanich, D.; Clarke, C.A.; Holmberg, L.; Yong, L.C.; Kolonel, L.N.; Gould, M.K.; et al. Lung cancer incidence in never-smoking. J. Clin. Oncol. 2007, 25, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Thriumani, R.; Zakaria, A.; Hashim, Y.Z.; Jeffree, A.I.; Helmy, K.M.; Kamarudin, L.M.; Omar, I.; Shakaff, A.Y.; Adom, A.H.; Persaud, K.C. A study on volatile organic compounds emitted by in-vitro lung cancer cultured cells using gas sensor array and SPME-GCMS. BMC Cancer 2018, 18, 362. [Google Scholar] [CrossRef] [PubMed]
- Kischkel, S.; Miekisch, W.; Sawacki, A.; Straker, E.M.; Trefz, P.; Amann, A.; Schubert, J.K. Breath biomarkers for lung cancer detection and assessment of smoking related effects—Confounding variables, influence of normalization and statistical algorithms. Clin. Chim. Acta 2010, 411, 1637–1644. [Google Scholar] [CrossRef]
- Sponring, A.; Filipiak, W.; Ager, C.; Schubert, J.; Miekisch, W.; Amann, A.; Troppmair, J. Analysis of volatile organic compounds (VOCs) in the headspace of NCI-H1666 lung cancer cells. Cancer Biomark. 2010, 7, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Castro, M.; Feller, J.F. An e-nose made of carbon nanotube based quantum resistive sensors for the detection of eighteen polar/nonpolar VOC biomarkers of lung cancer. J. Mater. Chem. B 2013, 1, 4563. [Google Scholar] [CrossRef]
- Hou, C.; Lei, J.; Huo, D.; Song, K.; Li, J.; Luo, X.; Yang, M.; Fa, H. Discrimination of lung cancer related volatile organic compounds with a colorimetric sensor array. Anal. Lett. 2013, 46, 2048–2059. [Google Scholar] [CrossRef]
- Xu, H.; Wei, Y.; Zhu, L.; Huang, J.; Li, Y.; Liu, F.; Wang, S.; Liu, S. Bifunctional magnetic nanoparticles for analysis of aldehyde metabolites in exhaled breath of lung cancer patients. J. Chromatogr. A 2014, 1324, 29–35. [Google Scholar] [CrossRef]
- Gregis, G.; Sanchez, J.B.; Bezverkhyy, I.; Weber, G.; Berger, F.; Fierro, V.; Bellat, J.P.; Celzard, A. Detection and quantification of lung cancer biomarkers by a micro-analytical device using a single metal oxide-based gas sensor. Sens. Actuators B Chem. 2018, 255, 391–400. [Google Scholar] [CrossRef]
- Saalberg, Y.; Bruhns, H.; Wolff, M. Photoacoustic Spectroscopy for the Determination of Lung Cancer Biomarkers—A Preliminary Investigation. Sensors 2017, 17, 210. [Google Scholar] [CrossRef]
- Darwiche, K.; Baumbach, J.I.; Sommerwerck, U.; Teschler, H.; Freitag, L. Bronchoscopically Obtained Volatile Biomarkers in Lung Cancer. Lung 2011, 189, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P.J.; Wang, X.F.; Xu, Y.; Mekhail, T.; Beukemann, M.C.; Na, J.; Kemling, W.J.; Suslick, K.S.; Sasidhar, M. Exhaled Breath Analysis with a Colorimetric Sensor Array for the Identification and Characterization of Lung Cancer. J. Thorac. Oncol. 2012, 7, 137–142. [Google Scholar] [CrossRef]
- Santonico, M.; Lucantoni, G.; Pennazza, G.; Capuano, R.; Galluccio, G.; Roscioni, C.; Delfa, G.L.; Consoli, D.; Martinelli, E.; Paolesse, R.; et al. In situ detection of lung cancer volatile fingerprints using bronchoscopic air-sampling. Lung Cancer 2012, 77, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Schmekel, B.; Winquist, F.; Vikström, A. Analysis of breath samples for lung cancer survival. Anal. Chim. Acta 2014, 840, 82–86. [Google Scholar] [CrossRef]
- Li, M.; Yang, D.; Brock, G.; Knipp, R.J.; Bousamra, M.; Nantz, M.H.; Fu, X.A. Breath carbonyl compounds as biomarkers of lung cancer. Lung Cancer 2015, 90, 92–97. [Google Scholar] [CrossRef]
- Phillips, M.; Bauer, T.L.; Cataneo, R.N.; Lebauer, C.; Mundada, M.; Pass, H.I.; Ramakrishna, N.; Rom, W.N.; Vallières, E. Blinded Validation of Breath Biomarkers of Lung Cancer, a Potential Ancillary to Chest CT Screening. PLoS ONE 2015, 10, e0142484. [Google Scholar] [CrossRef]
- Callol-Sanchez, L.; Munoz-Lucas, M.A.; Gomez-Martin, O.; Maldonado-Sanz, J.A.; Civera-Tejuca, A.; Gutierrez-Ortega, C.; Rodriguez-Trigo, G.; Jareno-Esteban, J. Observation of nonanoic acid and aldehydes in exhaled breath of patients with lung cancer. J. Breath Res. 2017, 11, 026004. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, Y.; Sun, C.; Liu, H.; Wang, Y.; Jiang, X. Analysis of volatile organic compounds from patients and cell lines for the validation of lung cancer biomarkers by proton-transfer-reaction mass spectrometry. Anal. Methods 2019, 11, 3188–3197. [Google Scholar] [CrossRef]
- Saidi, T.; Moufid, M.; Beleño-Saenz, K.J.; Welearegay, T.G.; Bari, N.E.; Jaimes-Mogollon, A.L.; Ionescu, R.; Bourkadi, J.E.; Benamor, J.; Ftouh, M.E.; et al. Non-invasive prediction of lung cancer histological types through exhaled breath analysis by UV-irradiated electronic nose and GC/QTOF/MS. Sens. Actuators B Chem. 2020, 311, 127932. [Google Scholar] [CrossRef]
- Fuchs, P.; Loeseken, C.; Schubert, J.K.; Mieksich, W. Breath gas aldehydes as biomarkers of lung cancer. Int. J. Cancer 2010, 126, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Poli, D.; Goldoni, M.; Corradi, M.; Acampa, O.; Carbognani, P.; Internullo, E.; Casalini, A.; Mutti, A. Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber-derivatisation SPME-GC/MS. J. Chromatogr. B 2010, 878, 2643–2651. [Google Scholar] [CrossRef]
- Rudnicka, J.; Kowalkowski, T.; Ligor, T.; Buszewski, B. Determination of volatile organic compounds as biomarkers of lung cancer by SPME-GC-TOF/MS and chemometrics. J. Chromatogr. B 2011, 879, 3360–3366. [Google Scholar] [CrossRef]
- Buszewski, B.; Ligor, T.; Jezierski, T.; Wenda-Piesik, A.; Walczak, M.; Rudnicka, J. Identification of volatile lung cancer markers by gas chromatography-mass spectrometry: Comparison with discrimination by canines. Anal. Bioanal. Chem. 2012, 404, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Ulanowska, A.; Kowalkowski, T.; Cieslinski, K. Investigation of lung cancer biomarkers by hyphenated separation techniques and chemometrics. Clin. Chem. Lab. Med. 2011, 50, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.A.; Li, M.; Knipp, R.J.; Nantz, M.H.; Bousamra, M. Noninvasive detection of lung cancer using exhaled breath. Cancer Med. 2014, 3, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Li, X.; Chen, J.; Wang, H.; Cheng, T.; Chen, K.; Xu, S. Analysis of human breath samples of lung cancer patients and healthy controls with solid-phase microextraction (SPME) and flow-modulated comprehensive two-dimensional gas chromatography (GC-GC). Anal. Methods 2014, 6, 6841–6849. [Google Scholar] [CrossRef]
- Handa, H.; Usuba, A.; Maddula, S.; Baumbach, J.I.; Mineshita, M.; Miyazawa, T. Exhaled Breath Analysis for Lung Cancer Detection Using Ion Mobility Spectrometry. PLoS ONE 2014, 9, e114555. [Google Scholar] [CrossRef]
- Ligor, T.; Pater, L.; Buszewski, B. Application of an artificial neural network model for selection of potential lung cancer biomarkers. J. Breath Res. 2015, 9, 027106. [Google Scholar] [CrossRef] [PubMed]
- Jouyban, A.; Djozan, D.; Mohammadandashti, P.; Alizadeh-Nabil, A.; Ghorbanpour, H.; Khoubnasabjafari, M.; Mohammadzadeh, M. Co-liquefaction with acetone and GC analysis of volatile compounds in exhaled breath as lung cancer biomarkers. Bioimpacts 2014, 7, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Key Statistics for Breast Cancer. Available online: https://www.cancer.org/cancer/types/breast-cancer/about/how-common-is-breast-cancer.html (accessed on 3 October 2023).
- Breast Cancer Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer (accessed on 3 October 2023).
- Benson, J.R.; Jatoi, I.; Keisch, M.; Esteva, F.J.; Makris, A.; Jordan, V.C. Early breast cancer. Lancet 2009, 373, 1463–1479. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. J. Am. Med. Assoc. 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Warner, E. Breast-Cancer Screening. N. Engl. J. Med. 2011, 365, 1025–1032. [Google Scholar] [CrossRef]
- Grimm, L.J.; Avery, C.S.; Hendrick, E.; Baker, J.A. Benefits and Risks of Mammography Screening in Women Ages 40 to 49 Years. J. Prim. Care Community Health 2022, 13, 21501327211058322. [Google Scholar] [CrossRef]
- Silva, C.L.; Passos, M.; Câmara, J.S. Solid phase microextraction, mass spectrometry and metabolomic approaches for detection of potential urinary cancer biomarkers—A powerful strategy for breast cancer diagnosis. Talanta 2012, 89, 360–368. [Google Scholar] [CrossRef]
- Byrnes, R.; Phillips, M.; Cataneo, R.N.; Chaturvedi, A.; Kaplan, P.D.; Libardoni, M.; Mehta, V.; Mundada, M.; Patel, U.; Ramakrishna, N.; et al. Detection of volatile biomarkers of therapeutic radiation in breath. J. Breath Res. 2013, 7, 036002. [Google Scholar]
- Taunk, K.; Taware, R.; More, T.H.; Porto-Figueira, P.; Pereira, J.A.M.; Mohapatra, R.; Soneji, D.; Câmara, J.S.; Nagarajaram, H.A.; Rapole, S. A non-invasive approach to explore the discriminatory potential of the urinary volatilome of invasive ductal carcinoma of the breast. RSC Adv. 2018, 8, 25040. [Google Scholar] [CrossRef]
- Woollam, M.; Teli, M.; Angarita-Rivera, P.; Liu, S.; Siegel, A.P.; Yokota, H.; Agarwal, M. Detection of Volatile Organic Compounds (VOCs) in Urine via Gas Chromatography—Mass Spectrometry QTOF to Differentiate Between Localized and Metastatic Models of Breast Cancer. Sci. Rep. 2019, 9, 2526. [Google Scholar] [CrossRef]
- Barash, O.; Zhang, W.; Halpern, J.M.; Hua, Q.L.; Pan, Y.Y.; Kayal, H.; Khoury, K.; Liu, H.; Davies, M.P.A.; Haick, H. Differentiation between genetic mutations of breast cancer by breath volatolomics. Oncotarget 2015, 6, 44864–88476. [Google Scholar] [CrossRef]
- Lavra, L.; Catini, A.; Ulivieri, A.; Capuano, R.; Salehi, L.B.; Sciacchitano, S.; Bartolazzi, A.; Nardis, S.; Paolesse, R.; Martinelli, E.; et al. Investigation of VOCs associated with different characteristics of breast cancer cells. Sci. Rep. 2015, 5, 13246. [Google Scholar] [CrossRef] [PubMed]
- Arshad, A.Z.; Munajat, Y.; Ibrahim, R.K.R.; Hamdan, S.; Mahmood, N.H. Volatolomics analysis using FTIR spectroscopy for breast cancer identification in vitro. In Proceedings of the 2014 IEEE Conference on Biomedical Engineering and Sciences (IECBES), Kuala Lumpur, Malaysia, 8–10 December 2014. [Google Scholar]
- Silva, C.L.; Perestrelo, R.; Silva, P.; Tomás, H.; Câmara, J.S. Volatile metabolomic signature of human breast cancer cell lines. Sci. Rep. 2017, 7, 43969. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Beatty, J.D.; Cataneo, R.N.; Huston, J.; Kaplan, P.D.; Lalisang, R.I.; Lambin, P.; Lobbes, M.B.I.; Mundada, M.; Pappas, N.; et al. Rapid Point-Of-Care Breath Test for Biomarkers of Breast Cancer and Abnormal Mammograms. PLoS ONE 2014, 9, e90226. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Peng, Y.; Liu, Y.; Li, W.; Jin, Y.; Tang, Z.; Duan, Y. Investigation of potential breath biomarkers for the early diagnosis of breast cancer using gas chromatography-mass spectrometry. Clin. Chim. Acta 2014, 436, 59–67. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.; Lebauer, C.; Mundada, M.; Saunders, C. Breath mass ion biomarkers of breast cancer. J. Breath Res. 2017, 11, 016004. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Cruz-Ramos, J.A.; Huston, J.; Ornelas, O.; Pappas, N.; Pathak, S. Prediction of breast cancer risk with volatile biomarkers in breath. Breast Cancer Res. Treat. 2018, 170, 343–350. [Google Scholar] [CrossRef]
- Yang, H.Y.; Wang, Y.C.; Peng, H.Y.; Huang, C.H. Breath biopsy of breast cancer using sensor array signals and machine learning analysis. Sci. Rep. 2021, 11, 103. [Google Scholar] [CrossRef]
- León-Martínez, L.D.; Rodríguez-Aguilar, M.; Gorocica-Rosete, P.; Domínguez-Reyes, C.A.; Martínez-Bustos, V.; Tenorio-Torres, J.A.; Ornelas-Rebolledo, O.; Cruz-Ramos, J.A.; Balderas-Segura, B.; Flores-Ramírez, R. Identification of profiles of volatile organic compounds in exhaled breath by means of an electronic nose as a proposal for a screening method for breast cancer: A case-control study. J. Breath Res. 2020, 14, 046009. [Google Scholar] [CrossRef]
- Rodríguez-Aguilar, M.; León-Martínez, L.D.; Gorocica-Rosete, P.; Pérez-Padilla, R.; Domínguez-Reyes, C.A.; Tenorio-Torres, J.A.; Ornelas-Rebolledo, O.; Mehta, G.; Zamora-Mendoza, B.N.; Flores-Ramírez, R. Application of chemoresistive gas sensors and chemometric analysis to differentiate the fingerprints of global volatile organic compounds from diseases. Preliminary results of COPD, lung cancer and breast cancer. Clin. Chim. Acta 2021, 518, 83–92. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Saunders, C.; Hope, P.; Schmitt, P.; Wai, J. Volatile biomarkers in the breath of women with breast cancer. J. Breath Res. 2010, 4, 026003. [Google Scholar] [CrossRef]
- Mandy, M.; Cornelia, F.; Malgorzata, L.; Oliver, S.; Achim, S.; Dorothee, S. Volatile organic compounds (VOCs) in exhaled breath of patients with breast cancer in a clinical setting. Ginekol. Pol. 2012, 83, 730–736. [Google Scholar]
- Xu, Y.; Lee, H.; Hu, Y.; Huang, J.; Kim, S.; Yun, M. Detection and Identification of Breast Cancer Volatile Organic Compounds Biomarkers Using Highly-Sensitive Single Nanowire Array on a Chip. J. Biomed. Nanotechnol. 2013, 9, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, Y.; Luo, Z.; Duan, Y. Investigation of biomarkers for discriminating breast cancer cell lines from normal mammary cell lines based on VOCs analysis and metabolomics. RSC Adv. 2016, 6, 41816–41824. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.; Qiu, Z.; Lv, Y.; Chen, G.; Li, E. Early diagnosis of breast cancer from exhaled breath by gas chromatography-mass spectrometry (GC/MS) analysis: A prospective cohort study. J. Clin. Lab. Anal. 2020, 34, e23526. [Google Scholar] [CrossRef]
- Gahleitner, F.; Guallar-Hoyas, C.; Beardsmore, C.S.; Pandaya, H.C.; Thomas, C.P. Metabolomics pilot study to identify volatile organic compounds markers of childhood asthma in exhaled breath. Bioanalysis 2013, 5, 2239–2247. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Wu, S.; Hua, Q.; Bao, C.; Liu, H. Volatile Organic Compounds in Human Exhaled Breath to Diagnose Gastrointestinal Cancer: A Metal-Analysis. Front. Oncol. 2021, 11, 606915. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Huang, J.; Cushnir, J.R.; Spanel, P.; Smith, D.; Hanna, G.B. Selected Ion Flow Tube-MS Analysis of Headspace Vapor from Gastric Content for the Diagnosis of Gastro-Esophageal Cancer. Anal. Chem. 2012, 84, 9550–9557. [Google Scholar] [CrossRef] [PubMed]
- Catalano, V.; Labianca, R.; Beretta, G.D.; Gatta, G.; Braud, F.; Cutsem, E. Gastric Cancer. Crit. Rev. Oncol./Hematol. 2009, 71, 127–164. [Google Scholar] [CrossRef]
- Key Statistics About Stomach Cancer. Available online: https://www.cancer.org/cancer/types/stomach-cancer/about/key-statistics.html (accessed on 3 October 2023).
- Bel’skaya, L.V.; Sarf, E.A.; Shalygin, S.P.; Postnova, T.V.; Kosenok, V.K. Identification of salivary volatile organic compounds as potential markers of stomach and colorectal cancer: A pilot study. J. Oral Biosci. 2020, 62, 212–221. [Google Scholar] [CrossRef]
- Schuermans, V.N.E.; Li, Z.; Jongen, A.; Wu, Z.; Shi, J.; Ji, J.; Bouvy, N.D. Pilot Study: Detection of Gastric Cancer From Exhaled Air Analyzed with an Electronic Nose in Chinese Patients. Surg. Innov. 2018, 25, 429–434. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, G.; Liu, H.; Fu, H.; Fan, J.; Wang, K.; Chen, Y.; Li, B.; Zhang, C.; Zhi, X.; et al. Identification of Volatile Biomarkers of Gastric Cancer Cells and Ultrasensitive Electrochemical Detection based on Sensing Interface of Au-Ag Alloy coated MWCNTs. Theranostics 2014, 4, 154–162. [Google Scholar] [CrossRef]
- Xu, Z.; Broza, Y.Y.; Ionsecu, R.; Tisch, U.; Ding, L.; Liu, H.; Song, Q.; Pan, Y.; Xiong, F.; Gu, K.; et al. A nanomaterial-based breath test for distinguishing gastric cancer from benign gastric conditions. Br. J. Cancer 2013, 108, 941–950. [Google Scholar] [CrossRef]
- Amal, H.; Leja, M.; Funka, K.; Skarpars, R.; Sivins, A.; Ancans, G.; Liepniece-Karele, I.; Kikuste, I.; Lasina, I.; Haick, H. Detection of precancerous gastric lesions and gastric cancer through exhaled breath. Gut 2016, 65, 400–407. [Google Scholar] [CrossRef]
- Jung, Y.J.; Seo, H.S.; Kim, J.H.; Song, K.Y.; Park, C.H.; Lee, H.H. Advanced Diagnostic Technology of Volatile Organic Compounds Real Tiem Analysis From Exhaled Breath of Gastric Cancer Patients Using Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry. Front. Oncol. 2021, 11, 560591. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Che, X.; Su, H.; Mai, Z.; Huang, Z.; Huang, W.; Chen, W.; Liu, S.; Gao, W.; Zhou, Z.; et al. Exhaled breath analysis using on-line preconcentration mass spectrometry for gastric cancer diagnosis. J. Mass Spectrom. 2021, 56, e4588. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Pan, F.; Liu, J.; Wang, K.; Zhang, C.; Cheng, S.; Lu, L.; Zhang, W.; Zhang, Z.; et al. Breath Analysis Based on Surface-Enhanced Raman Scattering Sensors Distinguishes Early and Advanced Gastric Cancer Patients from Healthy Persons. ACS Nano 2016, 10, 8169–8179. [Google Scholar] [CrossRef]
- Key Statistics for Colorectal Cancer. Available online: https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html (accessed on 3 October 2023).
- Mármol, I.; Sánchez-de-Diego, C.; Dieste, A.P.; Cerrada, E.; Yoldi, M.J.R. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef]
- Markowitz, S.D.; Bertagnolli, M.M. Molecular Basis of Colorectal Cancer. N. Engl. J. Med. 2009, 361, 2449–2460. [Google Scholar] [CrossRef]
- Oakley-Girvan, I.; Davis, S.W. Breath based volatile organic compounds in the detection of breast, lung, and colorectal cancers: A systematic review. Cancer Biomark. 2017, 21, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Lena, M.; Porcelli, F.; Altomare, D.F. Volatile organic compounds as new biomarkers for colorectal cancer: A review. Colorectal Dis. 2016, 18, 654–663. [Google Scholar] [CrossRef]
- Altomare, D.F.; Lena, M.D.; Porcelli, F.; Trizio, L.; Travaglio, E.; Tutino, M.; Dragonieri, S.; Memeo, V.; Gennaro, G. Exhaled volatile organic compounds identify patients with colorectal cancer. Br. J. Surg. 2013, 100, 144–150. [Google Scholar] [CrossRef]
- Altomare, D.F.; Lena, M.D.; Porcelli, F.; Travaglio, E.; Longobardi, F.; Tutino, M.; Depalma, N.; Tedesco, G.; Sardaro, A.; Memeo, R.; et al. Effects of Curative Colorectal Cancer Surgery on Exhaled Volatile Organic Compounds and Potential Implications in Clinical Follow-up. Ann. Surg. 2015, 262, 862–867. [Google Scholar] [CrossRef]
- Altomare, D.F.; Picciariello, A.; Rotelli, M.T.; Fazio, M.D.; Aresta, A.; Zambonin, C.G.; Vincenti, L.; Trerotoli, P.; Vietro, N.D. Chemical signature of colorectal cancer: Case-control study for profiling the breath print. BJS Open 2020, 4, 1189–1199. [Google Scholar] [CrossRef]
- Amal, H.; Leja, M.; Funka, K.; Lasina, I.; Skapars, R.; Sivins, A.; Ancans, G.; Kikuste, I.; Vanags, A.; Tolmanis, I.; et al. Breath testing as potential colorectal cancer screening tool. Int. J. Cancer 2016, 138, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Keulen, K.E.; Jansen, M.E.; Schrauwen, R.W.M.; Kolkman, J.M.; Siersema, P.D. Volatile organic compounds in breath can serve as a non-invasive diagnostic biomarker for the detection of advanced adenomas and colorectal cancer. Aliment. Pharmacol. Ther. 2020, 51, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Vietro, N.D.; Aresta, A.; Rotelli, M.T.; Zambonin, C.; Lippolis, C.; Picciariello, A.; Altomare, D.F. Relationship between cancer tissue derived and exhaled volatile organic compound from colorectal cancer patients. Preliminary results. J. Pharm. Biomed. Anal. 2020, 180, 113055. [Google Scholar] [CrossRef] [PubMed]
- About Prostate Cancer. Available online: https://prostatecanceruk.org/prostate-information-and-support/risk-and-symptoms/about-prostate-cancer (accessed on 3 October 2023).
- Key Statistics for Prostate Cancer. Available online: https://www.cancer.org/cancer/types/prostate-cancer/about/key-statistics.html (accessed on 3 October 2023).
- Asimakopoulos, A.D.; Fabbro, D.D.; Miano, R.; Santonico, M.; Capuano, R.; Pennazza, G.; D’Amico, A.; Finazzi-Agrò, E. Prostate cancer diagnosis through electronic nose in the urine headspace setting: A pilot study. Prostate Cancer Prostatic Dis. 2014, 17, 206–211. [Google Scholar] [CrossRef]
- Grönberg, H. Prostate cancer epidemiology. Lancet 2003, 361, 859–864. [Google Scholar] [CrossRef]
- Deev, V.; Solovieva, S.; Andreev, E.; Protoshchak, V.; Karpushchenko, E.; Sleptsov, A.; Kartsova, L.; Bessonova, E.; Legin, A.; Kirsanov, D. Prostate cancer screening using chemometric processing of GC-MS profiles obtained in the headspace above urine samples. J. Chromatogr. B 2020, 1155, 122298. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Pacheco, A.; Salinero-Bachiller, M.; Iribar, M.C.; López-Luque, A.; Miján-Ortiz, J.L.; Peinado, J.M. Furan and p-xylene as candidate biomarkers for prostate cancer. Urol. Oncol. 2018, 36, 243.e21–243.e27. [Google Scholar] [CrossRef]
- Waltman, C.G.; Marcelissen, T.A.T.; Roermund, J.G.H. Exhaled-breath Testing for Prostate Cancer Based on Volatile Organic Compound Profiling Using an Electronic Nose Device (Aeonose): A Preliminary Report. Eur. Urol. Focus 2020, 6, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.S.; Fill, E.; Strittmatter, F.; Volz, Y.; Sroka, R.; Apolonski, A. Towards reliable diagnostics of prostate cancer via breath. Sci. Rep. 2021, 11, 18381. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef]
- Grénman, R.; Chevalier, D.; Gregoire, V.; Myers, E.; Rogers, S. Treatment of head and neck cancer in the elderly. Eur. Arch. Oto-Rhino-L. 2010, 267, 1619–1621. [Google Scholar] [CrossRef]
- Bouza, M.; Gonzalez-Soto, J.; Pereiro, R.; Vicente, J.C.; Sanz-Medel, A. Exhaled breath and oral cavity VOCs as potential biomarkers in oral cancer patients. J. Breath Res. 2017, 11, 016015. [Google Scholar] [CrossRef]
- Lang, H.P.; Loizeau, F.; Hiou-Feige, A.; Rivals, J.P.; Romero, P.; Akiyama, T.; Gerber, C.; Meyer, E. Piezoresistive Membrane Surface Stress Sensors for Characterization of Breath Samples of Head and Neck Cancer Patients. Sensors 2016, 16, 1149. [Google Scholar] [CrossRef]
- Konings, E.; Stappers, S.; Geens, M.; Winter, B.Y.; Lamote, K.; Meerbeeck, J.P.; Specenier, P.; Vanderveken, O.M.; Ledeganck, K.J. A Literature Review of the Potential Diagnostic Biomarkers of Head and Neck Neoplasms. Front. Oncol. 2020, 10, 1020. [Google Scholar] [CrossRef]
- Shigeyama, H.; Wang, T.; Ichinose, M.; Ansai, T.; Lee, S.W. Identification of volatile metabolites in human saliva from patients with oral squamous cell carcinoma via zeolite-based thin-film microextraction coupled with GC-MS. J. Chromatogr. B 2019, 1104, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Leunis, N.; Boumans, M.L.; Kremer, M.B.; Din, S.; Stobberingh, E.; Kessels, A.G.H.; Kross, K.W. Application of an Electronic Nose in the Diagnosis of Head and Neck Cancer. Laryngoscope 2014, 124, 1377–1381. [Google Scholar] [CrossRef]
- Goor, R.; Leunis, N.; Hooren, M.; Francisca, E.; Masclee, A.; Kremer, B.; Kross, K.W. Feasibility of electronic nose technology for discriminating between head and neck, bladder, and colon carcinomas. Eur. Arch. Oto-Rhino-L. 2017, 274, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Goor, R.; Hardy, J.; Hooren, M.; Kremer, B.; Kross, K. Detecting recurrent head and neck cancer using electronic nose technology: A feasibility study. Head Neck-J. Sci. Spec. 2019, 41, 2983–2990. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, S.; Raguse, J.D.; Pfitzner, D.; Preissner, R.; Paris, S.; Preissner, S. Volatile Organic Compounds in the Breath of Oral Squamous Cell Carcinoma Patients: A Pilot Study. Otolaryngol. Head Neck Surg. 2017, 157, 981–987. [Google Scholar] [CrossRef]
- Optiz, P.; Herbarth, O. The volatilome—Investigation of volatile organic metabolites (VOM) as potential tumor markers in patients with head and neck squamous cell carcinoma (HNSCC). Otolaryngol. Head Neck Surg. 2018, 47, 42. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, C.; Zou, X.; Lu, Y.; Shen, C.; Ding, X.; Wang, H.; Jiang, H.; Chu, Y. Exhaled breath online measurement for cervical cancer patients and healthy subjects by proton transfer reaction mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 5603–5612. [Google Scholar] [CrossRef]
- Hakim, M.; Billan, S.; Tisch, U.; Peng, G.; Dvrokind, I.; Marom, O.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosis of head-and-neck cancer from exhaled breath. Br. J. Cancer 2011, 104, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Taware, R.; Taunk, K.; Pereira, J.A.M.; Shirolkar, M.; Soneji, D.; Câmara, J.S.; Nagarajaram, H.A.; Rapole, S. Volatilomic insight of head and neck cancer via the effects observed on saliva metabolites. Sci. Rep. 2018, 8, 17725. [Google Scholar] [CrossRef]
- Chandran, D.; Ooi, E.H.; Watson, D.I.; Kholmurodova, F.; Jaenisch, S.; Yazbeck, R. The Use of Selected Ion Flow Tube-Mass Spectrometry Technology to Identify Breath Volatile Organic Compounds for the Detection of Head and Neck Squamous Cell Carcinoma: A Pilot Study. Medicina 2019, 55, 306. [Google Scholar] [CrossRef]
- Gruber, M.; Tisch, U.; Jeries, R.; Amal, H.; Hakim, M.; Ronen, O.; Marshak, T.; Zimmerman, D.; Israel, O.; Amiga, E.; et al. Analysis of exhaled breath for diagnosing head and neck squamous cell carcinoma: A feasibility study. Br. J. Cancer 2014, 111, 790–798. [Google Scholar] [CrossRef]
- Santos, P.H.C.; Vassilenko, V.B.; Moura, P.C.; Conduto, C.; Fernandes, J.M.; Bonifácio, P. Instrumentation for differentiation of exhaled breath. In Proceedings of the SPIE 12126, Fifteenth International Conference on Correlation Optics, Chernivtsi, Ukraine, 13–16 September 2021; p. 121262L. [Google Scholar]
- Pereira, J.A.; Porto-Figueira, P.; Taware, R.; Sukul, P.; Rapole, S.; Câmara, J.S. Unravelling the Potential of Salivary Volatile Metabolites in Oral Diseases. A Review. Molecules 2020, 25, 3098. [Google Scholar] [CrossRef]
- Chu, E.A.; Kim, Y.J. Laryngeal Cancer: Diagnosis and Preoperative Work-up. Otolaryngol. Clin. N. Am. 2008, 41, 673–695. [Google Scholar] [CrossRef]
- Obid, R.; Redlich, M.; Tomeh, C. The Treatment of Laryngeal Cancer. Oral Maxillofac. Surg. Clin. 2019, 31, 1–11. [Google Scholar] [CrossRef]
- García, R.A.; Morales, V.; Martín, S.; Vilches, E.; Toledano, A. Volatile Organic Compounds Analysis in Breath Air in Healthy Volunteers and Patients Suffering Epidermoid Laryngeal Carcinomas. Chromatographia 2014, 77, 501–509. [Google Scholar] [CrossRef]
- Shoffel-Havakuk, H.; Frumin, I.; Lahav, Y.; Haviv, L.; Sobel, N.; Halperin, D. Increased Number of Volatile Organic Compounds Over Malignant Glottic Lesions. Laryngoscope 2016, 126, 1606–1611. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.; Vassilenko, V.; Conduto, C.; Fernandes, J.M.; Moura, P.C.; Bonifácio, P. Pilot Study for Validation and Differentiation of Alveolar and Esophageal Air. In Technological Innovation for Applied AI Systems. DoCEIS 2021. IFIP Advances in Information and Communication Technology; Springer International Publishing: Cham, Switzerland, 2021; pp. 331–338. [Google Scholar]
- Alsop, B.R.; Sharma, P. Esophageal Cancer. Gastroenterol. Clin. 2016, 45, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhou, W.; Lu, Y.; Shen, C.; Hu, Z.; Wang, H.; Jiang, H.; Chu, Y. Exhaled gases online measurements for esophageal cancer patients and healthy people by proton transfer reaction mass spectrometry. J. Gastroenterol. Hepatol. 2016, 31, 1837–1843. [Google Scholar] [CrossRef]
- Markar, S.R.; Wiggins, T.; Antonowicz, S.; Chin, S.T.; Romano, A.; Nikolic, K.; Evans, B.; Cunningham, D.; Mughal, M.; Lagergren, J.; et al. Assessment of a Noninvasive Exhaled Breath Test for the Diagnosis of Oesophagogastric Cancer. JAMA Oncol. 2018, 4, 970–976. [Google Scholar] [CrossRef]
- Kumar, S.; Huang, J.; Abbassi-Ghadi, N.; Spanel, P.; Smith, D.; Hanna, G.B. Selected Ion Flow Tube Mass Spectrometry Analysis of Exhaled Breath for Volatile Organic Compound Profiling of Esophago-Gastric Cancer. Anal. Chem. 2013, 85, 6121–6128. [Google Scholar] [CrossRef] [PubMed]
- Key Statistics for Bladder Cancer. Available online: https://www.cancer.org/cancer/types/bladder-cancer/about/key-statistics.html (accessed on 3 October 2023).
- Sanli, O.; Dobruch, J.; Knowles, M.A.; Burger, M.; Alemozaffar, M.; Nielsen, M.E.; Lotan, Y. Bladder cancer. Nat. Rev. Dis. Primers 2017, 3, 17022. [Google Scholar] [CrossRef] [PubMed]
- Jobu, K.; Sun, C.; Yoshioka, S.; Yokota, J.; Onogawa, M.; Kawada, C.; Inoue, K.; Shuin, T.; Sendo, T.; Miyamu, M. Metabolomics Study on the Biochemical Profiles of Odor Elements in Urine of Human with Bladder Cancer. Biol. Pharm. Bull. 2012, 35, 639–642. [Google Scholar] [CrossRef]
- Zhu, S.; Corsetti, S.; Wang, Q.; Li, C.; Huang, Z.; Nabi, G. Optical sensory arrays for the detection of urinary bladder cancer-related volatile organic compounds. J. Biophotonics 2018, 12, e201800165. [Google Scholar] [CrossRef] [PubMed]
- Gravitz, L. Liver cancer. Nature 2014, 516, S1. [Google Scholar] [CrossRef] [PubMed]
- Key Statistics About Liver Cancer. Available online: https://www.cancer.org/cancer/types/liver-cancer/about/what-is-key-statistics.html (accessed on 3 October 2023).
- Ferrandino, G.; Orf, I.; Smith, R.; Calcagno, M.; Thind, A.K.; Debiram-Beecham, I.; Williams, M.; Gandelman, O.; Saedeleer, A.; Kibble, G.; et al. Breath Biopsy Assessment of Liver Disease Using an Exogenous Volatile Organic Compound—Toward Improved Detection of Liver Impairment. Clin. Transl Gastroenterol. 2020, 11, e00239. [Google Scholar] [CrossRef] [PubMed]
- Amal, H.; Ding, L.; Liu, B.; Tisch, U.; Xu, Z.; Shi, D.; Zhao, Y.; Chen, J.; Sun, R.; Liu, H.; et al. The scent fingerprint of hepatocarcinoma: In-vitro metastasis prediction with volatile organic compounds (VOCs). Int. J. Nanomed. 2012, 7, 4135–4146. [Google Scholar]
- Bannaga, A.S.; Tyagi, H.; Daulton, H.; Covington, J.A.; Arasaradnam, R.P. Exploratory Study Using Urinary Volatile Organic Compounds for the Detection of Hepatocellular Carcinoma. Molecules 2021, 26, 2447. [Google Scholar] [CrossRef]
- Qin, T.; Liu, H.; Song, Q.; Song, G.; Wang, H.; Pan, Y.; Xiong, F.; Gu, K.; Sun, G.; Chen, Z. The screening of volatile markers for hepatocellular carcinoma. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2247–2253. [Google Scholar] [CrossRef]
- Miller-Atkins, G.; Acevedo-Moreno, L.A.; Grove, D.; Dweik, R.A.; Tonelli, A.R.; Brown, J.M.; Allende, D.S.; Aucejo, F.; Rotroff, D.M. Breath Metabolomics Provides an Accurate and Noninvasive Approach for Screening Cirrhosis, Primary, and Secondary Liver Tumors. Hepatol. Commun. 2020, 4, 1041–1055. [Google Scholar] [CrossRef]
- Key Statistics for Ovarian Cancer. Available online: https://www.cancer.org/cancer/types/ovarian-cancer/about/key-statistics.html (accessed on 3 October 2023).
- Lengyel, E. Ovarian Cancer Development and Metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Primers 2016, 2, 16061. [Google Scholar] [CrossRef] [PubMed]
- Niemi, R.J.; Roine, A.N.; Eräviita, E.; Kumpulainen, P.S.; Mäenpää, J.U.; Oksala, N. FAIMS analysis of urine gaseous headspace is capable of differentiating ovarian cancer. Gynecol. Oncol. 2018, 151, 519–524. [Google Scholar] [CrossRef]
- Amal, H.; Shi, D.Y.; Ionescu, R.; Zhang, W.; Hua, Q.L.; Pan, Y.Y.; Tao, L.; Liu, H.; Haick, H. Assessment of ovarian cancer conditions from exhaled breath. Int. J. Cancer 2015, 136, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Kahn, N.; Lavie, O.; Paz, M.; Segev, Y.; Haick, H. Dynamic Nanoparticle-Based Flexible Sensors: Diagnosis of Ovarian Carcinoma from Exhaled Breath. NANO Lett. 2015, 15, 7023–7028. [Google Scholar] [CrossRef] [PubMed]
- Maitra, A.; Hruban, R.H. Pancreatic Cancer. Ann. Rev. Pathol. 2008, 3, 157–188. [Google Scholar] [CrossRef] [PubMed]
- Kleeff, J.; Korc, M.; Apte, M.; Vecchia, C.L.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Arasaradnam, R.P.; Wicaksono, A.; O’Brien, H.; Kocher, H.M.; Covington, J.A.; Crnogorac-Jurcevic, T. Noninvasive Diagnosis of Pancreatic Cancer Through Detection of Volatile Organic Compounds in Urine. Gastroenterology 2018, 154, 485–487. [Google Scholar] [CrossRef]
- Daulton, E.; Wicaksono, A.N.; Tiele, A.; Kocher, H.M.; Debernardi, S.; Crnogorac-Jurcevic, T.; Covington, J.A. Volatile organic compounds (VOCs) for the non-invasive detection of pancreatic cancer from urine. Talanta 2021, 221, 121604. [Google Scholar] [CrossRef]
- Princivalle, A.; Monasta, L.; Butturini, G.; Bassi, C.; Perbellini, L. Pancreatic ductal adenocarcinoma can be detected by analysis of volatile organic compounds (VOCs) in alveolar air. BMC Cancer 2018, 15, 529. [Google Scholar] [CrossRef]
- Rahbari, R.; Zhang, L.; Kebebew, E. Thyroid cancer gender disparity. Future Oncol. 2010, 6, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Carling, T.; Udelsman, R. Thyroid Cancer. Annu. Rev. Med. 2014, 65, 125–137. [Google Scholar] [CrossRef]
- Bartolazzi, A.; Santonico, M.; Pennazza, G.; Martinelli, E.; Paolesse, R.; D’Amico, A.; Natale, C. A sensor array and GC study about VOCs and cancer cells. Sens. Actuators B Chem. 2010, 146, 483–488. [Google Scholar] [CrossRef]
| Volatile Organic Compounds | CAS Numbers | HMDB Numbers | References |
|---|---|---|---|
| Acetone | 67-64-1 | HMDB0001659 | [50,67,68,70] |
| Benzene | 71-43-2 | HMDB0001505 | [50,67] |
| Butanal | 123-72-8 | HMDB0003543 | [52,65,67] |
| Butane | 106-97-8 | --- | [72] |
| 2-Butanone | 78-93-3 | HMDB0000474 | [50,54,67,68,69] |
| Carbon disulphide | 75-15-0 | HMDB0036574 | [66,67] |
| Chloroform | 67-66-3 | HMDB0029596 | [50] |
| Cyclohexane | 110-82-7 | HMDB0029597 | [50,53] |
| Decane | 124-18-5 | HMDB0031450 | [50] |
| 2,4-Dimethylheptane | 2213-23-2 | --- | [72] |
| Dimethyl sulphide | 75-18-3 | HMDB0002303 | [68] |
| Ethanol | 64-17-5 | HMDB0000108 | [50,68] |
| Ethylbenzene | 100-41-4 | HMDB0059905 | [54,66,67] |
| Furan | 110-00-9 | HMDB0013785 | [67] |
| Heptanal | 111-71-7 | HMDB0031475 | [52,65] |
| Hexanal | 66-25-1 | HMDB0005994 | [51,52,54,64,65] |
| Hexene | 592-41-6 | --- | [50] |
| 2-Hydroxy acetaldehyde | 141-46-8 | HMDB0003344 | [69] |
| 3-Hydroxy-2-butanone | 513-86-0 | HMDB0003243 | [69] |
| 4-Hydroxyhexenal | 17427-21-3 | --- | [69] |
| Isoprene | 78-79-5 | HMDB0253673 | [50,51,54,70] |
| Isopropanol | 67-63-0 | HMDB0000863 | [50,66,67,68] |
| Methanol | 67-56-1 | HMDB0001875 | [50,70] |
| 2-Methylbutane | 78-78-4 | HMDB0253668 | [72] |
| 4-Methyloctane | 2216-34-4 | --- | [72] |
| Nonanal | 124-19-6 | HMDB0059835 | [52,64,65] |
| Octanal | 124-13-0 | HMDB0001140 | [52,64,65,73] |
| Pentanal | 110-62-3 | HMDB0031206 | [52,64,65] |
| Pentane | 109-66-0 | HMDB0029603 | [70] |
| 2-Pentanone | 107-87-9 | HMDB0034235 | [67,68,72] |
| Propanal | 123-38-6 | HMDB0003366 | [65,67,72] |
| Propane | 74-98-6 | HMDB0031630 | [50,72] |
| Propanol | 71-23-8 | HMDB0000820 | [50,53,54,67,68,70] |
| 2-Propenal | 107-02-8 | HMDB0041822 | [66,67] |
| Propene | 115-07-1 | HMDB0256839 | [66,72] |
| Styrene | 100-42-5 | HMDB0034240 | [50,51,54] |
| Toluene | 108-88-3 | HMDB0034168 | [50,53] |
| 1,2,4-Trimethylbenzene | 95-63-6 | HMDB0013733 | [50] |
| o-Xylene | 95-47-6 | HMDB0059851 | [50,53] |
| p-Xylene | 106-42-3 | HMDB0059924 | [51] |
| Volatile Organic Compounds | CAS Numbers | HMDB Numbers | References |
|---|---|---|---|
| Acetic Acid | 64-19-7 | HMDB0000042 | [84] |
| Acetophenone | 98-86-2 | HMDB0033910 | [97] |
| (+)-Aromadendrene | 489-39-4 | --- | [95] |
| Benzaldehyde | 100-52-7 | HMDB0006115 | [85] |
| 2-Butyloctanol | 3913-02-8 | HMDB0041288 | [95] |
| Caryophyllene | 87-44-5 | HMDB0036792 | [96] |
| Cyclohexanol | 108-93-0 | --- | [85,98] |
| Cyclohexanone | 108-94-1 | HMDB0003315 | [99] |
| Cyclopentane | 287-92-3 | --- | [84] |
| Cyclopentanone | 120-92-3 | HMDB0031407 | [99] |
| Decene | 872-05-9 | --- | [96] |
| 2,4-Dimethylbenzaldehyde | 15764-16-6 | HMDB0032142 | [85,98] |
| 1,3-Dimethylbenzene | 108-38-3 | HMDB0059810 | [84] |
| 2,2-Dimethylbutane | 75-83-2 | HMDB0245332 | [85] |
| 1,4-Dimethylcyclohexane | 589-90-2 | --- | [84] |
| 2,3-Dimethylhexane | 584-94-1 | HMDB0037617 | [85] |
| 2,6-Dimethyloctane | 2051-30-1 | --- | [99] |
| 2,4-Dimethylpentane | 108-08-7 | HMDB0245455 | [84] |
| 1,3-Di-ter-butylbenzene | 1014-60-4 | HMDB0061923 | [85] |
| 2,5-Ditert-butylcyclohexa-2,5-diene-1,4-dione | 2460-77-7 | --- | [95] |
| 2,6-Ditert-butylcyclohexa-2,5-diene-1,4-dione | 719-22-2 | HMDB0013817 | [95] |
| Dodecane | 112-40-3 | HMDB0031444 | [95] |
| 2-Dodecanone | 6175-49-1 | HMDB0031019 | [85] |
| Ethanol | 64-17-5 | HMDB0059905 | [84] |
| Ethyl acetate | 141-78-6 | HMDB0031217 | [87] |
| 1-Ethyl-3,5-dimethylbenzene | 934-74-7 | --- | [95] |
| Ethylene Carbonate | 96-49-1 | HMDB0252067 | [99] |
| Ethylidenecyclopropane | 18631-83-9 | --- | [95] |
| 2-Ethylhexanol | 104-76-7 | HMDB0031231 | [84,98,99] |
| Ethyl propanoate | 105-37-3 | HMDB0030058 | [87] |
| Ethyl-Tris(Trimethylsilyl)-Silicate | 18030-67-6 | --- | [95] |
| Heptanal | 111-71-7 | HMDB0031475 | [97] |
| Heptane | 142-82-5 | HMDB0031447 | [84,85] |
| 2-Heptanone | 110-43-0 | HMDB0003671 | [87] |
| Hexamethyldisilane | 1450-14-2 | --- | [99] |
| 2-Hexyloctanol | 19780-79-1 | --- | [95] |
| Isobutyric acid | 79-31-2 | HMDB0001873 | [85] |
| Isoprene | 78-79-5 | HMDB0253673 | [95] |
| Isopropanol | 67-63-0 | HMDB0000863 | [97] |
| Isopropylmyristate | 110-27-0 | HMDB0040392 | [97] |
| Limonene | 138-86-3 | HMDB0003375 | [95,100] |
| (+)-Longifolene | 475-20-7 | HMDB0302687 | [95] |
| Menthol | 89-78-1 | HMDB0003352 | [99] |
| Methanol | 67-56-1 | HMDB0001875 | [86] |
| 3-Methoxy-1,2-propanediol | 623-39-2 | --- | [99] |
| Methylacrylic acid | 79-41-4 | HMDB0254514 | [99] |
| 2-Methyl-1,2-bis(trimethylsiloxy)-propane | 6651-34-9 | --- | [99] |
| 2-Methylbutanoic acid | 116-53-0 | HMDB0002176 | [87] |
| 3-Methyl-3-butenol | 763-32-6 | HMDB0030126 | [87] |
| 4-Methyl-2-heptanone | 6137-06-0 | HMDB0013821 | [85] |
| 6-Methyl-5-hepten-2-one | 110-93-0 | HMDB0035915 | [84] |
| 3-Methylhexane | 589-34-4 | HMDB0245932 | [96] |
| (R)-1-Methyl-5-(1-methyl)cyclohexene | 1461-27-4 | --- | [95] |
| 3-Methylpyridine | 108-99-6 | HMDB0061887 | [99] |
| Naphthalene | 91-20-3 | HMDB0029751 | [96] |
| 2-Nonanone | 821-55-6 | HMDB0031266 | [85] |
| Octamethylcyclotetrasiloxane | 556-67-2 | --- | [95] |
| Pentadecane | 629-62-9 | HMDB0059886 | [95] |
| 1,4-Pentadiene | 591-93-5 | --- | [95] |
| 2-Pentanone | 107-87-9 | HMDB0034235 | [87] |
| 2-Phenyl-2-propanol | 617-94-7 | --- | [99] |
| Phenol | 108-95-2 | HMDB0000228 | [99] |
| α-Pinene | 80-56-8 | HMDB0006525 | [84] |
| 1,2-Propanediol | 57-55-6 | HMDB0001881 | [99] |
| 2-Propenoic acid | 79-10-7 | HMDB0031647 | [84] |
| Pyrrolidine | 123-75-1 | HMDB0031641 | [85] |
| Tetradecane | 629-59-4 | HMDB0059907 | [95] |
| 1,2,3,5-Tetramethylbenzene | 527-53-7 | HMDB0244133 | [95] |
| 1,2,4,5-Tetramethylbenzene | 95-93-2 | HMDB0244147 | [95] |
| Tetramethylsilicane | 75-76-3 | --- | [99] |
| 1,1,3,3-Tetramethylurea | 632-22-4 | HMDB0062789 | [99] |
| Toluene | 108-88-3 | HMDB0034168 | [84] |
| Trichlorethylene | 79-01-6 | HMDB0029593 | [96] |
| Tridecane | 629-50-5 | HMDB0034284 | [95] |
| Trifluoroacetic acid | 76-05-1 | HMDB0034284 | [95] |
| 2,6,11-Trimethyldodecane | 31295-56-4 | HMDB0302691 | [95] |
| 2,7,10-Trimethyldodecane | 74645-98-0 | HMDB0062790 | [95] |
| Undecane | 1120-21-4 | HMDB0031445 | [95] |
| p-Xylene | 106-42-3 | HMDB0059924 | [85,98] |
| Volatile Organic Compounds | CAS Numbers | HMDB Numbers | References |
|---|---|---|---|
| Acetic Acid | 64-19-7 | HMDB0000042 | [110] |
| Aceticamide | 60-35-5 | HMDB0031645 | [110] |
| Acetone | 67-64-1 | HMDB0001659 | [111,112] |
| 1,4-Butanediol | 110-63-4 | HMDB0244201 | [107] |
| 2-Butanone | 78-93-3 | HMDB0000474 | [107,109] |
| 4-Butoxybutanol | 4161-24-4 | --- | [107] |
| 2-Butoxyethanol | 111-76-2 | HMDB0031327 | [108,109] |
| 2,3-Dimethylpentane | 565-59-3 | --- | [112] |
| 1,3-Dioxolan-2-one | 96-49-1 | HMDB0252067 | [111] |
| Dodecane | 112-40-3 | HMDB0031444 | [112] |
| Ethylene | 74-85-1 | HMDB0029594 | [110] |
| Formic acid propylester | 110-74-7 | HMDB0040253 | [107] |
| Furfural | 98-01-1 | HMDB0032914 | [108,109] |
| Hexane | 110-54-3 | HMDB0029600 | [112] |
| Hexanol | 111-27-3 | HMDB0012971 | [112] |
| Isoprene | 78-79-5 | HMDB0253673 | [108,110,111,112] |
| 4-Isopropoxylbutanol | 42042-71-7 | --- | [107] |
| Menthol | 89-78-1 | HMDB0003352 | [112] |
| 6-Methyl-5-hepten-2-one | 110-93-0 | HMDB0035915 | [108] |
| 2-Methylhexane | 591-76-4 | HMDB0245230 | [112] |
| 3-Methylhexane | 589-34-4 | HMDB0245932 | [112] |
| Methylisobutylketone | 108-10-1 | HMDB0002939 | [110] |
| 4-Methyloctane | 2216-34-4 | --- | [109] |
| 2-Methylpentane | 107-83-5 | HMDB0061884 | [112] |
| 3-Methylpentane | 96-14-0 | HMDB0061885 | [112] |
| α-Methylstyrene | 98-83-9 | HMDB0059899 | [109] |
| Nonanol | 28473-21-4 | HMDB0031265 | [107] |
| 3-Octanone | 106-68-3 | --- | [107] |
| Phenol | 108-95-2 | HMDB0000228 | [111] |
| Phenyl acetate | 122-79-2 | HMDB0040733 | [111,112] |
| Pivalic acid | 75-98-9 | HMDB0041992 | [112] |
| Propanal | 123-38-6 | HMDB0003366 | [110] |
| 1,3-Propanediol | 504-63-2 | --- | [110] |
| 2-Propenenitrile | 107-13-1 | HMDB0247972 | [108,109] |
| Tetradecane | 629-59-4 | HMDB0059907 | [112] |
| Tolualdehyde | 1334-78-7 | HMDB0006236 | [110] |
| 1,2,3-Trimethylbenzene | 526-73-8 | HMDB0059901 | [109,111] |
| 1,3,5-Trimethylbenzene | 108-67-8 | HMDB0041924 | [110] |
| 2,6,11-Trimethyldodecane | 31295-56-4 | HMDB0302691 | [107] |
| m-Xylene | 108-38-3 | HMDB0059810 | [111] |
| Volatile Organic Compounds | CAS Numbers | HMDB Numbers | References |
|---|---|---|---|
| Acetone | 67-64-1 | HMDB0001659 | [121] |
| Benzaldehyde | 100-52-7 | HMDB0006115 | [120,123] |
| Benzoic acid | 65-85-0 | HMDB0001870 | [120] |
| Butanoic acid | 107-92-6 | HMDB0000039 | [123] |
| Butylatedhydroxytoluene | 128-37-0 | HMDB0033826 | [120] |
| 6-t-Butyl-2,2,9,9-tetramethyl-3,5-decadien-7-yne | --- | --- | [31] |
| Cyclohexane | 110-82-7 | HMDB0029597 | [118,119] |
| Cyclohexanone | 108-94-1 | HMDB0003315 | [31,119] |
| Cyclooctylmethanol | 3637-63-6 | --- | [31] |
| Decanal | 112-31-2 | HMDB0011623 | [118,119] |
| 1,3-Dimethylbenzene | 108-38-3 | HMDB0059810 | [118,119] |
| 1,4-Dimethylbenzene | 106-42-3 | HMDB0059924 | [118,119] |
| 2,2-Dimethyldecane | 17302-37-3 | HMDB0302690 | [31] |
| 6,10-Dimethyl-5,9-undecadien-2-one | 689-67-8 | HMDB0031846 | [120] |
| Dodecane | 112-40-3 | HMDB0031444 | [31,120] |
| Dodecanoic acid | 143-07-7 | HMDB0000638 | [123] |
| Ethanol | 64-17-5 | HMDB0000108 | [121] |
| Ethyl acetate | 141-78-6 | HMDB0031217 | [121] |
| Ethylaniline | 103-69-5 | HMDB0302429 | [31] |
| Ethylbenzene | 100-41-4 | HMDB0059905 | [120,123] |
| 4-Ethyl-1-octyn-3-ol | 5877-42-9 | --- | [31] |
| 3-Hydroxy-2,4,4-trimethylpentyl 2-methylpropanoate | 74367-34-3 | --- | [31] |
| Indole | 120-72-9 | HMDB0000738 | [123] |
| Methylbenzene | 108-88-3 | HMDB0034168 | [120,123] |
| 2-Methylbutane | 78-78-4 | HMDB0253668 | [118,119] |
| Methylcyclohexane | 108-87-2 | --- | [118,119] |
| Methylcyclopentane | 96-37-7 | HMDB0031542 | [118,119] |
| 4-Methyloctane | 2216-34-4 | --- | [118,121] |
| 2-Methylpentane | 107-83-5 | HMDB0061884 | [118] |
| 3-Methylpentane | 96-14-0 | HMDB0061885 | [118,119] |
| 4-methyl-2-pentanone | 108-10-1 | HMDB0002939 | [118,119] |
| 4-Methylundecane | 2980-69-0 | --- | [118] |
| Nonanal | 124-19-6 | HMDB0059835 | [118,119,123] |
| Octanoic acid | 124-07-2 | HMDB0040195 | [123] |
| 1,2-Pentadiene | 591-95-7 | --- | [118,119] |
| Pentanoic acid | 109-52-4 | HMDB0000892 | [123] |
| Phenol | 108-95-2 | HMDB0000228 | [123] |
| Tetradecane | 629-59-4 | HMDB0059907 | [120,123] |
| Trans-2-dodecenol | 69064-37-5 | --- | [31] |
| Tridecane | 629-50-5 | HMDB0034284 | [120] |
| Trimethyldecane | 98060-54-9 | --- | [118] |
| Volatile Organic Compounds | CAS Numbers | HMDB Numbers | References |
|---|---|---|---|
| Acetaldehyde | 75-07-0 | HMDB0000990 | [131] |
| Acetyl acetate | 108-24-7 | HMDB0031646 | [131] |
| Ethyl butyrate | 105-54-4 | HMDB0033889 | [131] |
| Ethyl vinyl ketone | 1629-58-9 | HMDB0031607 | [131] |
| Methyl butyrate | 623-42-7 | HMDB0033890 | [131] |
| Propyl propionate | 106-36-5 | HMDB0030059 | [131] |
| Volatile Organic Compounds | CAS Numbers | HMDB Numbers | References |
|---|---|---|---|
| Acetophenone | 98-86-2 | HMDB0033910 | [143] |
| Ammonium acetate | 631-61-8 | --- | [144] |
| Benzaldehyde | 100-52-7 | HMDB0006115 | [134] |
| 2,5-Bis-1,1-dimethylethylphenol | 5875-45-6 | --- | [145] |
| 2,3-Butanediol | 513-85-9 | HMDB0003156 | [152] |
| 2,3-Butanedione | 431-03-8 | HMDB0003407 | [143] |
| 2-Butanone | 78-93-3 | HMDB0000474 | [152] |
| Butyric Acid | 107-92-6 | HMDB0000039 | [153] |
| Decanal | 112-31-2 | HMDB0011623 | [134] |
| 1,2-Decanediol | 1119-86-4 | --- | [145] |
| E-3-Decen-2-ol | 18402-84-1 | HMDB0013810 | [145] |
| 1,4-Dichlorobenzene | 106-46-7 | HMDB0041971 | [145] |
| Dihydro-2(3H)-furanone | 96-48-0 | HMDB0000549 | [143] |
| 2,2-Dimethylbutane | 75-83-2 | HMDB0245332 | [143] |
| 2,3-Dimethylbutane | 79-29-8 | HMDB0245401 | [143] |
| 2,2-Dimethyldecane | 17302-37-3 | --- | [144] |
| 4,6-Dimethyl-dodecane | 61141-72-8 | --- | [144] |
| 2,4-Dimethylheptane | 2213-23-2 | HMDB0031416 | [144] |
| 4,5-Dimethylnonane | 17302-23-7 | --- | [134] |
| 2,2-Dimethylpropanoic acid | 75-98-9 | HMDB0041992 | [144] |
| 3,7-Dimethylundecane | 17301-29-0 | --- | [134] |
| Dodecane | 112-40-3 | HMDB0031444 | [134] |
| Ethanol | 64-17-5 | HMDB0000108 | [147,152] |
| Ethylphenol | 90-00-6 | HMDB0302562 | [158] |
| 2-Ethyltoluene | 611-14-3 | HMDB0059819 | [143] |
| 3-Ethyltoluene | 620-14-4 | HMDB0059848 | [143] |
| 4-Ethyltoluene | 622-96-8 | HMDB0059832 | [143] |
| Heptanoic Acid | 111-14-8 | HMDB0000666 | [153] |
| Hexadecane | 544-76-3 | HMDB0033792 | [134] |
| Hexanoic Acid | 142-62-1 | HMDB0000535 | [153,158] |
| Hydrogen cyanide | 74-90-8 | HMDB0060292 | [146] |
| Limonene | 138-86-3 | HMDB0003375 | [144] |
| 2-Methylbutanal | 96-17-3 | HMDB0031526 | [143] |
| 3-Methylbutanal | 590-86-3 | HMDB0006478 | [143] |
| 3-Methyl-2-butanone | 563-80-4 | --- | [143] |
| 3-Methylhexane | 589-34-4 | HMDB0245932 | [144] |
| 5-Methyl-3-hexanone | 623-56-3 | HMDB0031549 | [144] |
| 3-Methylnonane | 5911-04-6 | --- | [144] |
| 4-Methyloctane | 2216-34-4 | --- | [144] |
| 2-Methylpentane | 107-83-5 | HMDB0061884 | [143] |
| 3-Methylpentane | 96-14-0 | HMDB0061885 | [143] |
| Methylphenol | 620-17-7 | --- | [158] |
| 2-Methyltetrahydrofuran | 96-47-9 | HMDB0245240 | [143] |
| Octene | 111-66-0 | HMDB0032449 | [134] |
| Pentanal | 110-62-3 | HMDB0031206 | [143] |
| Pentanoic acid | 109-52-4 | HMDB0000892 | [153] |
| 2-Pentanone | 107-87-9 | HMDB0034235 | [143] |
| Phenol | 108-95-2 | HMDB0000228 | [158] |
| 2-Propenenitrile | 107-13-1 | HMDB0247972 | [147] |
| 9-Tetradecenol | 52957-16-1 | --- | [152] |
| Toluene | 108-88-3 | HMDB0034168 | [143] |
| Triglyceride | 32765-69-8 | HMDB0005474 | [143] |
| Trimethylamine | 75-50-3 | HMDB0000906 | [143] |
| 1,2,3-Trimethylbenzene | 526-73-8 | HMDB0059901 | [143] |
| 1,2,4-Trimethylbenzene | 95-63-6 | HMDB0013733 | [143] |
| 1,3,5-Trimethylbenzene | 108-67-8 | HMDB0041924 | [143] |
| 2,6,6-Trimethyloctane | 54166-32-4 | --- | [144] |
| Undecane | 1120-21-4 | HMDB0031445 | [134,147] |
| p-Xylene | 106-42-3 | HMDB0059924 | [144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vassilenko, V.; Moura, P.C.; Raposo, M. Diagnosis of Carcinogenic Pathologies through Breath Biomarkers: Present and Future Trends. Biomedicines 2023, 11, 3029. https://doi.org/10.3390/biomedicines11113029
Vassilenko V, Moura PC, Raposo M. Diagnosis of Carcinogenic Pathologies through Breath Biomarkers: Present and Future Trends. Biomedicines. 2023; 11(11):3029. https://doi.org/10.3390/biomedicines11113029
Chicago/Turabian StyleVassilenko, Valentina, Pedro Catalão Moura, and Maria Raposo. 2023. "Diagnosis of Carcinogenic Pathologies through Breath Biomarkers: Present and Future Trends" Biomedicines 11, no. 11: 3029. https://doi.org/10.3390/biomedicines11113029
APA StyleVassilenko, V., Moura, P. C., & Raposo, M. (2023). Diagnosis of Carcinogenic Pathologies through Breath Biomarkers: Present and Future Trends. Biomedicines, 11(11), 3029. https://doi.org/10.3390/biomedicines11113029








