Analysis of Membrane Type-1 Matrix Metalloproteinase (MT1-MMP, MMP14) in Eutopic and Ectopic Endometrium and in Serum and Endocervical Mucus of Endometriosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Sample Collection
2.2. Immunohistochemical Analysis and Quantification
2.3. MT1-MMP ELISA
2.4. Statistics
3. Results
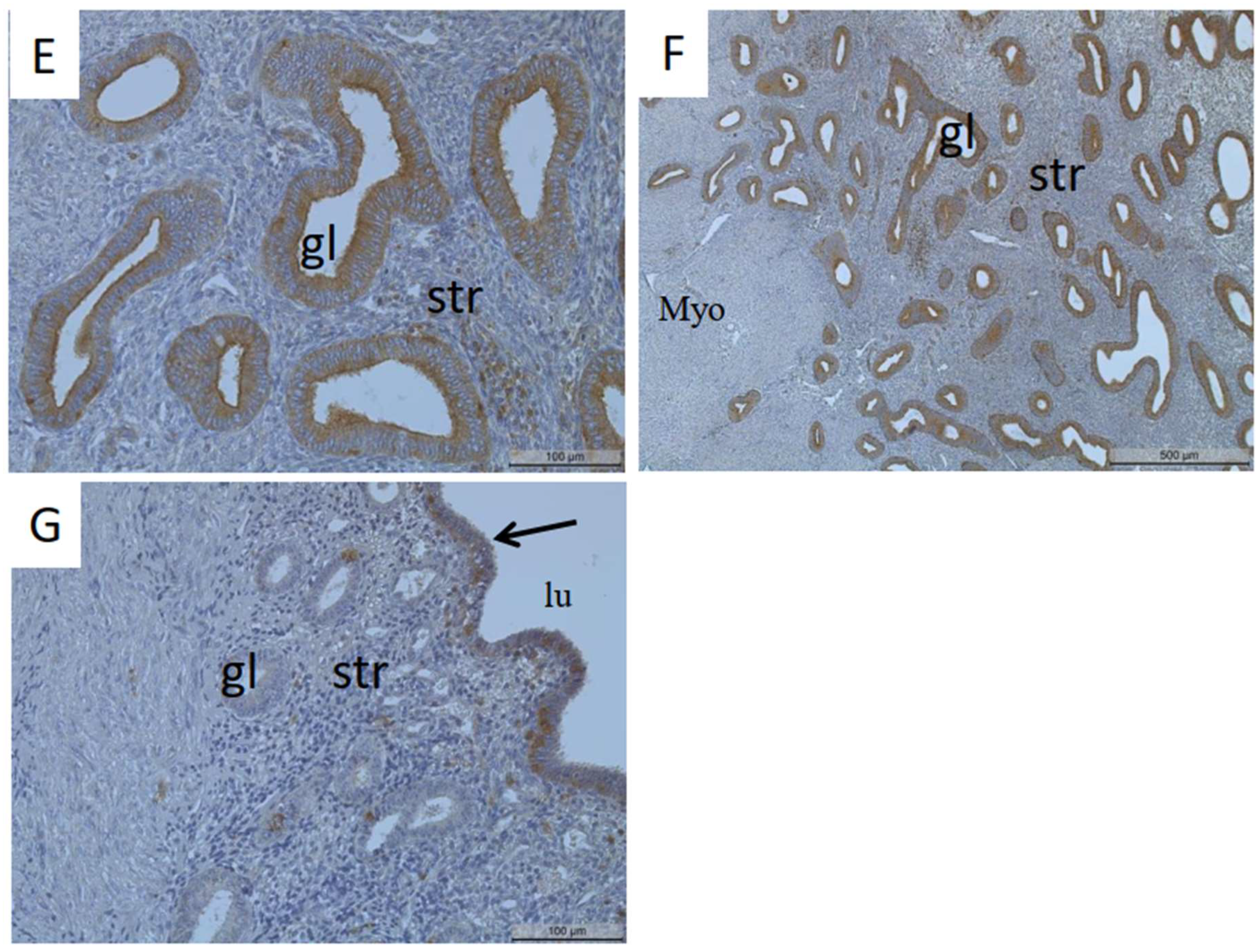
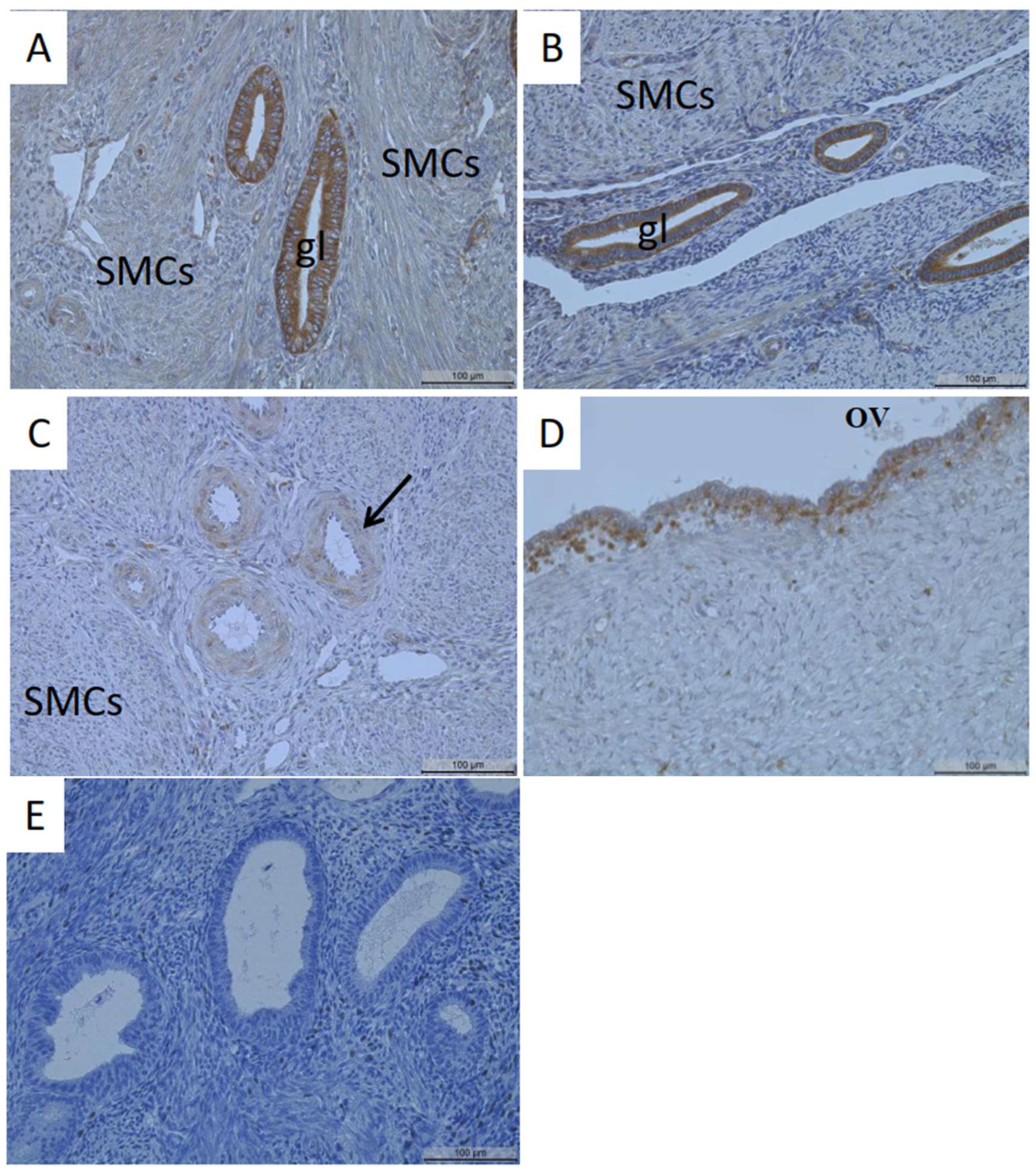
3.1. Identification of MT1-MMP Localization in the Human Uterus and Ovarian Endometriosis
3.2. Quantification of MT1-MMP in Serum and Endocervical Mucus Samples of Patients with and without Endometriosis
4. Discussion
4.1. Localization of MT1-MMP in the Uterus
4.2. Localization of MT1-MMP in Adenomyosis and Endometriosis
4.3. Serum Levels of MT1-MMP in Cases with Endometriosis
4.4. Endocervical Mucus Samples of MT1-MMP in Endometriosis
4.5. Correlation of Serum/Endocervical Mucus Levels of MT1-MMP with Clinical Parameters and Clinical Implications
4.6. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, M.; Kulkarni, M.T.; Missmer, S.A. Is endometriosis more common and more severe than it was 30 years ago? J. Minim. Invasive Gynecol. 2020, 27, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 1927, 14, 422–469. [Google Scholar] [CrossRef]
- Gordts, S.; Grimbizis, G.; Campo, R. Symptoms and classification of uterine adenomyosis, including the place of hysteroscopy in diagnosis. Fertil. Steril. 2018, 109, 380–388. [Google Scholar] [CrossRef]
- García-Solares, J.; Donnez, J.; Donnez, O.; Dolmans, M.M. Pathogenesis of uterine adenomyosis: Invagination or metaplasia? Fertil. Steril. 2018, 109, 371–379. [Google Scholar] [CrossRef]
- Zhai, J.; Vannuccini, S.; Petraglia, F.; Giudice, L.C. Adenomyosis: Mechanisms and pathogenesis. Semin. Reprod. Med. 2020, 38, 129–143. [Google Scholar] [CrossRef]
- Guo, S.W. Cracking the enigma of adenomyosis: An update on its pathogenesis and pathophysiology. Reproduction 2022, 164, R101–R121. [Google Scholar] [CrossRef]
- Choi, E.J.; Cho, S.B.; Lee, S.R.; Lim, Y.M.; Jeong, K.; Moon, H.S.; Chung, H. Comorbidity of gynecological and non-gynecological diseases with adenomyosis and endometriosis. Obstet. Gynecol. Sci. 2017, 60, 579–586. [Google Scholar] [CrossRef]
- Vannuccini, S.; Tosti, C.; Carmona, F.; Huang, S.J.; Chapron, C.; Guo, S.W.; Petraglia, F. Pathogenesis of adenomyosis: An update on molecular mechanisms. Reprod. Biomed. Online 2017, 35, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Yoshihara, K.; Suda, K.; Nakaoka, H.; Yachida, N.; Ueda, H.; Sugino, K.; Mori, Y.; Yamawaki, K.; Tamura, R.; et al. Three-dimensional understanding of the morphological complexity of the human uterine endometrium. iScience 2021, 24, 102258. [Google Scholar] [CrossRef] [PubMed]
- Gaide Chevronnay, H.P.; Selvais, C.; Emonard, H.; Galant, C.; Marbaix, E.; Henriet, P. Regulation of matrix metalloproteinases activity studied in human endometrium as a paradigm of cyclic tissue breakdown and regeneration. Biochim. Biophys. Acta 2012, 1824, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Ye, J.; Li, M.; Zhu, Z. The role of matrix metalloproteinases in endometriosis: A potential target. Biomolecules 2021, 11, 1739. [Google Scholar] [CrossRef]
- Marbaix, E.; Kokorine, I.; Moulin, P.; Donnez, J.; Eeckhout, Y.; Courtoy, P.J. Menstrual breakdown can be mimicked in vitro and is selectively and reversible blocked by inhibitors of matrix metalloproteinases. Proc. Natl. Acad. Sci. USA 1996, 93, 9120–9125. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and biological attributes of matrix metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar]
- Sahraei, S.S.; Asl, F.D.; Kalhor, N.; Sheykhhasan, M.; Fazaeli, H.; Moud, S.S.; Sheiholeslami, A. A comparative study of gene expression in menstrual blood-derived stromal cells between endometriosis and healthy women. BioMed Res. Int. 2022, 2022, 7053521. [Google Scholar] [CrossRef]
- Itoh, Y. Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biol. 2015, 44, 207–223. [Google Scholar] [CrossRef]
- Moracho, N.; Learte, A.I.; Muñoz-Sáez, E.; Marchena, M.A.; Cid, M.A.; Arroyo, A.G.; Sánchez-Camacho, C. Emerging roles of MT-MMPs in embryonic development. Dev. Dyn. 2022, 251, 240–275. [Google Scholar] [CrossRef]
- Luddi, A.; Marrocco, C.; Governini, L.; Semplici, B.; Pavone, V.; Luisi, S.; Petraglia, F.; Piomboni, P. Expression of matrix metalloproteinases and their inhibitors in endometrium: High levels in endometriotic lesions. Int. J. Mol. Sci. 2020, 21, 2840. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Apte, S.S.; Soininen, R.; Cao, R.; Baaklini, G.Y.; Rauser, R.W.; Wang, J.; Cao, Y.; Tryggvason, K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc. Natl. Acad. Sci. USA 2000, 97, 4052–4057. [Google Scholar] [CrossRef]
- Irie, K.; Komori, K.; Seiki, M.; Tsuruga, E.; Sakakura, Y.; Kaku, T.; Yajima, T. Impaired alveolization in mice deficient in membrane-type matrix metalloproteinase 1 (MT1-MMP). Med. Mol. Morphol. 2005, 38, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Fernández, A.; Soria-Valles, C.; Osorio, F.G.; Gutiérrez-Abril, J.; Garabaya, C.; Aguirre, A.; Fueyo, A.; Fernández-García, M.S.; Puente, X.S.; López-Otín, C. Loss of MT1-MMP causes cell senescence and nuclear defects which can be reversed by retinoic acid. EMBO J. 2015, 34, 1875–1888. [Google Scholar] [CrossRef] [PubMed]
- Turunen, S.P.; Tatti-Bugaeva, O.; Lehti, K. Membrane-type matrix metalloproteases as diverse effectors of cancer progression. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1974–1988. [Google Scholar] [CrossRef]
- Asthana, P.; Guo, X.; Wong, H.L.X. MT1-MMP–A potential drug target for the management of the obesity. Expert Opin. Ther. Targets 2022, 26, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hampton, A.L.; Nie, G.; Salamonsen, L.A. Progesterone inhibits activation of latent matrix metalloproteinase (MMP)-2 by membrane-type 1 MMP: Enzymes coordinately expressed in human endometrium. Biol. Reprod. 2000, 62, 85–94. [Google Scholar] [CrossRef]
- Plaisier, M.; Koolwijk, P.; Hanemaaijer, R.; Verwey, R.A.; van der Weiden, R.M.; Risse, E.K.; Jungerius, C.; Helmerhorst, F.M.; van Hinsbergh, V.W. Membrane-type matrix metalloproteinases and vascularization in human endometrium during the menstrual cycle. Mol. Hum. Reprod. 2006, 12, 11–18. [Google Scholar] [CrossRef]
- Londero, A.P.; Calcagno, A.; Grassi, T.; Marzinotto, S.; Orsaria, M.; Beltrami, C.A.; Marchesoni, D.; Mariuzzi, L. Survivin, MMP-2, MT1-MMP, and TIMP-2: Their impact on survival, implantation, and proliferation of endometriotic tissues. Virchows Arch. 2012, 461, 589–599. [Google Scholar] [CrossRef]
- Chung, H.W.; Lee, J.Y.; Moon, H.S.; Hur, S.E.; Park, M.H.; Wen, Y.; Polan, M.L. Matrix metalloproteinase-2, membranous type 1 matrix metalloproteinase, and tissue inhibitor of metalloproteinase-2 expression in ectopic and eutopic endometrium. Fertil. Steril. 2002, 78, 787–795. [Google Scholar] [CrossRef]
- Ueda, M.; Yamashita, Y.; Takehara, M.; Terai, Y.; Kumagai, K.; Ueki, K.; Kanda, K.; Hung, Y.C.; Ueki, M. Gene expression of adhesion molecules and matrix metalloproteinases in endometriosis. Gynecol. Endocrinol. 2002, 16, 391–402. [Google Scholar] [CrossRef]
- Laudanski, P.; Szamatowicz, J.; Ramel, P. Matrix metalloproteinase-13 and membrane type-1 matrix metalloproteinase in peritoneal fluid of women with endometriosis. Gynecol. Endocrinol. 2005, 21, 106–110. [Google Scholar] [CrossRef]
- Hoerscher, A.; Horné, F.; Dietze, R.; Berkes, E.; Oehmke, F.; Tinneberg, H.R.; Meinhold-Heerlein, I.; Konrad, L. Localization of claudin-2 and claudin-3 in eutopic and ectopic endometrium is highly similar. Arch. Gynecol. Obstet. 2020, 301, 1003–1011. [Google Scholar] [CrossRef]
- Haas, D.; Chvatal, R.; Habelsberger, A.; Wurm, P.; Schimetta, W.; Oppelt, P. Comparison of revised American Fertility Society and ENZIAN staging: A critical evaluation of classifications of endometriosis on the basis of our patient population. Fertil. Steril. 2011, 95, 1574–1578. [Google Scholar] [CrossRef] [PubMed]
- Mwaura, A.N.; Riaz, M.A.; Maoga, J.B.; Mecha, E.; Omwandho, C.O.; Scheiner-Bobis, G.; Meinhold-Heerlein, I.; Konrad, L. Role of betaglycan in TGF-β signaling and wound healing in human endometriotic epithelial cells and in endometriosis. Biology 2022, 11, 513. [Google Scholar] [CrossRef]
- Goffin, F.; Munaut, C.; Frankenne, F.; Perrier d’Hauterive, S.; Béliard, A.; Fridman, V.; Nervo, P.; Colige, A.; Foidart, J.M. Expression pattern of metalloproteinases and tissue inhibitors of matrix-metalloproteinases in cycling human endometrium. Biol. Reprod. 2003, 69, 976–984. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, N.; Zhang, G.; Fu, J.; Wu, Q. Matrix metalloproteinase-14 (MMP-14) downregulation inhibits esophageal squamous cell carcinoma cell migration, invasion, and proliferation. Thorac. Cancer 2020, 11, 3168–3174. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.C.; van der Wurff, A.A.; van Kuppevelt, T.H.; Massuger, L.F. The role of MMP-14 in ovarian cancer: A systematic review. J. Ovarian Res. 2021, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Wu, K.P.; Wu, A.B.; Yang, Z.C.; Li, J.M.; Mo, Y.L.; Xu, M.; Wu, B.; Yang, Z.X. MMP-14 overexpression correlates with poor prognosis in non-small cell lung cancer. Tumour Biol. 2014, 35, 9815–9821. [Google Scholar] [CrossRef]
- Cui, G.; Cai, F.; Ding, Z.; Gao, L. MMP14 predicts a poor prognosis in patients with colorectal cancer. Hum. Pathol. 2019, 83, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Meng, Q.; Wang, Y.; Gao, Y. Overexpression of MMP14 predicts the poor prognosis in gastric cancer: Meta-analysis and database validation. Medicine 2021, 100, e26545. [Google Scholar] [CrossRef]
- Matsuda, M.; Sasabe, H.; Adachi, Y.; Suzuki, T.; Mori, T. Increased invasion activity of endometrial stromal cells and elevated expression of matrix metalloproteinase messenger RNA in the uterine tissues of mice with experimentally induced adenomyosis. Am. J. Obstet. Gynecol. 2001, 185, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, N.; Ung, L.; Otani, T.; Wilkinson, D.; Lopata, A. Uterine cavity matrix metalloproteinases and cytokines in patients with leiomyoma, adenomyosis or endometrial polyp. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 111, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Tokyol, C.; Aktepe, F.; Dilek, F.H.; Sahin, O.; Arioz, D.T. Expression of cyclooxygenase-2 and matrix metalloproteinase-2 in adenomyosis and endometrial polyps and its correlation with angiogenesis. Int. J. Gynecol. Pathol. 2009, 28, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Konrad, L.; Dietze, R.; Riaz, M.A.; Scheiner-Bobis, G.; Behnke, J.; Horné, F.; Hoerscher, A.; Reising, C.; Meinhold-Heerlein, I. Epithelial–mesenchymal transition in endometriosis—When does it happen? J. Clin. Med. 2020, 9, 1915. [Google Scholar] [CrossRef] [PubMed]
- Maoga, J.B.; Riaz, M.A.; Mwaura, A.N.; Scheiner-Bobis, G.; Mecha, E.; Omwandho, C.O.; Meinhold-Heerlein, I.; Konrad, L. Impaired expression of membrane type-2 and type-3 matrix metalloproteinases in endometriosis but not in adenomyosis. Diagnostics 2022, 12, 779. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Barlow, D.; Kennedy, S. Implantation versus infiltration: The Sampson versus the endometriotic disease theory. Gynecol. Obstet. Investig. 1999, 47, 3–10. [Google Scholar] [CrossRef]
- Laudański, P.; Swiatecka, J.; Kozłowski, L.; Leśniewska, M.; Wojtukiewicz, M.; Wołczyński, S. Increased serum level of membrane type 1-matrix metalloproteinase (MT1-MMP/MMP-14) in patients with breast cancer. Folia Histochem. Cytobiol. 2010, 48, 101–103. [Google Scholar] [CrossRef]
- Kasurinen, A.; Tervahartiala, T.; Laitinen, A.; Kokkola, A.; Sorsa, T.; Böckelman, C.; Haglund, C. High serum MMP-14 predicts worse survival in gastric cancer. PLoS ONE 2018, 13, e0208800. [Google Scholar] [CrossRef]
- Sumawan, H. Maternal serum matrix metalloproteinase 14 (MMP14) in early onset preeclampsia and normal pregnancy. SHS Web. Conf. 2020, 86, 01004. [Google Scholar] [CrossRef]
- Dong, Z.; Sun, X.; Xu, J.; Han, X.; Xing, Z.; Wang, D.; Ge, J.; Meng, L.; Xu, X. Serum membrane type 1-matrix metalloproteinase (MT1-MMP) mRNA protected by exosomes as a potential biomarker for gastric cancer. Med. Sci. Monit. 2019, 25, 7770–7783. [Google Scholar] [CrossRef] [PubMed]
- Ovayolu, A.; Ovayolu, G.; Karaman, E.; Güler, S.; Doğan, İ.; Yüce, T. Analyses of soluble endoglin and matrix metalloproteinase 14 using enzyme-linked immunosorbent assay in the diagnosis and assessment of severity of early-and late-onset pre-eclampsia. J. Turk. Ger. Gynecol. Assoc. 2021, 22, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Konrad, L.; Hackethal, A.; Oehmke, F.; Berkes, E.; Engel, J.; Tinneberg, H.R. Analysis of clusterin and clusterin receptors in the endometrium and clusterin levels in cervical mucus of endometriosis. Reprod. Sci. 2016, 23, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Endrikat, J.; Parke, S.; Trummer, D.; Serrani, M.; Duijkers, I.; Klipping, C. Pituitary, ovarian and additional contraceptive effects of an estradiol-based combined oral contraceptive: Results of a randomized, open-label study. Contraception 2013, 87, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Becher, N.; Hein, M.; Danielsen, C.C.; Uldbjerg, N. Matrix metalloproteinases in the cervical mucus plug in relation to gestational age, plug compartment, and preterm labor. Reprod. Biol. Endocrinol. 2010, 8, 113. [Google Scholar] [CrossRef]
- Tsigkou, A.; Reis, F.M.; Ciarmela, P.; Lee, M.H.; Jiang, B.; Tosti, C.; Shen, F.R.; Shi, Z.; Chen, Y.G.; Petraglia, F. Expression levels of myostatin and matrix metalloproteinase 14 mRNAs in uterine leiomyoma are correlated with dysmenorrhea. Reprod. Sci. 2015, 22, 1597–1602. [Google Scholar] [CrossRef]
- Tanaka, N.; Sakamoto, T. MT1-MMP as a key regulator of metastasis. Cells 2023, 12, 2187. [Google Scholar] [CrossRef]

| Tissues | EM/EN−/AM− | EM/EN−/AM+ | EM EN+/AM− | EM EN+/AM+ | OV |
|---|---|---|---|---|---|
| All samples | n = 3 | n = 12 | n = 5 | n = 8 | n = 6 |
| Median age ± SD | 45 ± 4.4 | 41.5 ± 4.7 | 43 ± 2.8 | 46.5 ± 3.8 | 38 ± 8.4 |
| Proliferative | n = 2 | n = 7 | n = 2 | n = 6 | |
| Secretory | n = 1 | n = 2 | n = 2 | n = 1 | |
| Unknown | n = 3 | n = 1 | n = 1 |
| Serum Samples | Mucus Samples | |||
|---|---|---|---|---|
| EN− | EN+ | EN− | EN+ | |
| (n) | 61 | 71 | 87 | 106 |
| Median age ± SD | 27 ± 7.9 | 34 ± 7.1 | 28 ± 8.2 | 33 ± 7.1 |
| BMI (kg/m2) | 21.8 ± 6.3 | 23.7 ± 5.2 | 21.8 ± 4.6 | 22.9 ± 4.4 |
| Smoking n (%) | n = 12 (19.7) | n = 17 (23.9) | n = 23 (26.4) | n = 24 (22.6) |
| Allergy n (%) | n = 31 (50.8) | n = 34 (47.9) | n = 44 (50.6) | n = 58 (54.7) |
| Menstrual phase (n) | ||||
| Proliferative | 19 | 15 | 34 | 37 |
| Secretory | 28 | 16 | 47 | 48 |
| Menstruation | 8 | 9 | - | - |
| Unknown | 6 | 31 | 6 | 21 |
| Contraception use (n) | ||||
| Yes | 28 | 35 | 22 | 36 |
| No | 33 | 36 | 65 | 70 |
| Fertility | ||||
| Yes | 12 | 26 | 24 | 39 |
| No | 8 | 19 | 14 | 30 |
| Unknown | 41 | 26 | 49 | 37 |
| Pain (n) | ||||
| Dysmenorrhea | ||||
| Yes | 48 | 44 | 70 | 83 |
| No | 13 | 25 | 17 | 22 |
| Unknown | - | 2 | - | 1 |
| Dyspareunia | ||||
| Yes | 26 | 39 | 40 | 62 |
| No | 32 | 30 | 41 | 44 |
| Unknown | 3 | 2 | 6 | - |
| Dyschezia | ||||
| Yes | 18 | 26 | 27 | 44 |
| No | 43 | 45 | 56 | 62 |
| Unknown | - | - | 4 | - |
| Dysuria | ||||
| Yes | 11 | 15 | 19 | 29 |
| No | 50 | 56 | 68 | 77 |
| Unknown | - | - | - | - |
| EM a | OV b | Adenomyosis c | |
|---|---|---|---|
| HSCORE | |||
| Mean | 91 | 31 | 149 |
| SEM | 15.9 | 10.2 | 15.5 |
| p-values | 0.0325 a,b | 0.0003 b,c | 0.024 a,c |
| N | 16 | 6 | 20 |
| Age | 45 ± 6.6 | 38 ± 8.4 | 44.5 ± 5.0 |
| Percentage of stained glands | |||
| Mean | 65 | 32 | 92 |
| SEM | 9.2 | 3.0 | 10.5 |
| p-values | n.s. a,b | 0.0002 b,c | 0.037 a,c |
| N | 16 | 6 | 20 |
| Age | 45 ± 6.6 | 38 ± 8.4 | 44.5 ± 5.0 |
| Without Endometriosis | With Endometriosis | |||||
|---|---|---|---|---|---|---|
| Phases | Menstrual a | Proliferative b | Secretory c | Menstrual d | Proliferative e | Secretory f |
| Samples (n) | 8 | 18 | 29 | 9 | 15 | 16 |
| Median age | 26.5 ± 10.4 | 27 ± 8.7 | 28 ± 7.6 | 31 ± 6.3 | 37 ± 6.3 | 32 ± 6.6 |
| Mean (ng/mL) | 0.7 | 0.6 | 0.8 | 0.7 | 4.1 | 0.6 |
| SEM | 0.3 | 0.2 | 0.3 | 0.3 | 3.7 | 0.4 |
| Range (ng/mL) | 0.1–2.9 | 0–3.3 | 0–7.7 | 0–2.2 | 0–57.2 | 0–5.7 |
| p-values | n.s. a,b | n.s. a,c n.s. b,c | n.s. a,d | ≤0.05 d,e 0.042 b,e | ≤0.05 e,f n.s. d,f n.s. c,f | |
| A | Without Endometriosis | With Endometriosis | ||
|---|---|---|---|---|
| Samples (n) | 61 | 71 | ||
| Median age | 27 ± 7.9 | 34 ± 7.1 | ||
| Mean (ng/mL) | 0.7 | 1.3 | ||
| SEM | 0.2 | 0.8 | ||
| Range (ng/mL) | 0–7.7 | 0–57.2 | ||
| p-value | 0.0016 | |||
| B | Contraception | |||
| w/o EN, w/o c a | w/o EN, w c b | w EN, w/o c c | w EN, w c d | |
| Samples (n) | 33 | 28 | 36 | 35 |
| Median age | 28 ± 9.2 | 25 ± 4.9 | 34 ± 7.3 | 34 ± 6.9 |
| Mean (ng/mL) | 0.8 | 0.5 | 0.7 | 2.0 |
| SEM | 0.3 | 0.2 | 0.2 | 1.6 |
| Range (ng/mL) | 0–7.7 | 0–3.3 | 0–5.7 | 0–57.2 |
| p-values | n.s. a,b | n.s. b,c 0.043 a,c | n.s. c,d 0.030 b,d | |
| Without Endometriosis | With Endometriosis | |||
|---|---|---|---|---|
| Proliferative a | Secretory b | Proliferative c | Secretory d | |
| Samples (n) | 32 | 49 | 34 | 51 |
| Median age | 31 ± 9.1 | 27 ± 7.4 | 30.5 ± 6.4 | 34 ± 6.5 |
| Mean (ng/mL) | 4.1 | 2.8 | 4.6 | 3.5 |
| SEM | 0.3 | 0.2 | 0.4 | 0.3 |
| Range (ng/mL) | 0–7.6 | 0–6.9 | 0–14 | 0–9.3 |
| p-values | 0.0006 a,b | n.s. b,c n.s. a,c | 0.01 c,d n.s. b,d | |
| A | Without Endometriosis | With Endometriosis | ||
|---|---|---|---|---|
| Samples (n) | 87 | 106 | ||
| Median age | 28 ± 8.2 | 33 ± 7.1 | ||
| Mean (ng/mL) | 3.2 | 3.6 | ||
| SEM | 0.2 | 0.2 | ||
| Range (ng/mL) | 0–7.6 | 0–14 | ||
| p-value | n.s. | |||
| B | Contraception | |||
| w/o EN, w/o c a | w/o EN, w c b | w EN, w/o c c | w EN, w c d | |
| Samples (n) | 65 | 22 | 70 | 36 |
| Median age | 30 ± 8.5 | 27 ± 7.2 | 32.5 ± 7.2 | 33 ± 6.8 |
| Mean (ng/mL) | 3.4 | 2.7 | 3.8 | 3.2 |
| Range (ng/mL) | 0–7.6 | 0–5.4 | 0–9.3 | 0–14 |
| SEM | 0.2 | 0.4 | 0.2 | 0.4 |
| p-values | n.s. a,b | n.s. a,c n.s. b,c | 0.003 c,d n.s. b,d | |
| A | Serum MT1-MMP Levels | |||||
|---|---|---|---|---|---|---|
| Mean (ng/mL) ± SEM | ||||||
| W/o EN | W EN | p-Values | Without Pain | With Pain | p-Values | |
| BMI (kg/m2) | 23.9 ± 0.8 | 24.4 ± 0.6 | n.s. | |||
| Age | 28.9 ± 1.0 | 34.8 ± 0.8 | 0.0001 | |||
| Dysmenorrhea | n = 130 | 0.97 ± 0.3 | 1.10 ± 0.7 | n.s. | ||
| Dysuria | n = 131 | 1.18 ± 0.5 | 0.22 ± 0.1 | n.s. | ||
| Dyschezia | n = 132 | 1.24 ± 0.6 | 0.52 ± 0.2 | n.s. | ||
| Dyspareunia | n = 127 | 0.77 ± 0.2 | 1.46 ± 1.1 | n.s. | ||
| B | Endocervical Mucus MT1-MMP Levels | |||||
| Mean (ng/mL) ± SEM | ||||||
| W/o EN | W EN | p-Values | Without Pain | With Pain | p-Values | |
| BMI (kg/m2) | 22.9 ± 0.5 | 23.4 ± 0.4 | n.s. | |||
| Age | 30.8 ± 0.9 | 33.9 ± 0.7 | n.s. | |||
| Dysmenorrhea | n = 192 | 3.18 ± 0.3 | 3.49 ± 0.2 | n.s. | ||
| Dysuria | n = 193 | 3.39 ± 0.2 | 3.52 ± 0.3 | n.s. | ||
| Dyschezia | n = 189 | 3.46 ± 0.2 | 3.50 ± 0.2 | n.s. | ||
| Dyspareunia | n = 187 | 3.56 ± 0.2 | 3.39 ± 0.2 | n.s. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maoga, J.B.; Riaz, M.A.; Mwaura, A.N.; Mecha, E.; Omwandho, C.O.A.; Scheiner-Bobis, G.; Meinhold-Heerlein, I.; Konrad, L. Analysis of Membrane Type-1 Matrix Metalloproteinase (MT1-MMP, MMP14) in Eutopic and Ectopic Endometrium and in Serum and Endocervical Mucus of Endometriosis. Biomedicines 2023, 11, 2730. https://doi.org/10.3390/biomedicines11102730
Maoga JB, Riaz MA, Mwaura AN, Mecha E, Omwandho COA, Scheiner-Bobis G, Meinhold-Heerlein I, Konrad L. Analysis of Membrane Type-1 Matrix Metalloproteinase (MT1-MMP, MMP14) in Eutopic and Ectopic Endometrium and in Serum and Endocervical Mucus of Endometriosis. Biomedicines. 2023; 11(10):2730. https://doi.org/10.3390/biomedicines11102730
Chicago/Turabian StyleMaoga, Jane B., Muhammad A. Riaz, Agnes N. Mwaura, Ezekiel Mecha, Charles O. A. Omwandho, Georgios Scheiner-Bobis, Ivo Meinhold-Heerlein, and Lutz Konrad. 2023. "Analysis of Membrane Type-1 Matrix Metalloproteinase (MT1-MMP, MMP14) in Eutopic and Ectopic Endometrium and in Serum and Endocervical Mucus of Endometriosis" Biomedicines 11, no. 10: 2730. https://doi.org/10.3390/biomedicines11102730
APA StyleMaoga, J. B., Riaz, M. A., Mwaura, A. N., Mecha, E., Omwandho, C. O. A., Scheiner-Bobis, G., Meinhold-Heerlein, I., & Konrad, L. (2023). Analysis of Membrane Type-1 Matrix Metalloproteinase (MT1-MMP, MMP14) in Eutopic and Ectopic Endometrium and in Serum and Endocervical Mucus of Endometriosis. Biomedicines, 11(10), 2730. https://doi.org/10.3390/biomedicines11102730









