Low Plasma Levels of Soluble Endoglin and Cardiovascular Events in Patients Undergoing Coronary Angiography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Patients
2.2. Measurements of Plasma sEng and C-Reactive Protein (CRP) Levels
2.3. Baseline Coronary Angiography and Clinical Follow-Up Results for Cardiovascular Events
2.4. Statistical Analysis
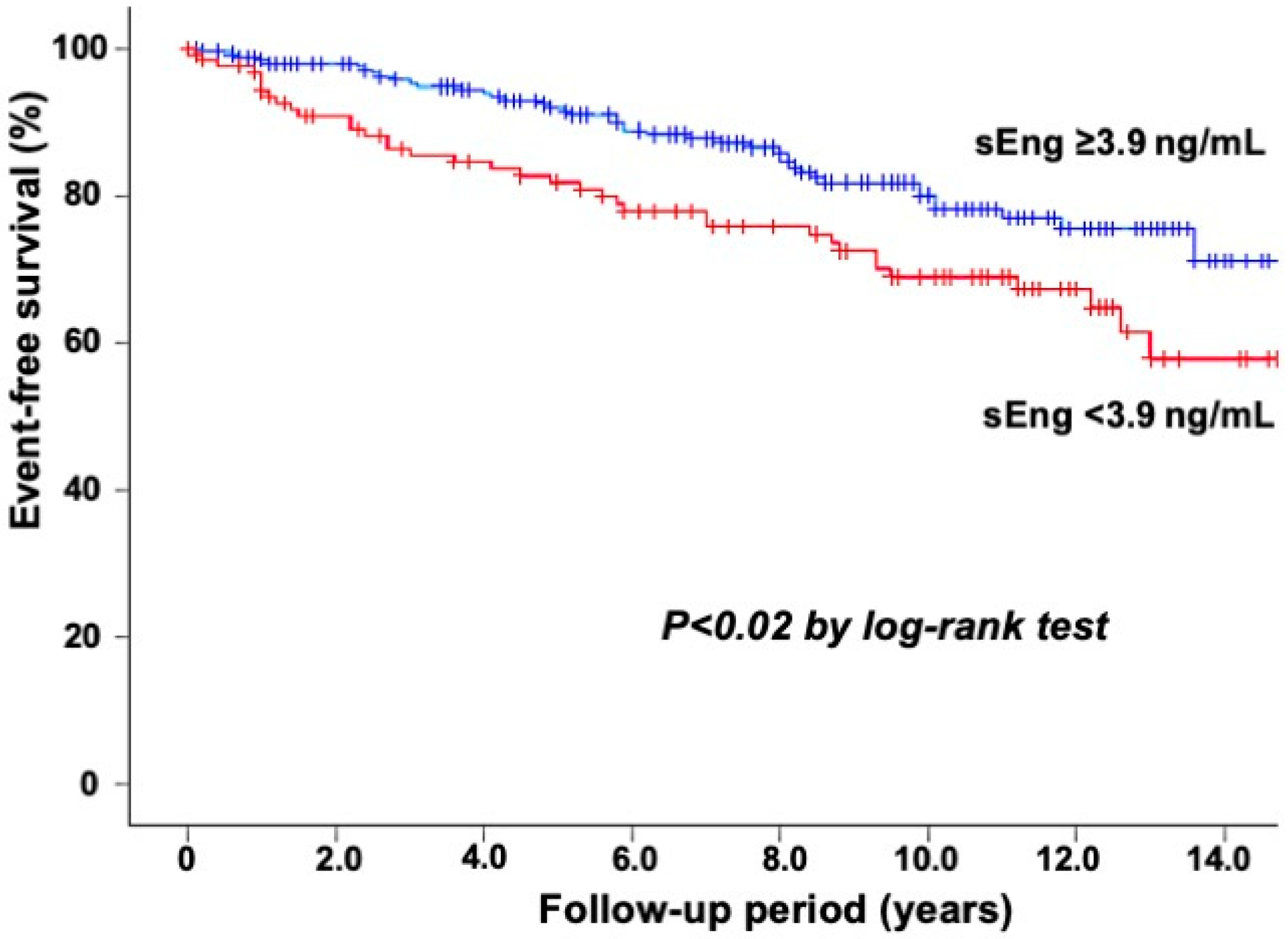
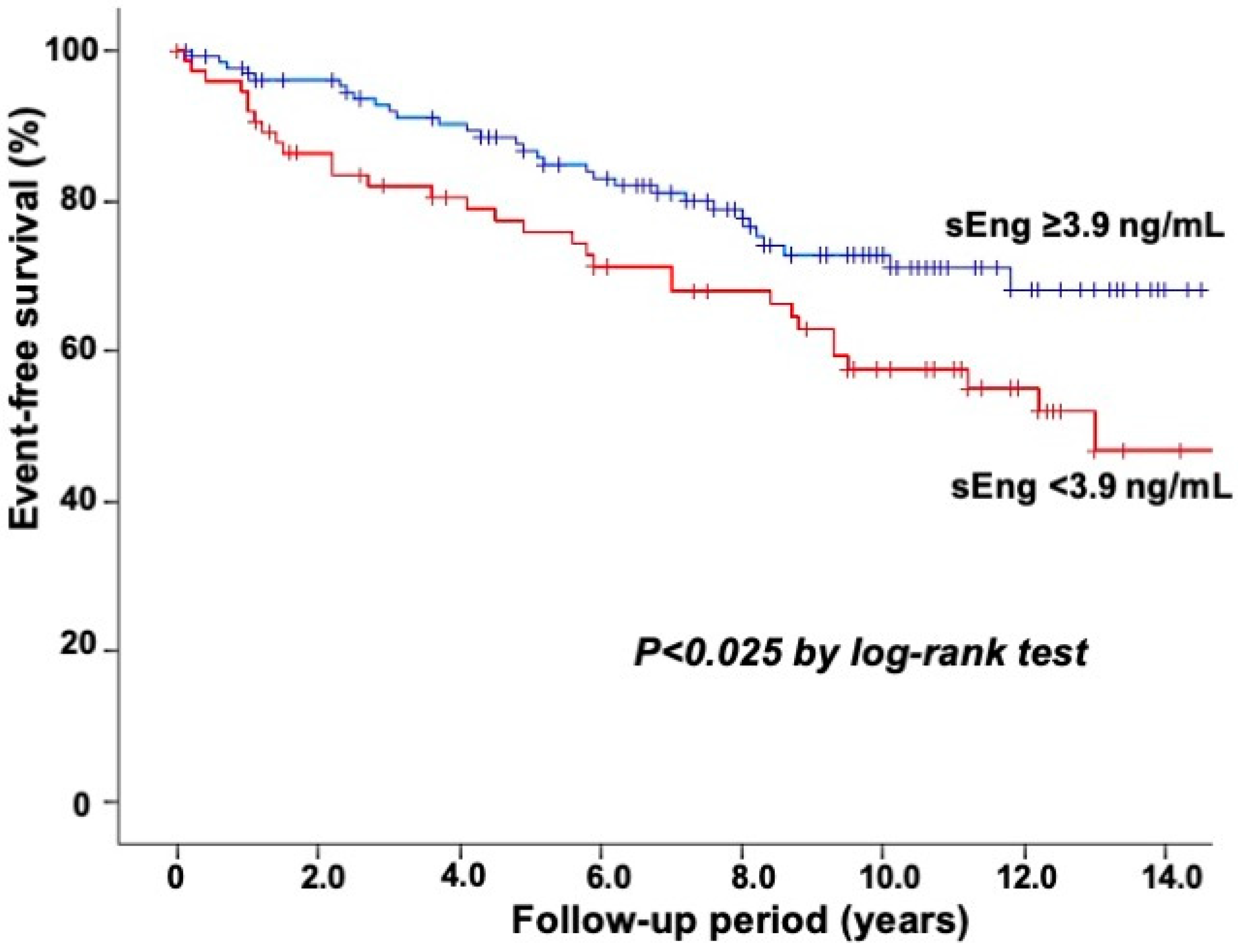
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jang, Y.S.; Choi, I.H. Contrasting roles of different endoglin forms in atherosclerosis. Immune Netw. 2014, 14, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.N.; Ramji, D.P. The role of transforming growth factor-beta in atherosclerosis. Cytokine Growth Factor Rev. 2006, 17, 487–499. [Google Scholar] [CrossRef]
- Gamble, J.R.; Khew-Goodall, Y.; Vadas, M.A. Transforming growth factor-beta inhibits E-selectin expression on human endothelial cells. J. Immunol. 1993, 150, 4494–4503. [Google Scholar] [CrossRef]
- Mallat, Z.; Gojova, A.; Marchiol-Foumigault, C.; Esposito, B.; Kamaté, C.; Merval, R.; Fradelizi, D.; Tedgui, A. Inhibition of transforming growth factor-β signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ. Res. 2001, 89, 930–934. [Google Scholar] [CrossRef]
- Rathouska, J.; Jezkova, K.; Nemeckova, I.; Nachtigal, P. Soluble endoglin, hypercholesterolemia and endothelial dysfunction. Atherosclerosis 2015, 243, 383–388. [Google Scholar] [CrossRef]
- Santibanez, J.F.; Quintanilla, M.; Bernabeu, C. TGF-beta/TGF-beta receptor system and its role in physiological and pathological conditions. Clin. Sci. 2011, 121, 233–251. [Google Scholar] [CrossRef]
- Li, C.; Hampson, I.N.; Hampson, L.; Kumar, P.; Bernabeu, C.; Kumar, S. CD105 antagonizes the inhibitory signaling of transforming growth factor-β1 on human vascular endothelial cell. FASEB. J. 1999, 14, 55–64. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Sun, B.; Bao, X.; Tang, Y.; Huang, F.; Zhu, S.; Xu, J. Negative correlation between endoglin levels and coronary atherosclerosis. Lipids Health Dis. 2021, 20, 127. [Google Scholar] [CrossRef] [PubMed]
- Jerkic, M.; Rivas-Elena, J.V.; Prieto, M.; Carrón, R.; Sanz-Rodríguez, F.; Pérez-Barriocanal, F.; Rodríguez-Barbero, A.; Bernabéu, C.; López-Novoa, J.M. Endoglin regulates nitric oxide-dependent vasodilatation. FASEB J. 2004, 18, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Conley, B.A.; Smith, J.D.; Guerrero-Esteo, M.; Bernabeu, C.; Vary, C.P. Endoglin, a TGF-beta receptor-associated protein, is expressed by smooth muscle cells in human atherosclerotic plaques. Atherosclerosis 2000, 153, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.; Tokunaga, O. Significant expression of endoglin, TGFβ-1 and TGFβR-2 in the atherosclerotic aorta: An immune-histological study. J. Atheroscler. Thromb. 2006, 13, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Bot, P.T.; Hoefer, I.E.; Sluijter, J.P.; van Vliet, P.; Smits, A.M.; Lebrin, F.; Moll, F.; de Vries, J.P.; Doevendans, P.; Piek, J.J.; et al. Increased expression of the transforming growth factor-beta signaling pathway, endoglin, and early growth response-1 in stable plaques. Stroke 2009, 40, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Saita, E.; Miura, K.; Suzuki-Sugihara, N.; Miyata, K.; Ikemura, N.; Ohmori, R.; Ikegami, Y.; Kishimoto, Y.; Kondo, K.; Momiyama, Y. Plasma soluble endoglin levels are inversely associated with the severity of coronary atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Blann, A.D.; Wang, J.M.; Wilson, P.B.; Kumar, S. Serum levels of the TGF-beta receptor are increased in atherosclerosis. Atherosclerosis 1996, 120, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Li, C.G.; Bethell, H.; Wilson, P.B.; Bhatnagar, D.; Walker, M.G.; Kumar, S. The significance of CD105, TGFβ and CD105/TGFβ complexes in coronary artery disease. Atherosclerosis 2000, 152, 249–256. [Google Scholar] [CrossRef]
- Hamm, C.W.; Braunwald, E. A classification of unstable angina revisited. Circulation 2000, 102, 118–122. [Google Scholar] [CrossRef]
- Kapur, N.K.; Heffernan, K.S.; Yunis, A.A.; Parpos, P.; Kiernan, M.S.; Sahasrabudhe, N.A.; Kimmelstiel, C.D.; Kass, D.A.; Karas, R.H.; Mendelsohn, M.E. Usefulness of soluble endoglin as a noninvasive measure of left ventricular filling pressure in heart failure. Am. J. Cardiol. 2010, 106, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.K.; Morine, K.J.; Letarte, M. Endoglin: A critical mediator of cardiovascular health. Vasc. Health Risk Manag. 2013, 9, 195–206. [Google Scholar] [CrossRef]
- Arima, H.; Kubo, M.; Yonemoto, K.; Doi, Y.; Ninomiya, T.; Tanizaki, Y.; Hata, J.; Matsumura, K.; Iida, M.; Kiyohara, Y. High-sensitivity C-reactive protein and coronary heart disease in a general population of Japanese: The Hisayama Study. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1385–1391. [Google Scholar] [CrossRef]
- Momiyama, Y.; Kawaguchi, A.; Kajiwara, I.; Ohmori, R.; Okada, K.; Saito, I.; Konishi, M.; Nakamura, M.; Sato, S.; Kokubo, Y.; et al. Prognostic value of plasma high-sensitivity C-reactive protein levels in Japanese patients with stable coronary artery disease: The Japan NCVC-Collaborative Inflammation Cohort Study. Atherosclerosis 2009, 207, 272–276. [Google Scholar] [CrossRef]
- Strasky, Z.; Vecerova, L.; Rathouska, J.; Slanarova, M.; Brcakova, E.; Kudlackova, Z.; Andrys, C.; Micuda, S.; Nachtigal, P. Cholesterol effects on endoglin and its downstream pathways in ApoE/LDLR double knockout mice. Circ. J. 2011, 75, 1747–1755. [Google Scholar] [CrossRef]
- Blaha, M.; Cermanova, M.; Blaha, V.; Jarolim, P.; Andrys, C.; Blazek, M.; Maly, J.; Smolej, L.; Zajic, J.; Masin, V.; et al. Elevated serum soluble endoglin (sCD105) decreased during extracorporeal elimination therapy for familial hypercholesterolemia. Atherosclerosis 2008, 197, 264–270. [Google Scholar] [CrossRef]
- Li, Q.; Lin, F.; Ke, D.; Cheng, Q.; Gui, Y.; Zhou, Y.; Wu, Y.; Wang, Y.; Zhu, P. Combination of endoglin and ASCVD risk assessment improves carotid subclinical atherosclerosis recognition. J. Atheroscler. Thromb. 2020, 27, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, T.; Hojo, Y.; Kondo, H.; Takahashi, N.; Hirose, M.; Nishimura, Y.; Katsuki, T.; Shimada, K.; Kario, K. Plasma endoglin as a marker to predict cardiovascular events in patients with chronic coronary artery diseases. Heart Vessels 2012, 27, 344–351. [Google Scholar] [CrossRef]
- Cruz-Gonzalez, I.; Pabón, P.; Rodríguez-Barbero, A.; Martín-Moreiras, J.; Pericacho, M.; Sánchez, P.L.; Ramirez, V.; Sánchez-Ledesma, M.; Martín-Herrero, F.; Jiménez-Candil, J.; et al. Identification of serum endoglin as a novel prognostic marker after acute myocardial infarction. J. Cell. Mol. Med. 2008, 12, 955–961. [Google Scholar] [CrossRef]
- He, X.W.; Ke, S.F.; Bao, Y.Y.; Hong, W.J.; Shen, Y.G.; Li, C.; Zhu, F.; Wang, E.; Jin, X.P. Serum levels of endocan and endoglin are associated with large-artery atherosclerotic stroke. Clin. Chim. Acta 2018, 478, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Pericacho, M.; Kauskot, A.; Gamella-Pozuelo, L.; Reboul, E.; Leuci, A.; Egido-Turrion, C.; El Hamaoui, D.; Marchelli, A.; Fernández, F.J.; et al. Soluble endoglin reduces thrombus formation and platelet aggregation via interaction with αIIbβ3 integrin. J. Thromb. Haemost. 2023, 21, 1943–1956. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Bernabeu, C. Novel vascular roles of human endoglin in pathophysiology. J. Thromb. Haemost. 2023, 21, 2327–2338. [Google Scholar] [CrossRef] [PubMed]
- Januszek, R.; Mika, P.; Nowobilski, R.; Nowak, W.; Kusienicka, A.; Klóska, D.; Maga, P.; Niżankowski, R. Soluble endoglin as a prognostic factor of the claudication distance improvement in patients with peripheral artery disease undergoing supervised treadmill training program. J. Am. Soc. Hypertens. 2017, 11, 553–564. [Google Scholar] [CrossRef]
- Pasterkamp, G.; Goumans, M.J. The microvasculature: The next battlefield where transforming growth factor-β and endoglin draw their double-edged swords? Arterioscler. Thromb. Vasc. Biol. 2017, 37, 10–12. [Google Scholar] [CrossRef]
- Jezkova, K.; Rathouska, J.; Nemeckova, I.; Fikrova, P.; Dolezelova, E.; Varejckova, M.; Vitverova, B.; Tysonova, K.; Serwadczak, A.; Buczek, E.; et al. High levels of soluble endoglin induce a proinflammatory and oxidative-stress phenotype associated with preserved NO-dependent vasodilatation in aortas from mice fed a high-fat diet. J. Vasc. Res. 2016, 53, 149–162. [Google Scholar] [CrossRef] [PubMed]
| All | ||||
|---|---|---|---|---|
| (n = 403) | CAD (−) (n = 194) | (−) vs. (+) | CAD (+) (n = 209) | |
| Age (years) | 67 ± 11 | 65 ± 12 | <0.001 | 69 ± 10 |
| Sex (male) | 281 (70%) | 120 (62%) | <0.005 | 161 (77%) |
| Hypertension | 285 (71%) | 119 (61%) | <0.001 | 166 (79%) |
| Hyperlipidemia | 206 (51%) | 78 (40%) | <0.001 | 128 (61%) |
| LDL cholesterol (mg/dL) | 114 ± 31 | 111 ± 29 | NS | 116 ± 33 |
| HDL cholesterol (mg/dL) | 55 ± 15 | 58 ± 16 | <0.001 | 51 ± 13 |
| Statin use | 144 (36%) | 46 (24%) | <0.001 | 98 (47%) |
| Diabetes mellitus | 99 (25%) | 26 (13%) | <0.001 | 73 (35%) |
| Smoking | 173 (43%) | 68 (35%) | <0.005 | 105 (50%) |
| LVEF (%) | 63 ± 10 | 63 ± 9 | <0.05 | 61 ± 12 |
| hsCRP level (mg/L) | 0.63 [0.30, 1.53] | 0.53 [0.27, 1.31] | <0.01 | 0.80 [0.37, 1.74] |
| sEng level (ng/mL) | 4.33 [3.74, 5.06] | 4.41 [3.85, 5.18] | <0.025 | 4.26 [3.57, 4.97] |
| All | ||||
|---|---|---|---|---|
| (n = 403) | Events (−) (n = 324) | (−) vs. (+) | Events (+) (n = 79) | |
| Age (years) | 67 ± 11 | 66 ± 11 | <0.005 | 70 ± 11 |
| Sex (male) | 281 (70%) | 222 (69%) | NS | 59 (75%) |
| Hypertension | 285 (71%) | 223 (69%) | NS | 62 (78%) |
| Hyperlipidemia | 206 (51%) | 161 (50%) | NS | 45 (57%) |
| LDL cholesterol (mg/dL) | 114 ± 31 | 112 ± 30 | NS | 119 ± 33 |
| HDL cholesterol (mg/dL) | 55 ± 15 | 55 ± 15 | <0.02 | 51 ± 14 |
| Statin use | 144 (36%) | 108 (33%) | NS | 36 (46%) |
| Diabetes mellitus | 99 (25%) | 79 (24%) | NS | 20 (25%) |
| Smoking | 173 (43%) | 133 (41%) | NS | 40 (51%) |
| CAD | 209 (52%) | 147 (45%) | <0.001 | 62 (78%) |
| Number of stenotic vessels | 1.0 ± 1.1 | 0.8 ± 1.0 | <0.001 | 1.7 ± 1.1 |
| LVEF (%) | 63 ± 10 | 63 ± 9 | <0.05 | 61 ± 12 |
| hsCRP level (mg/L) | 0.63 [0.30, 1.53] | 0.60 [0.29, 1.49] | <0.05 | 0.82 [0.45, 1.86] |
| hsCRP > 1.0 mg/L | 46 (36%) | 113 (35%) | NS | 33 (42%) |
| sEng level (ng/mL) | 4.33 [3.74, 5.06] | 4.39 [3.83, 5.08] | <0.02 | 3.95 [3.49, 4.84] |
| sEng < 3.9 ng/mL | 129 (32%) | 92 (28%) | <0.005 | 37 (47%) |
| Hazard Ratio | (95% CI) | p Value | |
|---|---|---|---|
| Age (per 10 year increase) | 1.38 | (1.07–1.77) | <0.02 |
| CAD | 2.80 | (1.61–4.88) | <0.001 |
| LVEF (<50%) | 2.80 | (1.47–5.34) | <0.005 |
| sEng level (<3.9 ng/mL) | 1.59 | (1.01–2.49) | <0.05 |
| Hazard Ratio | (95% CI) | p Value | |
|---|---|---|---|
| LVEF (<50%) | 3.27 | (1.62–6.62) | <0.002 |
| sEng level (<3.9 ng/mL) | 2.07 | (1.24–3.45) | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saita, E.; Kishimoto, Y.; Aoyama, M.; Ohmori, R.; Kondo, K.; Momiyama, Y. Low Plasma Levels of Soluble Endoglin and Cardiovascular Events in Patients Undergoing Coronary Angiography. Biomedicines 2023, 11, 2975. https://doi.org/10.3390/biomedicines11112975
Saita E, Kishimoto Y, Aoyama M, Ohmori R, Kondo K, Momiyama Y. Low Plasma Levels of Soluble Endoglin and Cardiovascular Events in Patients Undergoing Coronary Angiography. Biomedicines. 2023; 11(11):2975. https://doi.org/10.3390/biomedicines11112975
Chicago/Turabian StyleSaita, Emi, Yoshimi Kishimoto, Masayuki Aoyama, Reiko Ohmori, Kazuo Kondo, and Yukihiko Momiyama. 2023. "Low Plasma Levels of Soluble Endoglin and Cardiovascular Events in Patients Undergoing Coronary Angiography" Biomedicines 11, no. 11: 2975. https://doi.org/10.3390/biomedicines11112975
APA StyleSaita, E., Kishimoto, Y., Aoyama, M., Ohmori, R., Kondo, K., & Momiyama, Y. (2023). Low Plasma Levels of Soluble Endoglin and Cardiovascular Events in Patients Undergoing Coronary Angiography. Biomedicines, 11(11), 2975. https://doi.org/10.3390/biomedicines11112975





