Blackcurrant Anthocyanins Improve Blood Lipids and Biomarkers of Inflammation and Oxidative Stress in Healthy Women in Menopause Transition without Changing Body Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
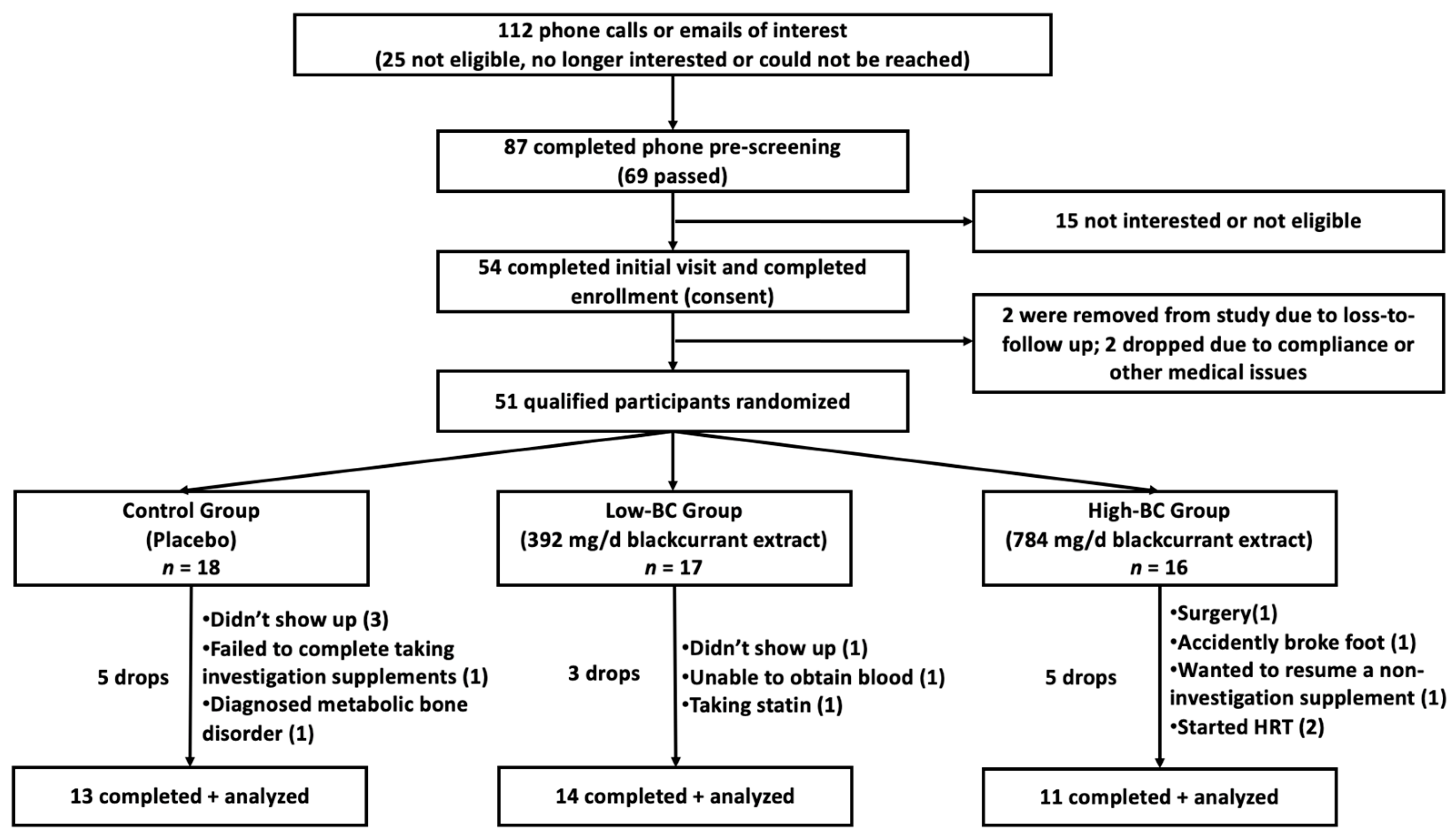
2.1.1. Study Overview, Recruitment and Eligibility
2.1.2. Blackcurrant Intervention
2.2. Anthropometric Assessments
2.3. Assessment of Fasting Blood Lipids and Biomarkers of Inflammation, Oxidative Stress, and Antioxidant Status
2.3.1. Blood Collection and Pretreatment
2.3.2. Measurement of Fasting Blood Lipids
2.3.3. Measurement of Blood Biomarkers of Inflammation and Oxidative Stress
2.3.4. Measurement of Blood Biomarkers of Antioxidant Status
2.4. Statistical Analysis
3. Results
3.1. Baseline
3.2. Anthropometric Measurements
3.3. Lipid Profile
3.4. Inflammatory Biomarkers
3.5. Biomarkers of Oxidative Stress, Antioxidant Capacity, and Antioxidant Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vogel, B.; Acevedo, M.; Appelman, Y.; Bairey Merz, C.N.; Chieffo, A.; Figtree, G.A.; Guerrero, M.; Kunadian, V.; Lam, C.S.P.; Maas, A.; et al. The Lancet women and cardiovascular disease Commission: Reducing the global burden by 2030. Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Hjortland, M.C.; McNamara, P.M.; Gordon, T. Menopause and risk of cardiovascular disease: The Framingham study. Ann. Intern. Med. 1976, 85, 447–452. [Google Scholar] [CrossRef] [PubMed]
- El Khoudary, S.R.; Aggarwal, B.; Beckie, T.M.; Hodis, H.N.; Johnson, A.E.; Langer, R.D.; Limacher, M.C.; Manson, J.E.; Stefanick, M.L.; Allison, M.A. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement From the American Heart Association. Circulation 2020, 142, e506–e532. [Google Scholar] [CrossRef] [PubMed]
- Farahmand, M.; Bahri Khomamid, M.; Rahmati, M.; Azizi, F.; Ramezani Tehrani, F. Aging and changes in adiposity indices: The impact of menopause. J. Endocrinol. Investig. 2022, 45, 69–77. [Google Scholar] [CrossRef]
- El Khoudary, S.R. Gaps, limitations and new insights on endogenous estrogen and follicle stimulating hormone as related to risk of cardiovascular disease in women traversing the menopause: A narrative review. Maturitas 2017, 104, 44–53. [Google Scholar] [CrossRef]
- Schwartz, J.B. Gender-specific implications for cardiovascular medication use in the elderly optimizing therapy for older women. Cardiol. Rev. 2003, 11, 275–298. [Google Scholar] [CrossRef]
- Scott, P.E.; Unger, E.F.; Jenkins, M.R.; Southworth, M.R.; McDowell, T.Y.; Geller, R.J.; Elahi, M.; Temple, R.J.; Woodcock, J. Participation of Women in Clinical Trials Supporting FDA Approval of Cardiovascular Drugs. J. Am. Coll. Cardiol. 2018, 71, 1960–1969. [Google Scholar] [CrossRef]
- Hair, R.; Sakaki, J.R.; Chun, O.K. Anthocyanins, Microbiome and Health Benefits in Aging. Molecules 2021, 26, 537. [Google Scholar] [CrossRef]
- Lee, S.G.; Vance, T.; Nam, T.; Kim, D.; Koo, S.; Chun, O.K. Contribution of anthocyanin composition to total antioxidant capacity of berries. Plant Foods Hum. Nutr. 2015, 70, 427–432. [Google Scholar] [CrossRef]
- Lister, C.; Wilson, P.; Sutton, K.; Morrison, S. Understanding the health benefits of blackcurrants. In Proceedings of the 8th International Rubus and Ribes Symposium, Dundee, UK, 4 July 2002; pp. 443–449. [Google Scholar]
- Gopalan, A.; Reuben, S.C.; Ahmed, S.; Darvesh, A.S.; Hohmann, J.; Bishayee, A. The health benefits of blackcurrants. Food Funct. 2012, 3, 795–809. [Google Scholar] [CrossRef]
- Zhao, F.-l.; Wu, Z.-y.; Hou, Y.; Zhang, T.; Li, K. Efficacy of blackcurrant oil soft capsule, a Chinese herbal drug, in hyperlipidemia treatment. Phytother. Res. 2010, 24 (Suppl. 2), S209–S213. [Google Scholar] [CrossRef]
- McGhie, T.; Walton, M.; Barnett, L.; Vather, R.; Martin, H.; Au, J.; Alspach, P.; Booth, C.; Kruger, M. Boysenberry and blackcurrant drinks increased the plasma antioxidant capacity in an elderly population but had little effect on other markers of oxidative stress. J. Sci. Food Agric. 2007, 87, 2519–2527. [Google Scholar] [CrossRef]
- Moller, P.; Loft, S.; Alfthan, G.; Freese, R. Oxidative DNA damage in circulating mononuclear blood cells after ingestion of blackcurrant juice or anthocyanin-rich drink. Mutat. Res. 2004, 551, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, Y.; Shackelford, A.; Mascioli, E.A.; Babayan, V.K.; Bistrian, B.R.; Blackburn, G.L. The response to endotoxin in guinea pigs after intravenous black currant seed oil. Lipids 1990, 25, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, Y.; Kawaguchi, K.; Takimoto, H. Immunomodulating effects of flavonoids on acute and chronic inflammatory responses caused by tumor necrosis factor alpha. Curr. Pharm. Des. 2006, 12, 4271–4279. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Kim, B.; Yang, Y.; Pham, T.X.; Park, Y.K.; Manatou, J.; Koo, S.I.; Chun, O.K.; Lee, J.Y. Berry anthocyanins suppress the expression and secretion of proinflammatory mediators in macrophages by inhibiting nuclear translocation of NF-κB independent of NRF2-mediated mechanism. J. Nutr. Biochem. 2014, 25, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Nosal, B.M.; Sakaki, J.R.; Macdonald, Z.; Mahoney, K.; Kim, K.; Madore, M.; Thornton, S.; Tran, T.D.B.; Weinstock, G.; Lee, E.C.; et al. Blackcurrants Reduce the Risk of Postmenopausal Osteoporosis: A Pilot Double-Blind, Randomized, Placebo-Controlled Clinical Trial. Nutrients 2022, 14, 4971. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Lee, S.G.; Wang, T.; Vance, T.M.; Hubert, P.; Kim, D.O.; Koo, S.I.; Chun, O.K. Validation of Analytical Methods for Plasma Total Antioxidant Capacity by Comparing with Urinary 8-Isoprostane Level. J. Microbiol. Biotechnol. 2017, 27, 388–394. [Google Scholar] [CrossRef]
- Sardu, C.; Gatta, G.; Pieretti, G.; Viola, L.; Sacra, C.; Di Grezia, G.; Musto, L.; Minelli, S.; La Forgia, D.; Capodieci, M.; et al. Pre-Menopausal Breast Fat Density Might Predict MACE During 10 Years of Follow-Up: The BRECARD Study. JACC Cardiovasc. Imaging 2021, 14, 426–438. [Google Scholar] [CrossRef]
- Sardu, C.; Gatta, G.; Pieretti, G.; Onofrio, N.; Balestrieri, M.L.; Scisciola, L.; Cappabianca, S.; Ferraro, G.; Nicoletti, G.F.; Signoriello, G.; et al. SGLT2 breast expression could affect the cardiovascular performance in pre-menopausal women with fatty vs. non fatty breast via over-inflammation and sirtuins’ down regulation. Eur. J. Intern. Med. 2023, 113, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Stone, N.J.; Ballantyne, C.; Bittner, V.; Criqui, M.H.; Ginsberg, H.N.; Goldberg, A.C.; Howard, W.J.; Jacobson, M.S.; Kris-Etherton, P.M.; et al. Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2011, 123, 2292–2333. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Danesh, J.; Eiriksdottir, G.; Sigurdsson, G.; Wareham, N.; Bingham, S.; Boekholdt, S.M.; Khaw, K.T.; Gudnason, V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007, 115, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Sandhu, M.S.; Ricketts, S.L.; Butterworth, A.S.; Di Angelantonio, E.; Boekholdt, S.M.; Ouwehand, W.; Watkins, H.; Triglyceride Coronary Disease Genetics Consortium; Emerging Risk Factors, Consortium; et al. Triglyceride-mediated pathways and coronary disease: Collaborative analysis of 101 studies. Lancet 2010, 375, 1634–1639. [Google Scholar] [CrossRef]
- Do, R.; Willer, C.J.; Schmidt, E.M.; Sengupta, S.; Gao, C.; Peloso, G.M.; Gustafsson, S.; Kanoni, S.; Ganna, A.; Chen, J.; et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat. Genet. 2013, 45, 1345–1352. [Google Scholar] [CrossRef]
- Teslovich, T.M.; Musunuru, K.; Smith, A.V.; Edmondson, A.C.; Stylianou, I.M.; Koseki, M.; Pirruccello, J.P.; Ripatti, S.; Chasman, D.I.; Willer, C.J.; et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 2010, 466, 707–713. [Google Scholar] [CrossRef]
- Willer, C.J.; Schmidt, E.M.; Sengupta, S.; Peloso, G.M.; Gustafsson, S.; Kanoni, S.; Ganna, A.; Chen, J.; Buchkovich, M.L.; Mora, S.; et al. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 2013, 45, 1274–1283. [Google Scholar] [CrossRef]
- Varbo, A.; Benn, M.; Tybjaerg-Hansen, A.; Jorgensen, A.B.; Frikke-Schmidt, R.; Nordestgaard, B.G. Remnant cholesterol as a causal risk factor for ischemic heart disease. J. Am. Coll. Cardiol. 2013, 61, 427–436. [Google Scholar] [CrossRef]
- Varbo, A.; Benn, M.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation 2013, 128, 1298–1309. [Google Scholar] [CrossRef]
- Twait, C.M.; Slavin, J.L. Grape powder lowers serum triglycerides in postmenopausal women. J. Appl. Res. 2007, 7, 196–203. [Google Scholar]
- Neyestani, T.R.; Yari, Z.; Rasekhi, H.; Nikooyeh, B. How effective are anthocyanins on healthy modification of cardiometabolic risk factors: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2023, 15, 106. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, J.; Zuo, Y.; Ma, K.Y.; Jiang, Y.; Huang, Y.; Chen, Z.Y. Blueberry anthocyanins at doses of 0.5 and 1% lowered plasma cholesterol by increasing fecal excretion of acidic and neutral sterols in hamsters fed a cholesterol-enriched diet. Eur. J. Nutr. 2013, 52, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xia, M.; Ma, J.; Hao, Y.; Liu, J.; Mou, H.; Cao, L.; Ling, W. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 2009, 90, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006, 83, 456s–460s. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.N.; Siddiqui, S.A.; Ibrahim, M.; Hakim, M.L.; Ahammed, M.S.; Kabir, A.; Sultana, F. Inflammatory cytokines in the pathogenesis of cardiovascular disease and cancer. SAGE Open Med. 2020, 8, 205031320965752. [Google Scholar] [CrossRef]
- Hou, D.X.; Yanagita, T.; Uto, T.; Masuzaki, S.; Fujii, M. Anthocyanidins inhibit cyclooxygenase-2 expression in LPS-evoked macrophages: Structure-activity relationship and molecular mechanisms involved. Biochem. Pharmacol. 2005, 70, 417–425. [Google Scholar] [CrossRef]
- Sakaki, J.; Melough, M.; Lee, S.G.; Kalinowski, J.; Koo, S.I.; Lee, S.K.; Chun, O.K. Blackcurrant Supplementation Improves Trabecular Bone Mass in Young but Not Aged Mice. Nutrients 2018, 10, 1671. [Google Scholar] [CrossRef]
- Strugala, P.; Dudra, A.; Gabrielska, J. Activity of Blackcurrant and Chokeberry Extracts and Two Major Cyanidin Glycosides against Lipid Membrane Oxidation and Their Binding Properties to Albumin. Acta Pol. Pharm. 2017, 74, 679–687. [Google Scholar]
- Trevisan, M.; Browne, R.; Ram, M.; Muti, P.; Freudenheim, J.; Carosella, A.M.; Armstrong, D. Correlates of Markers of Oxidative Status in the General Population. Am. J. Epidemiol. 2001, 154, 348–356. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef] [PubMed]
- Rannevik, G.; Jeppsson, S.; Johnell, O.; Bjerre, B.; Laurell-Borulf, Y.; Svanberg, L. A longitudinal study of the perimenopausal transition: Altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas 1995, 21, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef]
- Schmitt, E.; Stopper, H. Estrogenic activity of naturally occurring anthocyanidins. Nutr. Cancer 2001, 41, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Chen, W.; Tu, P.; Jia, R.; Liu, Y.; Li, Y.; Tang, Q.; Zheng, X.; Chu, Q. Food-derived cyanidin-3-O-glucoside alleviates oxidative stress: Evidence from the islet cell line and diabetic db/db mice. Food Funct. 2021, 12, 11599–11610. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, S.; Porri, D.; De Giuseppe, R.; Manuelli, M.; Alessio, F.; Cena, H. The controversial role of vitamin D as an antioxidant: Results from randomised controlled trials. Nutr. Res. Rev. 2019, 32, 99–105. [Google Scholar] [CrossRef]
- Ricca, C.; Aillon, A.; Bergandi, L.; Alotto, D.; Castagnoli, C.; Silvagno, F. Vitamin D Receptor Is Necessary for Mitochondrial Function and Cell Health. Int. J. Mol. Sci. 2018, 19, 1672. [Google Scholar] [CrossRef]
- Piotrowska, A.; Wierzbicka, J.; Ślebioda, T.; Woźniak, M.; Tuckey, R.C.; Slominski, A.T.; Żmijewski, M.A. Vitamin D derivatives enhance cytotoxic effects of H2O2 or cisplatin on human keratinocytes. Steroids 2016, 110, 49–61. [Google Scholar] [CrossRef]
- Harvey, R.E.; Coffman, K.E.; Miller, V.M. Women-specific factors to consider in risk, diagnosis and treatment of cardiovascular disease. Womens Health 2015, 11, 239–257. [Google Scholar] [CrossRef]
- Renke, G.; Starling-Soares, B.; Baesso, T.; Petronio, R.; Aguiar, D.; Paes, R. Effects of Vitamin D on Cardiovascular Risk and Oxidative Stress. Nutrients 2023, 15, 769. [Google Scholar] [CrossRef]
- De la Guía-Galipienso, F.; Martínez-Ferran, M.; Vallecillo, N.; Lavie, C.J.; Sanchis-Gomar, F.; Pareja-Galeano, H. Vitamin D and cardiovascular health. Clin. Nutr. 2021, 40, 2946–2957. [Google Scholar] [CrossRef] [PubMed]
- Derakhshandeh-Rishehri, S.M.; Ghobadi, S.; Akhlaghi, M.; Faghih, S. The effect of calcium supplement intake on lipid profile: A systematic review and meta-analysis of randomized controlled clinical trials. Crit. Rev. Food Sci. Nutr. 2022, 62, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell 2018, 9, 416–431. [Google Scholar] [CrossRef] [PubMed]
| All (n = 38) | Control (Placebo, n = 13) | Low BC (392 mg/day, n = 14) | High BC (784 mg/day, n = 11) | p-Value | |
|---|---|---|---|---|---|
| Age, (y) | 52.9 ± 4.2 | 54.3 ± 3.8 | 53.1 ± 4.6 | 50.9 ± 4.2 | 0.155 |
| Race/ethnicity, n (%) | |||||
| Caucasian | 35 (92.1) | 13 (100) | 11 (78.6) | 11 (100) | 0.272 |
| Hispanic | 1 (2.6) | 1 (7.1) | |||
| Asian American | 2 (5.3) | 2 (14.3) | |||
| Menopause, n (%) | 26 (68.4) | 8 (61.5) | 11 (78.6) | 7 (63.6) | 0.677 |
| SBP (mmHg) | 114.5 ± 14.6 | 115.7 ± 14.1 | 117.0 ± 15.9 | 109.9 ± 13.5 | 0.458 |
| DPB (mmHg) | 78.9 ± 7.6 | 80.5 ± 7.1 | 79.4 ± 8.3 | 76.5 ± 7.4 | 0.432 |
| WC (cm) | 84.3 ± 13.5 | 85.5 ± 15.6 | 85.2 ± 12.4 | 81.8 ± 12.0 | 0.758 |
| BMI (kg/m2) | 26.5 ± 5.7 | 27.0 ± 7.2 | 26.4 ± 4.5 | 25.8 ± 4.9 | 0.878 |
| Lean tissue % | 60.1 ± 6.6 | 61.7 ± 7.2 | 59.3 ± 6.3 | 61.0 ± 6.1 | 0.616 |
| Body fat % | 36.1 ± 7.0 | 35.0 ± 7.7 | 37.5 ± 6.6 | 35.5 ± 6.5 | 0.608 |
| FMI (kg/m2) | 9.8 ± 4.0 | 9.9 ± 4.9 | 10.1 ± 3.2 | 9.4 ± 3.5 | 0.909 |
| Android fat % | 37.2 ± 11.2 | 34.9 ± 12.3 | 40.3 ± 11.3 | 35.9 ± 9.0 | 0.412 |
| Gynoid fat % | 42.1 ± 5.8 | 41.1 ± 5.8 | 42.8 ± 5.1 | 42.3 ± 6.4 | 0.728 |
| A/G ratio | 0.9 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.2 | 0.8 ± 0.1 | 0.280 |
| TFM/TLM | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.666 |
| TC (mg/dL) | 183.3 ± 35.7 | 178.1 ± 31.0 | 179.7 ± 34.4 | 194.0 ± 42.0 | 0.501 |
| HDL (mg/dL) | 67.8 ± 15.6 | 71.5 ± 17.2 | 62.0 ± 15.3 | 70.8 ± 13.9 | 0.227 |
| LDL (mg/dL) | 97.96 ± 31.0 | 92.86 ± 25.70 | 95.16 ± 28.95 | 107.56 ± 38.49 | 0.475 |
| TG (mg/dL) | 87.6 ± 40.5 | 68.4 ± 30.2 | 112.8 ± 57.0 | 78.1 ± 20.7 | <0.05 |
| hs-CRP (mg/L) | 2.0 ± 2.6 | 1.3 ± 1.6 | 2.8 ± 3.7 | 1.6 ± 1.6 | 0.304 |
| IL-1β (pg/mL) * | 17.0 ± 6.9 | 18.0 ± 5.8 | 17.5 ± 7.6 | 15.3 ± 7.4 | 0.679 |
| TBARS (uM) | 0.61 ± 0.2 | 0.51 ± 0.2 | 0.59 ± 0.2 | 0.76 ± 0.2 | <0.05 |
| oxLDL (ng/mL) | 236.0 ± 59.1 | 223.3 ± 58.6 | 242.5 ± 56.9 | 242.8 ± 62.2 | 0.635 |
| TAC (mg VCE/100 mL) | 9.62 ± 1.63 | 9.99 ± 1.59 | 9.38 ± 1.09 | 9.50 ± 2.18 | 0.606 |
| CAT (nmol/min/mL) | 3.37 ± 1.41 | 3.53 ± 1.65 | 3.00 ± 1.05 | 3.66 ± 1.52 | 0.461 |
| Marker | Month | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Control (Placebo, n = 13) | Low BC (392 mg/Day, n = 14) | High BC (784 mg/Day, n = 11) | Group (Low Dose vs. High Dose vs. Placebo | Time (Baseline vs. 3 Months vs. 6 Months) | Time × Group (Interaction) | ||
| SBP (mm Hg) | Baseline | 115.7 ± 4.1 | 117.0± 3.9 | 109.9.8 ± 4.4 | 0.798 | 0.931 | 0.279 |
| 3 | 115.6 ± 4.2 | 111.8 ± 4.0 | 113.3 ± 4.6 | ||||
| 6 | 115.2 ± 3.6 | 114.0 ± 3.4 | 112.6 ± 3.8 | ||||
| DBP (mmHg) | Baseline | 80.5 ± 2.1 | 79.4± 2.0 | 76.5 ± 2.3 | 0.632 | 0.918 | 0.777 |
| 3 | 79.9 ± 2.6 | 77.8 ± 2.5 | 77.6 ± 2.8 | ||||
| 6 | 79.6 ± 2.8 | 80.4 ± 2.7 | 76.8 ± 3.0 | ||||
| BMI (kg/m2) | Baseline | 27.0 ± 1.6 | 26.4 ± 1.5 | 25.8 ± 1.7 | 0.848 | 0.100 | 0.787 |
| 3 | 27.4 ± 1.6 | 26.6 ± 1.6 | 26.1 ± 1.8 | ||||
| 6 | 27.3 ± 1.6 | 26.5 ± 1.6 | 25.8 ± 1.8 | ||||
| WC (cm) | Baseline | 86.5 ± 3.7 | 85.2 ± 3.6 | 81.8 ± 4.1 | 0.738 | <0.01 | 0.578 |
| 3 | 86.1 ± 3.8 | 87.5 ± 3.7 | 83.4 ± 4.2 | ||||
| 6 | 86.3 ± 3.8 | 88.2 ± 3.6 | 83.5 ± 4.1 | ||||
| Lean tissue % | Baseline | 61.7 ± 1.8 | 59.3 ± 1.7 | 61.0 ± 2.0 | 0.720 | 0.681 | 0.609 |
| 6 | 61.1 ± 2.0 | 59.5 ± 1.9 | 60.9 ± 2.1 | ||||
| Body fat % | Baseline | 35.0 ± 1.9 | 37.5 ± 1.9 | 35.5 ± 2.1 | 0.711 | 0.689 | 0.564 |
| 6 | 35.6 ± 2.1 | 37.3 ± 2.0 | 35.6 ± 2.3 | ||||
| FMI (kg/m2) | Baseline | 9.9 ± 1.1 | 10.1 ± 1.1 | 9.4 ± 1.2 | 0.910 | 0.476 | 0.551 |
| 6 | 10.1 ± 1.2 | 10.1 ± 1.1 | 9.4 ± 1.3 | ||||
| Andriod fat % | Baseline | 34.9 ± 3.1 | 40.3 ± 3.0 | 35.9 ± 3.3 | 0.511 | 0.884 | 0.768 |
| 6 | 35.3 ± 3.4 | 39.7 ± 3.3 | 36.3 ± 3.7 | ||||
| Gynoid fat % | Baseline | 41.1 ± 1.6 | 42.8 ± 1.5 | 42.3 ± 1.7 | 0.867 | 0.774 | 0.387 |
| 6 | 42.0 ± 1.7 | 42.7 ± 1.7 | 41.9 ± 1.9 | ||||
| A/G ratio | Baseline | 0.8 ± 0.0 | 0.9 ± 0.0 | 0.8 ± 0.1 | 0.358 | 0.860 | 0.763 |
| 6 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | ||||
| TFM/TLM | Baseline | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.771 | 0.436 | 0.631 |
| 6 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | ||||
| Marker | Month | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Control (Placebo, n = 13) | Low BC (392 mg/Day, n = 14) | High BC (784 mg/Day, n = 11) | Group (Low Dose vs. High Dose vs. Placebo | Time (Baseline vs. 3 Months vs. 6 Months) | Time × Group (Interaction) | ||
| TC (mg/dL) | Baseline | 178.1 ± 9.9 | 179.7 ± 9.5 | 194.0 ± 10.8 | 0.330 | 0.658 | 0.205 |
| 3 | 180.5 ± 9.4 | 177.5 ± 9.1 | 196.7 ± 10.2 | ||||
| 6 | 184.0 ± 10.1 | 167.6 ± 9.7 | 194.2 ± 11.0 | ||||
| HDL (mg/dL) | Baseline | 71.5 ± 4.3 | 62.0 ± 4.2 | 70.8 ± 4.7 | 0.161 | 0.807 | 0.516 |
| 3 | 72.3 ± 4.8 | 62.9 ± 4.7 | 71.6 ± 5.3 | ||||
| 6 | 72.8 ± 4.6 | 59.9 ± 4.5 | 73.5 ± 5.0 | ||||
| LDL (mg/dL) | Baseline | 92.9 ± 4.8 | 95.2 ± 4.7 | 107.6 ± 5.2 | 0.315 | 0.734 | 0.406 |
| 3 | 93.1 ± 4.2 | 93.3 ± 4.2 | 107.6 ± 4.7 | ||||
| 6 | 96.3 ± 4.5 | 85.3 ± 4.3 | 107.0 ± 4.9 | ||||
| TG (mg/dL) | Baseline | 68.4 ± 11.2 | 112.8 ± 10.8 | 78.1 ± 12.2 | <0.05 | 0.507 | 0.134 |
| 3 | 75.5 ± 10.0 | 106.5 ± 9.6 | 87.4 ± 10.9 | ||||
| 6 | 74.2 ± 9.2 | 112.3 ± 8.8 | 68.9 ± 10.0 | ||||
| hs-CRP (mg/L) | Baseline | 1.3 ± 0.7 | 2.8 ± 0.7 | 1.6 ± 0.8 | 0.271 | 0.622 | 0.355 |
| 3 | 1.2 ± 0.8 | 3.1 ± 0.8 | 1.3 ± 0.9 | ||||
| 6 | 1.3 ± 0.6 | 2.2 ± 0.6 | 1.6 ± 0.6 | ||||
| IL-1β (pg/mL) * | Baseline | 18.0 ± 2.0 | 17.5 ± 2.8 | 15.4 ± 2.3 | 0.636 | 0.663 | <0.05 |
| 3 | 17.9 ± 2.0 | 17.4 ± 1.8 | 15.4 ± 2.3 | ||||
| 6 | 18.4 ± 2.0 | 17.3 ± 2.8 | 15.2 ± 2.3 | ||||
| TBARS (uM) | Baseline | 0.50 ± 0.04 | 0.59 ± 0.03 | 0.76 ± 0.04 | <0.05 | 0.997 | 0.140 |
| 3 | 0.65 ± 0.03 | 0.54 ± 0.03 | 0.65 ± 0.04 | ||||
| 6 | 0.53 ± 0.04 | 0.56 ± 0.03 | 0.73 ± 0.04 | ||||
| oxLDL (ng/mL) | Baseline | 216.9 ± 9.4 | 242.5 ± 8.7 | 242.9 ± 9.9 | 0.861 | 0.694 | 0.489 |
| 3 | 228.6 ± 8.7 | 252.7 ± 8.7 | 244.3 ± 9.9 | ||||
| 6 | 238.7 ± 7.9 | 233.8 ± 7.4 | 239.4 ± 8.3 | ||||
| TAC(mg VCE/100 mL) | Baseline | 9.9 ± 0.3 | 9.4 ± 0.2 | 9.5 ± 0.3 | 0.282 | 0.657 | 0.420 |
| 3 | 10.0 ± 0.2 | 9.6 ± 0.2 | 8.7 ± 0.3 | ||||
| 6 | 10.3 ± 0.3 | 9.3 ± 0.2 | 9.6 ± 0.3 | ||||
| CAT (nmol/min/mL) | Baseline | 3.5 ± 0.2 | 3.0 ± 0.2 | 3.7 ± 0.2 | 0.141 | <0.01 | 0.384 |
| 3 | 3.4 ± 0.3 | 3.8 ± 0.3 | 5.0 ± 0.3 | ||||
| 6 | 4.0 ± 0.3 | 4.5 ± 0.3 | 5.3 ± 0.3 | ||||
| ∆TC | ∆HDL | ∆LDL | ∆TG | ∆hs-CRP | ∆IL-1β | ∆TBARS | ∆oxLDL | ∆TAC | ∆CAT | |
|---|---|---|---|---|---|---|---|---|---|---|
| ∆TC | 1.0 | |||||||||
| ∆HDL | 0.57061 (0.0002) | 1.0 | ||||||||
| ∆LDL | 0.91909 (<0.0001) | 0.28388 (0.0841) | 1.0 | |||||||
| ∆TG | −0.02880 (0.8637) | −0.13880 (0.4059) | −0.24332 (0.1410) | 1.0 | ||||||
| ∆hs-CRP | 0.25663 (0.1199) | 0.24334 (0.1410) | 0.08352 (0.6181) | 0.41675 (0.0092) | 1.0 | |||||
| ∆IL-1β | 0.10998 (0.5110) | 0.03787 (0.8214) | 0.04476 (0.7896) | 0.24585 (0.1368) | 0.44174 (0.0055) | 1.0 | ||||
| ∆TBARS | −0.06238 (0.7099) | −0.02323 (0.8899) | 0.02688 (0.8728) | −0.33275 (0.0412) | −0.11608 (0.4877) | 0.00680 (0.9677) | 1.0 | |||
| ∆oxLDL | 0.02847 (0.8653) | −0.09624 (0.5654) | 0.05442 (0.7458) | 0.06411 (0.7022) | 0.20878 (0.2084) | −0.08622 (0.6068) | −0.16624 (0.3185) | 1.0 | ||
| ∆TAC | −0.06189 (0.7121) | −0.04986 (0.7663) | −0.11270 (0.5005) | 0.23321 (0.1588) | 0.15710 (0.3462) | 0.08225 (0.6235) | −0.07633 (0.6488) | −0.34222 (0.0355) | 1.0 | |
| ∆CAT | −0.07445 (0.6569) | 0.13326 (0.4216) | −0.17861 (0.2833) | 0.14689 (0.3788) | −0.05463 (0.7446) | −0.05902 (0.7249) | −0.09861 (0.5599) | −0.33915 (0.0372) | 0.11525 (0.4908) | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nosal, B.M.; Sakaki, J.R.; Mofrad, M.D.; Macdonald, Z.; Mahoney, K.J.; Thornton, S.N.; Patel, D.; Drossman, J.; Lee, E.C.-H.; Chun, O.K. Blackcurrant Anthocyanins Improve Blood Lipids and Biomarkers of Inflammation and Oxidative Stress in Healthy Women in Menopause Transition without Changing Body Composition. Biomedicines 2023, 11, 2834. https://doi.org/10.3390/biomedicines11102834
Nosal BM, Sakaki JR, Mofrad MD, Macdonald Z, Mahoney KJ, Thornton SN, Patel D, Drossman J, Lee EC-H, Chun OK. Blackcurrant Anthocyanins Improve Blood Lipids and Biomarkers of Inflammation and Oxidative Stress in Healthy Women in Menopause Transition without Changing Body Composition. Biomedicines. 2023; 11(10):2834. https://doi.org/10.3390/biomedicines11102834
Chicago/Turabian StyleNosal, Briana M., Junichi R. Sakaki, Manije Darooghegi Mofrad, Zachary Macdonald, Kyle J. Mahoney, Staci N. Thornton, Dave Patel, Joseph Drossman, Elaine Choung-Hee Lee, and Ock K. Chun. 2023. "Blackcurrant Anthocyanins Improve Blood Lipids and Biomarkers of Inflammation and Oxidative Stress in Healthy Women in Menopause Transition without Changing Body Composition" Biomedicines 11, no. 10: 2834. https://doi.org/10.3390/biomedicines11102834
APA StyleNosal, B. M., Sakaki, J. R., Mofrad, M. D., Macdonald, Z., Mahoney, K. J., Thornton, S. N., Patel, D., Drossman, J., Lee, E. C.-H., & Chun, O. K. (2023). Blackcurrant Anthocyanins Improve Blood Lipids and Biomarkers of Inflammation and Oxidative Stress in Healthy Women in Menopause Transition without Changing Body Composition. Biomedicines, 11(10), 2834. https://doi.org/10.3390/biomedicines11102834







