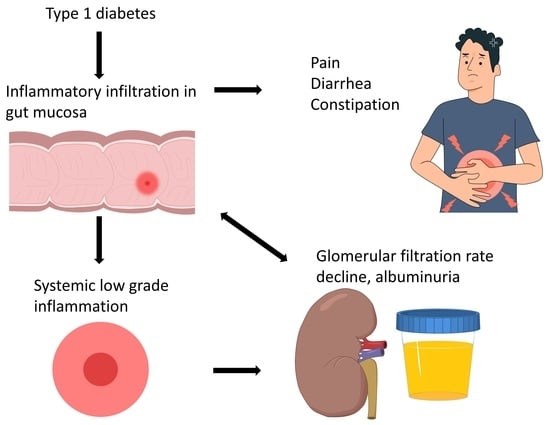
Progression of Diabetic Kidney Disease and Gastrointestinal Symptoms in Patients with Type I Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Ethics
2.2. Clinical Investigation and Monitoring of Diabetic Complications and Co-Morbidities
2.3. Blood Samples and Faecal Collection
2.4. Monitoring of Gastrointestinal Disease and Symptoms
2.5. Endoscopy in Patients with Indications and “Red Flag” Symptoms
2.6. Statistical Analysis
3. Results
3.1. Description of this Study Groups and Inflammatory Markers
3.2. Gastrointestinal Diseases and Symptoms
3.3. Correlation between Gastrointestinal Symptom Scores with Clinical Markers and Regression Analysis
3.4. Endoscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Valo, E.; McGurnaghan, S.J.; Sandholm, N.; Blackbourn, L.A.K.; Dalton, R.N.; Dunger, D.; Groop, P.H.; McKeigue, P.M.; Forsblom, C.; et al. Biomarker panels associated with progression of renal disease in type 1 diabetes. Diabetologia 2019, 62, 1616–1627. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.T.; Rayner, C.K.; Jones, K.L.; Talley, N.J.; Horowitz, M. Gastrointestinal Symptoms in Diabetes: Prevalence, Assessment, Pathogenesis, and Management. Diabetes Care 2018, 41, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Lomer, M.C. Review article: The aetiology, diagnosis, mechanisms and clinical evidence for food intolerance. Aliment. Pharmacol. Ther. 2015, 41, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Leeds, J.S.; Hadjivassiliou, M.; Tesfaye, S.; Sanders, D.S. Lower gastrointestinal symptoms are associated with worse glycemic control and quality of life in type 1 diabetes mellitus. BMJ Open Diabetes Res. Care 2018, 6, e000514. [Google Scholar] [CrossRef]
- Porter, J.A.; MacKenzie, K.E.; Darlow, B.A.; Pearson, J.F.; Day, A.S. A questionnaire-based assessment of gastrointestinal symptoms in children with type 1 diabetes mellitus. Transl. Pediatr. 2020, 9, 743–749. [Google Scholar] [CrossRef]
- Lehto, M.; Groop, P.H. The Gut-Kidney Axis: Putative Interconnections Between Gastrointestinal and Renal Disorders. Front. Endocrinol. 2018, 9, 553. [Google Scholar] [CrossRef]
- Ni, Y.; Zheng, L.; Nan, S.; Ke, L.; Fu, Z.; Jin, J. Enterorenal crosstalks in diabetic nephropathy and novel therapeutics targeting the gut microbiota. Acta Biochim. Biophys. Sin. 2022, 54, 1406–1420. [Google Scholar] [CrossRef]
- Guo, W.; Song, Y.; Sun, Y.; Du, H.; Cai, Y.; You, Q.; Fu, H.; Shao, L. Systemic immune-inflammation index is associated with diabetic kidney disease in Type 2 diabetes mellitus patients: Evidence from NHANES 2011–2018. Front. Endocrinol. 2022, 13, 1071465. [Google Scholar] [CrossRef]
- Winther, S.A.; Mannerla, M.M.; Frimodt-Møller, M.; Persson, F.; Hansen, T.W.; Lehto, M.; Hörkkö, S.; Blaut, M.; Forsblom, C.; Groop, P.H.; et al. Faecal biomarkers in type 1 diabetes with and without diabetic nephropathy. Sci. Rep. 2021, 11, 15208. [Google Scholar] [CrossRef]
- Lassenius, M.I.; Fogarty, C.L.; Blaut, M.; Haimila, K.; Riittinen, L.; Paju, A.; Kirveskari, J.; Järvelä, J.; Ahola, A.J.; Gordin, D.; et al. Intestinal alkaline phosphatase at the crossroad of intestinal health and disease—A putative role in type 1 diabetes. J. Intern. Med. 2017, 281, 586–600. [Google Scholar] [CrossRef] [PubMed]
- D’Addio, F.; La Rosa, S.; Maestroni, A.; Jung, P.; Orsenigo, E.; Ben Nasr, M.; Tezza, S.; Bassi, R.; Finzi, G.; Marando, A.; et al. Circulating IGF-I and IGFBP3 Levels Control Human Colonic Stem Cell Function and Are Disrupted in Diabetic Enteropathy. Cell Stem Cell 2015, 17, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, I.; Segol, O.; Shental, R.; Shimoni, P.; Eldor, R. Antihyperglycemic therapy during colonoscopy preparation: A review and suggestions for practical recommendations. United Eur. Gastroenterol. J. 2019, 7, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, S.; Sordi, V.; Bolla, A.M.; Saita, D.; Ferrarese, R.; Canducci, F.; Clementi, M.; Invernizzi, F.; Mariani, A.; Bonfanti, R.; et al. Duodenal Mucosa of Patients with Type 1 Diabetes Shows Distinctive Inflammatory Profile and Microbiota. J. Clin. Endocrinol. Metab. 2017, 102, 1468–1477. [Google Scholar] [CrossRef]
- Paul, P.; Kaul, R.; Chaari, A. Renal Health Improvement in Diabetes through Microbiome Modulation of the Gut-Kidney Axis with Biotics: A Systematic and Narrative Review of Randomized Controlled Trials. Int. J. Mol. Sci. 2022, 23, 14838. [Google Scholar] [CrossRef]
- Ahola, A.J.; Radzeviciene, L.; Zaharenko, L.; Bulum, T.; Skrebinska, S.; Prakapiene, E.; Blaslov, K.; Roso, V.; Rovite, V.; Pirags, V.; et al. Association between symptoms of depression, diabetes complications and vascular risk factors in four European cohorts of individuals with type 1 diabetes—InterDiane Consortium. Diabetes Res. Clin. Pract. 2020, 170, 108495. [Google Scholar] [CrossRef]
- Tzivian, L.; Sokolovska, J.; Grike, A.E.; Kalcenaua, A.; Seidmann, A.; Benis, A.; Mednis, M.; Danovska, I.; Berzins, U.; Bogdanovs, A.; et al. Quantitative and qualitative analysis of the quality of life of Type 1 diabetes patients using insulin pumps and of those receiving multiple daily insulin injections. Health Qual. Life Outcomes 2022, 20, 120. [Google Scholar] [CrossRef]
- Svikle, Z.; Pahirko, L.; Zariņa, L.; Baumane, K.; Kardonaite, D.; Radzeviciene, L.; Daugintyte-Petrusiene, L.; Balciuniene, V.J.; Verkauskiene, R.; Tiščuka, A.; et al. Telomere Lengths and Serum Proteasome Concentrations in Patients with Type 1 Diabetes and Different Severities of Diabetic Retinopathy in Latvia and Lithuania. J. Clin. Med. 2022, 11, 2768. [Google Scholar] [CrossRef]
- Salna, I.; Salna, E.; Pahirko, L.; Skrebinska, S.; Krikova, R.; Folkmane, I.; Pīrāgs, V.; Sokolovska, J. Achievement of treatment targets predicts progression of vascular complications in type 1 diabetes. J. Diabetes Complicat. 2021, 35, 108072. [Google Scholar] [CrossRef]
- Rostoka, E.; Salna, I.; Dekante, A.; Pahirko, L.; Borisovs, V.; Celma, L.; Valeinis, J.; Sjakste, N.; Sokolovska, J. DNA damage in leukocytes and serum nitrite concentration are negatively associated in type 1 diabetes. Mutagenesis 2021, 36, 213–222. [Google Scholar] [CrossRef]
- Sokolovska, J.; Stefanovics, J.; Gersone, G.; Pahirko, L.; Valeinis, J.; Kalva-Vaivode, S.; Rovite, V.; Blumfelds, L.; Pirags, V.; Tretjakovs, P. Angiopoietin 2 and Neuropeptide Y are Associated with Diabetic Kidney Disease in Type 1 Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2020, 128, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Sokolovska, J.; Dekante, A.; Baumane, L.; Pahirko, L.; Valeinis, J.; Dislere, K.; Rovite, V.; Pirags, V.; Sjakste, N. Nitric oxide metabolism is impaired by type 1 diabetes and diabetic nephropathy. Biomed. Rep. 2020, 12, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.M.; Todd, J.N.; Sandholm, N.; Cole, J.B.; Chen, W.M.; Andrews, D.; Pezzolesi, M.G.; McKeigue, P.M.; Hiraki, L.T.; Qiu, C.; et al. Genome-Wide Association Study of Diabetic Kidney Disease Highlights Biology Involved in Glomerular Basement Membrane Collagen. J. Am. Soc. Nephrol. 2019, 30, 2000–2016. [Google Scholar] [CrossRef] [PubMed]
- Sviklāne, L.; Olmane, E.; Dzērve, Z.; Kupčs, K.; Pīrāgs, V.; Sokolovska, J. Fatty liver index and hepatic steatosis index for prediction of non-alcoholic fatty liver disease in type 1 diabetes. J. Gastroenterol. Hepatol. 2018, 33, 270–276. [Google Scholar] [CrossRef]
- Rovite, V.; Wolff-Sagi, Y.; Zaharenko, L.; Nikitina-Zake, L.; Grens, E.; Klovins, J. Genome Database of the Latvian Population (LGDB): Design, Goals, and Primary Results. J. Epidemiol. 2018, 28, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Quan, C.; Talley, N.J.; Cross, S.; Jones, M.; Hammer, J.; Giles, N.; Horowitz, M. Development and validation of the Diabetes Bowel Symptom Questionnaire. Aliment. Pharmacol. Ther. 2003, 17, 1179–1187. [Google Scholar] [CrossRef]
- Karahan, D.; Şahin, İ. Comparison of gastrointestinal symptoms and findings in renal replacement therapy modalities. BMC Nephrol. 2022, 23, 261. [Google Scholar] [CrossRef]
- Bouri, S.; Martin, J. Investigation of iron deficiency anaemia. Clin. Med. 2018, 18, 242–244. [Google Scholar] [CrossRef]
- Lewandowski, K.; Rydzewska, G.; Ledwoń, T.K. Preparation for endoscopic examinations in patients with diabetes and hypoglycaemia. Przegląd Gastroenterol. 2021, 16, 297–305. [Google Scholar] [CrossRef]
- Hassan, C.; Bretthauer, M.; Kaminski, M.F.; Polkowski, M.; Rembacken, B.; Saunders, B.; Benamouzig, R.; Holme, O.; Green, S.; Kuiper, T.; et al. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2013, 45, 142–150. [Google Scholar] [CrossRef]
- Hinkelbein, J.; Lamperti, M.; Akeson, J.; Santos, J.; Costa, J.; De Robertis, E.; Longrois, D.; Novak-Jankovic, V.; Petrini, F.; Struys, M.; et al. European Society of Anaesthesiology and European Board of Anaesthesiology guidelines for procedural sedation and analgesia in adults. Eur. J. Anaesthesiol. 2018, 35, 6–24. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, J.R.; Cash, B.D.; Pasha, S.F.; Early, D.S.; Muthusamy, V.R.; Khashab, M.A.; Chathadi, K.V.; Fanelli, R.D.; Chandrasekhara, V.; Lightdale, J.R.; et al. Bowel preparation before colonoscopy. Gastrointest. Endosc. 2015, 81, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Y.; Zou, L.; Gong, R. Prognostic nutritional index as a risk factor for diabetic kidney disease and mortality in patients with type 2 diabetes mellitus. Acta Diabetol. 2023, 60, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Aktas, G.; Yilmaz, S.; Kantarci, D.B.; Duman, T.T.; Bilgin, S.; Balci, S.B.; Atak Tel, B.M. Is serum uric acid-to-HDL cholesterol ratio elevation associated with diabetic kidney injury? Postgrad. Med. 2023, 135, 519–523. [Google Scholar] [CrossRef]
- Schön, E.-M.; Winter, D.; Escalona, M.J.; Thomaschewski, J. Key Challenges in Agile Requirements Engineering; Springer: Cham, Switzerland, 2017; pp. 37–51. [Google Scholar]
- Shi, J.T.; Chen, N.; Xu, J.; Goyal, H.; Wu, Z.Q.; Zhang, J.X.; Xu, H.G. Diagnostic Accuracy of Fecal Calprotectin for Predicting Relapse in Inflammatory Bowel Disease: A Meta-Analysis. J. Clin. Med. 2023, 12, 1206. [Google Scholar] [CrossRef]
- Rezazadeh Ardabili, A.; Goudkade, D.; Wintjens, D.; Romberg-Camps, M.; Winkens, B.; Pierik, M.; Grabsch, H.I.; Jonkers, D. Histopathological Features in Colonic Biopsies at Diagnosis Predict Long-term Disease Course in Patients with Crohn’s Disease. J. Crohn’s Colitis 2021, 15, 1885–1897. [Google Scholar] [CrossRef]
| DKD Stable, N = 73 | DKD Progressive, N = 27 | p Value | |
|---|---|---|---|
| Male gender, N (%) | 31 (42.5%) | 7 (25.9%) | 0.99 |
| Age, years, | 44.0 (31.5–52.0) | 39.0 (34.0–50.0) | 0.86 |
| BMI, kg/m2 | 24.85 (22.63–28.15) | 25.40 (22.60–28.40) | 0.95 |
| Smokers, N (%) | 13 (17.8%) | 6 (22.2%) | <0.01 |
| Hypertension, N (%) | 32 (43.8%) | 21 (77.8%) | <0.01 |
| Length of diabetes, years | 21 (13–31) | 27 (24–37) | <0.01 |
| Mean follow-up, years | 7.00 (5.50–7.00) | 7.00 (3.00–7.00) | 0.72 |
| Retinopathy, N (%) | 30 (41.1%) | 22 (81.5%) | <0.01 |
| Cardiovascular disease, N (%) | 7 (9.6%) | 9 (33.3%) | <0.01 |
| On ACEI/ARB, N (%) | 12 (16.4%) | 8 (29.6%) | 0.14 |
| On lipid lowering medication, N (%) | 17 (23.3%) | 10 (37.0%) | 0.16 |
| Autoimmune thyroid disease, N (%) | 18 (24.7%) | 9 (33.3%) | 0.27 |
| Other autoimmune disease, N (%) | 11 (15.1%) | 5 (18.5%) | 0.67 |
| Haemoglobin A1C, % | 7.60 (7.00–8.70) | 8.90 (07.54–10.30) | 0.02 |
| Haemoglobin A1C, mmol/mol | 59.56 (53.00–71.58) | 73.77 (58.90–89.07) | 0.02 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 107.98(92.52–117.63) | 72.81(40.72–105.27) | <0.01 |
| End stage renal disease, N (%) | 0 (0%) | 4 (14.8%) | <0.01 |
| Albumin/creatinine ratio in urine, mg/mmol | 0.45(0.19–0.99) | 19.65 (4.98–112.50) | <0.01 |
| C-reactive protein, mg/L | 0.9 (0.5–2.8) | 1.5 (0.5–3.48) | 0.72 |
| Faecal calprotectin, µg/g | 7.60 (2.75–23.20) | 16.20 (5.80–22.00) | 0.21 |
| Faecal calprotectin > 50 µg/g, N (%) | 8 (11.0%) | 2 (7.4%) | 0.59 |
| Total cholesterol, mmol/L | 5.02(4.30–5.79) | 4.96(4.12–5.88) | 0.95 |
| Low density lipoproteins, mmol/L | 2.87(2.10–3.40) | 3.04(2.10–3.46) | 0.41 |
| Triglycerides, mmol/L | 1.14(0.85–1.45) | 1.31(0.86–2.11) | 0.14 |
| Alanine transaminase, U/L | 19.00(15.50–29.00) | 21.00(17.00–24.00) | 0.64 |
| Gamma-glutamyl Transferase, U/L | 16.00(13.00–25.50) | 17.00(15.00–26.00) | 0.41 |
| Bilirubin, µmol/L | 9.30(7.80–12.40) | 6.51(5.10–9.20) | 0.001 |
| Haemoglobin, g/L | 140.00(132.00–150.00) | 129.00(120.00–141.00) | 0.001 |
| Erythrocytes, 10 × 12/L | 4.70(4.40–5.00) | 4.40(4.00–4.73) | 0.002 |
| Leukocytes, 10 × 9/L | 6.13(5.01–7.22) | 6.53(5.71–7.64) | 0.14 |
| Thrombocytes, 10 × 9/L | 259.00(228.00–284.00) | 231.00(200.00–299.00) | 0.21 |
| DKD Stable, N = 73 | DKD Progressive, N = 27 | p Value | |
|---|---|---|---|
| History of upper gastrointestinal disease, N (%) | 29 (39.7%) | 13 (48.1%) | 0.50 |
| History of lower gastrointestinal disease, N (%) | 24 (32.9%) | 12 (44.4%) | 0.28 |
| History of gastrointestinal malignancy, N (%) | 0 (0.0%) | 1 (3.7%) | 0.10 |
| History of liver and pancreas disease, N (%) | 27 (37.0%) | 9 (33.3%) | 0.74 |
| History of abdominal surgery, N (%) | 21 (28.8%) | 13 (48.1%) | 0.07 |
| Mean value of gastrointestinal symptom score | 1.05 (0.73–1.35) | 1.27 (1.00–1.82) | 0.019 |
| Bowel movement disorders, N (%) | 16 (21.9%) | 14 (51.9%) | <0.01 |
| Usage of medications for gastrointestinal disorders | 0.16 (0.00–0.50) | 0.33 (0.00–0.66) | 0.54 |
| Variable | Model | Odds Ratio, OR | 95% Confidence Interval (CI) | p Value |
|---|---|---|---|---|
| Mean Symptoms | 1 | 3.086 | 1.20; 7.87 | 0.02 |
| Mean Symptoms | 2 | 2.77 | 1.06; 7.24 | 0.04 |
| Mean symptoms | 3 | 2.394 | 0.886; 6.467 | 0.085 |
| Mean Symptoms | 4 | 1.99 | 0.71; 5.54 | 0.18 |
| Procedure | Macroscopic Lesions | Histopathologic Lesions |
|---|---|---|
| Upper endoscopy, N = 10 | (1) Hyperaemic gastropathy (n = 4) (2) Hyperaemic duodenopathy (n = 1) (3) oesophageal candidiasis (n = 1) (4) gastric intestinal metaplasia (n = 1) | (1) active gastritis (N = 3) (2) chronic atrophic gastritis (N = 3) |
| Colonoscopy, N = 21 | (1) polyps (n = 2) (2) diverticulosis (n = 1) (3) erosion of sigmoid colon (n = 1) (4) perianal papilloma (n = 1) | (1) eosinophilic infiltration (n = 8, 38%) (2) lymphoid follicles/lymphoid aggregates (n = 7, 33%) (3) lymphoplasmacytic infiltration (n = 5, 24%). (4) lymphocyte and macrophage infiltration (n = 3, 14%) (5) stromal fibrosis (n = 3, 14%) (6) mononuclear cell infiltration (n = 2, 9.5%) (7) tubular adenomas (n = 2, 9.5%). (8) Active inflammation 6 (29%) (9) active colitis 1 (4.76%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedulovs, A.; Tzivian, L.; Zalizko, P.; Ivanova, S.; Bumane, R.; Janeviča, J.; Krūzmane, L.; Krustins, E.; Sokolovska, J. Progression of Diabetic Kidney Disease and Gastrointestinal Symptoms in Patients with Type I Diabetes. Biomedicines 2023, 11, 2679. https://doi.org/10.3390/biomedicines11102679
Fedulovs A, Tzivian L, Zalizko P, Ivanova S, Bumane R, Janeviča J, Krūzmane L, Krustins E, Sokolovska J. Progression of Diabetic Kidney Disease and Gastrointestinal Symptoms in Patients with Type I Diabetes. Biomedicines. 2023; 11(10):2679. https://doi.org/10.3390/biomedicines11102679
Chicago/Turabian StyleFedulovs, Aleksejs, Lilian Tzivian, Polina Zalizko, Santa Ivanova, Renāte Bumane, Jana Janeviča, Lelde Krūzmane, Eduards Krustins, and Jelizaveta Sokolovska. 2023. "Progression of Diabetic Kidney Disease and Gastrointestinal Symptoms in Patients with Type I Diabetes" Biomedicines 11, no. 10: 2679. https://doi.org/10.3390/biomedicines11102679
APA StyleFedulovs, A., Tzivian, L., Zalizko, P., Ivanova, S., Bumane, R., Janeviča, J., Krūzmane, L., Krustins, E., & Sokolovska, J. (2023). Progression of Diabetic Kidney Disease and Gastrointestinal Symptoms in Patients with Type I Diabetes. Biomedicines, 11(10), 2679. https://doi.org/10.3390/biomedicines11102679