Peritumoral Edema in Gliomas: A Review of Mechanisms and Management
Abstract
1. Introduction
2. Clinical Features of Glioma-Related Edema
2.1. Clinical Importance
2.2. Intracranial Pathophysiology of the Pressure–Volume Relationship and Cerebral Blood Flow (CBF) in Peritumoral Edema
2.3. Imaging Findings
3. Mechanisms Underlying the Formation of Glioma-Related Edema
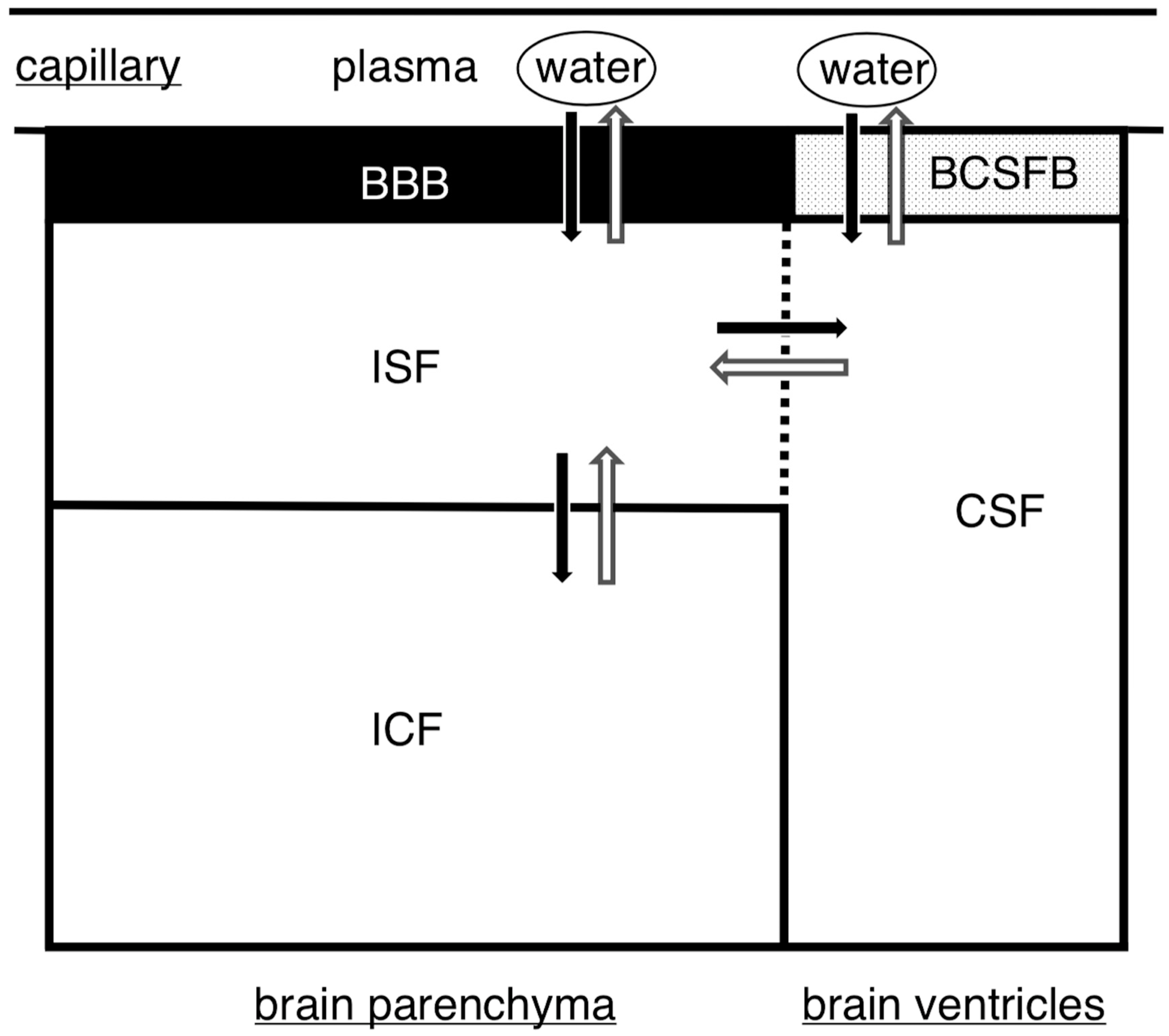
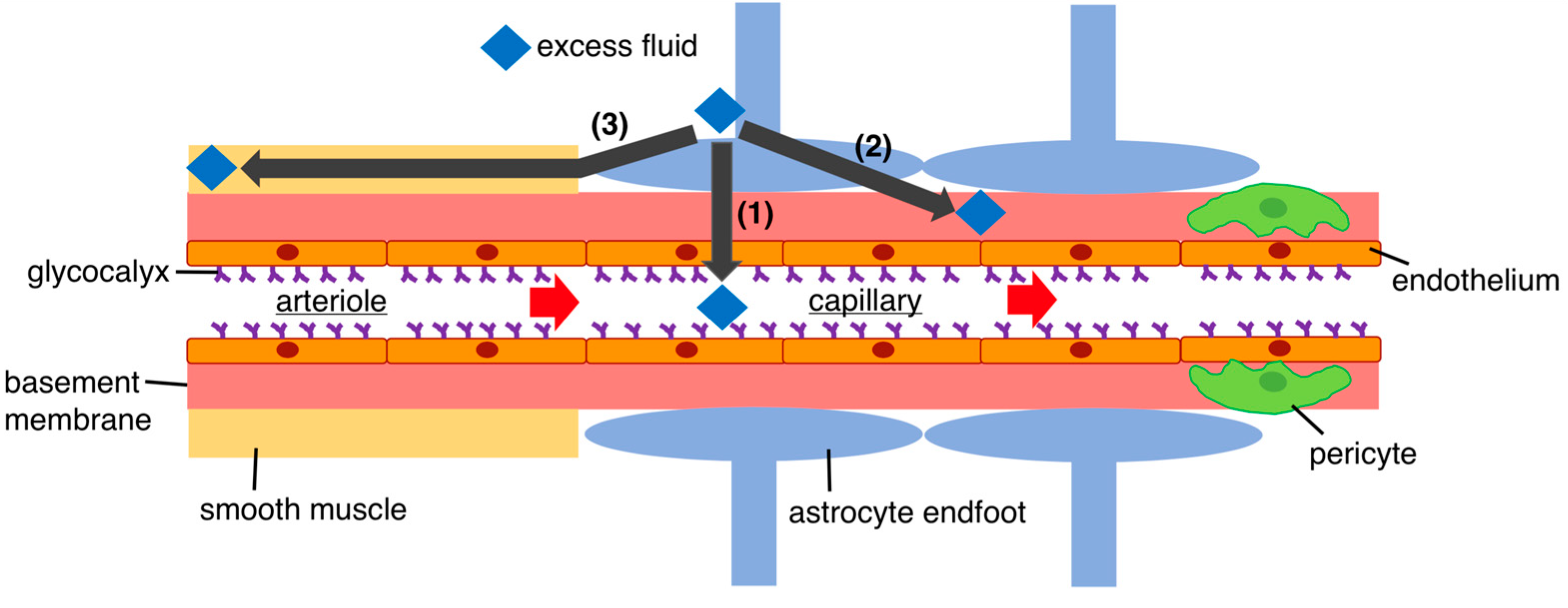
3.1. Fluid Regulation in the Brain
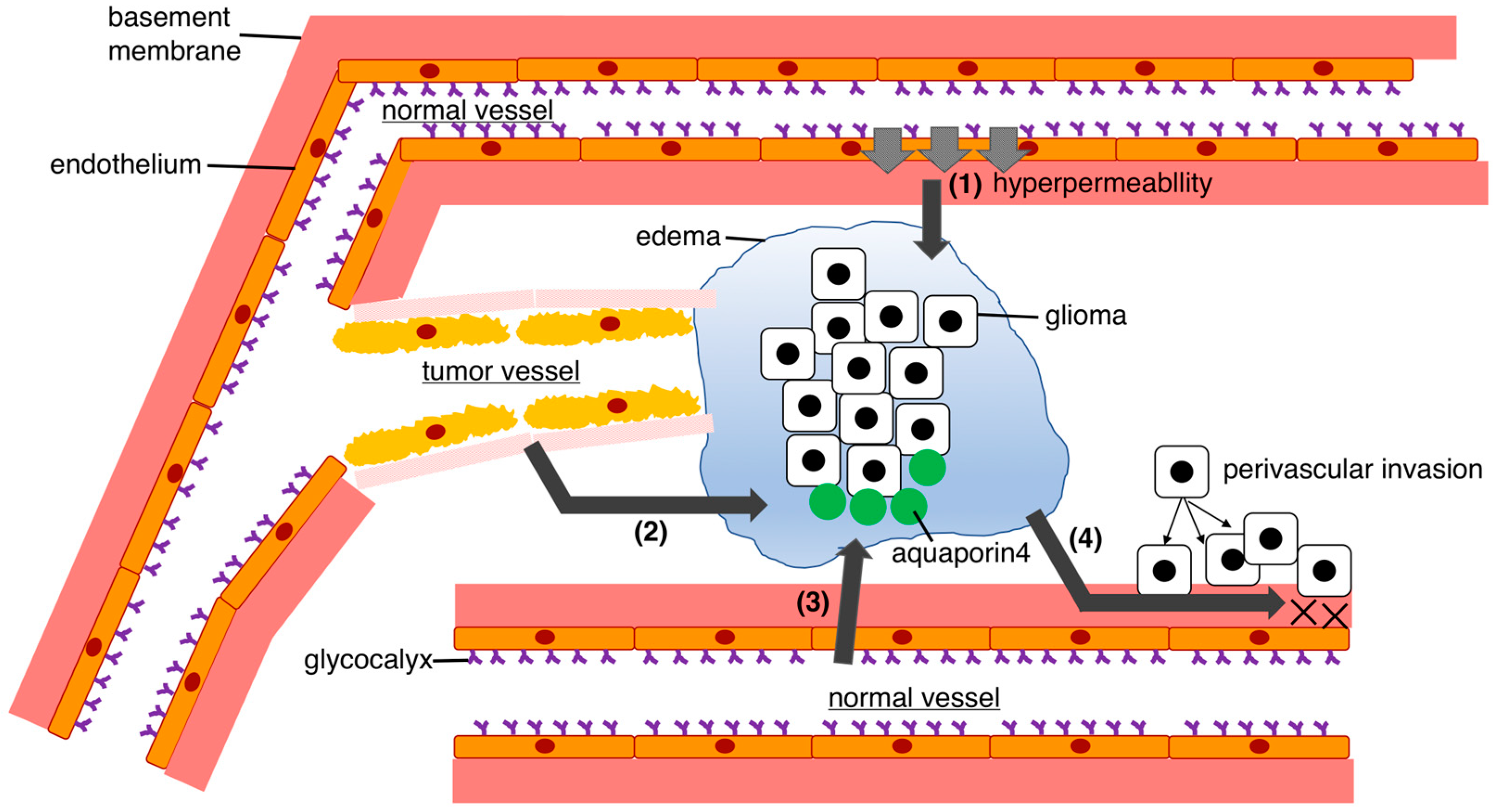
3.2. Mechanisms of Glioma-Related Edema Formation
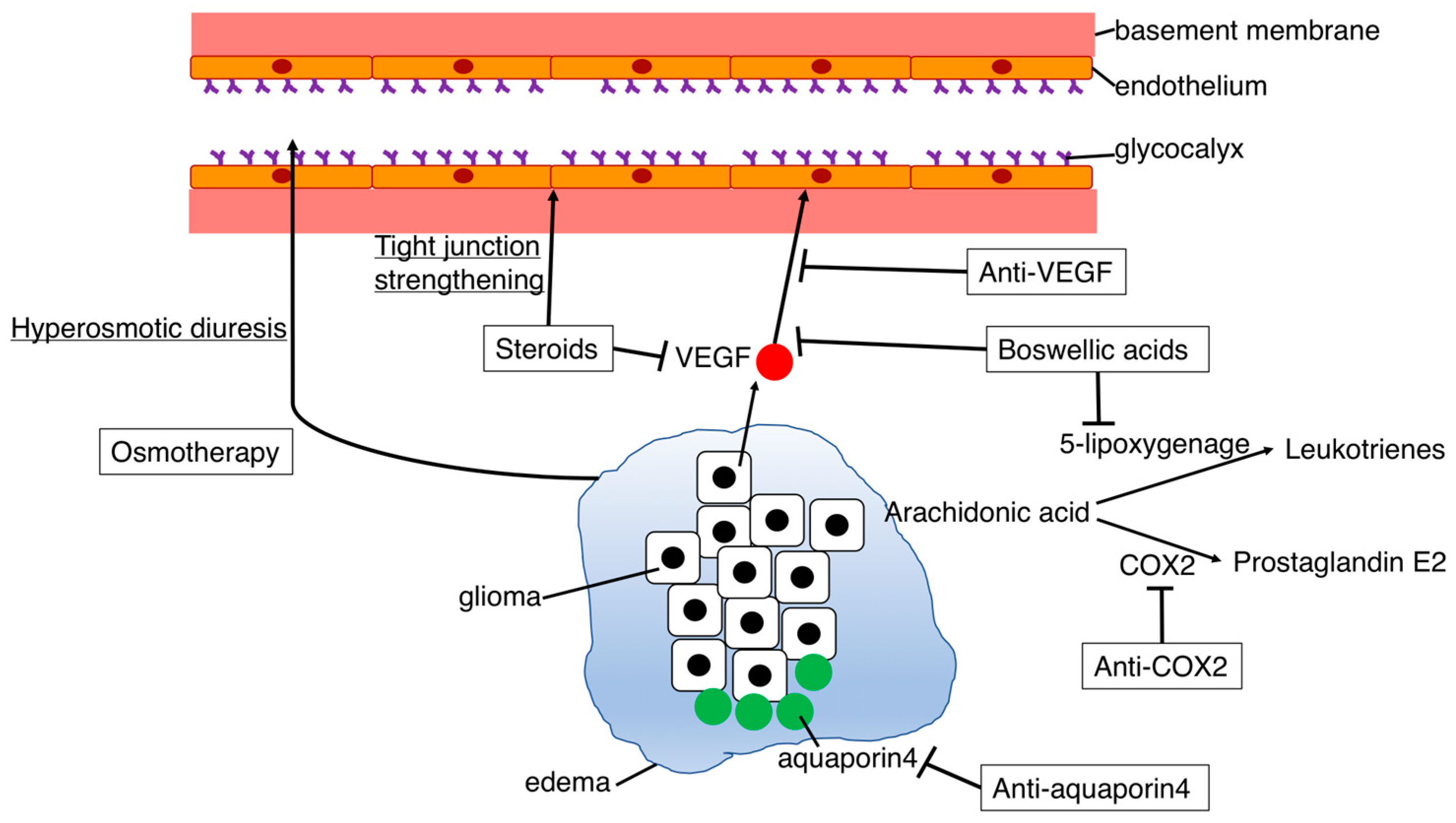
4. Prospects for Treatment of Cerebral Edema
4.1. Osmotherapy
4.2. Steroids
4.3. Anti-VEGF Agents
4.4. Development of New Therapeutic Agents
4.4.1. COX-2 Inhibitors
4.4.2. Aquaporin Inhibitors
4.4.3. Boswellic Acids
4.5. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faraj, C.A.; Snyder, R.I.; McCutcheon, I.E. Intracranial emergencies in neurosurgical oncology: Pathophysiology and clinical management. Emerg. Cancer Care 2022, 1, 13. [Google Scholar] [CrossRef]
- Dalby, T.; Wohl, E.; Dinsmore, M.; Unger, Z.; Chowdhury, T.; Venkatraghavan, L. Pathophysiology of Cerebral Edema—A Comprehensive Review. J. Neuroanaesth. Crit. Care 2020, 8, 163–172. [Google Scholar] [CrossRef]
- Zoccarato, M.; Nardetto, L.; Basile, A.M.; Giometto, B.; Zagonel, V.; Lombardi, G. Seizures, Edema, Thrombosis, and Hemorrhages: An Update Review on the Medical Management of Gliomas. Front. Oncol. 2021, 11, 617966. [Google Scholar] [CrossRef]
- Mahajan, S.; Bhagat, H. Cerebral oedema: Pathophysiological mechanisms and experimental therapies. J. Neuroanaesth. Crit. Care 2016, 3, S22–S28. [Google Scholar] [CrossRef]
- Hladky, S.B.; Barrand, M.A. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS 2016, 13, 19. [Google Scholar] [CrossRef]
- Osborn, A.G.; Louis, D.N.; Poussaint, T.Y.; Linscott, L.L.; Salzman, K.L. The 2021 World Health Organization Classification of Tumors of the Central Nervous System: What Neuroradiologists Need to Know. Am. J. Neuroradiol. 2022, 43, 928–937. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Perry, J.R.; Laperriere, N.; O’Callaghan, C.J.; Brandes, A.A.; Menten, J.; Phillips, C.; Fay, M.; Nishikawa, R.; Cairncross, J.G.; Roa, W.; et al. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N. Engl. J. Med. 2017, 376, 1027–1037. [Google Scholar] [CrossRef]
- Lin, Z.X. Glioma-related edema: New insight into molecular mechanisms and their clinical implications. Chin. J. Cancer 2013, 32, 49–52. [Google Scholar] [CrossRef]
- Cook, A.M.; Morgan Jones, G.; Hawryluk, G.W.J.; Mailloux, P.; McLaughlin, D.; Papangelou, A.; Samuel, S.; Tokumaru, S.; Venkatasubramanian, C.; Zacko, C.; et al. Guidelines for the Acute Treatment of Cerebral Edema in Neurocritical Care Patients. Neurocrit. Care 2020, 32, 647–666. [Google Scholar] [CrossRef]
- Qin, X.; Liu, R.; Akter, F.; Qin, L.; Xie, Q.; Li, Y.; Qiao, H.; Zhao, W.; Jian, Z.; Liu, R.; et al. Peri-tumoral brain edema associated with glioblastoma correlates with tumor recurrence. J. Cancer 2021, 12, 2073–2082. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, X.; Chen, Y.; Lin, G.; Mei, W.; Chen, J.; Liu, Y.; Lin, Z.; Zhang, S. Histopathological findings in the peritumoral edema area of human glioma. Histol. Histopathol. 2015, 30, 1101–1109. [Google Scholar] [CrossRef]
- Silbergeld, D.L.; Rostomily, R.C.; Alvord, E.C., Jr. The cause of death in patients with glioblastoma is multifactorial: Clinical factors and autopsy findings in 117 cases of supratentorial glioblastoma in adults. J. Neurooncol. 1991, 10, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Drumm, M.R.; Dixit, K.S.; Grimm, S.; Kumthekar, P.; Lukas, R.V.; Raizer, J.J.; Stupp, R.; Chheda, M.G.; Kam, K.L.; McCord, M.; et al. Extensive brainstem infiltration, not mass effect, is a common feature of end-stage cerebral glioblastomas. Neuro-Oncology 2020, 22, 470–479. [Google Scholar] [CrossRef]
- Saito, T.; Mizumoto, M.; Liang, H.K.; Nakai, K.; Sumiya, T.; Iizumi, T.; Kohzuki, H.; Numajiri, H.; Makishima, H.; Tsurubuchi, T.; et al. Factors Involved in Preoperative Edema in High-Grade Gliomas. Cureus 2022, 14, e31379. [Google Scholar] [CrossRef]
- Wick, W.; Küker, W. Brain edema in neurooncology: Radiological assessment and management. Onkologie 2004, 27, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qiu, H.; Chen, R.; Huang, J.; Chen, L.; Wan, J.; Chen, Q.; Zhang, L. Peritumor Edema Serves as an Independent Predictive Factor of Recurrence Patterns and Recurrence-Free Survival for High-Grade Glioma. Comput. Math. Methods Med. 2022, 2022, 9547166. [Google Scholar] [CrossRef]
- Wu, C.X.; Lin, G.S.; Lin, Z.X.; Zhang, J.D.; Chen, L.; Liu, S.Y.; Tang, W.L.; Qiu, X.X.; Zhou, C.F. Peritumoral edema on magnetic resonance imaging predicts a poor clinical outcome in malignant glioma. Oncol. Lett. 2015, 10, 2769–2776. [Google Scholar] [CrossRef]
- Giambra, M.; Di Cristofori, A.; Valtorta, S.; Manfrellotti, R.; Bigiogera, V.; Basso, G.; Moresco, R.M.; Giussani, C.; Bentivegna, A. The peritumoral brain zone in glioblastoma: Where we are and where we are going. J. Neurosci. Res. 2023, 101, 199–216. [Google Scholar] [CrossRef]
- Li, A.Y.; Iv, M. Conventional and Advanced Imaging Techniques in Post-treatment Glioma Imaging. Front. Radiol. 2022, 2, 883293. [Google Scholar] [CrossRef]
- Pavlisa, G.; Rados, M.; Pavlisa, G.; Pavic, L.; Potocki, K.; Mayer, D. The differences of water diffusion between brain tissue infiltrated by tumor and peritumoral vasogenic edema. Clin. Imaging 2009, 33, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Hoefnagels, F.W.; De Witt Hamer, P.; Sanz-Arigita, E.; Idema, S.; Kuijer, J.P.; Pouwels, P.J.; Barkhof, F.; Vandertop, W.P. Differentiation of edema and glioma infiltration: Proposal of a DTI-based probability map. J. Neurooncol. 2014, 120, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Esquenazi, Y.; Lo, V.P.; Lee, K. Critical Care Management of Cerebral Edema in Brain Tumors. J. Intensive Care Med. 2017, 32, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Horska, A.; Barker, P.B. Imaging of brain tumors: MR spectroscopy and metabolic imaging. Neuroimaging Clin. N. Am. 2010, 20, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Chow, D.S.; Horenstein, C.I.; Canoll, P.; Lignelli, A.; Hillman, E.M.; Filippi, C.G.; Grinband, J. Glioblastoma Induces Vascular Dysregulation in Nonenhancing Peritumoral Regions in Humans. Am. J. Roentgenol. 2016, 206, 1073–1081. [Google Scholar] [CrossRef]
- Kinoshita, M.; Goto, T.; Arita, H.; Okita, Y.; Isohashi, K.; Kagawa, N.; Fujimoto, Y.; Kishima, H.; Shimosegawa, E.; Saitoh, Y.; et al. Imaging 18F-fluorodeoxy glucose/11C-methionine uptake decoupling for identification of tumor cell infiltration in peritumoral brain edema. J. Neurooncol. 2012, 106, 417–425. [Google Scholar] [CrossRef]
- Miyake, K.; Ogawa, D.; Okada, M.; Hatakeyama, T.; Tamiya, T. Usefulness of positron emission tomographic studies for gliomas. Neurol. Med. Chir. 2016, 56, 396–408. [Google Scholar] [CrossRef]
- Rathore, S.; Akbari, H.; Doshi, J.; Shukla, G.; Rozycki, M.; Bilello, M.; Lustig, R.; Davatzikos, C. Radiomic signature of infiltration in peritumoral edema predicts subsequent recurrence in glioblastoma: Implications for personalized radiotherapy planning. J. Med. Imaging 2018, 5, 021219. [Google Scholar] [CrossRef]
- Artzi, M.; Liberman, G.; Blumenthal, D.T.; Aizenstein, O.; Bokstein, F.; Ben Bashat, D. Differentiation between vasogenic edema and infiltrative tumor in patients with high-grade gliomas using texture patch-based analysis. J. Magn. Reson. Imaging 2018, 48, 729–736. [Google Scholar] [CrossRef]
- Abdel Razek, A.A.K.; Alksas, A.; Shehata, M.; AbdelKhalek, A.; Abdel Baky, K.; El-Baz, A.; Helmy, E. Clinical applications of artificial intelligence and radiomics in neuro-oncology imaging. Insights Imaging 2021, 12, 152. [Google Scholar] [CrossRef]
- Weller, R.O.; Djuanda, E.; Yow, H.Y.; Carare, R.O. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009, 117, 1–14. [Google Scholar] [CrossRef]
- Agarwal, N.; Contarino, C.; Toro, E.F. Neurofluids: A holistic approach to their physiology, interactive dynamics and clinical implications for neurological diseases. Veins Lymphat. 2019, 8, 49–58. [Google Scholar] [CrossRef]
- Bulat, M.; Klarica, M. Recent insights into a new hydrodynamics of the cerebrospinal fluid. Brain Res. Rev. 2011, 65, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Weed, L.H. Studies on Cerebro-Spinal Fluid. No. IV: The dual Source of Cerebro-Spinal Fluid. J. Med. Res. 1914, 31, 93–118. [Google Scholar]
- Yamada, S. Cerebrospinal fluid physiology: Visualization of cerebrospinal fluid dynamics using the magnetic resonance imaging Time-Spatial Inversion Pulse method. Croat. Med. J. 2014, 55, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Asgari, M.; de Zelicourt, D.; Kurtcuoglu, V. Glymphatic solute transport does not require bulk flow. Sci. Rep. 2016, 6, 38635. [Google Scholar] [CrossRef]
- Abbott, N.J.; Pizzo, M.E.; Preston, J.E.; Janigro, D.; Thorne, R.G. The role of brain barriers in fluid movement in the CNS: Is there a ‘glymphatic’ system? Acta Neuropathol. 2018, 135, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Hladky, S.B.; Barrand, M.A. Elimination of substances from the brain parenchyma: Efflux via perivascular pathways and via the blood-brain barrier. Fluids Barriers CNS 2018, 15, 30. [Google Scholar] [CrossRef]
- Morris, A.W.; Sharp, M.M.; Albargothy, N.J.; Fernandes, R.; Hawkes, C.A.; Verma, A.; Weller, R.O.; Carare, R.O. Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol. 2016, 131, 725–736. [Google Scholar] [CrossRef]
- Walter, B.A.; Valera, V.A.; Takahashi, S.; Ushiki, T. The olfactory route for cerebrospinal fluid drainage into the peripheral lymphatic system. Neuropathol. Appl. Neurobiol. 2006, 32, 388–396. [Google Scholar] [CrossRef]
- Nedergaard, M. Neuroscience. Garbage truck of the brain. Science 2013, 340, 1529–1530. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, B.; Vajkoczy, P.; Weller, R.O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 2017, 18, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, J.; Shi, Y. Scanning electron microscopy of human cerebral meningeal stomata. Ann. Anat. 1996, 178, 259–261. [Google Scholar] [CrossRef]
- Dobrogowska, D.H.; Lossinsky, A.S.; Tarnawski, M.; Vorbrodt, A.W. Increased blood-brain barrier permeability and endothelial abnormalities induced by vascular endothelial growth factor. J. Neurocytol. 1998, 27, 163–173. [Google Scholar] [CrossRef]
- Stummer, W. Mechanisms of tumor-related brain edema. Neurosurg. Focus 2007, 22, E8. [Google Scholar] [CrossRef]
- Jain, R.K.; di Tomaso, E.; Duda, D.G.; Loeffler, J.S.; Sorensen, A.G.; Batchelor, T.T. Angiogenesis in brain tumours. Nat. Rev. Neurosci. 2007, 8, 610–622. [Google Scholar] [CrossRef]
- Guyon, J.; Chapouly, C.; Andrique, L.; Bikfalvi, A.; Daubon, T. The Normal and Brain Tumor Vasculature: Morphological and Functional Characteristics and Therapeutic Targeting. Front. Physiol. 2021, 12, 622615. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, X.; Zhen, S.; Zhang, S.; Kang, D.; Lin, Z. Aquaporin-4 upregulated expression in glioma tissue is a reaction to glioma-associated edema induced by vascular endothelial growth factor. Oncol. Rep. 2012, 28, 1633–1638. [Google Scholar] [CrossRef]
- Roth, P.; Regli, L.; Tonder, M.; Weller, M. Tumor-associated edema in brain cancer patients: Pathogenesis and management. Expert Rev. Anticancer Ther. 2013, 13, 1319–1325. [Google Scholar] [CrossRef]
- Tamura, R.; Tanaka, T.; Miyake, K.; Yoshida, K.; Sasaki, H. Bevacizumab for malignant gliomas: Current indications, mechanisms of action and resistance, and markers of response. Brain Tumor Pathol. 2017, 34, 62–77. [Google Scholar] [CrossRef]
- Wu, Y.; Peng, Z.; Wang, H.; Xiang, W. Identifying the Hub Genes of Glioma Peritumoral Brain Edema Using Bioinformatical Methods. Brain Sci. 2022, 12, 805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Y.; Wang, H.; Xu, L.; Yu, Y. MMP-2 expression and correlation with pathology and MRI of glioma. Oncol. Lett. 2019, 17, 1826–1832. [Google Scholar] [CrossRef]
- Tsai, J.C.; Goldman, C.K.; Gillespie, G.Y. Vascular endothelial growth factor in human glioma cell lines: Induced secretion by EGF, PDGF-BB, and bFGF. J. Neurosurg. 1995, 82, 864–873. [Google Scholar] [CrossRef]
- Jiang, J.; Qiu, J.; Li, Q.; Shi, Z. Prostaglandin E2 Signaling: Alternative Target for Glioblastoma? Trends Cancer 2017, 3, 75–78. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, T.; Yu, S.; Liu, C.; He, M.; Hu, C. Prostaglandin E2 increases migration and proliferation of human glioblastoma cells by activating transient receptor potential melastatin 7 channels. J. Cell. Mol. Med. 2018, 22, 6327–6337. [Google Scholar] [CrossRef] [PubMed]
- Dubois, L.G.; Campanati, L.; Righy, C.; D’Andrea-Meira, I.; Spohr, T.C.; Porto-Carreiro, I.; Pereira, C.M.; Balca-Silva, J.; Kahn, S.A.; DosSantos, M.F.; et al. Gliomas and the vascular fragility of the blood brain barrier. Front. Cell Neurosci. 2014, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S. Ultrastructure of capillary walls in human brain tumors. Acta Neuropathol. 1989, 78, 561–571. [Google Scholar] [CrossRef]
- Egorova, A.V.; Baranich, T.I.; Brydun, A.V.; Glinkina, V.V.; Sukhorukov, V.S. Morphological and Histophysiological Features of the Brain Capillary Endothelium. J. Evol. Biochem. Physiol. 2022, 58, 755–768. [Google Scholar] [CrossRef]
- Bar-Sella, P.; Front, D.; Hardoff, R.; Peyser, E.; Borovich, B.; Nir, I. Ultrastructural basis for different pertechnetate uptake patterns by various human brain tumours. J. Neurol. Neurosurg. Psychiatry 1979, 42, 924–930. [Google Scholar] [CrossRef]
- Ribatti, D.; Ranieri, G.; Annese, T.; Nico, B. Aquaporins in cancer. Biochim. Biophys. Acta 2014, 1840, 1550–1553. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin-4 and brain edema. Pediatr. Nephrol. 2007, 22, 778–784. [Google Scholar] [CrossRef]
- Yool, A.J.; Brown, E.A.; Flynn, G.A. Roles for novel pharmacological blockers of aquaporins in the treatment of brain oedema and cancer. Clin. Exp. Pharmacol. Physiol. 2010, 37, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Valente, O.; Messina, R.; Ingravallo, G.; Bellitti, E.; Zimatore, D.S.; de Gennaro, L.; Abbrescia, P.; Pati, R.; Palazzo, C.; Nicchia, G.P.; et al. Alteration of the translational readthrough isoform AQP4ex induces redistribution and downregulation of AQP4 in human glioblastoma. Cell Mol. Life Sci. 2022, 79, 140. [Google Scholar] [CrossRef] [PubMed]
- Mou, K.; Chen, M.; Mao, Q.; Wang, P.; Ni, R.; Xia, X.; Liu, Y. AQP-4 in peritumoral edematous tissue is correlated with the degree of glioma and with expression of VEGF and HIF-alpha. J. Neurooncol. 2010, 100, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Kaal, E.C.; Vecht, C.J. The management of brain edema in brain tumors. Curr. Opin. Oncol. 2004, 16, 593–600. [Google Scholar] [CrossRef]
- Palma, L.; Bruni, G.; Fiaschi, A.I.; Mariottini, A. Passage of mannitol into the brain around gliomas: A potential cause of rebound phenomenon. A study on 21 patients. J. Neurosurg. Sci. 2006, 50, 63–66. [Google Scholar] [PubMed]
- Cenciarini, M.; Valentino, M.; Belia, S.; Sforna, L.; Rosa, P.; Ronchetti, S.; D’Adamo, M.C.; Pessia, M. Dexamethasone in Glioblastoma Multiforme Therapy: Mechanisms and Controversies. Front. Mol. Neurosci. 2019, 12, 65. [Google Scholar] [CrossRef]
- Dubinski, D.; Hattingen, E.; Senft, C.; Seifert, V.; Peters, K.G.; Reiss, Y.; Devraj, K.; Plate, K.H. Controversial roles for dexamethasone in glioblastoma—Opportunities for novel vascular targeting therapies. J. Cereb. Blood Flow Metab. 2019, 39, 1460–1468. [Google Scholar] [CrossRef]
- Lee, E.Q.; Wen, P.Y. Corticosteroids for peritumoral edema: Time to overcome our addiction? Neuro-Oncology 2016, 18, 1191–1192. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; De Stefani, A.; Ghidini, A.; Bruschieri, L.; Riboldi, V.; Dottorini, L.; Iaculli, A.; Zaniboni, A.; Trevisan, F. Steroids use and survival in patients with glioblastoma multiforme: A pooled analysis. J. Neurol. 2021, 268, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Bastin, M.E.; Carpenter, T.K.; Armitage, P.A.; Sinha, S.; Wardlaw, J.M.; Whittle, I.R. Effects of dexamethasone on cerebral perfusion and water diffusion in patients with high-grade glioma. Am. J. Neuroradiol. 2006, 27, 402–408. [Google Scholar] [PubMed]
- Dubinski, D.; Won, S.Y.; Gessler, F.; Quick-Weller, J.; Behmanesh, B.; Bernatz, S.; Forster, M.T.; Franz, K.; Plate, K.H.; Seifert, V.; et al. Dexamethasone-induced leukocytosis is associated with poor survival in newly diagnosed glioblastoma. J. Neurooncol. 2018, 137, 503–510. [Google Scholar] [CrossRef]
- Reichman, H.R.; Farrell, C.L.; Del Maestro, R.F. Effects of steroids and nonsteroid anti-inflammatory agents on vascular permeability in a rat glioma model. J. Neurosurg. 1986, 65, 233–237. [Google Scholar] [CrossRef]
- Ly, K.I.; Wen, P.Y. Clinical Relevance of Steroid Use in Neuro-Oncology. Curr. Neurol. Neurosci. Rep. 2017, 17, 5. [Google Scholar] [CrossRef]
- Gerstner, E.R.; Duda, D.G.; di Tomaso, E.; Ryg, P.A.; Loeffler, J.S.; Sorensen, A.G.; Ivy, P.; Jain, R.K.; Batchelor, T.T. VEGF inhibitors in the treatment of cerebral edema in patients with brain cancer. Nat. Rev. Clin. Oncol. 2009, 6, 229–236. [Google Scholar] [CrossRef]
- Wang, X.F.; Lin, G.S.; Lin, Z.X.; Chen, Y.P.; Chen, Y.; Zhang, J.D.; Tan, W.L. Association of pSTAT3-VEGF signaling pathway with peritumoral edema in newly diagnosed glioblastoma: An immunohistochemical study. Int. J. Clin. Exp. Pathol. 2014, 7, 6133–6140. [Google Scholar]
- Kamoun, W.S.; Ley, C.D.; Farrar, C.T.; Duyverman, A.M.; Lahdenranta, J.; Lacorre, D.A.; Batchelor, T.T.; di Tomaso, E.; Duda, D.G.; Munn, L.L.; et al. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J. Clin. Oncol. 2009, 27, 2542–2552. [Google Scholar] [CrossRef]
- Reardon, D.A.; Turner, S.; Peters, K.B.; Desjardins, A.; Gururangan, S.; Sampson, J.H.; McLendon, R.E.; Herndon, J.E., 2nd; Jones, L.W.; Kirkpatrick, J.P.; et al. A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. J. Natl. Compr. Cancer Netw. 2011, 9, 414–427. [Google Scholar] [CrossRef]
- Trevisan, E.; Bertero, L.; Bosa, C.; Magistrello, M.; Pellerino, A.; Ruda, R.; Soffietti, R. Antiangiogenic therapy of brain tumors: The role of bevacizumab. Neurol. Sci. 2014, 35, 507–514. [Google Scholar] [CrossRef]
- Beppu, T.; Sato, Y.; Yamada, N.; Terasaki, K.; Sasaki, T.; Sugai, T.; Ogasawara, K. Impacts on Histological Features and (11)C-Methyl-L-methionine Uptake after “One-Shot” Administration with Bevacizumab before Surgery in Newly Diagnosed Glioblastoma. Transl. Oncol. 2019, 12, 1480–1487. [Google Scholar] [CrossRef]
- de Groot, J.F.; Lamborn, K.R.; Chang, S.M.; Gilbert, M.R.; Cloughesy, T.F.; Aldape, K.; Yao, J.; Jackson, E.F.; Lieberman, F.; Robins, H.I.; et al. Phase II study of aflibercept in recurrent malignant glioma: A North American Brain Tumor Consortium study. J. Clin. Oncol. 2011, 29, 2689–2695. [Google Scholar] [CrossRef]
- Keunen, O.; Johansson, M.; Oudin, A.; Sanzey, M.; Rahim, S.A.; Fack, F.; Thorsen, F.; Taxt, T.; Bartos, M.; Jirik, R.; et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc. Natl. Acad. Sci. USA 2011, 108, 3749–3754. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Shi, Z.; Jiang, J. Cyclooxygenase-2 in glioblastoma multiforme. Drug Discov. Today 2017, 22, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Portnow, J.; Suleman, S.; Grossman, S.A.; Eller, S.; Carson, K. A cyclooxygenase-2 (COX-2) inhibitor compared with dexamethasone in a survival study of rats with intracerebral 9L gliosarcomas. Neuro-Oncology 2002, 4, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Yeung, W.L.; Zhang, P.; Li, N.; Kiang, M.Y.; Leung, G.K.K. Progesterone Is More Effective than Dexamethasone in Prolonging Overall Survival and Preserving Neurologic Function in Experimental Animals with Orthotopic Glioblastoma Allografts. World Neurosurg. 2019, 125, e497–e507. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.; Wang, Y.; Liu, X.; Liu, Y.; Li, Y.; Chen, H.; Fan, C.; Wu, D.; Yang, J. Inhibition of COX-2, mPGES-1 and CYP4A by isoliquiritigenin blocks the angiogenic Akt signaling in glioma through ceRNA effect of miR-194-5p and lncRNA NEAT1. J. Exp. Clin. Cancer Res. 2019, 38, 371. [Google Scholar] [CrossRef]
- Recht, L.; Mechtler, L.L.; Wong, E.T.; O’Connor, P.C.; Rodda, B.E. Steroid-sparing effect of corticorelin acetate in peritumoral cerebral edema is associated with improvement in steroid-induced myopathy. J. Clin. Oncol. 2013, 31, 1182–1187. [Google Scholar] [CrossRef]
- Khan, R.B.; Krasin, M.J.; Kasow, K.; Leung, W. Cyclooxygenase-2 inhibition to treat radiation-induced brain necrosis and edema. J. Pediatr. Hematol. Oncol. 2004, 26, 253–255. [Google Scholar] [CrossRef]
- Fosslien, E. Cardiovascular complications of non-steroidal anti-inflammatory drugs. Ann. Clin. Lab. Sci. 2005, 35, 347–385. [Google Scholar]
- Jorgacevski, J.; Zorec, R.; Potokar, M. Insights into Cell Surface Expression, Supramolecular Organization, and Functions of Aquaporin 4 Isoforms in Astrocytes. Cells 2020, 9, 2622. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Zhou, Y.; Sun, K.; Jiang, W.; Li, W.; Liu, X.; Tian, C.; Li, Z.; Ying, G.; Fu, L.; et al. Knockdown a water channel protein, aquaporin-4, induced glioblastoma cell apoptosis. PLoS ONE 2013, 8, e66751. [Google Scholar] [CrossRef] [PubMed]
- Nico, B.; Ribatti, D. Role of aquaporins in cell migration and edema formation in human brain tumors. Exp. Cell Res. 2011, 317, 2391–2396. [Google Scholar] [CrossRef]
- Ding, T.; Ma, Y.; Li, W.; Liu, X.; Ying, G.; Fu, L.; Gu, F. Role of aquaporin-4 in the regulation of migration and invasion of human glioma cells. Int. J. Oncol. 2011, 38, 1521–1531. [Google Scholar] [PubMed]
- Kwon, S.; Jin, C.; Cho, K.H. Oreongsan, an herbal medicine prescription developed as a new alternative treatment in patients with chronic subdural hematoma: A narrative review. Integr. Med. Res. 2019, 8, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Nakao, J.; Marushima, A.; Fujita, K.; Fujimori, H.; Mashiko, R.; Kamezaki, T.; Sato, N.; Shibata, Y.; Takano, S.; Ishikawa, E. Conservative Treatment of Chronic Subdural Hematoma with Gorei-san. Neurol. Med. Chir. 2023, 63, 31–36. [Google Scholar] [CrossRef]
- Lee, B.; Leem, J.; Kim, H.; Jo, H.G.; Kwon, C.Y. Herbal Medicine for Traumatic Brain Injury: A Systematic Review and Meta-Analysis of Randomized Controlled Trials and Limitations. Front. Neurol. 2020, 11, 772. [Google Scholar] [CrossRef]
- Jin, W.R.; Zhang, F.E.; Diao, B.Z.; Zhang, Y.Y. Clinical Outcomes of Wulingsan Subtraction Decoction Treatment of Postoperative Brain Edema and Fever as a Complication of Glioma Neurosurgery. Evid. Based Complement. Alternat. Med. 2016, 2016, 5078689. [Google Scholar] [CrossRef]
- Nakano, T.; Nishigami, C.; Irie, K.; Shigemori, Y.; Sano, K.; Yamashita, Y.; Myose, T.; Tominaga, K.; Matsuo, K.; Nakamura, Y.; et al. Goreisan Prevents Brain Edema after Cerebral Ischemic Stroke by Inhibiting Aquaporin 4 Upregulation in Mice. J. Stroke Cerebrovasc. Dis. 2018, 27, 758–763. [Google Scholar] [CrossRef]
- Shimizu, T.; Murakami, K.; Matsumoto, C.; Kido, T.; Isohama, Y. Goreisan alleviates cerebral edema: Possibility of its involvement in inhibiting aquaporin-4 function. Tradit. Kampo Med. 2023, 10, 168–176. [Google Scholar] [CrossRef]
- Yano, Y.; Yano, H.; Takahashi, H.; Yoshimoto, K.; Tsuda, S.; Fujiyama, K.; Izumo-Shimizu, Y.; Motoie, R.; Ito, M.; Tanaka, J.; et al. Goreisan Inhibits Upregulation of Aquaporin 4 and Formation of Cerebral Edema in the Rat Model of Juvenile Hypoxic-Ischemic Encephalopathy. Evid. Based Complement. Alternat. Med. 2017, 2017, 3209219. [Google Scholar] [CrossRef]
- Lan, Y.L.; Nie, T.; Zou, S. Identification of the prognostic and immunological roles of aquaporin 4: A potential target for survival and immunotherapy in glioma patients. Front. Cell Neurosci. 2022, 16, 1061428. [Google Scholar] [CrossRef]
- Warnick, R.E. Treatment of adverse radiation effects with Boswellia serrata after failure of pentoxifylline and vitamin E: Illustrative cases. J. Neurosurg. Case Lessons 2023, 5, CASE22488. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Jiang, X.; Yang, S.; Huang, Y.; Hong, J.; Ma, Y.; Fang, X.; Fang, Y.; Wu, J. The Biological Activity of 3-O-Acetyl-11-keto-β-Boswellic Acid in Nervous System Diseases. Neuromol. Med. 2022, 24, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Glaser, T.; Winter, S.; Groscurth, P.; Safayhi, H.; Sailer, E.R.; Ammon, H.P.; Schabet, M.; Weller, M. Boswellic acids and malignant glioma: Induction of apoptosis but no modulation of drug sensitivity. Br. J. Cancer 1999, 80, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.; Weller, M. Boswellic acid activity against glioblastoma stem-like cells. Oncol. Lett. 2016, 11, 4187–4192. [Google Scholar] [CrossRef]
- Kirste, S.; Treier, M.; Wehrle, S.J.; Becker, G.; Abdel-Tawab, M.; Gerbeth, K.; Hug, M.J.; Lubrich, B.; Grosu, A.L.; Momm, F. Boswellia serrata acts on cerebral edema in patients irradiated for brain tumors: A prospective, randomized, placebo-controlled, double-blind pilot trial. Cancer 2011, 117, 3788–3795. [Google Scholar] [CrossRef]
- Di Pierro, F.; Simonetti, G.; Petruzzi, A.; Bertuccioli, A.; Botta, L.; Bruzzone, M.G.; Cuccarini, V.; Fariselli, L.; Lamperti, E. A novel lecithin-based delivery form of Boswellic acids as complementary treatment of radiochemotherapy-induced cerebral edema in patients with glioblastoma multiforme: A longitudinal pilot experience. J. Neurosurg. Sci. 2019, 63, 286–291. [Google Scholar] [CrossRef]
| Drug (Example) | Mechanism | Edema-Improving Effect | Side Effects |
|---|---|---|---|
| Osmotherapy (mannitol) | Hyperosmotic diuresis | Immediate effect | A temporary effect only, rebound |
| Steroids (dexamethasone) | Tight junction strengthening and suppression of increased vascular permeability | Standard treatment | Infection, weight gain, many side effects |
| Anti-VEGF (bevacizumab) | Inhibits VEGF → suppresses vascular permeability and tumor angiogenesis | Strong effect | Hemorrhage, embolism, high drug cost |
| Anti-COX-2 (celecoxib) | Inhibition of prostaglandin production → suppresses increased vascular permeability | Unknown | Cardiovascular disorder |
| Anti-aquaporin 4 (goreisan) | Water accumulation control? | Unknown | Unknown |
| Boswellic acids (5-Loxin) | Interference with the VEGF pathway and leukotriene formation | Unknown | Gastrointestinal side effects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohmura, K.; Tomita, H.; Hara, A. Peritumoral Edema in Gliomas: A Review of Mechanisms and Management. Biomedicines 2023, 11, 2731. https://doi.org/10.3390/biomedicines11102731
Ohmura K, Tomita H, Hara A. Peritumoral Edema in Gliomas: A Review of Mechanisms and Management. Biomedicines. 2023; 11(10):2731. https://doi.org/10.3390/biomedicines11102731
Chicago/Turabian StyleOhmura, Kazufumi, Hiroyuki Tomita, and Akira Hara. 2023. "Peritumoral Edema in Gliomas: A Review of Mechanisms and Management" Biomedicines 11, no. 10: 2731. https://doi.org/10.3390/biomedicines11102731
APA StyleOhmura, K., Tomita, H., & Hara, A. (2023). Peritumoral Edema in Gliomas: A Review of Mechanisms and Management. Biomedicines, 11(10), 2731. https://doi.org/10.3390/biomedicines11102731






