Identification of Potential Drug Targets for Antiplatelet Therapy Specifically Targeting Platelets of Old Individuals through Proteomic Analysis
Abstract
:1. Introduction
2. Material and Methods
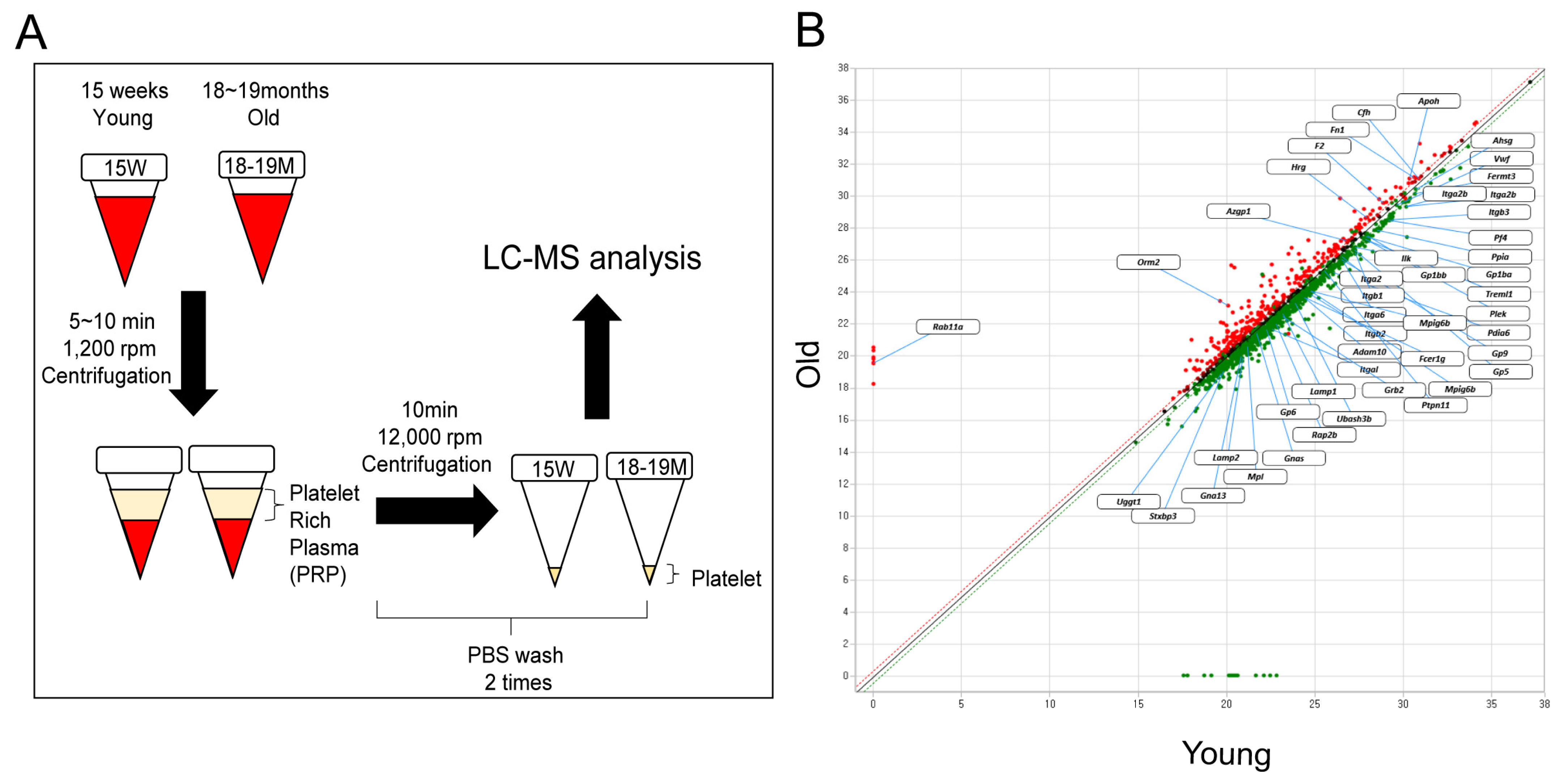
2.1. Preparation of Aged Mice
2.2. CD62p ELISA
2.3. Platelet Isolation
2.4. Western Blotting
2.5. Immunostaining
2.6. Proteomics
- Protein digestion: the samples were suspended in lysis buffer (8 M urea–0.1 M Tris-HCl buffer, pH 8.5) and protease inhibitor cocktail, followed by sonication for 20 min at 15 °C using Covaris S2 Focused Ultrasonicator (Covaris, Woburn, MA, USA). The Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, IL, USA) quantified the protein concentration. The digestion step was performed using filter-aided sample preparation on a Microcon 30 K centrifugal filter device (Millipore, Billerica, MA, USA). Each sample was reduced by incubating with Tris (2-carboxyethyl) phosphine (TCEP) at 37 °C for 30 min and alkylated with iodoacetic acid (IAA) at 25 °C for an hour in dark conditions. After washing with lysis buffer and 50 mM ammonium bicarbonate (ABC) sequentially, the proteins were digested with trypsin (enzyme to protein ratio of 1:50; w/w) at 37 °C for 18 h. The resulting peptide mixtures were transferred into new tubes, and trypsin was inactivated by acidifying with 15 μL of formic acid (Honeywell, Charlotte, NC, USA). The digested peptides were desalted using C18 spin columns (Harvard Apparatus, Holliston, MA, USA), and the peptides were eluted with 80% acetonitrile in 0.1% formic acid in water.
- LC–MS/MS analysis: The prepared samples were resuspended in 0.1% formic acid in water and analyzed using a Q-Exactive Orbitrap hybrid mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) along with an Ultimate 3000 system (Thermo Fisher Scientific, Waltham, MA, USA). We used a 2 cm × 75 μm ID trap column packed with 3 μm C18 resin and a 50 cm × 75 μm ID analytical column packed with 2 μm C18 resin for the peptides depending on the peptides’ hydrophobicity. The mobile phase solvents consisted of (A) 0.1% formic acid in water and (B) 0.1% formic acid in 90% acetonitrile, whereas the flow rate was fixed at 300 nL/min. The gradient of the mobile phase was as follows: 4% solvent B for 14 min, 4%–15% solvent B for 61 min, 15%–28% solvent B for 50 min, 28%–40% solvent B for 20 min, 40%–96% solvent B for 2 min, holding at 96% of solvent B for 13 min, 96%–4% solvent B for 1 min, and 4% solvent B for 24 min. A data-dependent acquisition method was adopted, and the top 10 precursor peaks were selected and isolated for fragmentation. Ions were scanned in high resolution (70,000 in MS1, 17,500 in MS2 at m/z 400), and the MS scan range was 400–2000 m/z in both the MS1 and MS2 levels. Precursor ions were fragmented with a normalized collisional energy of 27%. Dynamic exclusion was set to 30 s.
- Proteome data analysis: Thermo MS/MS raw files of each analysis were searched using the Proteome Discoverer™ software (ver. 2.5), and the mouse database was downloaded from UniProt. The appropriate consensus workflow included a peptide-spectrum match validation step and a SEQUEST HT process for detection as a database search algorithm. The search parameters were set up as follows: 10 ppm of tolerances of precursor ion masses, 0.02 Da fragment ion mass, and a maximum of two missed cleavages with trypsin enzyme. The dynamic modification on the peptide sequence was as follows: static carbamidomethylation of cysteine (+57.012 Da), variable modifications of methionine oxidation (+15.995 Da), acetylation of protein N-term (+42.011 Da), and carbamylation of protein in N-term (+43.0006 Da). After searching, the data results below 1% of FDR were selected and filtered for at least six more peptide lengths. Precursor abundance calculation was based on intensity. Fold change was calculated in the protein abundance-based ratio. Furthermore, the p-values were calculated for the reported quantification ratios using a t-test based on the background.
2.7. Statistics
3. Results
3.1. Identification of Proteins with Increased or Decreased Expression in Aged Mice Platelets
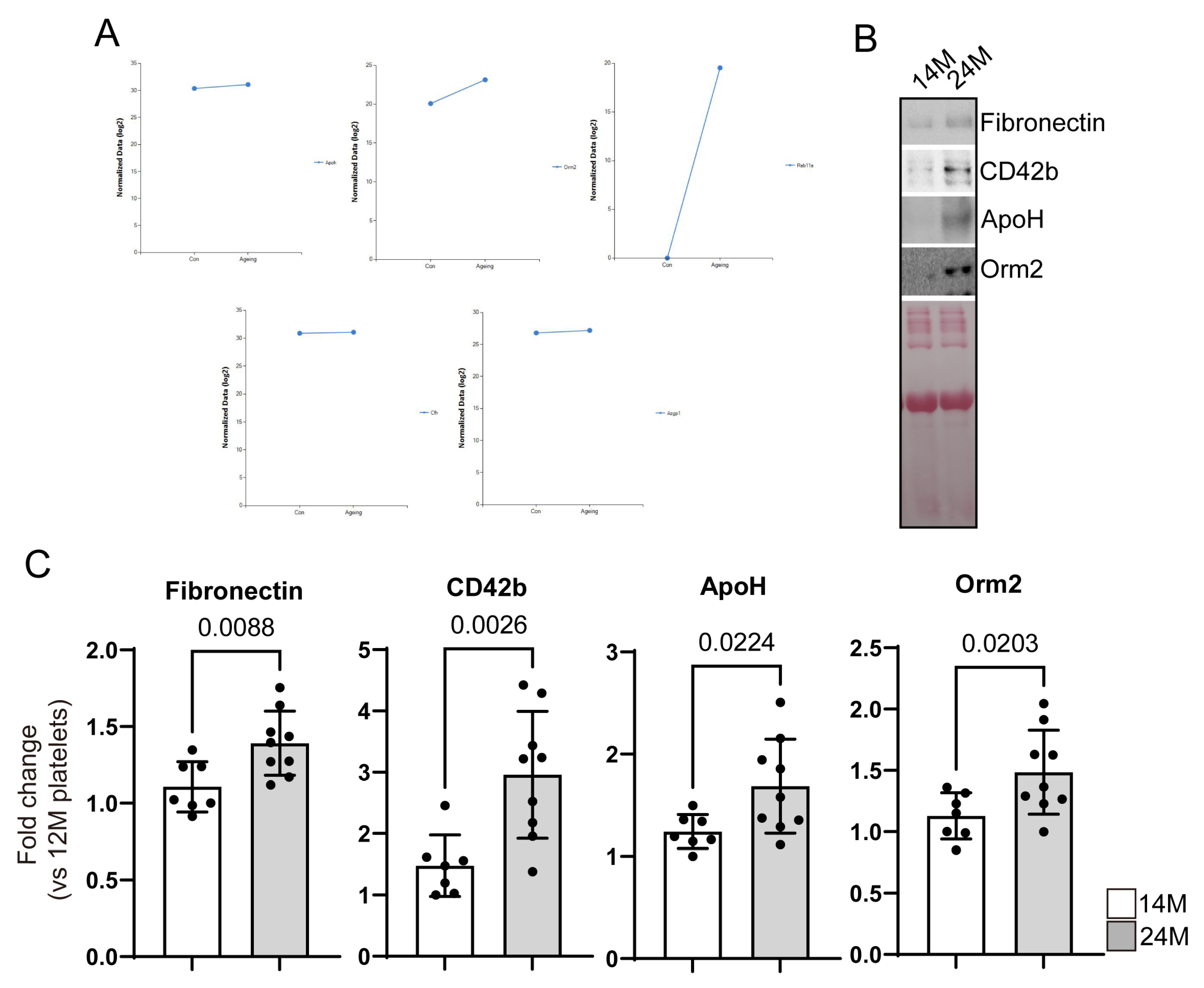
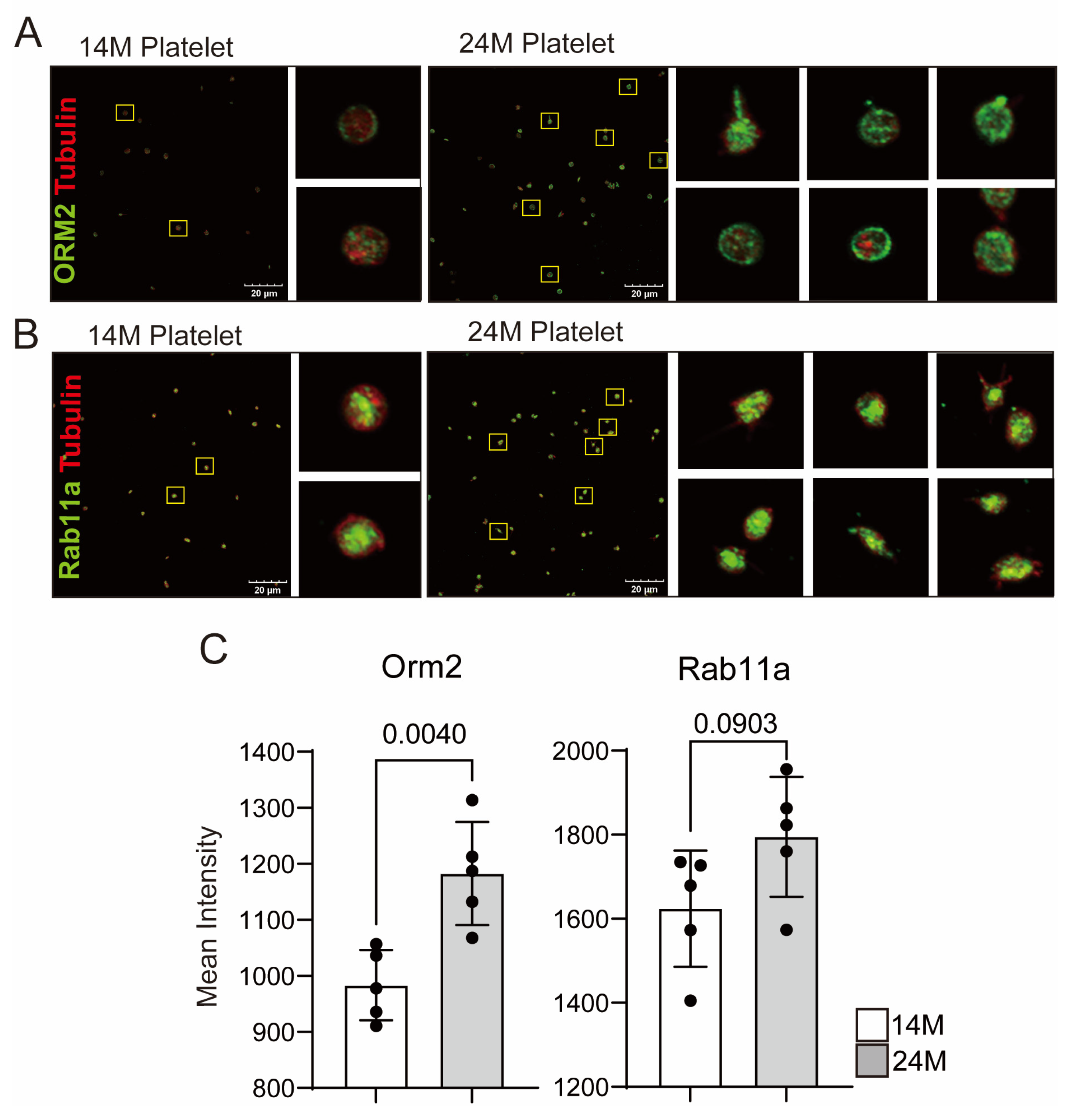
3.2. Reconfirmation of Target Protein Expression in Aged Mice Platelets
3.3. Potential Targets of Antithrombotic Agent
- Beta-2-glycoprotein1 (β2GP1, Apolipoprotein H (ApoH)
- 2.
- Alpha-1-acid glycoprotein2 (AGP-1, Orm2)
- 3.
- Ras-related protein Rab-11A (Rab11a)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Le Blanc, J.; Lordkipanidze, M. Platelet Function in Aging. Front. Cardiovasc. Med. 2019, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhou, X.; Fan, X.; Xiao, M.; Yang, D.; Liang, B.; Dai, M.; Shan, L.; Lu, J.; Lin, Z.; et al. mTORC1 promotes aging-related venous thrombosis in mice via elevation of platelet volume and activation. Blood 2016, 128, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, M.D.; Heit, J.A.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J. Trends in the incidence of deep vein thrombosis and pulmonary embolism: A 25-year population-based study. Arch. Intern. Med. 1998, 158, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.X.; Tyagi, T.; Jain, K.; Gu, V.W.; Lee, S.H.; Hwa, J.M.; Kwan, J.M.; Krause, D.S.; Lee, A.I.; Halene, S.; et al. Thrombocytopathy and endotheliopathy: Crucial contributors to COVID-19 thromboinflammation. Nat. Rev. Cardiol. 2021, 18, 194–209. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, S.; Du, J.; Jain, K.; Ding, M.; Kadado, A.J.; Atteya, G.; Jaji, Z.; Tyagi, T.; Kim, W.H.; et al. Mitochondrial MsrB2 serves as a switch and transducer for mitophagy. EMBO Mol. Med. 2019, 11, e10409. [Google Scholar] [CrossRef]
- Galli, M.; Barbui, T.; Comfurius, P.; Maassen, C.; Hemker, H.C.; Zwaal, R.F.; Bevers, E.M.; De Baets, M.H.; van Breda-Vriesman, P.J. Anticardiolipin antibodies (ACA) directed not to cardiolipin but to a plasma protein cofactor. Lancet 1990, 335, 1544–1547. [Google Scholar] [CrossRef] [PubMed]
- Mather, K.A.; Thalamuthu, A.; Oldmeadow, C.; Song, F.; Armstrong, N.J.; Poljak, A.; Holliday, E.G.; McEvoy, M.; Kwok, J.B.; Assareh, A.A.; et al. Genome-wide significant results identified for plasma apolipoprotein H levels in middle-aged and older adults. Sci. Rep. 2016, 6, 23675. [Google Scholar] [CrossRef] [PubMed]
- Sodin-Semrl, S.; Rozman, B. Beta2-glycoprotein I and its clinical significance: From gene sequence to protein levels. Autoimmun. Rev. 2007, 6, 547–552. [Google Scholar] [CrossRef]
- Athanasiadis, G.; Sabater-Lleal, M.; Buil, A.; Souto, J.C.; Borrell, M.; Lathrop, M.; Watkins, H.; Almasy, L.; Hamsten, A.; Soria, J.M. Genetic determinants of plasma beta(2)-glycoprotein I levels: A genome-wide association study in extended pedigrees from Spain. J. Thromb. Haemost. 2013, 11, 521–528. [Google Scholar] [CrossRef]
- Banzato, A.; Pengo, V. Clinical relevance of beta(2)-glycoprotein-I plasma levels in antiphospholipid syndrome (APS). Curr. Rheumatol. Rep. 2014, 16, 424. [Google Scholar] [CrossRef]
- Castro, A.; Lázaro, I.; Selva, D.M.; Céspedes, E.; Girona, J.; Guardiola, M.; Cabré, A.; Simó, R.; Masana, L. APOH is increased in the plasma and liver of type 2 diabetic patients with metabolic syndrome. Atherosclerosis 2010, 209, 201–205. [Google Scholar] [CrossRef]
- Miyakis, S.; Giannakopoulos, B.; Krilis, S.A. Beta 2 glycoprotein I--function in health and disease. Thromb. Res. 2004, 114, 335–346. [Google Scholar] [CrossRef]
- George, J.; Afek, A.; Gilburd, B.; Blank, M.; Levy, Y.; Aron-Maor, A.; Levkovitz, H.; Shaish, A.; Goldberg, I.; Kopolovic, J.; et al. Induction of early atherosclerosis in LDL-receptor-deficient mice immunized with beta2-glycoprotein I. Circulation 1998, 98, 1108–1115. [Google Scholar] [CrossRef]
- McDonnell, T.; Wincup, C.; Buchholz, I.; Pericleous, C.; Giles, I.; Ripoll, V.; Cohen, H.; Delcea, M.; Rahman, A. The role of beta-2-glycoprotein I in health and disease associating structure with function: More than just APS. Blood Rev. 2020, 39, 100610. [Google Scholar] [CrossRef]
- Cevik, O.; Baykal, A.T.; Sener, A. Platelets Proteomic Profiles of Acute Ischemic Stroke Patients. PLoS ONE 2016, 11, e0158287. [Google Scholar] [CrossRef] [PubMed]
- Zamanian-Azodi, M.; Arjmand, B.; Razzaghi, M.; Tavirani, M.R.; Ahmadzadeh, A.; Rostaminejad, M. Platelet and Haemostasis are the Main Targets in Severe Cases of COVID-19 Infection; a System Biology Study. Arch. Acad. Emerg. Med. 2021, 9, e27. [Google Scholar]
- Fournier, T.; Medjoubi, N.N.; Porquet, D. Alpha-1-acid glycoprotein. Biochim. Biophys. Acta 2000, 1482, 157–171. [Google Scholar] [CrossRef]
- Schmid, K.; Mao, S.; Kimura, A.; Hayashi, S.; Binette, J. Isolation and characterization of a serine-threonine-rich galactoglycoprotein from normal human plasma. J. Biol. Chem. 1980, 255, 3221–3226. [Google Scholar] [CrossRef]
- Adam, P.; Sobek, O.; Táborský, L.; Hildebrand, T.; Tutterová, O.; Žáček, P. CSF and serum orosomucoid (alpha-1-acid glycoprotein) in patients with multiple sclerosis: A comparison among particular subgroups of MS patients. Clin. Chim. Acta 2003, 334, 107–110. [Google Scholar] [CrossRef]
- Ceciliani, F.; Lecchi, C. The Immune Functions of alpha(1) Acid Glycoprotein. Curr. Protein Pept. Sci. 2019, 20, 505–524. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, T.W.; Van der Stelt, M.E.; Anbergen, M.G.; van Dijk, W. Inflammation-induced expression of sialyl Lewis X-containing glycan structures on alpha 1-acid glycoprotein (orosomucoid) in human sera. J. Exp. Med. 1993, 177, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Mackiewicz, A.; Marcinkowska-Pieta, R.; Ballou, S.; Mackiewicz, S.; Kushner, I. Microheterogeneity of alpha 1-acid glycoprotein in the detection of intercurrent infection in systemic lupus erythematosus. Arthritis Rheum. 1987, 30, 513–518. [Google Scholar] [CrossRef]
- Moule, S.K.; Peak, M.; Thompson, S.; Turner, G.A. Studies of the sialylation and microheterogeneity of human serum alpha 1-acid glycoprotein in health and disease. Clin. Chim. Acta 1987, 166, 177–185. [Google Scholar] [CrossRef]
- Ongay, S.; Neususs, C. Isoform differentiation of intact AGP from human serum by capillary electrophoresis-mass spectrometry. Anal. Bioanal. Chem. 2010, 398, 845–855. [Google Scholar] [CrossRef]
- Pawlowski, T.; Mackiewicz, S.H.; Mackiewicz, A. Microheterogeneity of alpha 1-acid glycoprotein in the detection of intercurrent infection in patients with rheumatoid arthritis. Arthritis Rheum. 1989, 32, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Sumanth, M.S.; Jacob, S.P.; Abhilasha, K.V.; Manne, B.K.; Basrur, V.; Lehoux, S.; Campbell, R.A.; Yost, C.C.; McIntyre, T.M.; Cummings, R.D.; et al. Different glycoforms of alpha-1-acid glycoprotein contribute to its functional alterations in platelets and neutrophils. J. Leukoc. Biol. 2021, 109, 915–930. [Google Scholar] [CrossRef]
- Higuchi, H.; Kamimura, D.; Jiang, J.J.; Atsumi, T.; Iwami, D.; Hotta, K.; Harada, H.; Takada, Y.; Kanno-Okada, H.; Hatanaka, K.C.; et al. Orosomucoid 1 is involved in the development of chronic allograft rejection after kidney transplantation. Int. Immunol. 2020, 32, 335–346. [Google Scholar] [CrossRef]
- Berntsson, J.; Östling, G.; Persson, M.; Smith, J.G.; Hedblad, B.; Engström, G. Orosomucoid, Carotid Plaque, and Incidence of Stroke. Stroke 2016, 47, 1858–1863. [Google Scholar] [CrossRef]
- Boncela, J.; Papiewska, I.; Fijalkowska, I.; Walkowiak, B.; Cierniewski, C.S. Acute phase protein alpha 1-acid glycoprotein interacts with plasminogen activator inhibitor type 1 and stabilizes its inhibitory activity. J. Biol. Chem. 2001, 276, 35305–35311. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, P.; Levander, L.; Pahlsson, P.; Grenegard, M. alpha(1)-acid glycoprotein (AGP)-induced platelet shape change involves the Rho/Rho kinase signalling pathway. Thromb. Haemost. 2009, 102, 694–703. [Google Scholar] [PubMed]
- Costello, M.; Fiedel, B.A.; Gewurz, H. Inhibition of platelet aggregation by native and desialised alpha-1 acid glycoprotein. Nature 1979, 281, 677–678. [Google Scholar] [CrossRef]
- Daemen, M.A.; Heemskerk, V.H.; van’t Veer, C.; Denecker, G.; Wolfs, T.G.; Vandenabeele, P.; Buurman, W.A. Functional protection by acute phase proteins alpha(1)-acid glycoprotein and alpha(1)-antitrypsin against ischemia/reperfusion injury by preventing apoptosis and inflammation. Circulation 2000, 102, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Fiedel, B.A.; Costello, M.; Gewurz, H.; Hussissian, E. Effects of heparin and alpha 1-acid glycoprotein on thrombin or Activated Thrombofax Reagent-induced platelet aggregation and clot formation. Haemostasis 1983, 13, 89–95. [Google Scholar]
- Osikov, M.V.; Makarov, E.V.; Krivokhizhina, L.V. Effects of alpha1-acid glycoprotein on hemostasis in experimental septic peritonitis. Bull. Exp. Biol. Med. 2007, 144, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Ciccosanti, F.; Antonioli, M.; Sacchi, A.; Notari, S.; Farina, A.; Beccacece, A.; Fusto, M.; Vergori, A.; D’offizi, G.; Taglietti, F.; et al. Proteomic analysis identifies a signature of disease severity in the plasma of COVID-19 pneumonia patients associated to neutrophil, platelet and complement activation. Clin. Proteomics 2022, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Aarts, C.E.M.; Downes, K.; Hoogendijk, A.J.; Sprenkeler, E.G.G.; Gazendam, R.P.; Favier, R.; Favier, M.; Tool, A.T.J.; van Hamme, J.L.; Kostadima, M.A.; et al. Neutrophil specific granule and NETosis defects in gray platelet syndrome. Blood Adv. 2021, 5, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lone, M.A.; Schneiter, R.; Chang, A. Orm1 and Orm2 are conserved endoplasmic reticulum membrane proteins regulating lipid homeostasis and protein quality control. Proc. Natl. Acad. Sci. USA 2010, 107, 5851–5856. [Google Scholar] [CrossRef]
- Sultana, P.; Novotny, J. Rab11 and Its Role in Neurodegenerative Diseases. ASN Neuro 2022, 14, 17590914221142360. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, Z.G.; Segev, N.; Hu, S.; Minshall, R.D.; Dull, R.O.; Zhang, M.; Malik, A.B.; Hu, G. Rab11a Mediates Vascular Endothelial-Cadherin Recycling and Controls Endothelial Barrier Function. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 339–349. [Google Scholar] [CrossRef]
- Gong, Y.; Hu, Y.; Huang, J.; Wang, H. RAB11A aggravates PDGF-BB-stimulated proliferation, migration, and inflammation of airway smooth muscle cells via affecting the NF-kappaB and PI3K/AKT pathways. Allergol. Immunopathol. 2022, 50, 147–154. [Google Scholar] [CrossRef]
- Law, Z.K.; Desborough, M.; Roberts, I.; Al-Shahi Salman, R.; England, T.J.; Werring, D.J.; Robinson, T.; Krishnan, K.; Dineen, R.; Laska, A.C.; et al. Outcomes in Antiplatelet-Associated Intracerebral Hemorrhage in the TICH-2 Randomized Controlled Trial. J. Am. Heart Assoc. 2021, 10, e019130. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Yang, T.; Xia, K.; Yan, Z.; Tan, J.; Li, L.; Qin, Y.; Shi, W. P-selectin (CD62P) and soluble TREM-like transcript-1 (sTLT-1) are associated with coronary artery disease: A case control study. BMC Cardiovasc. Disord. 2020, 20, 387. [Google Scholar] [CrossRef] [PubMed]
- Mannel, D.N.; Grau, G.E. Role of platelet adhesion in homeostasis and immunopathology. Mol. Pathol. 1997, 50, 175–185. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Cho, S.; Lee, J.Y.; Hong, J.Y.; Kim, S.; Jeong, M.-H.; Kim, W.-H. Identification of Potential Drug Targets for Antiplatelet Therapy Specifically Targeting Platelets of Old Individuals through Proteomic Analysis. Biomedicines 2023, 11, 2944. https://doi.org/10.3390/biomedicines11112944
Lee SH, Cho S, Lee JY, Hong JY, Kim S, Jeong M-H, Kim W-H. Identification of Potential Drug Targets for Antiplatelet Therapy Specifically Targeting Platelets of Old Individuals through Proteomic Analysis. Biomedicines. 2023; 11(11):2944. https://doi.org/10.3390/biomedicines11112944
Chicago/Turabian StyleLee, Seung Hee, Suyeon Cho, Jong Youl Lee, Jung Yeon Hong, Suji Kim, Myong-Ho Jeong, and Won-Ho Kim. 2023. "Identification of Potential Drug Targets for Antiplatelet Therapy Specifically Targeting Platelets of Old Individuals through Proteomic Analysis" Biomedicines 11, no. 11: 2944. https://doi.org/10.3390/biomedicines11112944
APA StyleLee, S. H., Cho, S., Lee, J. Y., Hong, J. Y., Kim, S., Jeong, M.-H., & Kim, W.-H. (2023). Identification of Potential Drug Targets for Antiplatelet Therapy Specifically Targeting Platelets of Old Individuals through Proteomic Analysis. Biomedicines, 11(11), 2944. https://doi.org/10.3390/biomedicines11112944





