Cross-Sectional and Longitudinal Associations between Skin Autofluorescence and Tubular Injury Defined by Urinary Excretion of Liver-Type Fatty Acid-Binding Protein in People with Type 2 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. SAF Measurement
2.3. Biochemical Analyses
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics of the Subjects
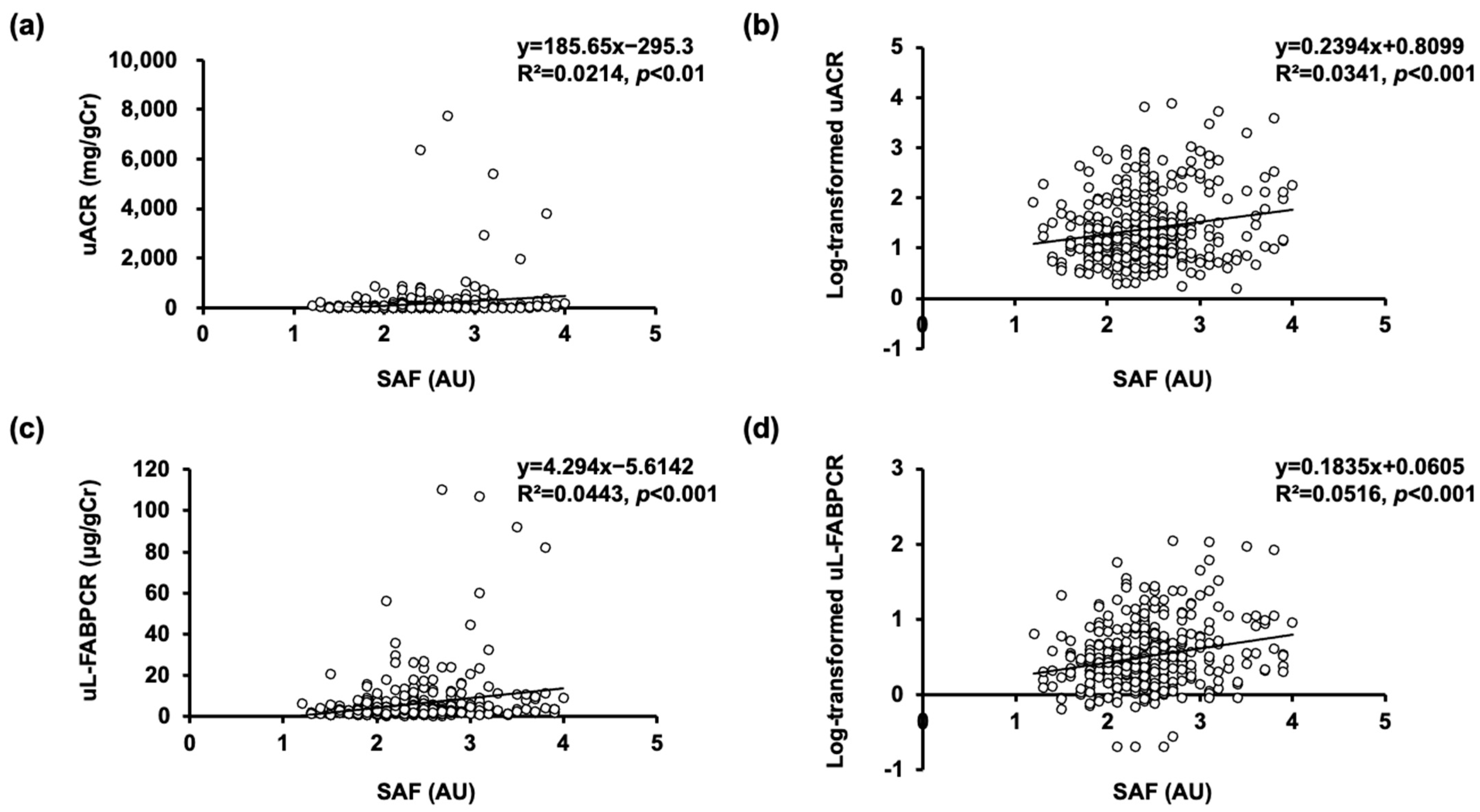
3.2. Associations of SAF with uACR and Log-Transformed uACR without Adjusting for Confounding Factors
3.3. Associations of SAF with uL-FABPCR and Log-Transformed uL-FABPCR without Adjusting for Confounding Factors
3.4. Associations of SAF with uACR and Log-Transformed uACR after Adjusting for Confounding Factors
3.5. Associations of SAF with uL-FABPCR and Log-Transformed uL-FABPCR after Adjusting for Confounding Factors
3.6. Associations of SAF with uL-FABPCR and Log-Transformed uL-FABPCR after Adjusting for Identified Confounding Factors and Medications Used
3.7. Association between Annual Changes in uL-FABPCR and Those in SAF
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.P.; Parving, H.H.; Hunsicker, L.G.; Ravid, M.; Remuzzi, G.; Lewis, J.B. Renal Dysfunction in the Presence of Normoalbuminuria in Type 2 Diabetes: Results from the DEMAND Study. Cardiorenal Med. 2012, 2, 1–10. [Google Scholar] [CrossRef]
- Yoshida, Y.; Kashiwabara, K.; Hirakawa, Y.; Tanaka, T.; Noso, S.; Ikegami, H.; Ohsugi, M.; Ueki, K.; Mita, T.; Watada, H.; et al. Conditions, pathogenesis, and progression of diabetic kidney disease and early decliner in Japan. BMJ Open Diabetes Res. Care 2020, 8, e000902. [Google Scholar] [CrossRef]
- Kramer, H.J.; Nguyen, Q.D.; Curhan, G.; Hsu, C.Y. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 2003, 289, 3273–3277. [Google Scholar] [CrossRef] [PubMed]
- Penno, G.; Solini, A.; Bonora, E.; Fondelli, C.; Orsi, E.; Zerbini, G.; Trevisan, R.; Vedovato, M.; Gruden, G.; Cavalot, F.; et al. Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J. Hypertens. 2011, 29, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Macisaac, R.J.; Jerums, G.; Weekes, A.; Moran, J.; Shaw, J.E.; Atkins, R.C. Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (national evaluation of the frequency of renal impairment cO-existing with NIDDM [NEFRON] 11). Diabetes Care 2009, 32, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Nicklett, E.J.; Ferrucci, L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 963–975. [Google Scholar] [CrossRef]
- Meertens, J.H.; Nienhuis, H.L.; Lefrandt, J.D.; Schalkwijk, C.G.; Nyyssönen, K.; Ligtenberg, J.J.; Smit, A.J.; Zijlstra, J.G.; Mulder, D.J. The Course of Skin and Serum Biomarkers of Advanced Glycation Endproducts and Its Association with Oxidative Stress, Inflammation, Disease Severity, and Mortality during ICU Admission in Critically Ill Patients: Results from a Prospective Pilot Study. PLoS ONE 2016, 11, e0160893. [Google Scholar] [CrossRef]
- Mulder, D.J.; Water, T.V.; Lutgers, H.L.; Graaff, R.; Gans, R.O.; Zijlstra, F.; Smit, A.J. Skin autofluorescence, a novel marker for glycemic and oxidative stress-derived advanced glycation endproducts: An overview of current clinical studies, evidence, and limitations. Diabetes Technol. Ther. 2006, 8, 523–535. [Google Scholar] [CrossRef]
- Lutgers, H.L.; Graaff, R.; Links, T.P.; Ubink-Veltmaat, L.J.; Bilo, H.J.; Gans, R.O.; Smit, A.J. Skin autofluorescence as a noninvasive marker of vascular damage in patients with type 2 diabetes. Diabetes Care 2006, 29, 2654–2659. [Google Scholar] [CrossRef]
- Lutgers, H.L.; Gerrits, E.G.; Graaff, R.; Links, T.P.; Sluiter, W.J.; Gans, R.O.; Bilo, H.J.; Smit, A.J. Skin autofluorescence provides additional information to the UK Prospective Diabetes Study (UKPDS) risk score for the estimation of cardiovascular prognosis in type 2 diabetes mellitus. Diabetologia 2009, 52, 789–797. [Google Scholar] [CrossRef]
- Januszewski, A.S.; Xu, D.; Cho, Y.H.; Benitez-Aguirre, P.Z.; O’Neal, D.N.; Craig, M.E.; Donaghue, K.C.; Jenkins, A.J. Skin autofluorescence in people with type 1 diabetes and people without diabetes: An eight-decade cross-sectional study with evidence of accelerated aging and associations with complications. Diabet. Med. 2021, 38, e14432. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, N.J.; Fluck, R.J.; McIntyre, C.W.; Taal, M.W. Skin autofluorescence and the association with renal and cardiovascular risk factors in chronic kidney disease stage 3. Clin. J. Am. Soc. Nephrol. 2011, 6, 2356–2363. [Google Scholar] [CrossRef]
- Skrha, J., Jr.; Soupal, J.; Loni Ekali, G.; Prázný, M.; Kalousová, M.; Kvasnička, J.; Landová, L.; Zima, T.; Skrha, J. Skin autofluorescence relates to soluble receptor for advanced glycation end-products and albuminuria in diabetes mellitus. J. Diabetes Res. 2013, 2013, 650694. [Google Scholar] [CrossRef] [PubMed]
- Fiseha, T.; Tamir, Z. Urinary Markers of Tubular Injury in Early Diabetic Nephropathy. Int. J. Nephrol. 2016, 2016, 4647685. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Graaff, R.; Oomen, P.H.N.; Links, T.P.; Jager, J.J.; Alderson, N.L.; Thorpe, S.R.; Baynes, J.W.; Gans, R.O.B.; Smit, A.J. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia 2004, 47, 1324–1330. [Google Scholar] [CrossRef]
- Kamijo-Ikemori, A.; Sugaya, T.; Kimura, K. Novel urinary biomarkers in early diabetic kidney disease. Curr. Diab Rep. 2014, 14, 513. [Google Scholar] [CrossRef]
- Suzuki, K.; Babazono, T.; Murata, H.; Iwamoto, Y. Clinical significance of urinary liver-type fatty acid-binding protein in patients with diabetic nephropathy. Diabetes Care 2005, 28, 2038–2039. [Google Scholar] [CrossRef]
- Kamijo-Ikemori, A.; Sugaya, T.; Yasuda, T.; Kawata, T.; Ota, A.; Tatsunami, S.; Kaise, R.; Ishimitsu, T.; Tanaka, Y.; Kimura, K. Clinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patients. Diabetes Care 2011, 34, 691–696. [Google Scholar] [CrossRef]
- Genevieve, M.; Vivot, A.; Gonzalez, C.; Raffaitin, C.; Barberger-Gateau, P.; Gin, H.; Rigalleau, V. Skin autofluorescence is associated with past glycaemic control and complications in type 1 diabetes mellitus. Diabetes Metab. 2013, 39, 349–354. [Google Scholar] [CrossRef]
- Gerrits, E.G.; Lutgers, H.L.; Kleefstra, N.; Graaff, R.; Groenier, K.H.; Smit, A.J.; Gans, R.O.; Bilo, H.J. Skin autofluorescence: A tool to identify type 2 diabetic patients at risk for developing microvascular complications. Diabetes Care 2008, 31, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Tanji, N.; Markowitz, G.S.; Fu, C.; Kislinger, T.; Taguchi, A.; Pischetsrieder, M.; Stern, D.; Schmidt, A.M.; D’Agati, V.D. Expression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal disease. J. Am. Soc. Nephrol. 2000, 11, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- McCance, D.R.; Dyer, D.G.; Dunn, J.A.; Bailie, K.E.; Thorpe, S.R.; Baynes, J.W.; Lyons, T.J. Maillard reaction products and their relation to complications in insulin-dependent diabetes mellitus. J. Clin. Investig. 1993, 91, 2470–2478. [Google Scholar] [CrossRef]
- Beisswenger, P.J.; Moore, L.L.; Brinck-Johnsen, T.; Curphey, T.J. Increased collagen-linked pentosidine levels and advanced glycosylation end products in early diabetic nephropathy. J. Clin. Investig. 1993, 92, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Matsui, T.; Takeuchi, M.; Yamagishi, S. Sodium-glucose cotransporter 2-mediated oxidative stress augments advanced glycation end products-induced tubular cell apoptosis. Diabetes Metab. Res. Rev. 2013, 29, 406–412. [Google Scholar] [CrossRef]
- Qi, W.; Niu, J.; Qin, Q.; Qiao, Z.; Gu, Y. Glycated albumin triggers fibrosis and apoptosis via an NADPH oxidase/Nox4-MAPK pathway-dependent mechanism in renal proximal tubular cells. Mol. Cell Endocrinol. 2015, 405, 74–83. [Google Scholar] [CrossRef]
- Horie, K.; Miyata, T.; Maeda, K.; Miyata, S.; Sugiyama, S.; Sakai, H.; van Ypersole de Strihou, C.; Monnier, V.M.; Witztum, J.L.; Kurokawa, K. Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. Implication for glycoxidative stress in the pathogenesis of diabetic nephropathy. J. Clin. Investig. 1997, 100, 2995–3004. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, I.M.; van de Zande, S.C.; Westra, J.; Zwerver, J.; Smit, A.J.; Mulder, D.J. The AGE Reader: A non-invasive method to assess long-term tissue damage. Methods 2022, 203, 533–541. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Hartog, J.W.; Graaff, R.; Huisman, R.J.; Links, T.P.; den Hollander, N.C.; Thorpe, S.R.; Baynes, J.W.; Navis, G.; Gans, R.O.; et al. Skin autofluorescence, a measure of cumulative metabolic stress and advanced glycation end products, predicts mortality in hemodialysis patients. J. Am. Soc. Nephrol. 2005, 16, 3687–3693. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Links, T.; Graaff, R.; Thorpe, S.R.; Baynes, J.W.; Hartog, J.; Gans, R.; Smit, A. Simple noninvasive measurement of skin autofluorescence. Ann. N. Y. Acad. Sci. 2005, 1043, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Ueda, Y.; Horie, K.; Nangaku, M.; Tanaka, S.; van Ypersele de Strihou, C.; Kurokawa, K. Renal catabolism of advanced glycation end products: The fate of pentosidine. Kidney Int. 1998, 53, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Waanders, F.; Greven, W.L.; Baynes, J.W.; Thorpe, S.R.; Kramer, A.B.; Nagai, R.; Sakata, N.; van Goor, H.; Navis, G. Renal accumulation of pentosidine in non-diabetic proteinuria-induced renal damage in rats. Nephrol. Dial. Transplant. 2005, 20, 2060–2070. [Google Scholar] [CrossRef] [PubMed]


| Total | Males | Females | p Value (Males vs. Females) | |
|---|---|---|---|---|
| Number of subjects | 350 | 198 | 152 | |
| Age (years) | 70 (61, 75) | 71 (61, 76) | 69 (61, 75) | 0.706 |
| BMI (kg/m2) | 24.2 (22.0, 26.9) | 24.2 (22.1, 26.4) | 24.2(21.6, 28.0) | 0.943 |
| SBP (mmHg) | 132.0 (120.3, 143.0) | 131.0 (120.0, 141.8) | 133.0 (121.8, 144.0) | 0.257 |
| TG (mmol/L) | 1.3 (0.9, 1.8) | 1.4 (0.9, 1.9) | 1.1 (0.8, 1.7) | 0.050 |
| HDL-C (mmol/L) | 1.3 (1.1, 1.6) | 1.3 (1.1, 1.5) | 1.5 (1.3, 1.7) | <0.001 |
| LDL-C (mmol/L) | 2.5 (2.1, 3.1) | 2.5 (2.0, 3.1) | 2.6 (2.1, 3.1) | 0.435 |
| Casual PG (mmol/L) | 7.5 (6.3, 9.7) | 7.7 (6.8, 10.4) | 6.7 (5.8, 8.7) | <0.001 |
| HbA1c (%) | 6.8 (6.4, 7.4) | 6.7 (6.3, 7.3) | 6.9 (6.5, 7.5) | 0.854 |
| HbA1c (mmol/mol) | 51 (46, 57) | 50 (45, 56) | 52 (48, 58) | 0.854 |
| UA (umol/L) | 297.4 (249.8, 355.4) | 321.2 (273.6, 368.8) | 258.7 (218.6, 304.8) | <0.001 |
| Cr (umol/L) | 66.7 (55.7, 82.2) | 75.1 (55.6, 83.8) | 55.7 (46.9, 63.9) | <0.001 |
| eGFR (mL/min) | 71.5 ± 20.1 | 70.2 ± 19.4 | 73.2 ± 20.9 | 0.171 |
| uACR (mg/gCr) | 16.5 (7.7, 53.0) | 15.6 (7.1, 72.4) | 16.7 (8.9, 37.8) | 0.880 |
| Log-transformed uACR | 1.22 (0.89, 1.72) | 1.19 (0.85, 1.86) | 1.22 (0.95, 1.58) | 0.880 |
| uL-FABPCR (µg/gCr) | 2.76 (1.74, 5.01) | 2.55 (1.47, 5.02) | 3.04 (2.06, 4.94) | 0.045 |
| Log-transformed uL-FABPCR | 0.44 (0.24, 0.70) | 0.41 (0.17, 0.70) | 0.48 (0.31, 0.69) | 0.045 |
| SAF (AU) | 2.4 (2.1, 2.7) | 2.4 (2.1, 2.8) | 2.3 (2.0, 2.6) | 0.025 |
| Current smoker (n, (%)) | 59 (16.9) | 53 (26.8) | 6 (3.9) | <0.001 |
| Hypertension (n, (%)) | 227 (64.9) | 121 (61.1) | 106 (69.7) | 0.114 |
| Dyslipidemia (n, (%)) | 260 (74.3) | 144 (72.7) | 116 (76.3) | 0.462 |
| Duration of T2D (years) | 10 (3, 19) | 10 (4, 18) | 10 (2, 19) | 0.632 |
| ARB or ACEi (n, (%)) | 142 (40.6) | 76 (38.4) | 66 (43.4) | 0.380 |
| CCB (n, (%)) | 130 (37.1) | 74 (37.4) | 56 (36.8) | 0.999 |
| β blocker (n, (%)) | 15 (4.3) | 9 (4.5) | 6 (3.9) | 0.999 |
| MR blocker (n, (%)) | 4 (1.1) | 3 (1.5) | 1 (0.7) | 0.636 |
| Statin (n, (%)) | 174 (49.7) | 87 (43.9) | 87 (57.2) | 0.018 |
| Ezetimibe (n, (%)) | 27 (7.7) | 14 (7.1) | 13 (8.6) | 0.596 |
| Other hypolipidemic drugs (n, (%)) | 21 (6.0) | 13 (6.6) | 8 (5.3) | 0.657 |
| Antiplatelets (n, (%)) | 35 (10.0) | 28 (14.1) | 7 (4.6) | 0.004 |
| SU or Glinide (n, (%)) | 66 (18.9) | 46 (23.2) | 20 (13.2) | 0.019 |
| Metformin (n, (%)) | 184 (52.6) | 106 (53.5) | 78 (51.3) | 0.746 |
| DPP-4i (n, (%)) | 209 (59.7) | 120 (60.6) | 89 (58.6) | 0.742 |
| SGLT2i (n, (%)) | 149 (42.6) | 87 (43.9) | 62 (40.8) | 0.587 |
| αGI (n, (%)) | 46 (13.1) | 25 (12.6) | 21 (13.8) | 0.752 |
| Pioglitazone (n, (%)) | 11 (3.1) | 5 (2.5) | 6 (3.9) | 0.542 |
| Insulin (n, (%)) | 73 (20.9) | 39 (19.7) | 34 (22.4) | 0.596 |
| GLP-1RA (n, (%)) | 35 (10.0) | 18 (9.1) | 17 (11.2) | 0.591 |
| Variables | uACR | Log-Transformed uACR | uL-FABPCR | Log-Transformed uL-FABPCR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t Value | VIF | p Value | t Value | VIF | p Value | t Value | VIF | p Value | t Value | VIF | p Value | |
| Age | −2.378 | 1.620 | 0.018 | 0.628 | 1.620 | 0.531 | −0.223 | 1.620 | 0.823 | 2.055 | 1.620 | 0.041 |
| Male | −2.960 | 1.539 | 0.003 | −1.913 | 1.539 | 0.057 | −3.164 | 1.539 | 0.002 | −3.250 | 1.539 | 0.001 |
| BMI | 1.557 | 1.556 | 0.121 | 3.21 | 1.556 | 0.002 | 2.003 | 1.556 | 0.046 | 2.105 | 1.556 | 0.036 |
| Current smoker | 1.311 | 1.228 | 0.191 | 2.16 | 1.228 | 0.032 | 1.899 | 1.228 | 0.058 | 2.074 | 1.228 | 0.039 |
| SBP | 2.210 | 1.309 | 0.028 | 1.765 | 1.309 | 0.079 | 1.023 | 1.309 | 0.307 | 0.469 | 1.309 | 0.639 |
| TG | 2.963 | 1.473 | 0.003 | 1.272 | 1.473 | 0.204 | 0.713 | 1.473 | 0.476 | −0.502 | 1.473 | 0.616 |
| HDL-C | 0.099 | 1.388 | 0.921 | −0.708 | 1.388 | 0.479 | 0.183 | 1.388 | 0.855 | −0.290 | 1.388 | 0.772 |
| LDL-C | 3.259 | 1.252 | 0.001 | 1.700 | 1.252 | 0.090 | 2.503 | 1.252 | 0.013 | 0.858 | 1.252 | 0.391 |
| Casual PG | 2.494 | 1.651 | 0.013 | 0.805 | 1.651 | 0.422 | 0.857 | 1.651 | 0.392 | 0.687 | 1.651 | 0.493 |
| HbA1c | −0.604 | 1.687 | 0.547 | 0.529 | 1.687 | 0.598 | 0.180 | 1.687 | 0.858 | 1.742 | 1.687 | 0.082 |
| UA | −2.772 | 1.506 | 0.006 | −2.144 | 1.506 | 0.033 | −2.409 | 1.506 | 0.017 | −2.189 | 1.506 | 0.029 |
| Cr | 7.308 | 1.660 | <0.001 | 4.779 | 1.660 | <0.001 | 6.406 | 1.660 | <0.001 | 4.472 | 1.660 | <0.001 |
| Hypertension | 0.506 | 1.329 | 0.613 | 2.982 | 1.329 | 0.003 | 1.023 | 1.329 | 0.307 | 2.017 | 1.329 | 0.045 |
| Duration of T2D | 3.539 | 1.260 | <0.001 | 3.443 | 1.260 | 0.001 | 3.680 | 1.260 | <0.001 | 2.857 | 1.260 | 0.005 |
| Dyslipidemia | −0.347 | 1.150 | 0.729 | −0.010 | 1.150 | 0.992 | −0.770 | 1.150 | 0.442 | 0.127 | 1.150 | 0.899 |
| SAF | 1.549 | 1.294 | 0.122 | 1.644 | 1.294 | 0.101 | 2.255 | 1.294 | 0.025 | 2.022 | 1.294 | 0.044 |
| Model 1 | Model 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | uL-FABPCR | Log-Transformed uL-FABPCR | uL-FABPCR | Log-Transformed uL-FABPCR | ||||||||
| t Value | VIF | p Value | T Value | VIF | p Value | t Value | VIF | p Value | t Value | VIF | p Value | |
| Age | - | - | - | 1.570 | 1.591 | 0.117 | - | - | - | 1.901 | 1.663 | 0.058 |
| Male | −2.842 | 1.370 | 0.005 | −3.570 | 1.511 | <0.001 | −2.670 | 1.347 | 0.008 | −3.398 | 1.532 | <0.001 |
| BMI | 2.669 | 1.257 | 0.008 | 2.445 | 1.483 | 0.015 | 2.424 | 1.340 | 0.016 | 1.988 | 1.577 | 0.048 |
| Current smoker | - | - | - | 2.008 | 1.221 | 0.045 | - | - | - | 2.224 | 1.213 | 0.027 |
| LDL-C | 3.135 | 1.279 | 0.002 | - | - | - | 2.877 | 1.089 | 0.004 | - | - | - |
| UA | −2.005 | 1.502 | 0.046 | −1.927 | 1.511 | 0.055 | −2.132 | 1.538 | 0.034 | −1.511 | 1.536 | 0.132 |
| Cr | 6.224 | 1.597 | <0.001 | 4.672 | 1.708 | <0.001 | 6.217 | 1.553 | <0.001 | 4.019 | 1.702 | <0.001 |
| Hypertension | - | - | - | 1.621 | 1.871 | 0.106 | - | - | - | 2.271 | 1.175 | 0.024 |
| Duration of T2D | 3.515 | 1.205 | <0.001 | 2.979 | 1.250 | 0.003 | 3.465 | 1.550 | <0.001 | 1.591 | 1.673 | 0.113 |
| SAF | 3.063 | 1.193 | 0.002 | 2.606 | 1.258 | 0.010 | 3.115 | 1.229 | 0.002 | 2.268 | 1.322 | 0.024 |
| ARB or ACEi | −0.004 | 1.344 | 0.997 | −0.862 | 1.695 | 0.390 | - | - | - | - | - | - |
| CCB | 3.107 | 1.385 | 0.002 | 1.842 | 1.525 | 0.066 | - | - | - | - | - | - |
| β blocker | −2.267 | 1.085 | 0.024 | −2.227 | 1.094 | 0.027 | - | - | - | - | - | - |
| MR blocker | −2.091 | 1.078 | 0.037 | −1.897 | 1.085 | 0.059 | - | - | - | - | - | - |
| Statin | −0.480 | 1.272 | 0.632 | −0.905 | 1.141 | 0.366 | - | - | - | - | - | - |
| Ezetimibe | 0.066 | 1.080 | 0.948 | 0.950 | 1.070 | 0.343 | - | - | - | - | - | - |
| Other hypolipidemic drugs | −0.372 | 1.049 | 0.710 | 0.253 | 1.064 | 0.800 | - | - | - | - | - | - |
| Antiplatelets | 0.149 | 1.189 | 0.882 | 0.276 | 1.200 | 0.783 | - | - | - | - | - | - |
| SU or Glinide | - | - | - | - | - | - | −0.569 | 1.297 | 0.570 | 0.897 | 1.300 | 0.370 |
| Metformin | - | - | - | - | - | - | 0.089 | 1.282 | 0.929 | −1.151 | 1.287 | 0.251 |
| DPP-4i | - | - | - | - | - | - | −1.121 | 1.505 | 0.263 | 0.028 | 1.531 | 0.978 |
| SGLT2i | - | - | - | - | - | - | 0.134 | 1.256 | 0.894 | 2.746 | 1.262 | 0.006 |
| αGI | - | - | - | - | - | - | −0.470 | 1.226 | 0.639 | 0.015 | 1.225 | 0.988 |
| Pioglitazone | - | - | - | - | - | - | 1.122 | 1.083 | 0.263 | −0.065 | 1.085 | 0.948 |
| Insulin | - | - | - | - | - | - | −0.221 | 1.241 | 0.825 | 1.011 | 1.342 | 0.313 |
| GLP-1RA | - | - | - | - | - | - | 0.705 | 1.496 | 0.482 | 0.201 | 1.482 | 0.841 |
| Variables | ⊿uL-FABPCR | ||
|---|---|---|---|
| t Value | VIF | p Value | |
| Age | −0.183 | 1.329 | 0.854 |
| Male | 0.282 | 1.159 | 0.778 |
| BMI | −0.558 | 1.349 | 0.577 |
| Hypertension | −0.249 | 1.131 | 0.804 |
| Duration of T2D | −1.820 | 1.173 | 0.070 |
| Dyslipidemia | 2.020 | 1.086 | 0.045 |
| Current smoker | −0.490 | 1.173 | 0.624 |
| ⊿SAF | 2.240 | 1.023 | 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamagami, H.; Hara, T.; Yasui, S.; Hosoki, M.; Hori, T.; Kaneko, Y.; Mitsui, Y.; Kurahashi, K.; Harada, T.; Yoshida, S.; et al. Cross-Sectional and Longitudinal Associations between Skin Autofluorescence and Tubular Injury Defined by Urinary Excretion of Liver-Type Fatty Acid-Binding Protein in People with Type 2 Diabetes. Biomedicines 2023, 11, 3020. https://doi.org/10.3390/biomedicines11113020
Yamagami H, Hara T, Yasui S, Hosoki M, Hori T, Kaneko Y, Mitsui Y, Kurahashi K, Harada T, Yoshida S, et al. Cross-Sectional and Longitudinal Associations between Skin Autofluorescence and Tubular Injury Defined by Urinary Excretion of Liver-Type Fatty Acid-Binding Protein in People with Type 2 Diabetes. Biomedicines. 2023; 11(11):3020. https://doi.org/10.3390/biomedicines11113020
Chicago/Turabian StyleYamagami, Hiroki, Tomoyo Hara, Saya Yasui, Minae Hosoki, Taiki Hori, Yousuke Kaneko, Yukari Mitsui, Kiyoe Kurahashi, Takeshi Harada, Sumiko Yoshida, and et al. 2023. "Cross-Sectional and Longitudinal Associations between Skin Autofluorescence and Tubular Injury Defined by Urinary Excretion of Liver-Type Fatty Acid-Binding Protein in People with Type 2 Diabetes" Biomedicines 11, no. 11: 3020. https://doi.org/10.3390/biomedicines11113020
APA StyleYamagami, H., Hara, T., Yasui, S., Hosoki, M., Hori, T., Kaneko, Y., Mitsui, Y., Kurahashi, K., Harada, T., Yoshida, S., Nakamura, S., Otoda, T., Yuasa, T., Kuroda, A., Endo, I., Matsuhisa, M., Abe, M., & Aihara, K.-i. (2023). Cross-Sectional and Longitudinal Associations between Skin Autofluorescence and Tubular Injury Defined by Urinary Excretion of Liver-Type Fatty Acid-Binding Protein in People with Type 2 Diabetes. Biomedicines, 11(11), 3020. https://doi.org/10.3390/biomedicines11113020







