SKAP2—A Molecule at the Crossroads for Integrin Signalling and Immune Cell Migration and Function
Abstract
1. Introduction
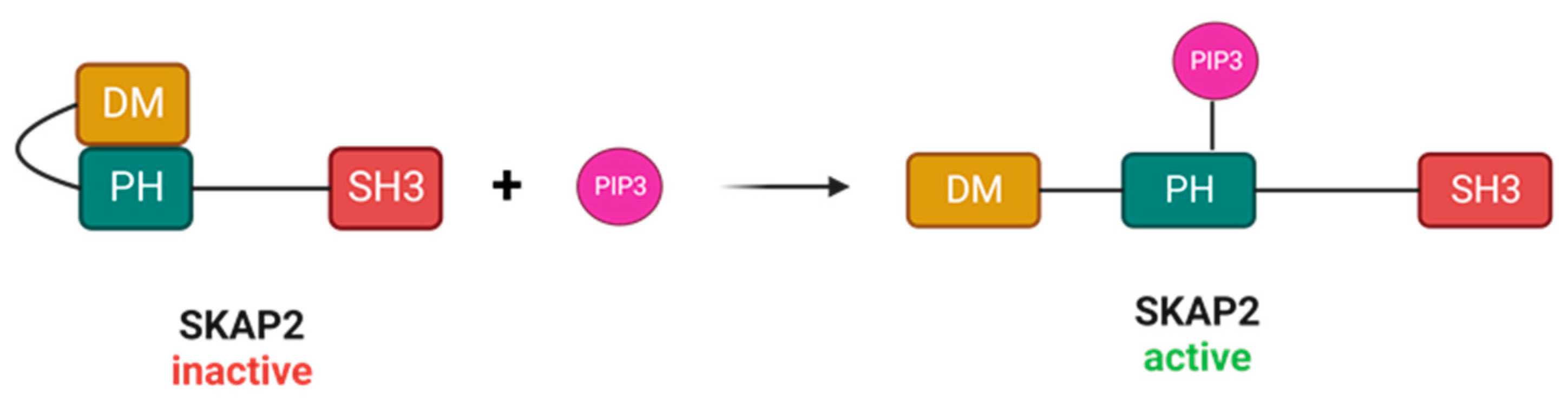
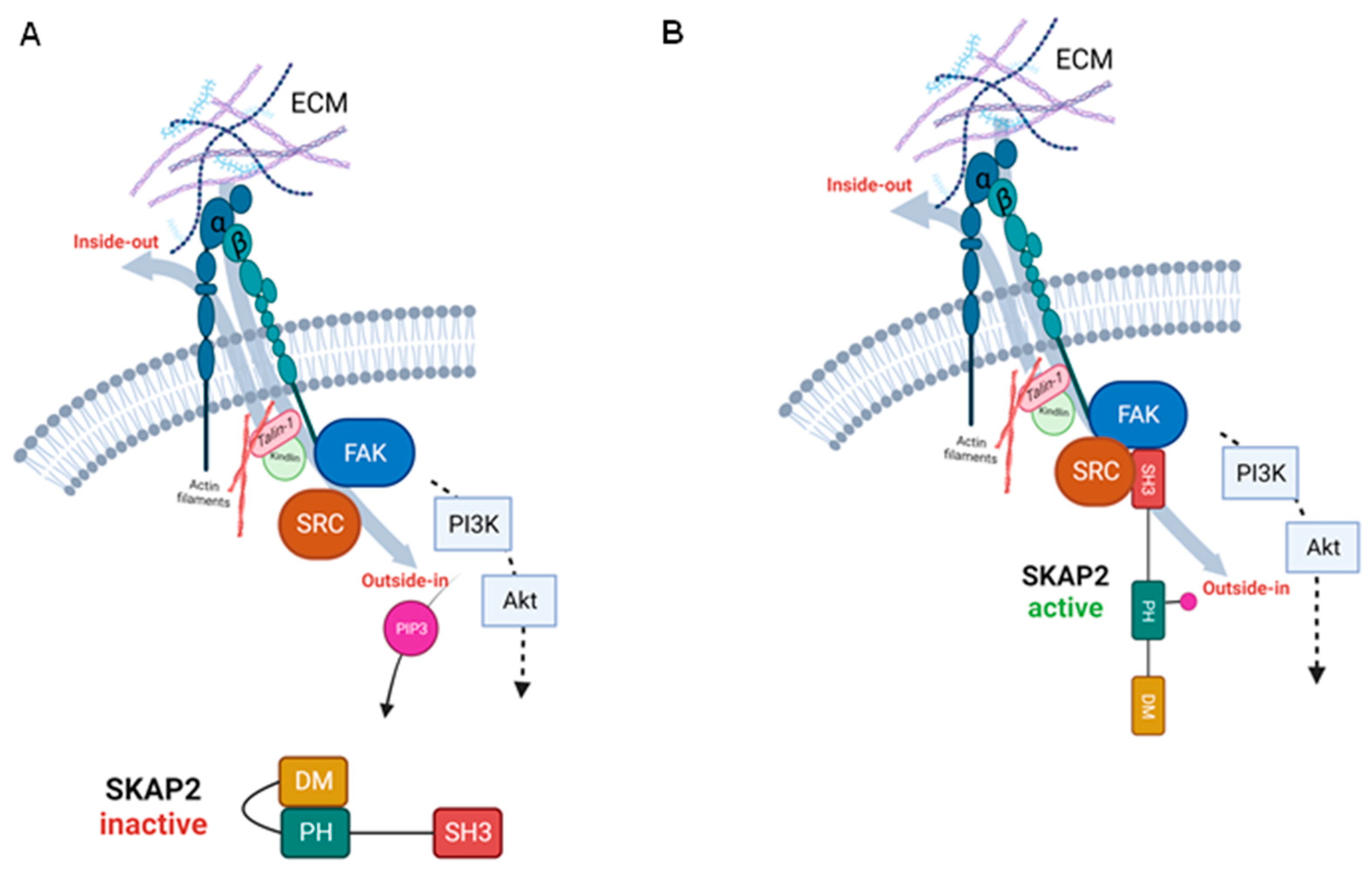
2. Structure of SKAP2
3. Binding Partners of SKAP2
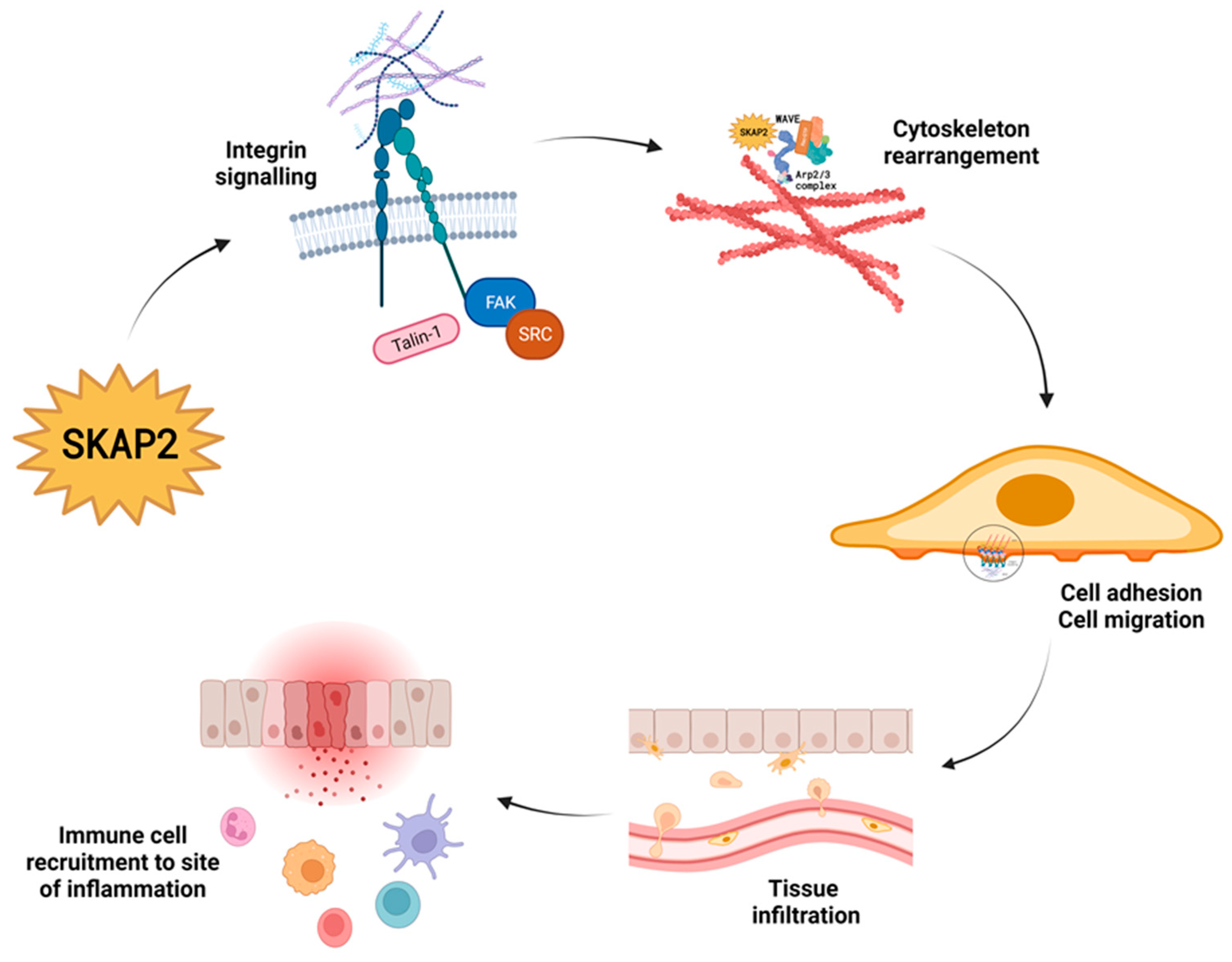
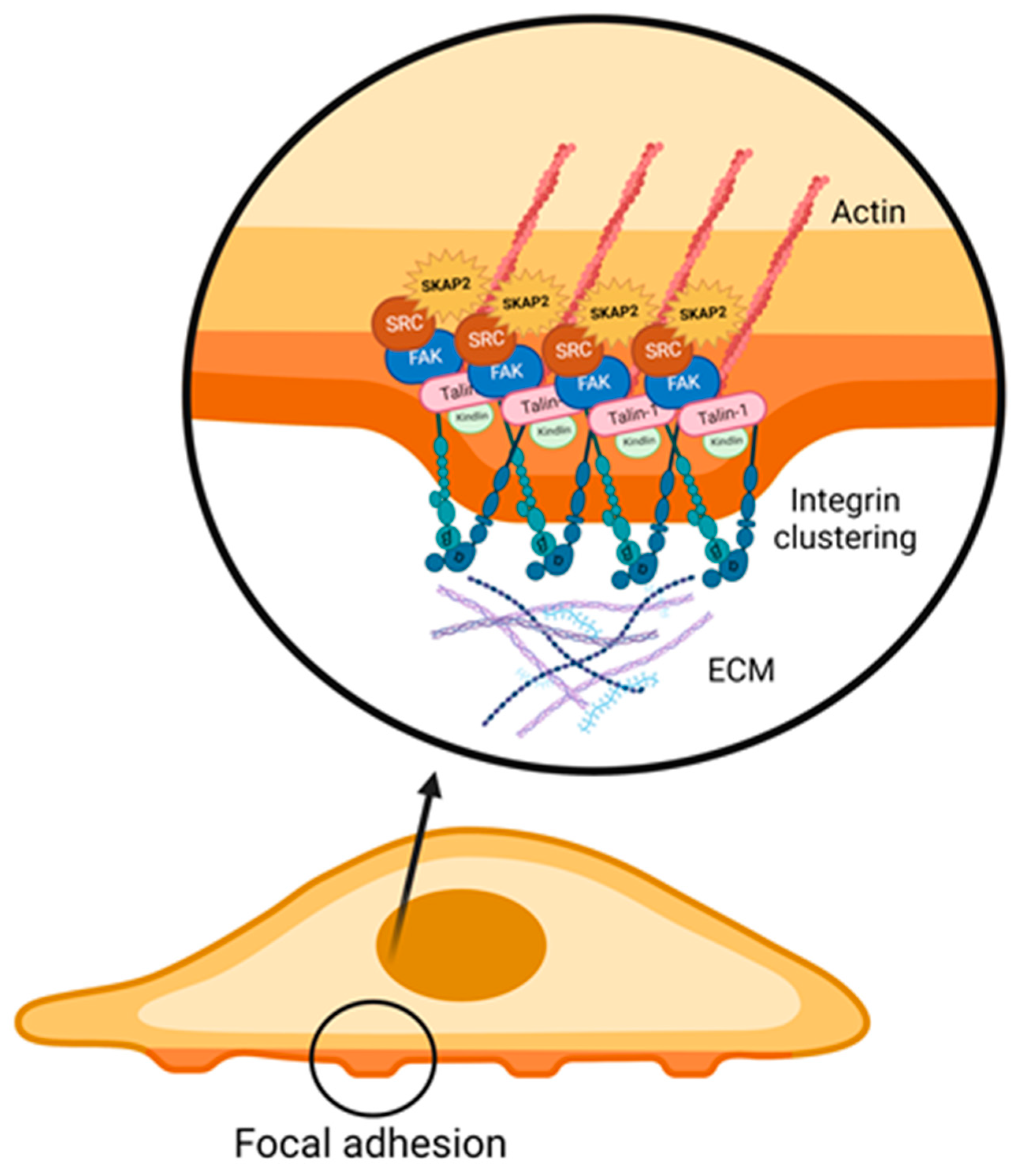
3.1. Integrin Activation and Integrin Signalling
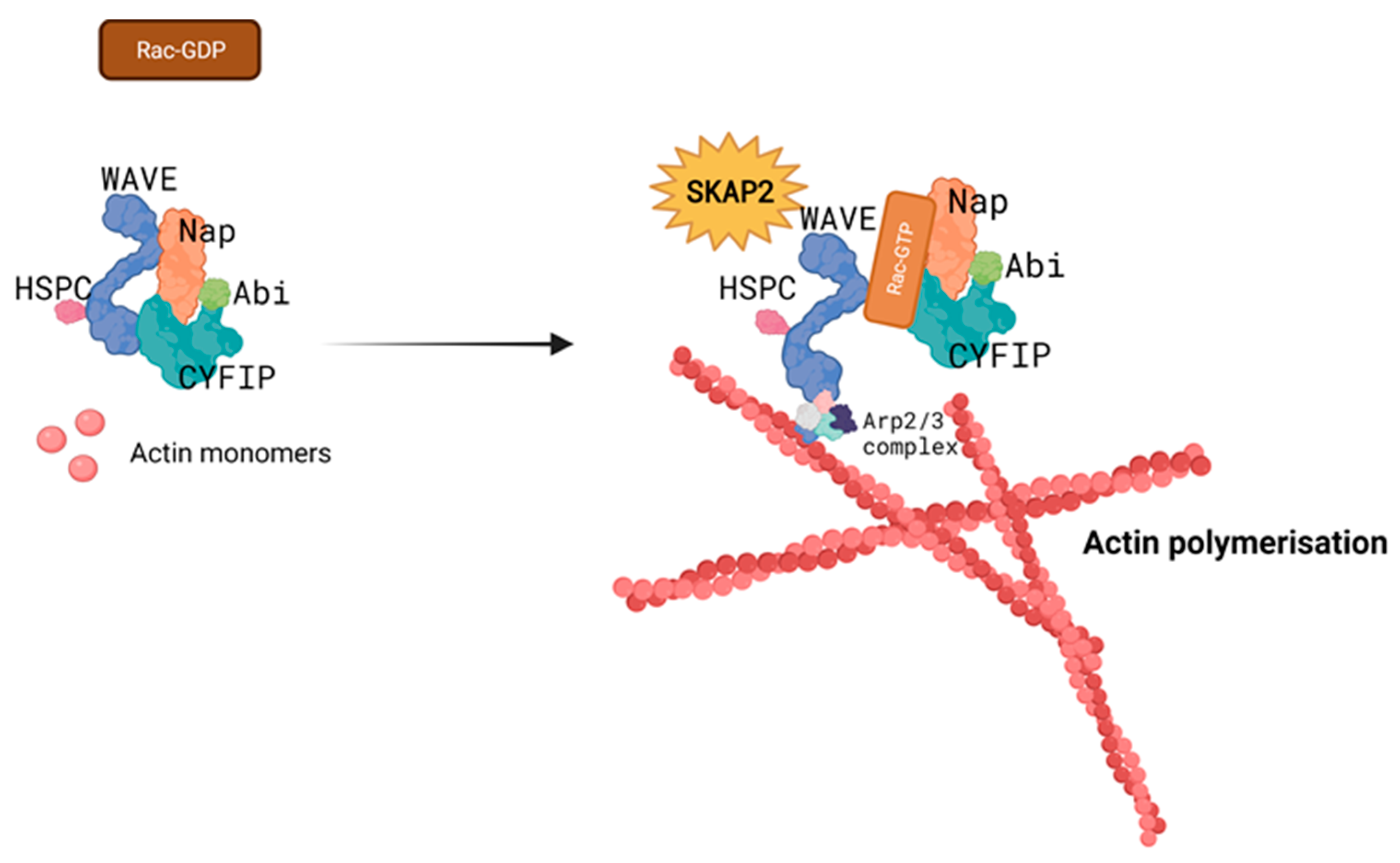
3.2. Interaction with the WAVE Complex Regulates Cytoskeleton Rearrangement
3.3. SKAP2 and Cell Migration
4. The Role of SKAP2 in Immune Cells
4.1. Neutrophils
4.2. Macrophages
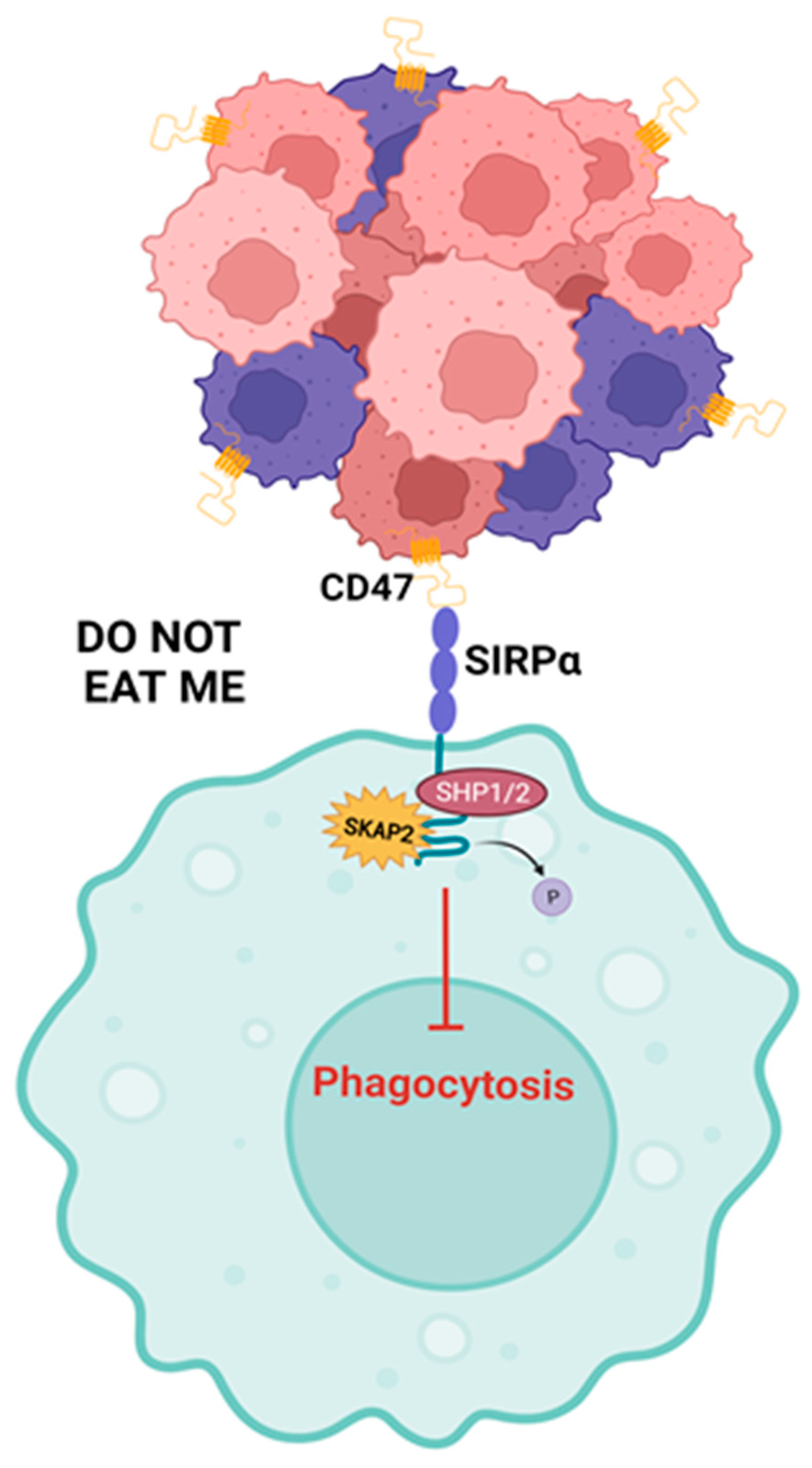
4.2.1. Effects on Phagocytosis in Macrophages
4.2.2. SKAP2 and the Differentiation of Macrophages
4.3. Lymphocytes
5. Relevance for Disease Development
5.1. Cancer
5.2. Inflammatory Disorders
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Valverde, A.; Nares, S.; Naqvi, A.R. Impaired cell migration and structural defects in myeloid cells overexpressing miR-30b and miR-142-3p. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194628. [Google Scholar] [CrossRef] [PubMed]
- Levillayer, L.; Cassonnet, P.; Declercq, M.; Santos, M.D.; Lebreton, L.; Danezi, K.; Demeret, C.; Sakuntabhai, A.; Jacob, Y.; Bureau, J.F. SKAP2 Modular Organization Differently Recognizes SRC Kinases Depending on Their Activation Status and Localization. Mol. Cell Proteom. 2023, 22, 100451. [Google Scholar] [CrossRef]
- Alenghat, F.J.; Baca, Q.J.; Rubin, N.T.; Pao, L.I.; Matozaki, T.; Lowell, C.A.; Golan, D.E.; Neel, B.G.; Swanson, K.D. Macrophages require Skap2 and Sirpα for integrin-stimulated cytoskeletal rearrangement. J. Cell Sci. 2012, 125, 5535–5545. [Google Scholar] [CrossRef]
- Reinhold, A.; Reimann, S.; Reinhold, D.; Schraven, B.; Togni, M. Expression of SKAP-HOM in DCs is required for an optimal immune response in vivo. J. Leukoc. Biol. 2009, 86, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Ellinghaus, E.; Ellinghaus, D.; Krusche, P.; Greiner, A.; Schreiber, C.; Nikolaus, S.; Gieger, C.; Strauch, K.; Lieb, W.; Rosenstiel, P.; et al. Genome-wide association analysis for chronic venous disease identifies EFEMP1 and KCNH8 as susceptibility loci. Sci. Rep. 2017, 7, 45652. [Google Scholar] [CrossRef] [PubMed]
- Rutsch, N.; Chamberlain, C.E.; Dixon, W.; Spector, L.; Letourneau-Freiberg, L.R.; Lwin, W.W.; Philipson, L.H.; Zarbock, A.; Saintus, K.; Wang, J.; et al. Diabetes With Multiple Autoimmune and Inflammatory Conditions Linked to an Activating SKAP2 Mutation. Diabetes Care 2021, 44, 1816–1825. [Google Scholar] [CrossRef]
- Momozawa, Y.; Dmitrieva, J.; Théâtre, E.; Deffontaine, V.; Rahmouni, S.; Charloteaux, B.; Crins, F.; Docampo, E.; Elansary, M.; Gori, A.S.; et al. IBD risk loci are enriched in multigenic regulatory modules encompassing putative causative genes. Nat. Commun. 2018, 9, 2427. [Google Scholar] [CrossRef]
- Garza-Hernandez, D.; Sepulveda-Villegas, M.; Garcia-Pelaez, J.; Aguirre-Gamboa, R.; Lakatos, P.L.; Estrada, K.; Martinez-Vazquez, M.; Trevino, V. A systematic review and functional bioinformatics analysis of genes associated with Crohn’s disease identify more than 120 related genes. BMC Genomics 2022, 23, 302. [Google Scholar] [CrossRef]
- Swanson, K.D.; Tang, Y.; Ceccarelli, D.F.; Poy, F.; Sliwa, J.P.; Neel, B.G.; Eck, M.J. The Skap-hom dimerization and PH domains comprise a 3’-phosphoinositide-gated molecular switch. Mol. Cell 2008, 32, 564–575. [Google Scholar] [CrossRef]
- Feng, J.; He, L.; Li, Y.; Xiao, F.; Hu, G. Modeling of PH Domains and Phosphoinositides Interactions and Beyond. Adv. Exp. Med. Biol. 2019, 1111, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Bai, F.; Ren, X.; Sun, R.; Guo, X.; Liu, W.; Wang, B.; Yang, Y.; Zhang, X.; Xu, Y.; et al. Phosphoinositide-Binding Protein TIPE1 Promotes Alternative Activation of Macrophages and Tumor Progression via PIP3/Akt/TGFβ Axis. Cancer Res. 2022, 82, 1603–1616. [Google Scholar] [CrossRef]
- Marie-Cardine, A.; Bruyns, E.; Eckerskorn, C.; Kirchgessner, H.; Meuer, S.C.; Schraven, B. Molecular cloning of SKAP55, a novel protein that associates with the protein tyrosine kinase p59fyn in human T-lymphocytes. J. Biol. Chem. 1997, 272, 16077–16080. [Google Scholar] [CrossRef] [PubMed]
- Marie-Cardine, A.; Verhagen, A.M.; Eckerskorn, C.; Schraven, B. SKAP-HOM, a novel adaptor protein homologous to the FYN-associated protein SKAP55. FEBS Lett. 1998, 435, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Boras, M.; Volmering, S.; Bokemeyer, A.; Rossaint, J.; Block, H.; Bardel, B.; Van Marck, V.; Heitplatz, B.; Kliche, S.; Reinhold, A.; et al. Skap2 is required for β(2) integrin-mediated neutrophil recruitment and functions. J. Exp. Med. 2017, 214, 851–874. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.D.; Humphries, M.J. Integrin structure, activation, and interactions. Cold Spring Harbor Perspect. Biol. 2011, 3, a004994. [Google Scholar] [CrossRef]
- Moreno-Layseca, P.; Icha, J.; Hamidi, H.; Ivaska, J. Integrin trafficking in cells and tissues. Nat. Cell Biol. 2019, 21, 122–132. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Chen, C.; Manso, A.M.; Ross, R.S. Talin and Kindlin as Integrin-Activating Proteins: Focus on the Heart. Pediatr. Cardiol. 2019, 40, 1401–1409. [Google Scholar] [CrossRef]
- Xiong, J.; Yan, L.; Zou, C.; Wang, K.; Chen, M.; Xu, B.; Zhou, Z.; Zhang, D. Integrins regulate stemness in solid tumor: An emerging therapeutic target. J. Hematol. Oncol. 2021, 14, 177. [Google Scholar] [CrossRef]
- Bouchard, V.; Demers, M.J.; Thibodeau, S.; Laquerre, V.; Fujita, N.; Tsuruo, T.; Beaulieu, J.F.; Gauthier, R.; Vézina, A.; Villeneuve, L.; et al. Fak/Src signaling in human intestinal epithelial cell survival and anoikis: Differentiation state-specific uncoupling with the PI3-K/Akt-1 and MEK/Erk pathways. J. Cell Physiol. 2007, 212, 717–728. [Google Scholar] [CrossRef]
- Thamilselvan, V.; Craig, D.H.; Basson, M.D. FAK association with multiple signal proteins mediates pressure-induced colon cancer cell adhesion via a Src-dependent PI3K/Akt pathway. FASEB J. 2007, 21, 1730–1741. [Google Scholar] [CrossRef]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef]
- Zhao, Y.; Lykov, N.; Tzeng, C. Talin-1 interaction network in cellular mechanotransduction (Review). Int. J. Mol. Med. 2022, 49, 60. [Google Scholar] [CrossRef]
- Han, K.A.; Ko, J. Orchestration of synaptic functions by WAVE regulatory complex-mediated actin reorganization. Exp. Mol. Med. 2023, 55, 1065–1075. [Google Scholar] [CrossRef]
- Dadwal, N.; Mix, C.; Reinhold, A.; Witte, A.; Freund, C.; Schraven, B.; Kliche, S. The Multiple Roles of the Cytosolic Adapter Proteins ADAP, SKAP1 and SKAP2 for TCR/CD3 -Mediated Signaling Events. Front. Immunol. 2021, 12, 703534. [Google Scholar] [CrossRef]
- Ghelman, J.; Grewing, L.; Windener, F.; Albrecht, S.; Zarbock, A.; Kuhlmann, T. SKAP2 as a new regulator of oligodendroglial migration and myelin sheath formation. Glia 2021, 69, 2699–2716. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, G.J.; Reumiller, C.M.; Ercan, H.; Resch, U.; Butt, E.; Heber, S.; Liutkevičiūte, Z.; Basílio, J.; Schmid, J.A.; Assinger, A.; et al. Comparative proteomics reveals unexpected quantitative phosphorylation differences linked to platelet activation state. Sci. Rep. 2019, 9, 19009. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, E.; Hall, A.; Scott, A.M.; Chagnon, M.J.; Miquel, G.; Hallé, M.; Noda, M.; Bikfalvi, A.; Tremblay, M.L. Regulation of the Src kinase-associated phosphoprotein 55 homologue by the protein tyrosine phosphatase PTP-PEST in the control of cell motility. J. Biol. Chem. 2013, 288, 25739–25748. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Z.; Zheng, Y.; Zhu, Y.; Wei, Z.; Xu, H.; Tang, Q.; Kong, X.; Hu, L. SKAP2, a novel target of HSF4b, associates with NCK2/F-actin at membrane ruffles and regulates actin reorganization in lens cell. J. Cell Mol. Med. 2011, 15, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Chen, Y.; Sheng, Z.; Wang, X.; Zhang, Z.; Huang, J.; Zhou, J.; Lin, F. SKAP2 is downregulated in the villous tissues of patients with missed abortion and regulates growth and migration in trophoblasts through the WAVE2-ARP2/3 signaling pathway. Placenta 2022, 128, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Shaban, L.; Nguyen, G.T.; Mecsas-Faxon, B.D.; Swanson, K.D.; Tan, S.; Mecsas, J. Yersinia pseudotuberculosis YopH targets SKAP2-dependent and independent signaling pathways to block neutrophil antimicrobial mechanisms during infection. PLoS Pathog. 2020, 16, e1008576. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.T.; Shaban, L.; Mack, M.; Swanson, K.D.; Bunnell, S.C.; Sykes, D.B.; Mecsas, J. SKAP2 is required for defense against K. pneumoniae infection and neutrophil respiratory burst. eLife 2020, 9, e56656. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.T.; Green, E.R.; Mecsas, J. Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance. Front. Cell Infect. Microbiol. 2017, 7, 373. [Google Scholar] [CrossRef] [PubMed]
- Coppolino, M.G.; Krause, M.; Hagendorff, P.; Monner, D.A.; Trimble, W.; Grinstein, S.; Wehland, J.; Sechi, A.S. Evidence for a molecular complex consisting of Fyb/SLAP, SLP-76, Nck, VASP and WASP that links the actin cytoskeleton to Fcgamma receptor signalling during phagocytosis. J. Cell Sci. 2001, 114, 4307–4318. [Google Scholar] [CrossRef]
- Wang, H.; Rudd, C.E. SKAP-55, SKAP-55-related and ADAP adaptors modulate integrin-mediated immune-cell adhesion. Trends Cell Biol. 2008, 18, 486–493. [Google Scholar] [CrossRef]
- Black, D.S.; Marie-Cardine, A.; Schraven, B.; Bliska, J.B. The Yersinia tyrosine phosphatase YopH targets a novel adhesion-regulated signalling complex in macrophages. Cell Microbiol. 2000, 2, 401–414. [Google Scholar] [CrossRef]
- Okazawa, H.; Motegi, S.; Ohyama, N.; Ohnishi, H.; Tomizawa, T.; Kaneko, Y.; Oldenborg, P.A.; Ishikawa, O.; Matozaki, T. Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J. Immunol. 2005, 174, 2004–2011. [Google Scholar] [CrossRef]
- Oldenborg, P.A.; Gresham, H.D.; Lindberg, F.P. CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J. Exp. Med. 2001, 193, 855–862. [Google Scholar] [CrossRef]
- Shi, L.; Kidder, K.; Bian, Z.; Chiang, S.K.T.; Ouellette, C.; Liu, Y. SIRPα sequesters SHP-2 to promote IL-4 and IL-13 signaling and the alternative activation of macrophages. Sci. Signal 2021, 14, eabb3966. [Google Scholar] [CrossRef]
- Takagane, K.; Umakoshi, M.; Itoh, G.; Kuriyama, S.; Goto, A.; Tanaka, M. SKAP2 suppresses inflammation-mediated tumorigenesis by regulating SHP-1 and SHP-2. Oncogene 2022, 41, 1087–1099. [Google Scholar] [CrossRef]
- Gauttier, V.; Pengam, S.; Durand, J.; Biteau, K.; Mary, C.; Morello, A.; Néel, M.; Porto, G.; Teppaz, G.; Thepenier, V.; et al. Selective SIRPα blockade reverses tumor T cell exclusion and overcomes cancer immunotherapy resistance. J. Clin. Investig. 2020, 130, 6109–6123. [Google Scholar] [CrossRef]
- Schroeder, A.; Hyatt, D.; Alenghat, F.J. Skap2 Regulates Atherosclerosis through Macrophage Polarization and Efferocytosis. Arterioscler. Thromb. Vasc. Biol. 2019, 38, A001. [Google Scholar] [CrossRef]
- Alenghat, D.H.a.F.J. Skap2 Regulates the Inflammatory Macrophage Response in Atherogenesis. Circulation 2015, 132, A18680. [Google Scholar] [CrossRef]
- Rivers, E.; Thrasher, A.J. Wiskott-Aldrich syndrome protein: Emerging mechanisms in immunity. Eur. J. Immunol. 2017, 47, 1857–1866. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.; Ismail, S. Spatiotemporal Regulation of Signaling: Focus on T Cell Activation and the Immunological Synapse. Int. J. Mol. Sci. 2020, 21, 3283. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Norton, D.D.; Precht, P.; Martindale, J.L.; Burkhardt, J.K.; Wange, R.L. Deficiency of ADAP/Fyb/SLAP-130 destabilizes SKAP55 in Jurkat T cells. J. Biol. Chem. 2005, 280, 23576–23583. [Google Scholar] [CrossRef]
- Togni, M.; Swanson, K.D.; Reimann, S.; Kliche, S.; Pearce, A.C.; Simeoni, L.; Reinhold, D.; Wienands, J.; Neel, B.G.; Schraven, B.; et al. Regulation of in vitro and in vivo immune functions by the cytosolic adaptor protein SKAP-HOM. Mol. Cell Biol. 2005, 25, 8052–8063. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jo, E.K.; Wang, H.; Rudd, C.E. An essential role for SKAP-55 in LFA-1 clustering on T cells that cannot be substituted by SKAP-55R. J. Exp. Med. 2005, 201, 1733–1739. [Google Scholar] [CrossRef]
- Su, W.; Wynne, J.; Pinheiro, E.M.; Strazza, M.; Mor, A.; Montenont, E.; Berger, J.; Paul, D.S.; Bergmeier, W.; Gertler, F.B.; et al. Rap1 and its effector RIAM are required for lymphocyte trafficking. Blood 2015, 126, 2695–2703. [Google Scholar] [CrossRef]
- Kuranami, S.; Yokobori, T.; Mogi, A.; Altan, B.; Yajima, T.; Onozato, R.; Azuma, Y.; Iijima, M.; Kosaka, T.; Kuwano, H. Src kinase-associated phosphoprotein2 expression is associated with poor prognosis in non-small cell lung cancer. Anticancer Res. 2015, 35, 2411–2415. [Google Scholar]
- Tanaka, M.; Shimamura, S.; Kuriyama, S.; Maeda, D.; Goto, A.; Aiba, N. SKAP2 Promotes Podosome Formation to Facilitate Tumor-Associated Macrophage Infiltration and Metastatic Progression. Cancer Res. 2016, 76, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Allison, E.; Edirimanne, S.; Matthews, J.; Fuller, S.J. Breast Cancer Survival Outcomes and Tumor-Associated Macrophage Markers: A Systematic Review and Meta-Analysis. Oncol. Ther. 2023, 11, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Obata, T.; Khan, Q.; Highshaw, R.A.; De Vere White, R.; Sweeney, C. The phosphatidylinositol-3 kinase pathway regulates bladder cancer cell invasion. BJU Int. 2004, 93, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Fløyel, T.; Meyerovich, K.; Prause, M.C.; Kaur, S.; Frørup, C.; Mortensen, H.B.; Nielsen, L.B.; Pociot, F.; Cardozo, A.K.; Størling, J. SKAP2, a Candidate Gene for Type 1 Diabetes, Regulates β-Cell Apoptosis and Glycemic Control in Newly Diagnosed Patients. Diabetes 2021, 70, 464–476. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilmink, M.; Spalinger, M.R. SKAP2—A Molecule at the Crossroads for Integrin Signalling and Immune Cell Migration and Function. Biomedicines 2023, 11, 2788. https://doi.org/10.3390/biomedicines11102788
Wilmink M, Spalinger MR. SKAP2—A Molecule at the Crossroads for Integrin Signalling and Immune Cell Migration and Function. Biomedicines. 2023; 11(10):2788. https://doi.org/10.3390/biomedicines11102788
Chicago/Turabian StyleWilmink, Marijn, and Marianne Rebecca Spalinger. 2023. "SKAP2—A Molecule at the Crossroads for Integrin Signalling and Immune Cell Migration and Function" Biomedicines 11, no. 10: 2788. https://doi.org/10.3390/biomedicines11102788
APA StyleWilmink, M., & Spalinger, M. R. (2023). SKAP2—A Molecule at the Crossroads for Integrin Signalling and Immune Cell Migration and Function. Biomedicines, 11(10), 2788. https://doi.org/10.3390/biomedicines11102788




