Ultrasound-Driven Healing: Unleashing the Potential of Chondrocyte-Derived Extracellular Vesicles for Chondrogenesis in Adipose-Derived Stem Cells
Abstract
:1. Introduction
2. Results
2.1. Characterization of ADSCs
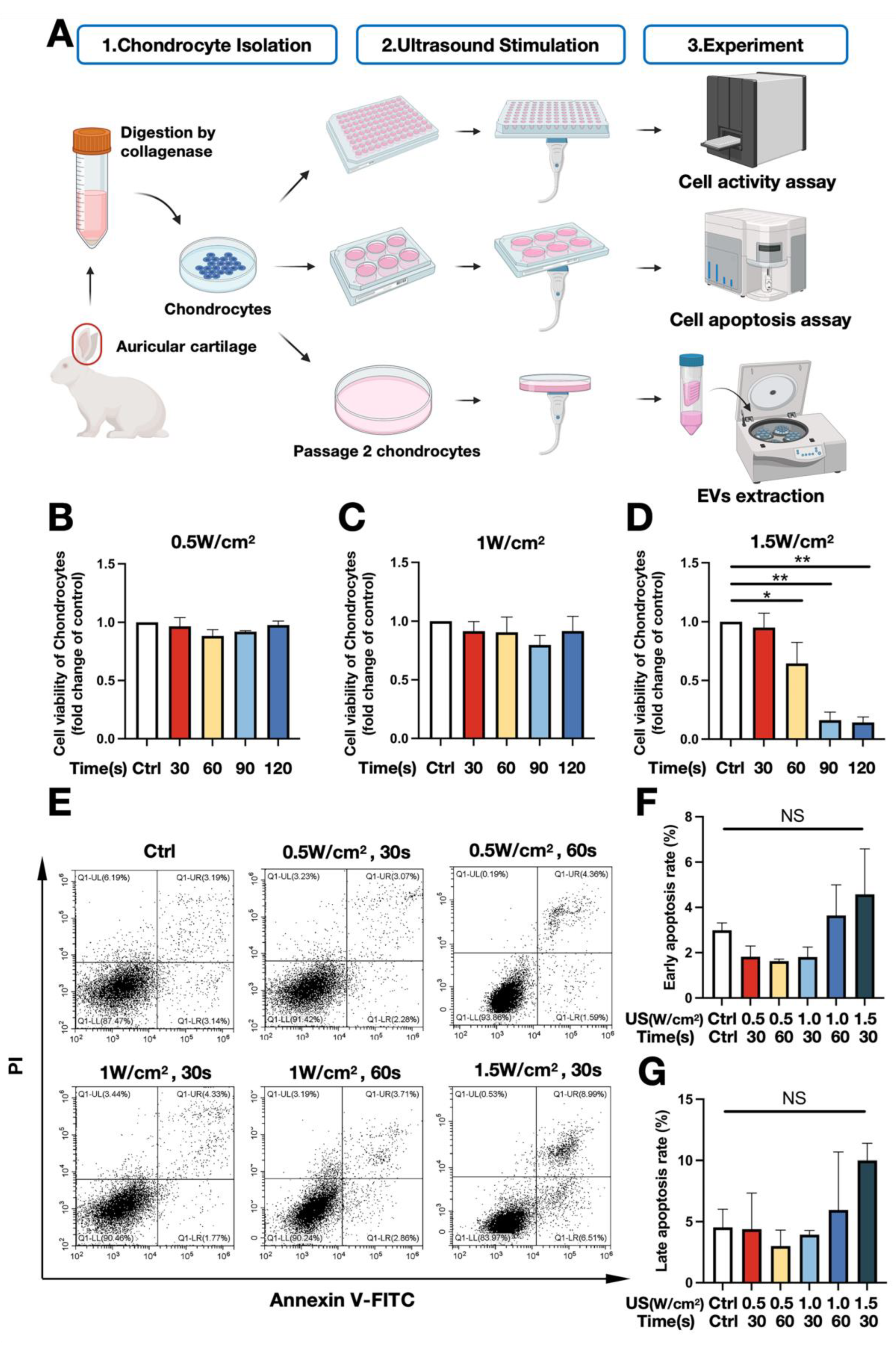
2.2. Optimization of Low-Intensity Ultrasound Parameters
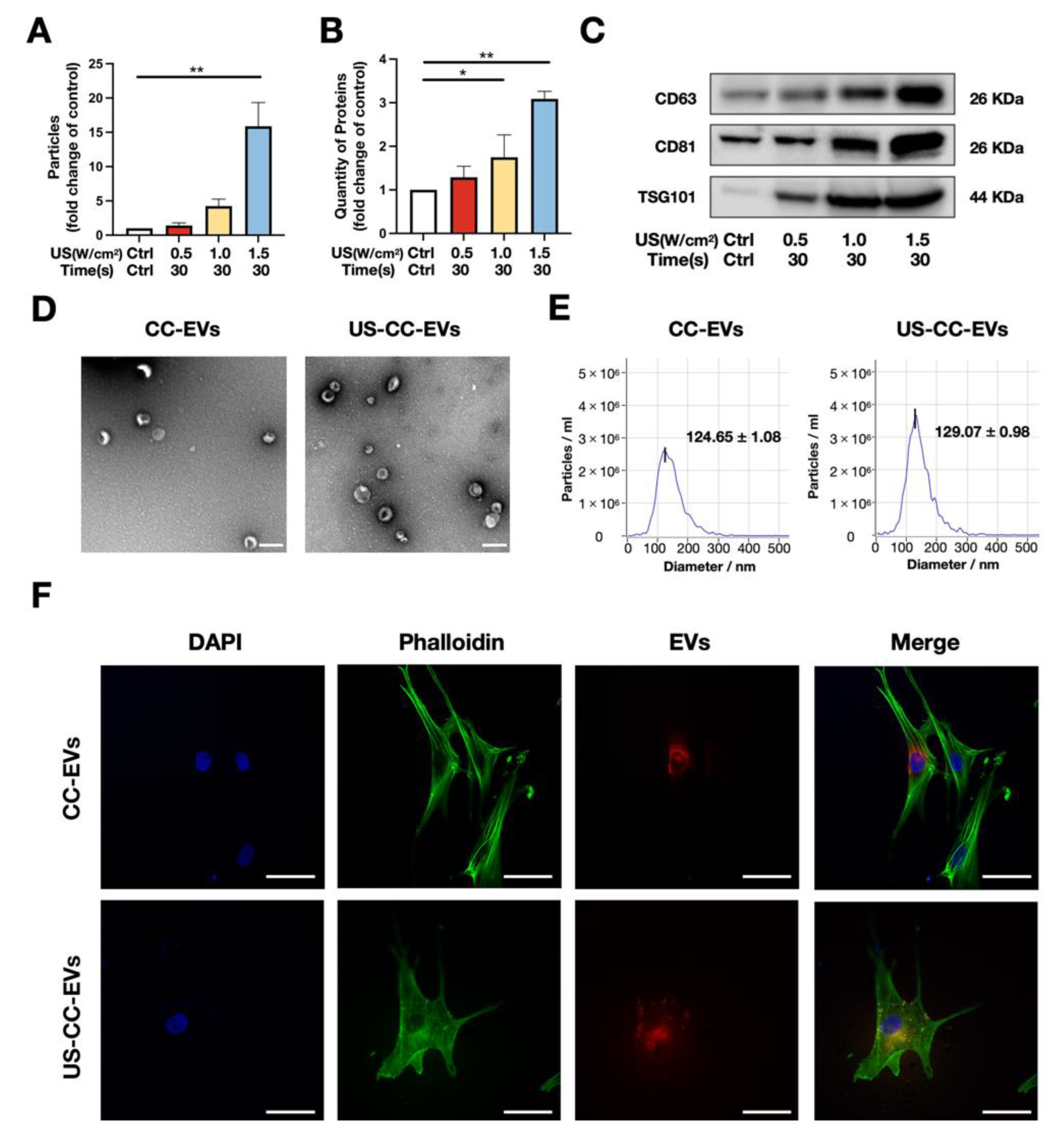
2.3. Appropriate Low-Intensity Ultrasound Significantly Promotes the Release of Extracellular Vesicles by Chondrocytes
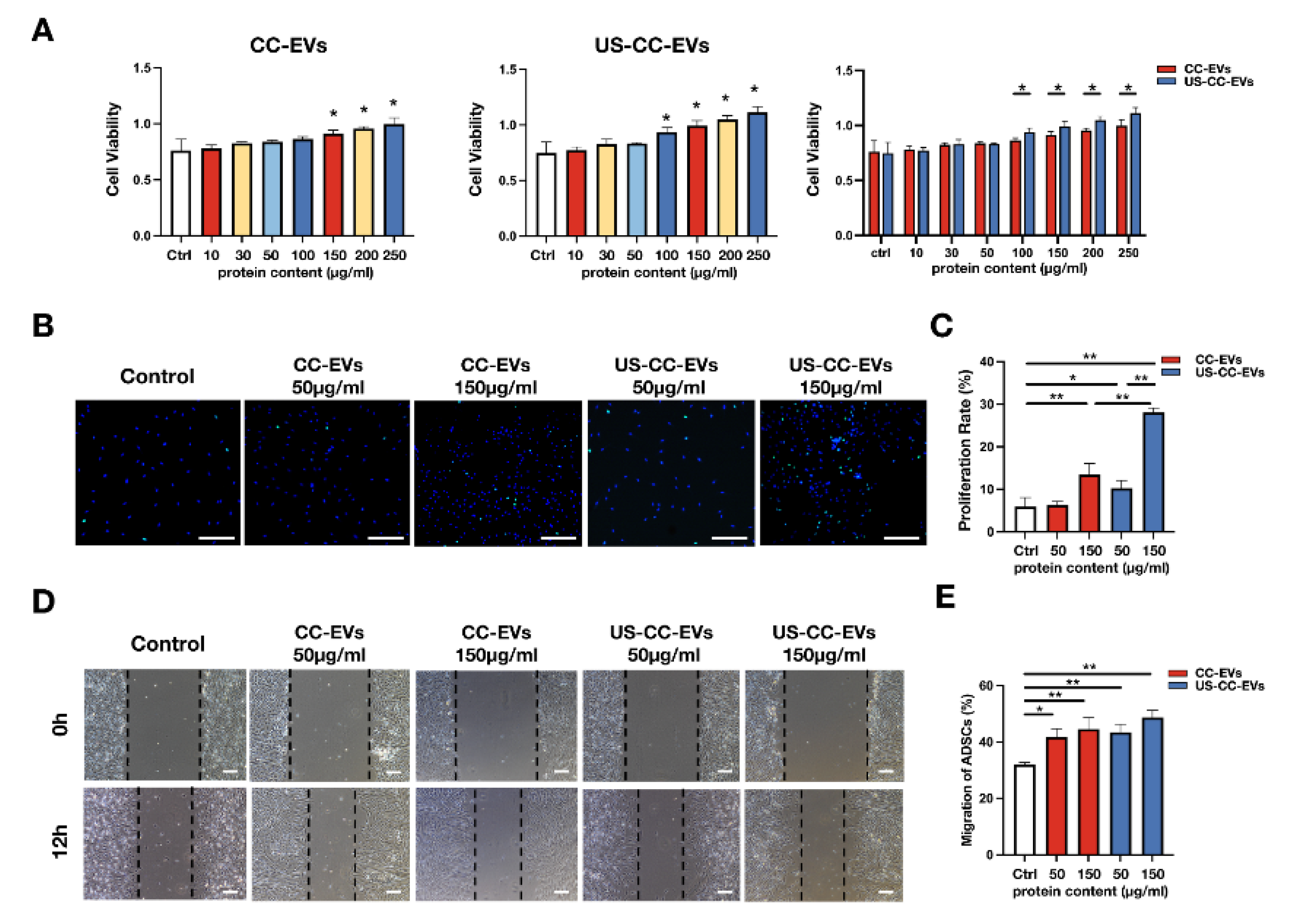
2.4. CC-EVs and US-CC-EVs Promote Cell Activity, Proliferation, and Migration of ADSCs
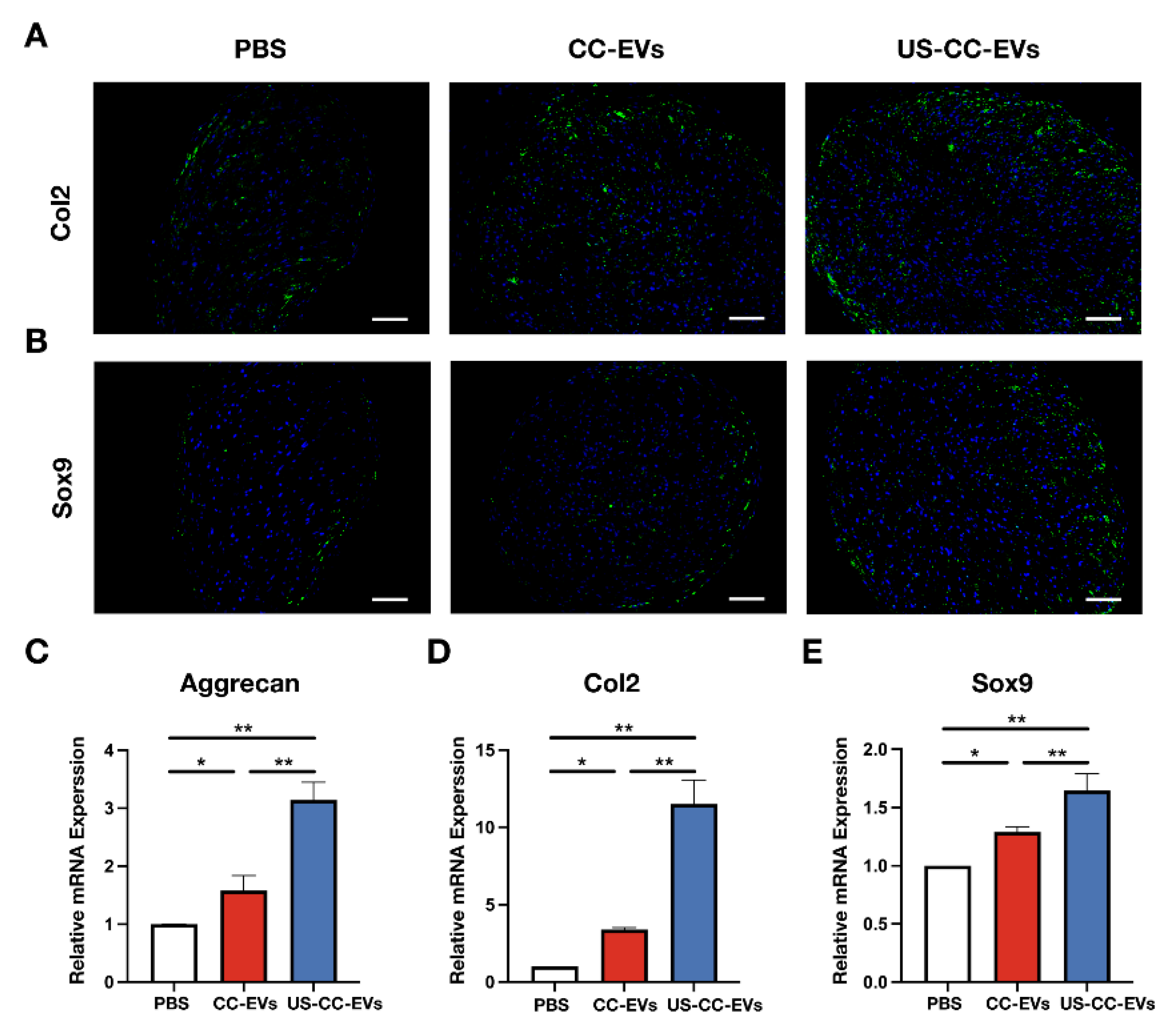
2.5. CC-EVs and US-CC-EVs Promote the Chondrogenic Differentiation of ADSCs
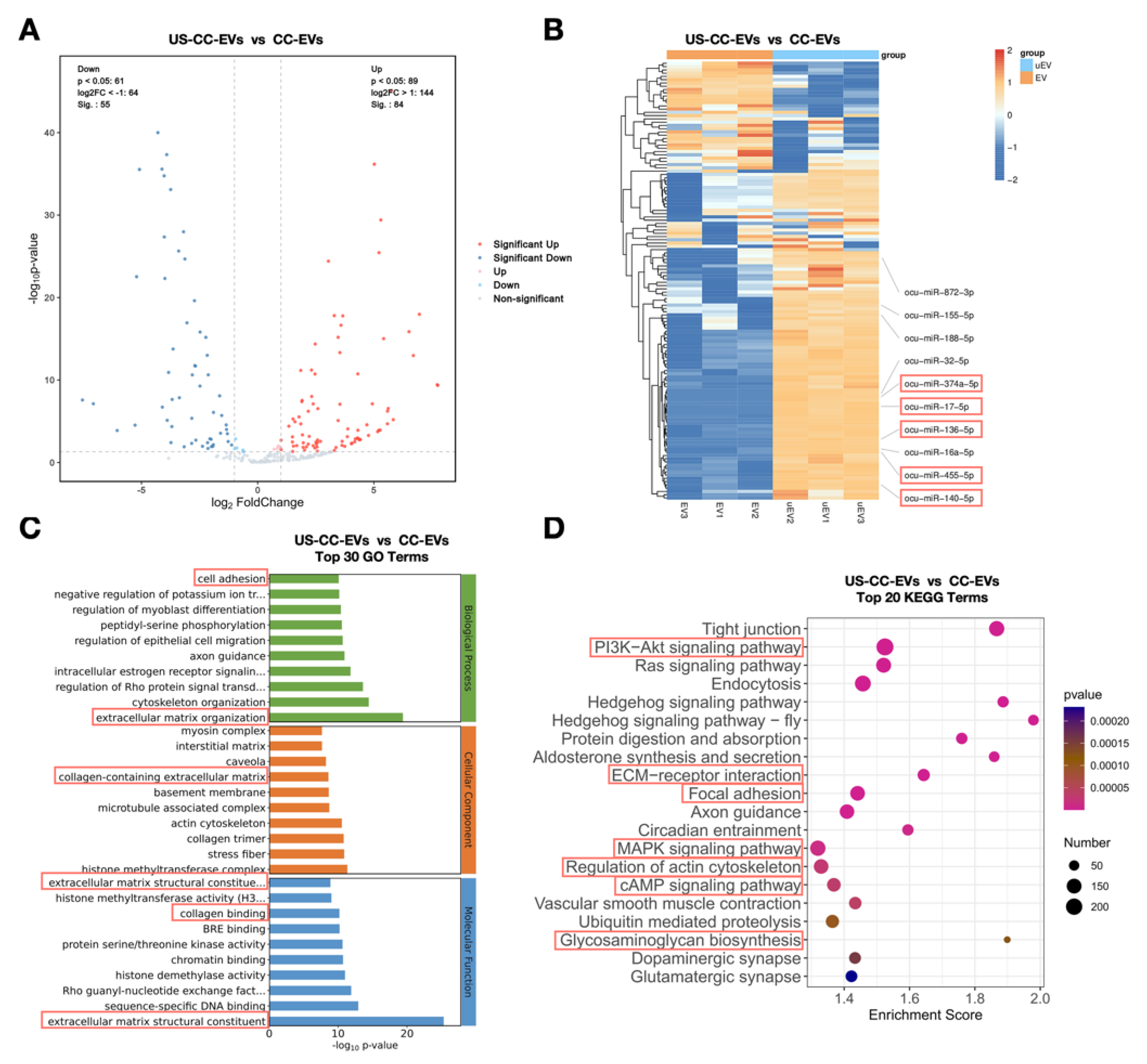
2.6. Expression Profiling of CC-EV and US-CC-EV miRNAs
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of Chondrocytes and ADSCs
4.2. Characterization of ADSCs
4.3. Chondrocyte Ultrasound Stimulation
4.4. Cell Viability Assay
4.5. Apoptosis Assay
4.6. Preparation of EVs
4.7. Cellular Uptake of EVs
4.8. Western Blotting
4.9. In Vitro Cell Proliferation Assay
4.10. Scratch Assay
4.11. In Vitro Chondrogenic Differentiation of ADSCs
4.12. Histological Analyses and Immunofluorescence Staining
4.13. Quantitative RT-PCR Analysis
4.14. RNA Extraction and Library Construction
4.15. Bioinformatics Analysis
4.16. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lories, R.J.; Luyten, F.P. The Bone-Cartilage Unit in Osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Muthu, S.; Korpershoek, J.V.; Novais, E.J.; Tawy, G.F.; Hollander, A.P.; Martin, I. Failure of Cartilage Regeneration: Emerging Hypotheses and Related Therapeutic Strategies. Nat. Rev. Rheumatol. 2023, 19, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, W.; Skinner, J.A.; Gooding, C.R.; Carrington, R.W.J.; Flanagan, A.M.; Briggs, T.W.R.; Bentley, G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: A Prospective, Randomised Study. J. Bone Jt. Surg. Br. Vol. 2005, 87-B, 640–645. [Google Scholar] [CrossRef]
- Liu, Y.; Dzidotor, G.; Le, T.T.; Vinikoor, T.; Morgan, K.; Curry, E.J.; Das, R.; McClinton, A.; Eisenberg, E.; Apuzzo, L.N.; et al. Exercise-Induced Piezoelectric Stimulation for Cartilage Regeneration in Rabbits. Sci. Transl. Med. 2022, 14, eabi7282. [Google Scholar] [CrossRef]
- Armiento, A.R.; Stoddart, M.J.; Alini, M.; Eglin, D. Biomaterials for Articular Cartilage Tissue Engineering: Learning from Biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Sun, M.; Hu, X.; Ren, B.; Cheng, J.; Li, C.; Duan, X.; Fu, X.; Zhang, J.; Chen, H.; et al. Structurally and Functionally Optimized Silk-Fibroin-Gelatin Scaffold Using 3D Printing to Repair Cartilage Injury In Vitro and In Vivo. Adv. Mater. 2017, 29, 1701089. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Tian, B.; Bai, B.; Ci, Z.; Liu, Y.; Zhang, Y.; Zhou, G.; Cao, Y. Dominant Role of in Situ Native Cartilage Niche for Determining the Cartilage Type Regenerated by BMSCs. Bioact. Mater. 2022, 13, 149–160. [Google Scholar] [CrossRef]
- Mohamed-Ahmed, S.; Fristad, I.; Lie, S.A.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S.B. Adipose-Derived and Bone Marrow Mesenchymal Stem Cells: A Donor-Matched Comparison. Stem Cell Res. Ther. 2018, 9, 168. [Google Scholar] [CrossRef]
- Pak, J.; Lee, J.H.; Pak, N.; Pak, Y.; Park, K.S.; Jeon, J.H.; Jeong, B.C.; Lee, S.H. Cartilage Regeneration in Humans with Adipose Tissue-Derived Stem Cells and Adipose Stromal Vascular Fraction Cells: Updated Status. Int. J. Mol. Sci. 2018, 19, 2146. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; Chen, Y.; Zhang, Z.; Saunders, L.; Schipani, E.; Chen, Q.; Ma, P.X. Suppressing Mesenchymal Stem Cell Hypertrophy and Endochondral Ossification in 3D Cartilage Regeneration with Nanofibrous Poly(l-Lactic Acid) Scaffold and Matrilin-3. Acta Biomater. 2018, 76, 29–38. [Google Scholar] [CrossRef]
- Guilak, F.; Estes, B.T.; Diekman, B.O.; Moutos, F.T.; Gimble, J.M. 2010 Nicolas Andry Award: Multipotent Adult Stem Cells from Adipose Tissue for Musculoskeletal Tissue Engineering. Clin. Orthop. Relat. Res. 2010, 468, 2530–2540. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and Tissue Engineering Techniques for Articular Cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Koga, H.; Muneta, T.; Nagase, T.; Nimura, A.; Ju, Y.-J.; Mochizuki, T.; Sekiya, I. Comparison of Mesenchymal Tissues-Derived Stem Cells for in Vivo Chondrogenesis: Suitable Conditions for Cell Therapy of Cartilage Defects in Rabbit. Cell Tissue Res. 2008, 333, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Kohli, N.; Wright, K.T.; Sammons, R.L.; Jeys, L.; Snow, M.; Johnson, W.E.B. An In Vitro Comparison of the Incorporation, Growth, and Chondrogenic Potential of Human Bone Marrow versus Adipose Tissue Mesenchymal Stem Cells in Clinically Relevant Cell Scaffolds Used for Cartilage Repair. Cartilage 2015, 6, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.-W.; Lasitschka, F.; Andrulis, M.; et al. A Complex Secretory Program Orchestrated by the Inflammasome Controls Paracrine Senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef]
- Leipziger, J.; Praetorius, H. Renal Autocrine and Paracrine Signaling: A Story of Self-Protection. Physiol. Rev. 2020, 100, 1229–1289. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, K.; Zhang, X.; Zheng, Z.; Liu, K. Exosomes Derived from Mature Chondrocytes Facilitate Subcutaneous Stable Ectopic Chondrogenesis of Cartilage Progenitor Cells. Stem Cell Res. Ther. 2018, 9, 318. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Xiang, S.; Zheng, Z.; Bian, Y.; Feng, B.; Weng, X. Chondrocytes-Derived Exosomal MiR-8485 Regulated the Wnt/β-Catenin Pathways to Promote Chondrogenic Differentiation of BMSCs. Biochem. Biophys. Res. Commun. 2020, 523, 506–513. [Google Scholar] [CrossRef]
- Hwang, N.S.; Im, S.G.; Wu, P.B.; Bichara, D.A.; Zhao, X.; Randolph, M.A.; Langer, R.; Anderson, D.G. Chondrogenic Priming Adipose-Mesenchymal Stem Cells for Cartilage Tissue Regeneration. Pharm. Res. 2011, 28, 1395–1405. [Google Scholar] [CrossRef]
- de Lucas, B.; Pérez, L.M.; Bernal, A.; Gálvez, B.G. Ultrasound Therapy: Experiences and Perspectives for Regenerative Medicine. Genes 2020, 11, 1086. [Google Scholar] [CrossRef] [PubMed]
- Heckman, J.D.; Ryaby, J.P.; McCabe, J.; Frey, J.J.; Kilcoyne, R.F. Acceleration of Tibial Fracture-Healing by Non-Invasive, Low-Intensity Pulsed Ultrasound. J. Bone Joint Surg. Am. 1994, 76, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.; Lin, S.; Pounder, N.; Mikuni-Takagaki, Y. Mode & Mechanism of Low Intensity Pulsed Ultrasound (LIPUS) in Fracture Repair. Ultrasonics 2016, 70, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, H.; Wang, Z.; Zhu, R.; Cheng, L.; Cheng, Q. Low-Intensity Pulsed Ultrasound Promotes Mesenchymal Stem Cell Transplantation-Based Articular Cartilage Regeneration via Inhibiting the TNF Signaling Pathway. Stem Cell Res. Ther. 2023, 14, 93. [Google Scholar] [CrossRef]
- Nishida, T.; Kubota, S.; Aoyama, E.; Yamanaka, N.; Lyons, K.M.; Takigawa, M. Low-Intensity Pulsed Ultrasound (LIPUS) Treatment of Cultured Chondrocytes Stimulates Production of CCN Family Protein 2 (CCN2), a Protein Involved in the Regeneration of Articular Cartilage: Mechanism Underlying This Stimulation. Osteoarthr. Cartil. 2017, 25, 759–769. [Google Scholar] [CrossRef]
- Yang, T.; Liang, C.; Chen, L.; Li, J.; Geng, W. Low-Intensity Pulsed Ultrasound Alleviates Hypoxia-Induced Chondrocyte Damage in Temporomandibular Disorders by Modulating the Hypoxia-Inducible Factor Pathway. Front. Pharmacol. 2020, 11, 689. [Google Scholar] [CrossRef]
- Sang, F.; Xu, J.; Chen, Z.; Liu, Q.; Jiang, W. Low-Intensity Pulsed Ultrasound Alleviates Osteoarthritis Condition Through Focal Adhesion Kinase-Mediated Chondrocyte Proliferation and Differentiation. Cartilage 2021, 13, 196S–203S. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, P.; Pan, C.; Wang, Y.; Liu, Z.; Chen, Y.; Chen, C.; Fu, S.; Xue, K.; Zhou, Q.; et al. Production and Biological Effects of Extracellular Vesicles from Adipose-Derived Stem Cells Were Markedly Increased by Low-Intensity Ultrasound Stimulation for Promoting Diabetic Wound Healing. Stem Cell Rev. Rep. 2023, 19, 784–806. [Google Scholar] [CrossRef]
- Jiang, X.; Savchenko, O.; Li, Y.; Qi, S.; Yang, T.; Zhang, W.; Chen, J. A Review of Low-Intensity Pulsed Ultrasound for Therapeutic Applications. IEEE Trans. Biomed. Eng. 2019, 66, 2704–2718. [Google Scholar] [CrossRef]
- Bachu, V.S.; Kedda, J.; Suk, I.; Green, J.J.; Tyler, B. High-Intensity Focused Ultrasound: A Review of Mechanisms and Clinical Applications. Ann. Biomed. Eng. 2021, 49, 1975–1991. [Google Scholar] [CrossRef]
- Uddin, S.M.Z.; Richbourgh, B.; Ding, Y.; Hettinghouse, A.; Komatsu, D.E.; Qin, Y.-X.; Liu, C.-J. Chondro-Protective Effects of Low Intensity Pulsed Ultrasound. Osteoarthr. Cartil. 2016, 24, 1989–1998. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-Y.; Jiang, T.; Huang, Z.-F.; Chu, B.; Gu, J.; Zhao, X.; Liu, H.; Fan, J.; Yu, L.-P.; Jiang, S.-H.; et al. Fatty Acids Derived from Apoptotic Chondrocytes Fuel Macrophages FAO through MSR1 for Facilitating BMSCs Osteogenic Differentiation. Redox Biol. 2022, 53, 102326. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Kou, X.; Chen, C.; Liu, S.; Liu, Y.; Yu, W.; Yu, T.; Yang, R.; Wang, R.; Zhou, Y.; et al. Circulating apoptotic bodies maintain mesenchymal stem cell homeostasis and ameliorate osteopenia via transferring multiple cellular factors. Cell Res. 2018, 28, 918–933. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, H.; Shen, H.; Wang, B.; Lei, G.; Tuan, R.S. Mesenchymal Stem Cell-Derived Extracellular Matrix Enhances Chondrogenic Phenotype of and Cartilage Formation by Encapsulated Chondrocytes in Vitro and in Vivo. Acta Biomater. 2018, 69, 71–82. [Google Scholar] [CrossRef]
- Wu, B.; Liu, D.-A.; Guan, L.; Myint, P.K.; Chin, L.; Dang, H.; Xu, Y.; Ren, J.; Li, T.; Yu, Z.; et al. Stiff Matrix Induces Exosome Secretion to Promote Tumour Growth. Nat. Cell Biol. 2023, 25, 415–424. [Google Scholar] [CrossRef]
- King, H.W.; Michael, M.Z.; Gleadle, J.M. Hypoxic Enhancement of Exosome Release by Breast Cancer Cells. BMC Cancer 2012, 12, 421. [Google Scholar] [CrossRef]
- Chen, T.; Guo, J.; Yang, M.; Zhu, X.; Cao, X. Chemokine-Containing Exosomes Are Released from Heat-Stressed Tumor Cells via Lipid Raft-Dependent Pathway and Act as Efficient Tumor Vaccine. J. Immunol. 2011, 186, 2219–2228. [Google Scholar] [CrossRef]
- Du, S.; Liang, C.; Sun, Y.; Ma, B.; Gao, W.; Geng, W. The Attenuating Effect of Low-Intensity Pulsed Ultrasound on Hypoxia-Induced Rat Chondrocyte Damage in TMJ Osteoarthritis Based on TMT Labeling Quantitative Proteomic Analysis. Front. Pharmacol. 2021, 12, 752734. [Google Scholar] [CrossRef]
- Liao, Q.; Li, B.J.; Li, Y.; Xiao, Y.; Zeng, H.; Liu, J.M.; Yuan, L.X.; Liu, G. Low-Intensity Pulsed Ultrasound Promotes Osteoarthritic Cartilage Regeneration by BMSC-Derived Exosomes via Modulating the NF-ΚB Signaling Pathway. Int. Immunopharmacol. 2021, 97, 107824. [Google Scholar] [CrossRef]
- Yu, X.; Odenthal, M.; Fries, J.W.U. Exosomes as MiRNA Carriers: Formation-Function-Future. Int. J. Mol. Sci. 2016, 17, 2028. [Google Scholar] [CrossRef]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-Derived Circulating MiRNAs Regulate Gene Expression in Other Tissues. Nature 2017, 542, 450–455. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Jin, P.; Shang, T.; Sun, R.; Lu, L.; Guo, K.; Liu, J.; Tong, Y.; Wang, J.; et al. Dual Functions of MicroRNA-17 in Maintaining Cartilage Homeostasis and Protection against Osteoarthritis. Nat. Commun. 2022, 13, 2447. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shi, Y.; Xue, P.; Ma, X.; Li, J.; Zhang, J. Mesenchymal Stem Cell-Derived Exosomal MicroRNA-136-5p Inhibits Chondrocyte Degeneration in Traumatic Osteoarthritis by Targeting ELF3. Arthritis Res. Ther. 2020, 22, 256. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.C.; Yuan, T.; Zhang, Y.L.; Yin, W.J.; Guo, S.C.; Zhang, C.Q. Exosomes Derived from MiR-140-5p-Overexpressing Human Synovial Mesenchymal Stem Cells Enhance Cartilage Tissue Regeneration and Prevent Osteoarthritis of the Knee in a Rat Model. Theranostics 2017, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic Signaling Pathways and Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Lee, W.; Nims, R.J.; Savadipour, A.; Zhang, Q.; Leddy, H.A.; Liu, F.; McNulty, A.L.; Chen, Y.; Guilak, F.; Liedtke, W.B. Inflammatory Signaling Sensitizes Piezo1 Mechanotransduction in Articular Chondrocytes as a Pathogenic Feed-Forward Mechanism in Osteoarthritis. Proc. Natl. Acad. Sci. USA 2021, 118, e2001611118. [Google Scholar] [CrossRef]
- Huber, A.K.; Patel, N.; Pagani, C.A.; Marini, S.; Padmanabhan, K.R.; Matera, D.L.; Said, M.; Hwang, C.; Hsu, G.C.-Y.; Poli, A.A.; et al. Immobilization after Injury Alters Extracellular Matrix and Stem Cell Fate. J. Clin. Investig. 2020, 130, 5444–5460. [Google Scholar] [CrossRef]
- Sun, K.; Luo, J.; Guo, J.; Yao, X.; Jing, X.; Guo, F. The PI3K/AKT/MTOR Signaling Pathway in Osteoarthritis: A Narrative Review. Osteoarthr. Cartil. 2020, 28, 400–409. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Q.; Chen, P.; Zhao, S.; Jiang, W.; Wang, F.; Li, P.; Zhang, Y.; Lu, W.; Zhong, T.P.; et al. A Novel Prostaglandin E Receptor 4 (EP4) Small Molecule Antagonist Induces Articular Cartilage Regeneration. Cell Discov. 2022, 8, 24. [Google Scholar] [CrossRef]
- Shen, S.; Yang, Y.; Shen, P.; Ma, J.; Fang, B.; Wang, Q.; Wang, K.; Shi, P.; Fan, S.; Fang, X. CircPDE4B Prevents Articular Cartilage Degeneration and Promotes Repair by Acting as a Scaffold for RIC8A and MID1. Ann. Rheum. Dis. 2021, 80, 1209–1219. [Google Scholar] [CrossRef]
- Li, Q.; Yu, H.; Sun, M.; Yang, P.; Hu, X.; Ao, Y.; Cheng, J. The Tissue Origin Effect of Extracellular Vesicles on Cartilage and Bone Regeneration. Acta Biomater. 2021, 125, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Maglio, M.; Tschon, M.; Aldini, N.N.; Fini, M. Adipose-Derived Mesenchymal Stem Cells for Cartilage Tissue Engineering: State-of-The-Art in in Vivo Studies. J. Biomed. Mater. Res. Part A 2014, 102, 2448–2466. [Google Scholar] [CrossRef]
- Masuoka, K.; Asazuma, T.; Hattori, H.; Yoshihara, Y.; Sato, M.; Matsumura, K.; Matsui, T.; Takase, B.; Nemoto, K.; Ishihara, M. Tissue Engineering of Articular Cartilage with Autologous Cultured Adipose Tissue-Derived Stromal Cells Using Atelocollagen Honeycomb-Shaped Scaffold with a Membrane Sealing in Rabbits. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 79B, 25–34. [Google Scholar] [CrossRef]
- Xue, K.; Xia, W.; Zhang, X.; Qi, L.; Zhou, J.; Xu, P.; Liu, K. Isolation and Identification of Stem Cells in Different Subtype of Cartilage Tissue. Expert. Opin. Biol. Ther. 2015, 15, 623–632. [Google Scholar] [CrossRef]
- Xue, K.; Qi, L.; Zhou, G.; Liu, K. A Two-Step Method of Constructing Mature Cartilage Using Bone Marrow-Derived Mesenchymal Stem Cells. Cells Tissues Organs 2013, 197, 484–495. [Google Scholar] [CrossRef]
- Xu, P.; Yu, Q.; Huang, H.; Zhang, W.J.; Li, W. Nanofat Increases Dermis Thickness and Neovascularization in Photoaged Nude Mouse Skin. Aesthetic Plast. Surg. 2018, 42, 343–351. [Google Scholar] [CrossRef]
- Xu, P.; Xin, Y.; Zhang, Z.; Zou, X.; Xue, K.; Zhang, H.; Zhang, W.; Liu, K. Extracellular Vesicles from Adipose-Derived Stem Cells Ameliorate Ultraviolet B-Induced Skin Photoaging by Attenuating Reactive Oxygen Species Production and Inflammation. Stem Cell Res. Ther. 2020, 11, 264. [Google Scholar] [CrossRef]
- Mao, G.; Zhang, Z.; Hu, S.; Zhang, Z.; Chang, Z.; Huang, Z.; Liao, W.; Kang, Y. Exosomes Derived from MiR-92a-3p-Overexpressing Human Mesenchymal Stem Cells Enhance Chondrogenesis and Suppress Cartilage Degradation via Targeting WNT5A. Stem Cell Res. Ther. 2018, 9, 247. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and Memory-Efficient Alignment of Short DNA Sequences to the Human Genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef]
- Griffiths-Jones, S.; Bateman, A.; Marshall, M.; Khanna, A.; Eddy, S.R. Rfam: An RNA Family Database. Nucleic Acids Res. 2003, 31, 439–441. [Google Scholar] [CrossRef]
- Griffiths-Jones, S.; Saini, H.K.; van Dongen, S.; Enright, A.J. MiRBase: Tools for MicroRNA Genomics. Nucleic Acids Res. 2008, 36, D154–D158. [Google Scholar] [CrossRef] [PubMed]
- Friedländer, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. MiRDeep2 Accurately Identifies Known and Hundreds of Novel MicroRNA Genes in Seven Animal Clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Enright, A.J.; John, B.; Gaul, U.; Tuschl, T.; Sander, C.; Marks, D.S. MicroRNA Targets in Drosophila. Genome Biol. 2003, 5, R1. [Google Scholar] [CrossRef] [PubMed]

| Target Genes | Forward | Reverse |
|---|---|---|
| GAPDH | ATGGTGAAGGTCGGAGTGA | AACATCCACTTTGCCAGATTA |
| Aggrecan | CACCCCGGAATCAAATGGA | TGGGCAGCGAGACCTTGT |
| Col2 | TCCTGTGCGAGACATAATCT | GCAGTGGCGAGGTCAGTAG |
| Sox9 | GGCTCCGACACCGAGAATA | TCCTCTTCGCTCTCCTTCTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, Z.; Pan, C.; Zheng, Y.; Chen, Y.; Lian, X.; Jiang, Y.; Chen, C.; Xue, K.; Zhang, Y.; et al. Ultrasound-Driven Healing: Unleashing the Potential of Chondrocyte-Derived Extracellular Vesicles for Chondrogenesis in Adipose-Derived Stem Cells. Biomedicines 2023, 11, 2836. https://doi.org/10.3390/biomedicines11102836
Wang Y, Liu Z, Pan C, Zheng Y, Chen Y, Lian X, Jiang Y, Chen C, Xue K, Zhang Y, et al. Ultrasound-Driven Healing: Unleashing the Potential of Chondrocyte-Derived Extracellular Vesicles for Chondrogenesis in Adipose-Derived Stem Cells. Biomedicines. 2023; 11(10):2836. https://doi.org/10.3390/biomedicines11102836
Chicago/Turabian StyleWang, Yikai, Zibo Liu, Chuqiao Pan, Yi Zheng, Yahong Chen, Xiang Lian, Yu Jiang, Chuhsin Chen, Ke Xue, Yuanyuan Zhang, and et al. 2023. "Ultrasound-Driven Healing: Unleashing the Potential of Chondrocyte-Derived Extracellular Vesicles for Chondrogenesis in Adipose-Derived Stem Cells" Biomedicines 11, no. 10: 2836. https://doi.org/10.3390/biomedicines11102836
APA StyleWang, Y., Liu, Z., Pan, C., Zheng, Y., Chen, Y., Lian, X., Jiang, Y., Chen, C., Xue, K., Zhang, Y., Xu, P., & Liu, K. (2023). Ultrasound-Driven Healing: Unleashing the Potential of Chondrocyte-Derived Extracellular Vesicles for Chondrogenesis in Adipose-Derived Stem Cells. Biomedicines, 11(10), 2836. https://doi.org/10.3390/biomedicines11102836








