Renal Papillary Necrosis (RPN) in an African Population: Disease Patterns, Relevant Pathways, and Management
Abstract
1. Introduction
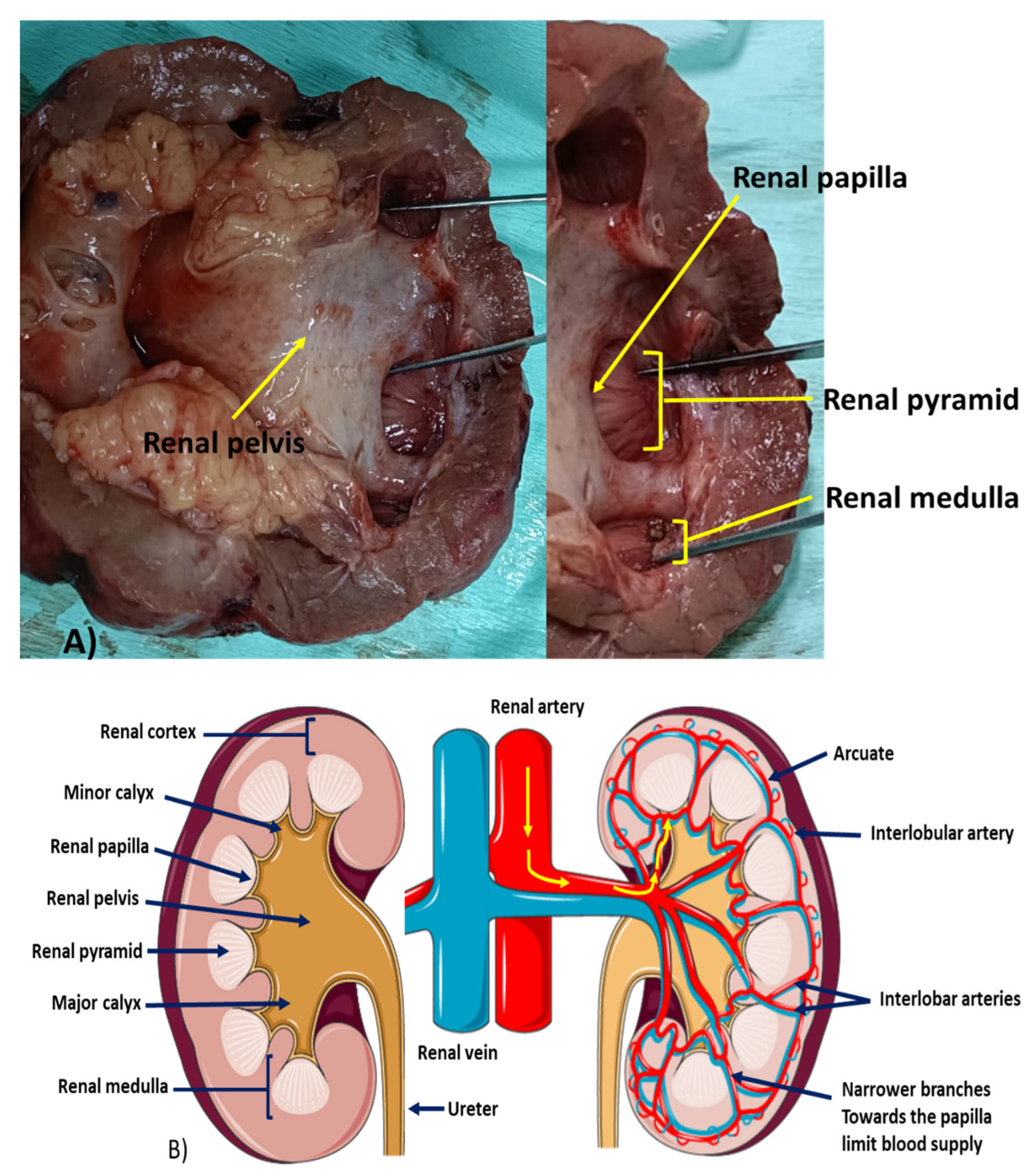
2. Renal Pyramid, Renal Papilla, and Vasa Recta: A Unique Morphological Entity
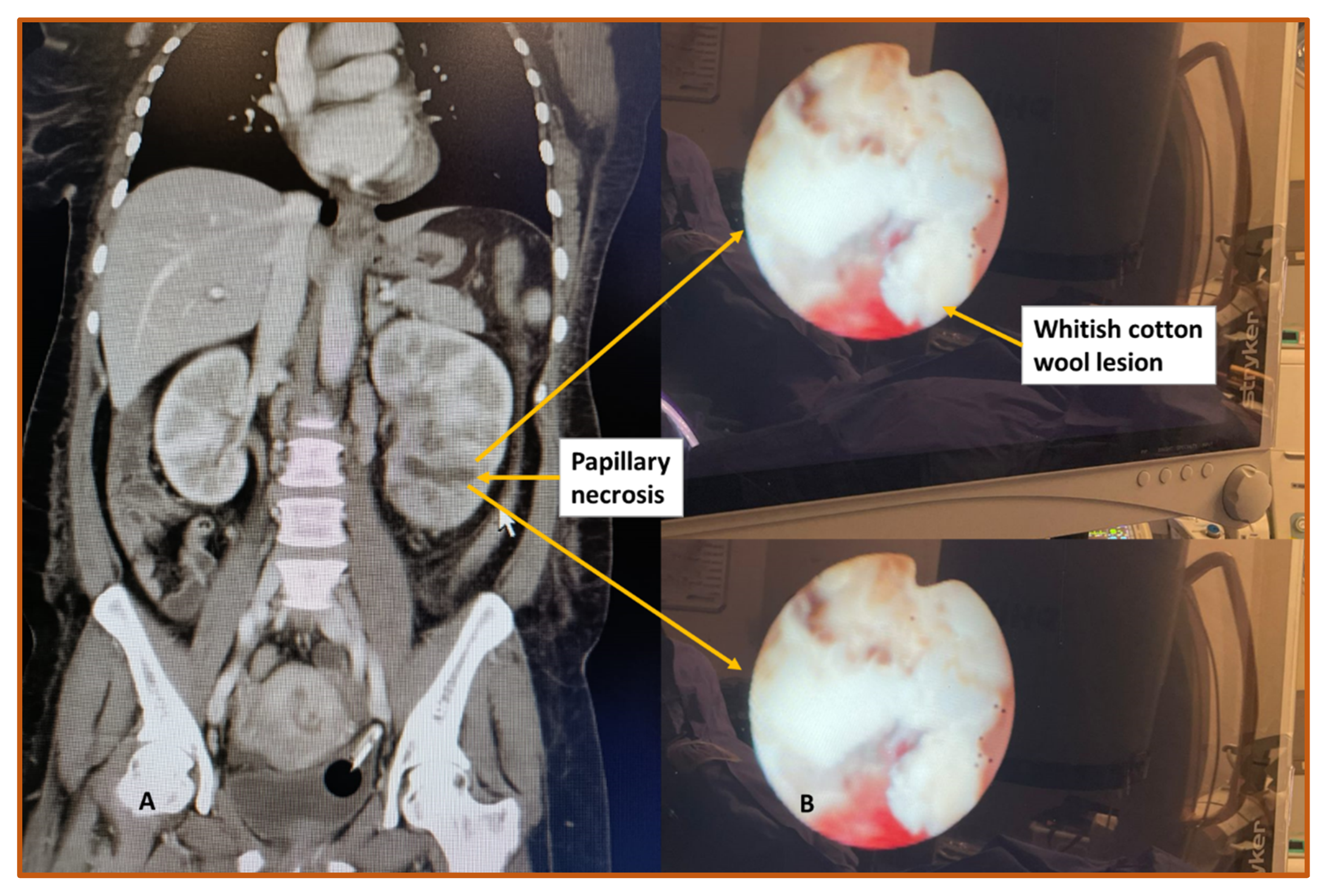
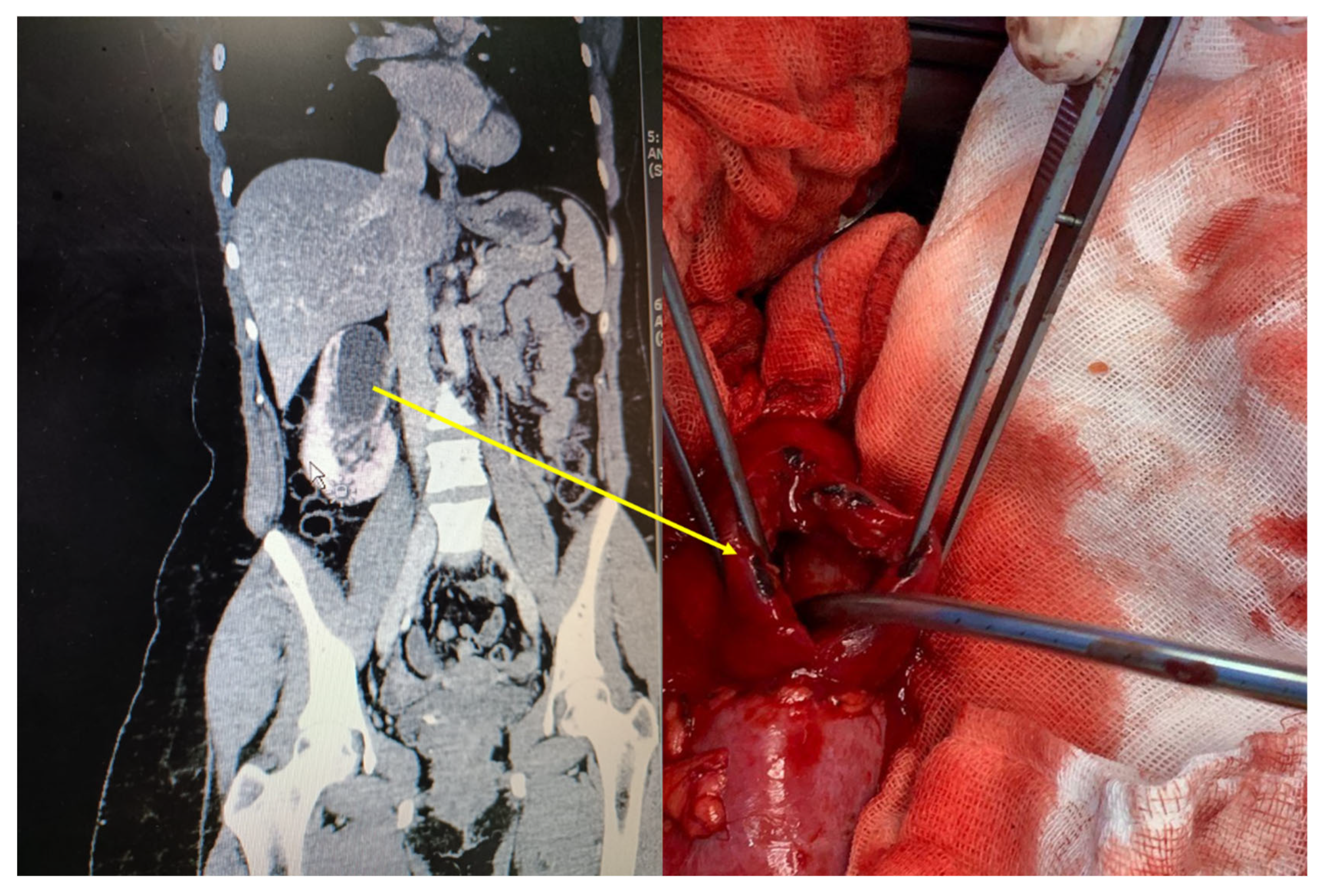
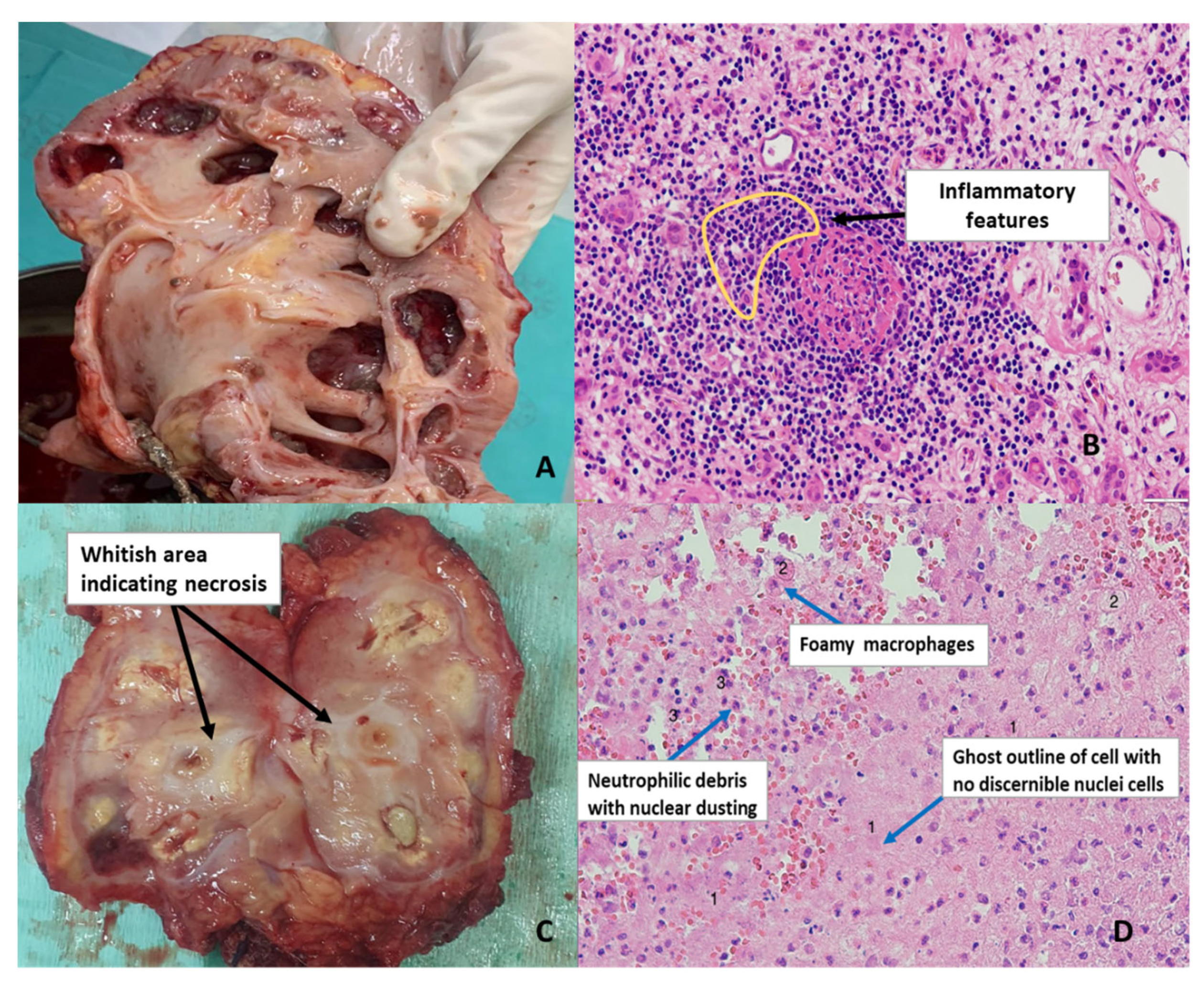
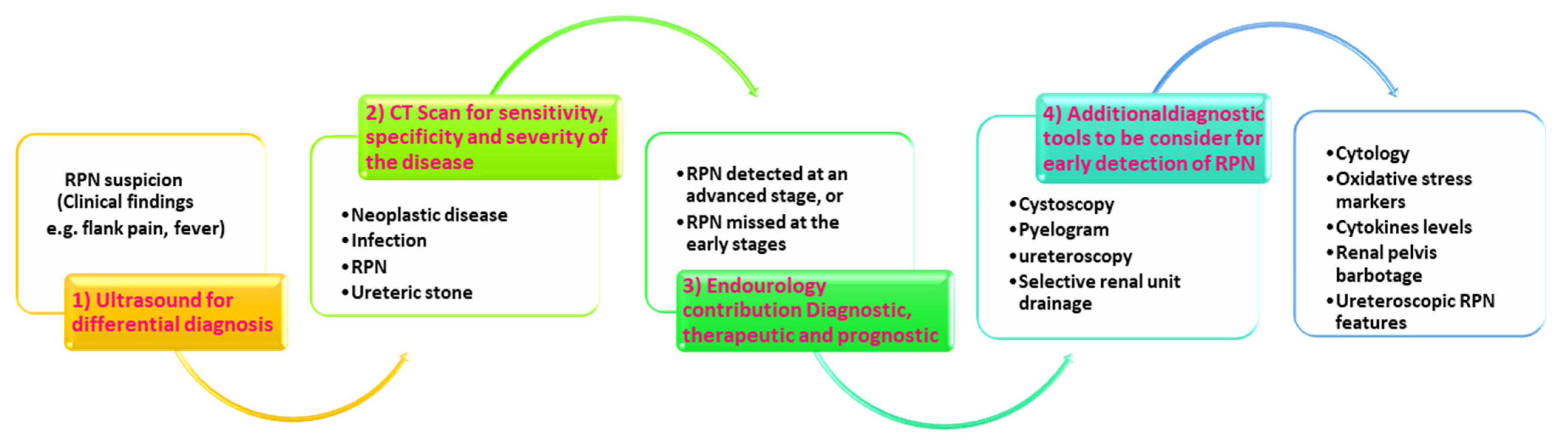
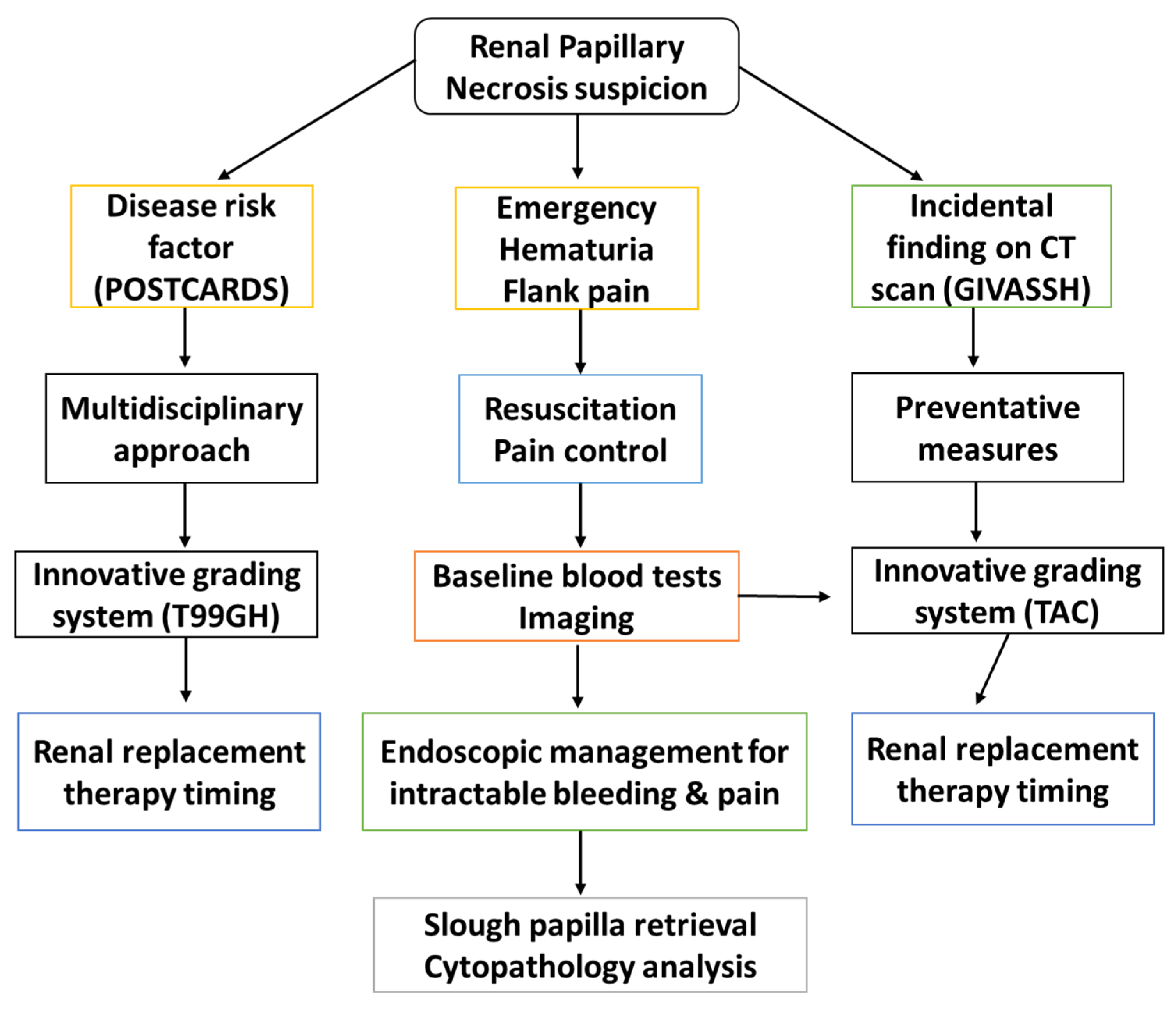
3. Diagnostic Shortcomings
- A.
- Clinical Features
- B.
- Imaging
- C.
- Cytopathology
4. Potential Diagnostic Paradigm for RPN
5. RPN Epidemiology in The World and Sub-Saharan Africa
6. RPN Etiologies and Risk Factors
- A. Etiology of Renal Papillary Necrosis
- G-Genetic
- I-Iatrogenic
- V-Viral
- A-Antimicrobials
- S-Schistosomiasis
- S-Substance Abuse
- H-Hypertension
7. Contributing Pathways to RPN
7.1. Acute Tubular Necrosis Cumulative Effect Theory
7.2. Pathogenesis of RPN in the Course of Analgesic Nephropathy
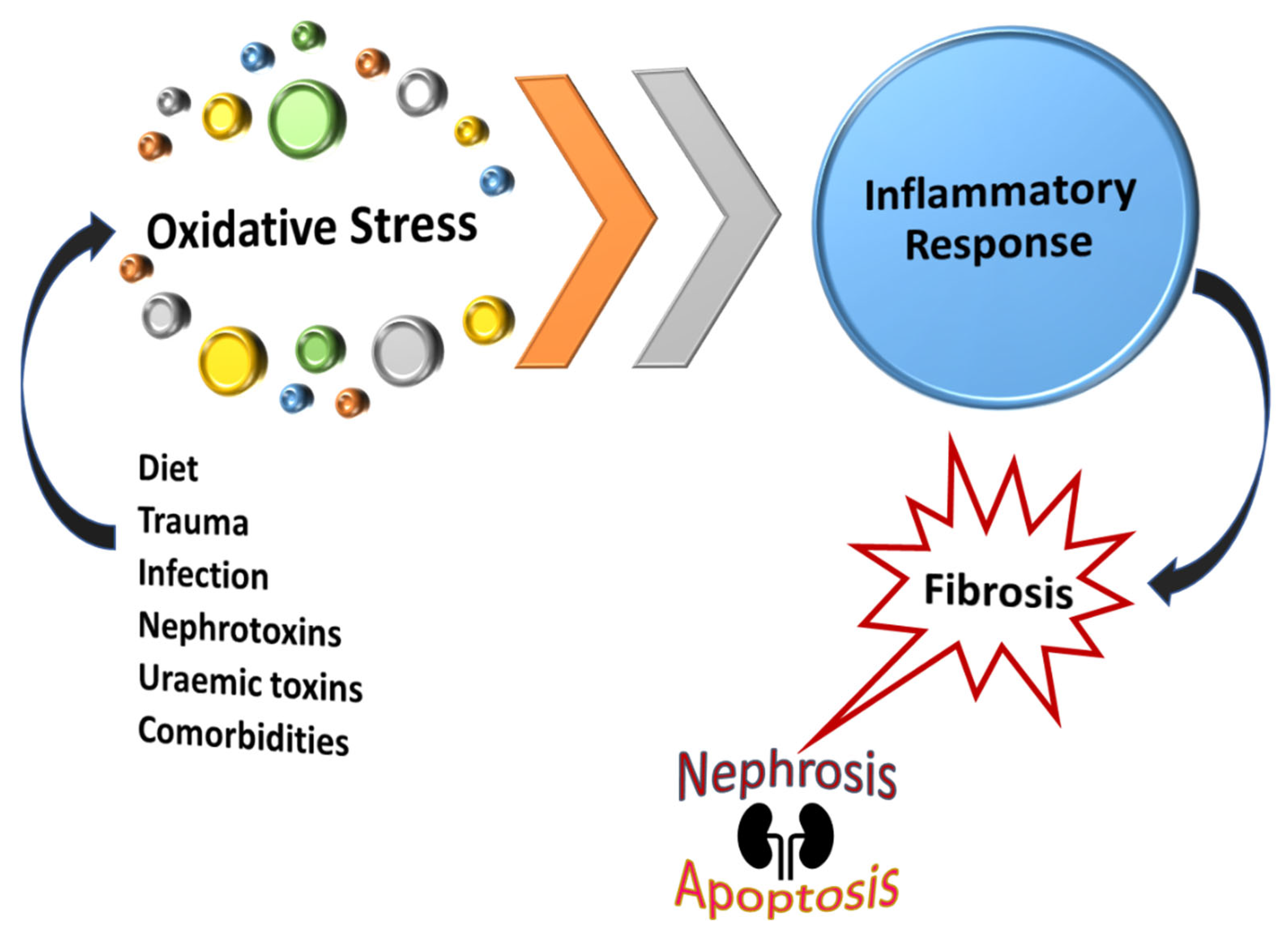
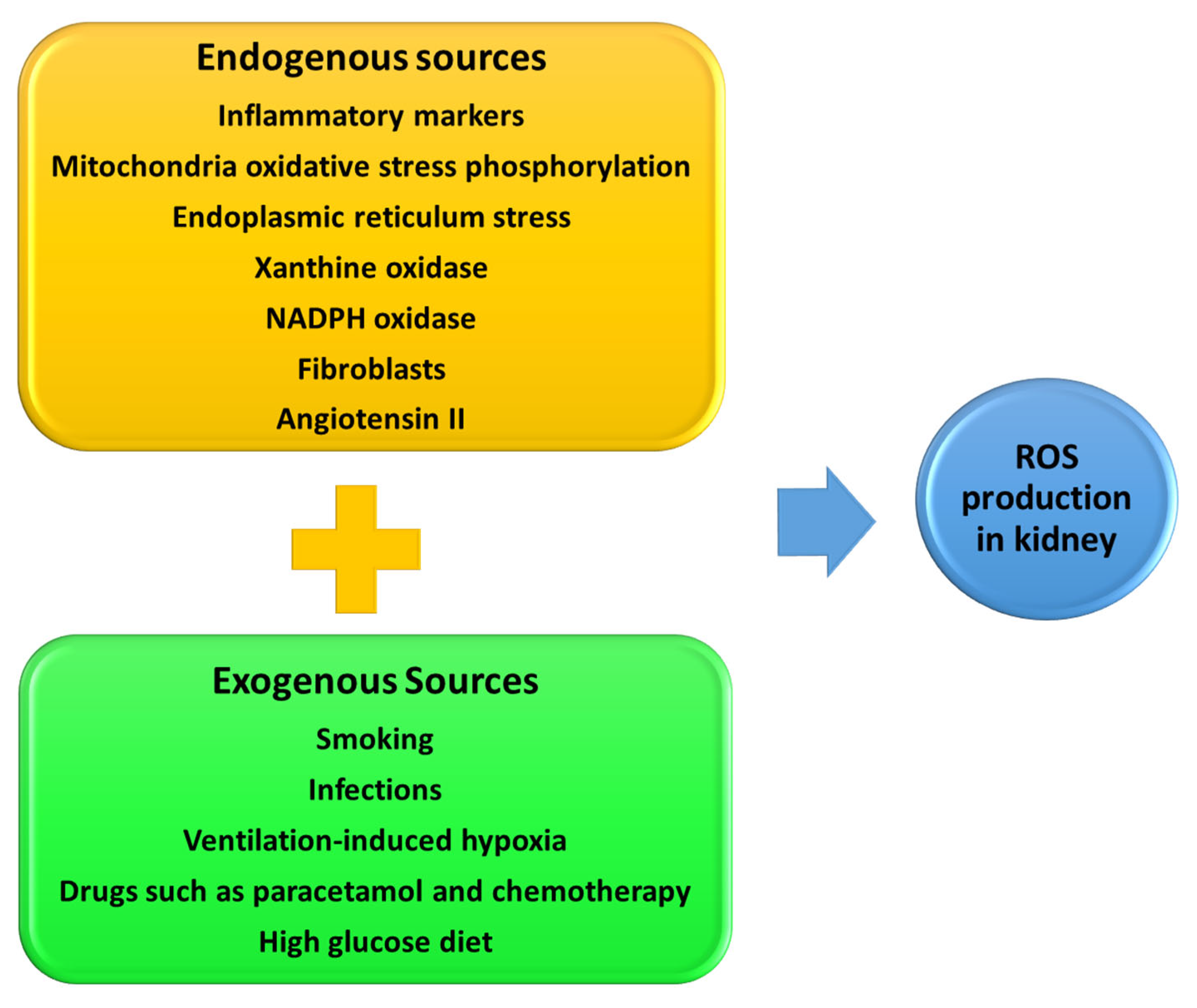
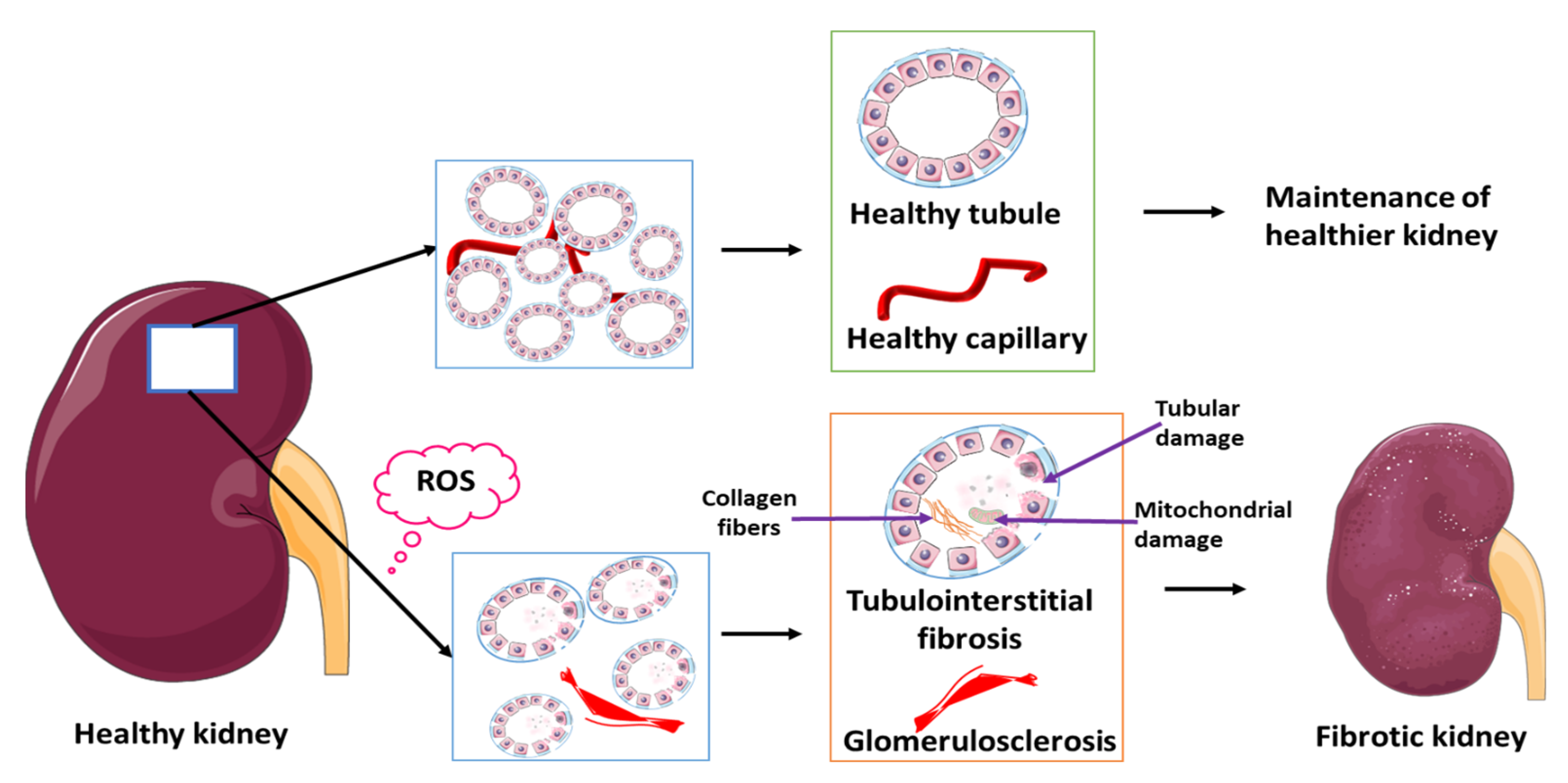
8. Reactive Oxygen Species and RPN
9. RPN Management; Medical and Surgical Therapy
10. Summary, Conclusions and Future Prospects
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedreich, N. Ueber necrose der nierenpapillen bei hydronephrose. In Band 69; De Gruyter: Berlin, Germany, 1877; Volume 69, pp. 308–312. [Google Scholar]
- Froboese, C. Uber sequestrierende Marknekrosen der Nieren bei Diabetes mellitus. Verh. Dtsch. Ges. Pathol. 1937, 30, 431–443. [Google Scholar]
- Günther, G. Die papillennekrosen der niere bei diabetes. München Med. Wchnschr. 1937, 84, 1695. [Google Scholar]
- Mandel, E. Renal medullary necrosis. Am. J. Med. 1952, 13, 322–327. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oiseth, S.J. Beethoven’s autopsy revisited: A pathologist sounds a final note. J. Med. Biogr. 2017, 25, 139–147. [Google Scholar] [CrossRef]
- Kayange, N.M.; Smart, L.R.; Tallman, J.E.; Chu, E.Y.; Fitzgerald, D.W.; Pain, K.J.; Peck, R.N. Kidney disease among children in sub-Saharan Africa: Systematic review. Pediatr. Res. 2015, 77, 272–281. [Google Scholar] [CrossRef]
- Kahindo, C.K.; Mukuku, O.; Wembonyama, S.O.; Tsongo, Z.K. Prevalence and Factors Associated with Acute Kidney Injury in Sub-Saharan African Adults: A Review of the Current Literature. Int. J. Nephrol. 2022, 2022, 5621665. [Google Scholar] [CrossRef] [PubMed]
- Ebah, L.M. Renal involvement in sickle cell disease: An African perspective for an African condition. Clin. Kidney. J. 2013, 6, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Madu, A.J.; Okoye, A.E.; Ajuba, I.C.; Madu, K.A.; Anigbo, C.; Agu, K. Prevalence and associations of symptomatic renal papillary necrosis in sickle cell anemia patients in South-Eastern Nigeria. Niger. J. Clin. Pr. 2016, 19, 471–474. [Google Scholar] [CrossRef]
- Uwaezuoke, S.N.; Odimegwu, C.L.; Mbanefo, N.R.; Eze, I.C. Posterior urethral valve in children: Using novel biomarkers as an early predictive tool for the onset and progression of chronic kidney disease. Front. Urol. 2022, 2, 904452. [Google Scholar] [CrossRef]
- Alebiosu, C.O. Renal papillary necrosis as first presentation of a Nigerian sickle cell patient. West. Afr. J. Med. 2002, 21, 168–169. [Google Scholar] [PubMed]
- Aeddula, N.R.; Bardhan, M.; Baradhi, K.M. Sickle Cell Nephropathy. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Moeckel, G.W. Pathologic Perspectives on Acute Tubular Injury Assessment in the Kidney Biopsy. Semin. Nephrol. 2018, 38, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.C.; Nordquist, L.; Larsson, T.E.; Carrero, J.J.; Larsson, A.; Lind, L.; Ärnlöv, J. Soluble Tumor Necrosis Factor Receptor 1 Is Associated with Glomerular Filtration Rate Progression and Incidence of Chronic Kidney Disease in Two Community-Based Cohorts of Elderly Individuals. Cardiorenal. Med. 2015, 5, 278–288. [Google Scholar] [CrossRef]
- Barr, E.L.M.; Barzi, F.; Hughes, J.T.; Jerums, G.; Hoy, W.E.; O’Dea, K.; Jones, G.R.D.; Lawton, P.D.; Brown, A.D.H.; Thomas, M.; et al. High Baseline Levels of Tumor Necrosis Factor Receptor 1 Are Associated With Progression of Kidney Disease in Indigenous Australians With Diabetes: The eGFR Follow-up Study. Diabetes Care 2018, 41, 739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Brown, R.P.; Shaw, M.; Vaidya, V.S.; Zhou, Y.; Espandiari, P.; Sadrieh, N.; Stratmeyer, M.; Keenan, J.; Kilty, C.G.; et al. Immunolocalization of Kim-1, RPA-1, and RPA-2 in Kidney of Gentamicin-, Mercury-, or Chromium-Treated Rats: Relationship to Renal Distributions of iNOS and Nitrotyrosine. Toxicol. Pathol. 2008, 36, 397–409. [Google Scholar] [CrossRef]
- Price, S.A.; Davies, D.; Rowlinson, R.; Copley, C.G.; Roche, A.; Falkenberg, F.W.; Riccardi, D.; Betton, G.R. Characterization of renal papillary antigen 1 (RPA-1), a biomarker of renal papillary necrosis. Toxicol. Pathol. 2010, 38, 346–358. [Google Scholar] [CrossRef]
- Xue, W.; Xie, Y.; Wang, Q.; Xu, W.; Mou, S.; Ni, Z. Diagnostic performance of urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin for acute kidney injury in an obstructive nephropathy patient. Nephrology 2014, 19, 186–194. [Google Scholar] [CrossRef]
- Ransley, P.; Risdon, R. Renal papillary morphology in infants and young children. Urol. Res. 1975, 3, 111–113. [Google Scholar] [CrossRef]
- Anderson, J.; Kabalin, J.N.; Cadeddu, J. Surgical Anatomy of the Retroperitoneum, Adrenals, Kidneys, and Ureters. Campbell.-Walsh. Urol. 2007, 3–37. [Google Scholar]
- Jung, D.C.; Kim, S.H.; Jung, S.I.; Hwang, S.I.; Kim, S.H. Renal papillary necrosis: Review and comparison of findings at multi–detector row CT and intravenous urography. Radiographics 2006, 26, 1827–1836. [Google Scholar] [CrossRef]
- Ishimoto, Y.; Tanaka, T.; Yoshida, Y.; Inagi, R. Physiological and pathophysiological role of reactive oxygen species and reactive nitrogen species in the kidney. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Eroschenko, V.P. Di. Fiore’s Atlas of Histology with Functional Correlations, 11th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Geller, S.A.; de Campos, F.P.F. Renal papillary necrosis. Autops. Case. Rep. 2013, 3, 69–71. [Google Scholar] [CrossRef]
- Martínez, J.L. A Study of the Clinical Features of Renal Papillary Necrosis; Yale University: New Haven, CT, USA, 1978. [Google Scholar]
- Shah, V.B.; Khade, A. Bilateral Renal Papillary Necrosis: An autopsy study of 4 cases. J. Case Rep. 2016, 6, 288–292. [Google Scholar] [CrossRef]
- Edelstein, C.L. Biomarkers of acute kidney injury. Adv. Chronic Kidney Dis. 2008, 15, 222–234. [Google Scholar] [CrossRef]
- Kim, H.; Cheigh, J.S.; Ham, H.W. Urinary stones following renal transplantation. Korean J. Intern. Med. 2001, 16, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, S.B.; Kandasamy, S.V.; Arul, M.; Prabhakar, M. Sonographic features of necrosed renal papillae causing hydronephrosis. J. Ultrasound. Med. 2003, 22, 951–956. [Google Scholar] [CrossRef][Green Version]
- Lang, E.; Macchia, R.; Thomas, R.; Davis, R.; Ruiz-Deya, G.; Watson, R.; Richter, F.; Gayle, B. Detection of medullary and papillary necrosis at an early stage by multiphasic helical computerized tomography. J. Urol. 2003, 170, 94–98. [Google Scholar] [CrossRef]
- Fadel, M.G.; Carey, M.; Bolgeri, M. Infected and obstructed kidney secondary to sloughed necrotic renal papilla. Case Rep. 2018, 2018, bcr–2018–227403. [Google Scholar] [CrossRef]
- Kamath, S.; Moody, M.; Hammonds, J.; Wells, I. Papillary necrosis causing hydronephrosis in renal allograft treated by percutaneous retrieval of sloughed papilla. Br. J. Radiol. 2005, 78, 346–348. [Google Scholar] [CrossRef]
- Cachofeiro, V.; Goicochea, M.; de Vinuesa, S.G.; Oubiña, P.; Lahera, V.; Luño, J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease: New strategies to prevent cardiovascular risk in chronic kidney disease. Kidney Int. 2008, 74, S4–S9. [Google Scholar] [CrossRef]
- Jay, A.P.; Nicol, D.L. The Pathophysiology of Upper Tract Obstruction. In Ureteric Stenting; Wiley: Hoboken, NJ, USA, 2017; pp. 16–28. [Google Scholar]
- American Urological Association Renal Papillary Necrosis. Available online: https://www.auanet.org/education/auauniversity/education-products-and-resources/pathology-for-urologists/kidney/inflammatory/necrotic-renal-lesions/renal-papillary-necrosis (accessed on 11 August 2020).
- Fourie, I.J.; Pauw, F.H.; de Bruin, B. Analgesic nephropathy. Local experience in Bloemfontein. S. Afr. Med. J. 1982, 62, 351–353. [Google Scholar]
- Santos, J.; Chaves, J.; Araújo, H.; Vale, N.; Costa, J.M.; Brindley, P.J.; Lopes, C.; Naples, J.; Shiff, C.; Dupret, J.; et al. Comparison of findings using ultrasonography and cystoscopy in urogenital schistosomiasis in a public health centre in rural Angola. S. Afr. Med. J. 2015, 105, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Brix, A.E. Renal Papillary Necrosis. Toxicol. Pathol. 2002, 30, 672–674. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharm. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Donnan, P.T.; Bell, S.; Guthrie, B. Non-steroidal anti-inflammatory drug induced acute kidney injury in the community dwelling general population and people with chronic kidney disease: Systematic review and meta-analysis. BMC Nephrol. 2017, 18, 256. [Google Scholar] [CrossRef]
- Odita, J.C.; Ugbodaga, C.I.; Okafor, L.A.; Ojogwu, L.I.; Ogisi, O.A. Urographic changes in homozygous sickle cell disease. Diagn. Imaging 1983, 52, 259–263. [Google Scholar] [PubMed]
- Pandya, K.K.; Koshy, M.; Brown, N.; Presman, D. Renal papillary necrosis in sickle cell hemoglobinopathies. J. Urol. 1976, 115, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.; Kleess, L.; Yeh, J.; Berko, C.; Kuehl, S. Sickle cell trait: Not as benign as once thought. J. Community Hosp. Intern. Med. Perspect. 2014, 4, 25418. [Google Scholar] [CrossRef]
- Ndeezi, G.; Kiyaga, C.; Hernandez, A.G.; Munube, D.; Howard, T.A.; Ssewanyana, I.; Nsungwa, J.; Kiguli, S.; Ndugwa, C.M.; Ware, R.E.; et al. Burden of sickle cell trait and disease in the Uganda Sickle Surveillance Study (US3): A cross-sectional study. Lancet. Glob. Health 2016, 4, e195–e200. [Google Scholar] [CrossRef]
- Wonkam, A.; Ponde, C.; Nicholson, N.; Fieggen, K.; Ramessar, R.; Davidson, A. The burden of sickle cell disease in Cape Town. S. Afr. Med. J. 2012, 102, 752–754. [Google Scholar] [CrossRef][Green Version]
- Rao, V.C.; Bhat, S.S.; Vijayan, P.; Ramamurthy, S. Endoscopic intervention in obstructive renal papillary necrosis. Indian J. Urol. 2004, 20, 29. [Google Scholar]
- Leão, R.; Pereira, B.J.; Coelho, H. Benign prostate hyperplasia and chronic kidney disease. Chronic. Kidney Dis. 2012, 347–350. [Google Scholar] [CrossRef]
- Corfield, V.; Chauke, G.; Bardien, S.; Moolman-Smook, H.; Freeman, V.; Makubalo, Z.; Brink, P. Novel mutations identified within the 3’ region of the PKD1 gene in South African polycystic kidney disease patients : Research letter. S. Afr. J. Sci. 2006, 102, 317–321. [Google Scholar]
- Lambie, L.; Amin, R.; Essop, F.; Cnaan, A.; Krause, A.; Guay-Woodford, L.M. Clinical and genetic characterization of a founder PKHD1 mutation in Afrikaners with ARPKD. Pediatr. Nephrol. 2015, 30, 273–279. [Google Scholar] [CrossRef]
- Imam, T.H.; Patail, H.; Patail, H. Medullary sponge kidney: Current perspectives. Int. J. Nephrol. Renov. Dis. 2019, 12, 213–218. [Google Scholar] [CrossRef]
- Both, T.; Zietse, R.; Hoorn, E.J.; van Hagen, P.M.; Dalm, V.A.; van Laar, J.A.; van Daele, P.L. Everything you need to know about distal renal tubular acidosis in autoimmune disease. Rheumatol. Int. 2014, 34, 1037–1045. [Google Scholar] [CrossRef]
- Murugapoopathy, V.; Gupta, I.R. A Primer on Congenital Anomalies of the Kidneys and Urinary Tracts (CAKUT). Clin. J. Am. Soc. Nephrol. 2020, 15, 723–731. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Govender, M.A.; Brandenburg, J.-T.; Winkler, C.A. Kidney disease and APOL1. Hum. Mol. Genet. 2021, 30, R129–R137. [Google Scholar] [CrossRef] [PubMed]
- George, C.; Yako, Y.Y.; Okpechi, I.G.; Matsha, T.E.; Kaze Folefack, F.J.; Kengne, A.P. An African perspective on the genetic risk of chronic kidney disease: A systematic review. BMC Med. Genet. 2018, 19, 187. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, G.N.; Chauhan, K.; Verghese, D.A.; Parikh, C.R.; Do, R.; Horowitz, C.R.; Bottinger, E.P.; Coca, S.G. Plasma biomarkers are associated with renal outcomes in individuals with APOL1 risk variants. Kidney Int. 2018, 93, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.; Keys, T.; Mufarrij, P.; Assimos, D.G. Impact of stone removal on renal function: A review. Rev. Urol. 2011, 13, 73–89. [Google Scholar] [PubMed]
- Schnaper, H.W. Remnant nephron physiology and the progression of chronic kidney disease. Pediatr. Nephrol. 2014, 29, 193–202. [Google Scholar] [CrossRef]
- Mitropoulos, D.; Artibani, W.; Graefen, M.; Remzi, M.; Rouprêt, M.; Truss, M. Reporting and grading of complications after urologic surgical procedures: An ad hoc EAU guidelines panel assessment and recommendations. Eur. Urol. 2012, 61, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Chadban, S.J.; Ahn, C.; Axelrod, D.A.; Foster, B.J.; Kasiske, B.L.; Kher, V.; Kumar, D.; Oberbauer, R.; Pascual, J.; Pilmore, H.L.; et al. Summary of the Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation. Transplantation 2020, 104, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, A.Y.; Fouda, W.B.; Mosharafa, A.A.; Abelrasoul, M.A.; Fayyad, A.; Fawzi, K. Forgotten ureteral stents: Risk factors, complications and management. Afr. J. Urol. 2018, 24, 28–33. [Google Scholar] [CrossRef]
- Cassell, A., 3rd; Jalloh, M.; Ndoye, M.; Mbodji, M.; Gaye, O.; Thiam, N.M.; Diallo, A.; Labou, I.; Niang, L.; Gueye, S. Surgical Management of Urolithiasis of the Upper Tract—Current Trend of Endourology in Africa. Res. Rep. Urol. 2020, 12, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Karolin, A.; Genitsch, V.; Sidler, D. Calcineurin Inhibitor Toxicity in Solid Organ Transplantation. Pharmacology 2021, 106, 347–355. [Google Scholar] [CrossRef]
- Muller, W.K.; Dandara, C.; Manning, K.; Mhandire, D.; Ensor, J.; Barday, Z.; Freercks, R. CYP3A5 polymorphisms and their effects on tacrolimus exposure in an ethnically diverse South African renal transplant population. S. Afr. Med. J. 2020, 110, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Risenga, M. Statistical Release: Mid-Year Population Estimates. Available online: https://www.statssa.gov.za/publications/P0302/P03022019.pdf (accessed on 11 December 2020).
- Li, X.; Zhuang, S. Acute Kidney Injury in HIV Infection. J. Trop. Dis. 2013, 1, 101. [Google Scholar]
- Campos, P.; Ortiz, A.; Soto, K. HIV and kidney diseases: 35 years of history and consequences. Clin. Kidney J. 2016, 9, 772–781. [Google Scholar] [CrossRef]
- Eastwood, J.B.; Kerry, S.M.; Plange-Rhule, J.; Micah, F.B.; Antwi, S.; Boa, F.G.; Banerjee, D.; Emmett, L.; Miller, M.A.; Cappuccio, F.P. Assessment of GFR by four methods in adults in Ashanti, Ghana: The need for an eGFR equation for lean African populations. Nephrol. Dial. Transplant. 2010, 25, 2178–2187. [Google Scholar] [CrossRef] [PubMed]
- Okparavero, A.A.; Tighiouart, H.; Krishnasami, Z.; Wyatt, C.M.; Graham, H.; Hellinger, J.; Inker, L.A. Use of glomerular filtration rate estimating equations for drug dosing in HIV-positive patients. Antivir 2013, 18, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Zulfiqar, M.; Siddiqui, M.A.; Shetty, A.S.; Haq, A.; Varela, C.; Siegel, C.; Menias, C.O. Imaging Manifestations of Genitourinary Tuberculosis. Radiographics 2021, 41, 1123–1143. [Google Scholar] [CrossRef] [PubMed]
- Gaut, J.P.; Liapis, H. Acute kidney injury pathology and pathophysiology: A retrospective review. Clin. Kidney J. 2021, 14, 526–536. [Google Scholar] [CrossRef]
- Tallai, B.; Gulistan, T.G.; Alrayashi, M.N.A.B.; Al Mughalles, S.A.A.; Kamkoum, H.M.; Ebrahim, M.A.A.; Abdelkarim, M.A.A.; Salah, M.A. A Rare Presentation of Renal Papillary Necrosis in a COVID-19-Positive Patient. Case Rep. Urol. 2021, 2021, 6611861. [Google Scholar] [CrossRef]
- Fabrizi, F. Hepatitis C Virus, Cryoglobulinemia, and Kidney: Novel Evidence. Scientifica 2012, 2012, 128382. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Soares, D.; Cavalcante, M.G.; Ribeiro, S.M.V.; Leitão, R.C.; Vieira, A.P.F.; da Justa Pires Neto, R.; da Silva, G.B., Jr.; de Francesco Daher, E. Acute kidney injury in HIV-infected children: Comparison of patients according to the use of highly active antiretroviral therapy. J. Pediatr. 2016, 92, 631–637. [Google Scholar] [CrossRef][Green Version]
- Pazhayattil, G.S.; Shirali, A.C. Drug-induced impairment of renal function. Int. J. Nephrol. Renov. Dis. 2014, 7, 457–468. [Google Scholar]
- Bird, S.T.; Etminan, M.; Brophy, J.M.; Hartzema, A.G.; Delaney, J.A. Risk of acute kidney injury associated with the use of fluoroquinolones. Can. Med. Assoc. J. 2013, 185, E475–E482. [Google Scholar] [CrossRef]
- Adamsick, M.L.; Gandhi, R.G.; Bidell, M.R.; Elshaboury, R.H.; Bhattacharyya, R.P.; Kim, A.Y.; Nigwekar, S.; Rhee, E.P.; Sise, M.E. Remdesivir in Patients with Acute or Chronic Kidney Disease and COVID-19. J. Am. Soc. Nephrol. 2020, 31, 1384–1386. [Google Scholar] [CrossRef]
- Daher, E.D.F.; da Silva Junior, G.B.; Trivedi, M.; Fayad, T.; Srisawat, N.; Nair, S.; Siriyasatien, P.; de Lacerda, M.V.G.; Baptista, M.A.S.F.; Vankalakunti, M.; et al. Kidney complications of parasitic diseases. Nat. Rev. Nephrol. 2022, 18, 396–406. [Google Scholar] [CrossRef]
- Magaisa, K.; Taylor, M.; Kjetland, E.F.; Naidoo, P.J. A review of the control of schistosomiasis in South Africa. S. Afr. J. Sci. 2015, 111, 1–6. [Google Scholar] [CrossRef]
- Barsoum, R.S. Schistosomiasis and glomerular disease. Semin Nephrol. 2003, 23, 34–41. [Google Scholar] [CrossRef]
- Bamgbola, O.F. Urinary schistosomiasis. Pediatr. Nephrol. 2014, 29, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- Antwi, S.; Aboah, K.E.; Sarpong, C.K. The unacknowledged impact of urinary schistosomiasis in children: 5 cases from Kumasi, Ghana. Ghana. Med. J. 2014, 48, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Oyediran, A.B. Renal disease due to schistosomiasis of the lower urinary tract. Kidney Int. 1979, 16, 15–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duarte, D.B.; Vanderlei, L.A.; de Azevêdo Bispo, R.K.; Pinheiro, M.E.; da Silva Junior, G.B.; De Francesco Daher, E. Acute kidney injury in schistosomiasis: A retrospective cohort of 60 patients in Brazil. J. Parasitol. 2015, 101, 244–247. [Google Scholar] [CrossRef]
- Pan, H.H.; Luo, Y.J.; Zhu, Q.G.; Ye, L.F. Renal papillary necrosis with urinary tract obstruction: A case report. World J. Clin. Cases 2022, 10, 5400–5405. [Google Scholar] [CrossRef]
- Xia, J.; Wang, L.; Ma, Z.; Zhong, L.; Wang, Y.; Gao, Y.; He, L.; Su, X. Cigarette smoking and chronic kidney disease in the general population: A systematic review and meta-analysis of prospective cohort studies. Nephrol. Dial. Transplant. 2017, 32, 475–487. [Google Scholar] [CrossRef]
- Reddy, P.; Zuma, K.; Shisana, O.; Jonas, K.; Sewpaul, R. Prevalence of tobacco use among adults in South Africa: Results from the first South African National Health and Nutrition Examination Survey. S. Afr. Med. J. 2015, 105, 648–655. [Google Scholar] [CrossRef]
- Van Heerden, M.S.; Grimsrud, A.T.; Seedat, S.; Myer, L.; Williams, D.R.; Stein, D.J. Patterns of substance use in South Africa: Results from the South African Stress and Health study. S. Afr. Med. J. 2009, 99, 358–366. [Google Scholar]
- Buettner, M.; Toennes, S.W.; Buettner, S.; Bickel, M.; Allwinn, R.; Geiger, H.; Bratzke, H.; Amann, K.; Jung, O. Nephropathy in illicit drug abusers: A postmortem analysis. Am. J. Kidney Dis. 2014, 63, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Rein, J.L. The nephrologist’s guide to cannabis and cannabinoids. Curr. Opin. Nephrol. Hypertens 2020, 29, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Mokwena, K.E. Nyaope addiction: The despair of a lost generation. Afr. J. Drug. Alcohol. Stud. 2020, 19, 37–51. [Google Scholar]
- Mthembi, P.M.; Mwenesongole, E.M.; Cole, M.D. Chemical profiling of the street cocktail drug ‘nyaope’ in South Africa using GC–MS II: Stability studies of the cannabinoid, opiate and antiretroviral components during sample storage. Forensic. Sci. Int. 2019, 300, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Röling, J.; Schmid, H.; Fischereder, M.; Draenert, R.; Goebel, F.D. HIV-Associated Renal Diseases and Highly Active Antiretroviral Therapy—Induced Nephropathy. Clin. Infect. Dis. 2006, 42, 1488–1495. [Google Scholar] [CrossRef]
- Gómez-Olivé, F.X.; Ali, S.A.; Made, F.; Kyobutungi, C.; Nonterah, E.; Micklesfield, L.; Alberts, M.; Boua, R.; Hazelhurst, S.; Debpuur, C. Regional and sex differences in the prevalence and awareness of hypertension: An H3Africa AWI-Gen study across 6 sites in sub-Saharan Africa. Glob. Heart 2017, 12, 81–90. [Google Scholar] [CrossRef]
- Kincaid-Smith, P. Renal pathology in hypertension and the effects of treatment. Br. J. Clin. Pharmacol. 1982, 13, 107–115. [Google Scholar] [CrossRef]
- Akbarpour, S.; Khalili, D.; Zeraati, H.; Mansournia, M.A.; Ramezankhani, A.; Ahmadi Pishkuhi, M.; Rostami Gooran, S.; Fotouhi, A. Relationship between lifestyle pattern and blood pressure—Iranian national survey. Sci. Rep. 2019, 9, 15194. [Google Scholar] [CrossRef]
- Sharma, J.R.; Mabhida, S.E.; Myers, B.; Apalata, T.; Nicol, E.; Benjeddou, M.; Muller, C.; Johnson, R. Prevalence of Hypertension and Its Associated Risk Factors in a Rural Black Population of Mthatha Town, South Africa. Int. J. Environ. Res. Public Health 2021, 18, 1215. [Google Scholar] [CrossRef]
- Tozawa, M.; Iseki, K.; Iseki, C.; Kinjo, K.; Ikemiya, Y.; Takishita, S. Blood Pressure Predicts Risk of Developing End-Stage Renal Disease in Men and Women. Hypertension 2003, 41, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Barri, Y.M. Hypertension and kidney disease: A deadly connection. Curr. Hypertens Rep. 2008, 10, 39–45. [Google Scholar] [CrossRef]
- Mitchell, H.; Rosner, M.D.O. Pathogenesis and Etiology of Ischemic Acute Tubular Necrosis. Available online: https://www.uptodate.com/contents/pathogenesis-and-etiology-of-ischemic-acute-tubular-necrosis (accessed on 3 June 2022).
- Wen, Y.; Yang, C.; Menez, S.P.; Rosenberg, A.Z.; Parikh, C.R. A Systematic Review of Clinical Characteristics and Histologic Descriptions of Acute Tubular Injury. Kidney Int. Rep. 2020, 5, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- Salliot, C.; Guichard, I.; Daugas, E.; Lagrange, M.; Verine, J.; Molina, J.M. Acute kidney disease due to immune reconstitution inflammatory syndrome in an HIV-infected patient with tuberculosis. J. Int. Assoc. Physicians AIDS Care Chic. 2008, 7, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, U.; Keen, N.R.A. Analgesic Nephropathy; StatPearls Publishing, National Library of Medicine: Bethesda, MD, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541101/?report=classic (accessed on 31 October 2022).
- Chapter 14—Tubulointerstitial Diseases. In Pocket Companion to Brenner and Rector’s The Kidney, 8th ed.; Clarkson, M.R., Magee, C.N., Brenner, B.M., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2011; pp. 297–312. [Google Scholar]
- Nanra, R.S.; Stuart-Taylor, J.; de Leon, A.H.; White, K.H. Analgesic nephropathy: Etiology, clinical syndrome, and clinicopathologic correlations in Australia. Kidney Int. 1978, 13, 79–92. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sampathkumar, K.; Rajiv, A.; Sampathkumar, D. Analgesic Nephropathy–-A Painful Progression. Clin. Med. Insights. Urol. 2016, 9, CMU.S13179. [Google Scholar] [CrossRef][Green Version]
- Naicker, S. Patterns of renal disease in South Africa. Nephrology 1998, 4, S21–S24. [Google Scholar] [CrossRef]
- Madala, N.D.; Thusi, G.P.; Assounga, A.G.H.; Naicker, S. Characteristics of South African patients presenting with kidney disease in rural KwaZulu-Natal: A cross sectional study. BMC Nephrol. 2014, 15, 61. [Google Scholar] [CrossRef][Green Version]
- Okwuonu, C.; Chukwuonye, I.; Adejumo, O.; Agaba, E.; Ojogwu, L. Prevalence of chronic kidney disease and its risk factors among adults in a semi-urban community of South-East Nigeria. Niger. Postgrad. Med. J. 2017, 24, 81–87. [Google Scholar] [CrossRef]
- Uduagbamen, P.; Salako, B.; Hamzat, M.; Kadiri, S.; Arogundade, F. Kidney Function in Frequent Users of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). Open J. Intern. Med. 2020, 10, 69. [Google Scholar]
- Okpechi, I.; Swanepoel, C.; Duffield, M.; Mahala, B.; Wearne, N.; Alagbe, S.; Barday, Z.; Arendse, C.; Rayner, B. Patterns of renal disease in Cape Town South Africa: A 10-year review of a single-centre renal biopsy database. Nephrol. Dial. Transpl. 2011, 26, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.C.; Breyer, M.D. Update on Cyclooxygenase-2 Inhibitors. Clin. J. Am. Soc. Nephrol. 2006, 1, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-F.; Harris, R.C. Cyclooxygenases, the Kidney, and Hypertension. Hypertension 2004, 43, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Kirkby, N.S.; Sampaio, W.; Etelvino, G.; Alves, D.T.; Anders, K.L.; Temponi, R.; Shala, F.; Nair, A.S.; Ahmetaj-Shala, B.; Jiao, J.; et al. Cyclooxygenase-2 Selectively Controls Renal Blood Flow Through a Novel PPARβ/δ-Dependent Vasodilator Pathway. Hypertension 2018, 71, 297–305. [Google Scholar] [CrossRef]
- Gyurászová, M.; Gurecká, R.; Bábíčková, J.; Tóthová, Ľ. Oxidative Stress in the Pathophysiology of Kidney Disease: Implications for Noninvasive Monitoring and Identification of Biomarkers. Oxidative Med. Cell Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef]
- Ueda, N.; Shah, S.V. Tubular cell damage in acute renal failure—Apoptosis, necrosis, or both. Nephrol. Dial. Transpl. 2000, 15, 318–323. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant Mechanisms in Renal Injury and Disease. Antioxid Redox. Signal 2016, 25, 119–146. [Google Scholar] [CrossRef]
- Chang, J.; Yan, J.; Li, X.; Liu, N.; Zheng, R.; Zhong, Y. Update on the Mechanisms of Tubular Cell Injury in Diabetic Kidney Disease. Front. Med. 2021, 8, 661076. [Google Scholar] [CrossRef]
- Small, D.M.; Coombes, J.S.; Bennett, N.; Johnson, D.W.; Gobe, G.C. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrol. Carlton. 2012, 17, 311–321. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Developmental Origins of Kidney Disease: Why Oxidative Stress Matters? Antioxidants 2020, 10, 33. [Google Scholar] [CrossRef]
- Siu, Y.P.; Leung, K.T.; Tong, M.K.; Kwan, T.H. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am. J. Kidney Dis. 2006, 47, 51–59. [Google Scholar] [CrossRef]
- Abdel-Rahman Mohamed, A.; Khater, S.I.; Metwally, M.M.M.; Bin Emran, T.; Nassan, M.A.; Abd El-Emam, M.M.; Mostafa-Hedeab, G.; El-Shetry, E.S. TGF-β1, NAG-1, and antioxidant enzymes expression alterations in Cisplatin-induced nephrotoxicity in a rat model: Comparative modulating role of Melatonin, Vit. E and Ozone. Gene 2022, 820, 146293. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Tanaka, T.; Nangaku, M. Nuclear factor erythroid 2-related factor 2 as a treatment target of kidney diseases. Curr. Opin. Nephrol. Hypertens 2020, 29, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Sourris, K.C.; Harcourt, B.E.; Tang, P.H.; Morley, A.L.; Huynh, K.; Penfold, S.A.; Coughlan, M.T.; Cooper, M.E.; Nguyen, T.V.; Ritchie, R.H.; et al. Ubiquinone (coenzyme Q10) prevents renal mitochondrial dysfunction in an experimental model of type 2 diabetes. Free Radic. Biol. Med. 2012, 52, 716–723. [Google Scholar] [CrossRef]
- Ozgur, R.; Turkan, I.; Uzilday, B.; Sekmen, A.H. Endoplasmic reticulum stress triggers ROS signalling, changes the redox state, and regulates the antioxidant defence of Arabidopsis thaliana. J. Exp. Bot. 2014, 65, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Inagi, R. Endoplasmic Reticulum Stress in the Kidney as a Novel Mediator of Kidney Injury. Nephron. Exp. Nephrol. 2009, 112, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Cybulsky, A.V.; Takano, T.; Papillon, J.; Bijian, K. Role of the endoplasmic reticulum unfolded protein response in glomerular epithelial cell injury. J. Biol. Chem. 2005, 280, 24396–24403. [Google Scholar] [CrossRef]
- Okamura, M.; Takano, Y.; Hiramatsu, N.; Hayakawa, K.; Kasai, A.; Yao, J.; Kitamura, M. Suppression of cytokine response in podocytes by indomethacin: Involvement of unfolded protein response. Am. J. Physiol.-Ren. Physiol. 2007, 295, F1495–F1503. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Li, M.; Cai, Q.; Wu, S.; Li, X.; He, Q.; Hu, Y. Roles of NRF2 in Fibrotic Diseases: From Mechanisms to Therapeutic Approaches. Front. Physiol. 2022, 13, 889792. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Seok, Y.M.; Jung, K.-J.; Park, K.M. Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am. J. Physiol.-Ren. Physiol. 2009, 297, F461–F470. [Google Scholar] [CrossRef] [PubMed]
- Irazabal, M.V.; Torres, V.E. Reactive Oxygen Species and Redox Signaling in Chronic Kidney Disease. Cells 2020, 9, 1342. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Kietzmann, T. Reactive oxygen species and fibrosis: Further evidence of a significant liaison. Cell Tissue Res. 2016, 365, 591–605. [Google Scholar] [CrossRef]
- Qi, R.; Yang, C. Renal tubular epithelial cells: The neglected mediator of tubulointerstitial fibrosis after injury. Cell Death Dis. 2018, 9, 1126. [Google Scholar] [CrossRef]
- Gilbert, R.E. Proximal tubulopathy: Prime mover and key therapeutic target in diabetic kidney disease. Diabetes 2017, 66, 791–800. [Google Scholar] [CrossRef]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression Through Oxidative Stress. Front. Physiol. 2021, 12, 627837. [Google Scholar] [CrossRef]
- Mostrom, M.S. Chapter 48—Grapes and Raisins. In Small Animal Toxicology, 3rd ed.; Peterson, M.E., Talcott, P.A., Eds.; W.B. Saunders: Saint Louis, MI, USA, 2013; pp. 569–572. [Google Scholar]
- Bülow, R.D.; Boor, P. Extracellular matrix in kidney fibrosis: More than just a scaffold. J. Histochem. Cytochem. 2019, 67, 643–661. [Google Scholar] [CrossRef]
- Amann, K.; Haas, C.S. What you should know about the work-up of a renal biopsy. Nephrol. Dial. Transplant. 2006, 21, 1157–1161. [Google Scholar] [CrossRef]
- Rasmussen, D.G.K.; Boesby, L.; Nielsen, S.H.; Tepel, M.; Birot, S.; Karsdal, M.A.; Kamper, A.-L.; Genovese, F. Collagen turnover profiles in chronic kidney disease. Sci. Rep. 2019, 9, 16062. [Google Scholar] [CrossRef]
- Lv, W.; Booz, G.W.; Fan, F.; Wang, Y.; Roman, R.J. Oxidative stress and renal fibrosis: Recent insights for the development of novel therapeutic strategies. Front. Physiol. 2018, 9, 105. [Google Scholar] [CrossRef]
- Bhattu, A.; Joshi, U.; Bethur, S. MP26-16 Endourological management of renal papillary necrosis. J. Urol. 2016, 195, e360. [Google Scholar] [CrossRef]
- Okafor, U.H.; Aneke, E. Outcome and challenges of kidney transplant in patients with sickle cell disease. J. Transpl. 2013, 2013, 614610. [Google Scholar] [CrossRef][Green Version]
- López Revuelta, K.; Ricard Andrés, M.P. Kidney abnormalities in sickle cell disease. Nefrologia 2011, 31, 591–601. [Google Scholar] [PubMed]
- Kheswa, J.G. Exploring the factors and effects of non-adherence to antiretroviral treatment by people living with HIV/AIDS. Indo-Pac. J. Phenomenol. 2017, 17, 1–11. [Google Scholar] [CrossRef]
- Azia, I.N.; Mukumbang, F.C.; van Wyk, B. Barriers to adherence to antiretroviral treatment in a regional hospital in Vredenburg, Western Cape, South Africa. S. Afr. J. HIV Med. 2016, 17, 476. [Google Scholar] [CrossRef]
- Micanovic, R.; LaFavers, K.; Garimella, P.S.; Wu, X.R.; El-Achkar, T.M. Uromodulin (Tamm-Horsfall protein): Guardian of urinary and systemic homeostasis. Nephrol. Dial. Transpl. 2020, 35, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Neyra, J.A.; Li, X.; Canepa-Escaro, F.; Adams-Huet, B.; Toto, R.D.; Yee, J.; Hedayati, S.S.; Acute Kidney Injury in Critical Illness Study, G. Cumulative Fluid Balance and Mortality in Septic Patients With or Without Acute Kidney Injury and Chronic Kidney Disease. Crit. Care Med. 2016, 44, 1891–1900. [Google Scholar] [CrossRef]
- Coccolini, F.; Moore, E.E.; Kluger, Y.; Biffl, W.; Leppaniemi, A.; Matsumura, Y.; Kim, F.; Peitzman, A.B.; Fraga, G.P.; Sartelli, M.; et al. Kidney and uro-trauma: WSES-AAST guidelines. World J. Emerg. Surg. 2019, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Mitropoulos, D.; Artibani, W.; Biyani, C.S.; Bjerggaard Jensen, J.; Rouprêt, M.; Truss, M. Validation of the Clavien-Dindo Grading System in Urology by the European Association of Urology Guidelines Ad Hoc Panel. Eur. Urol. Focus 2018, 4, 608–613. [Google Scholar] [CrossRef]
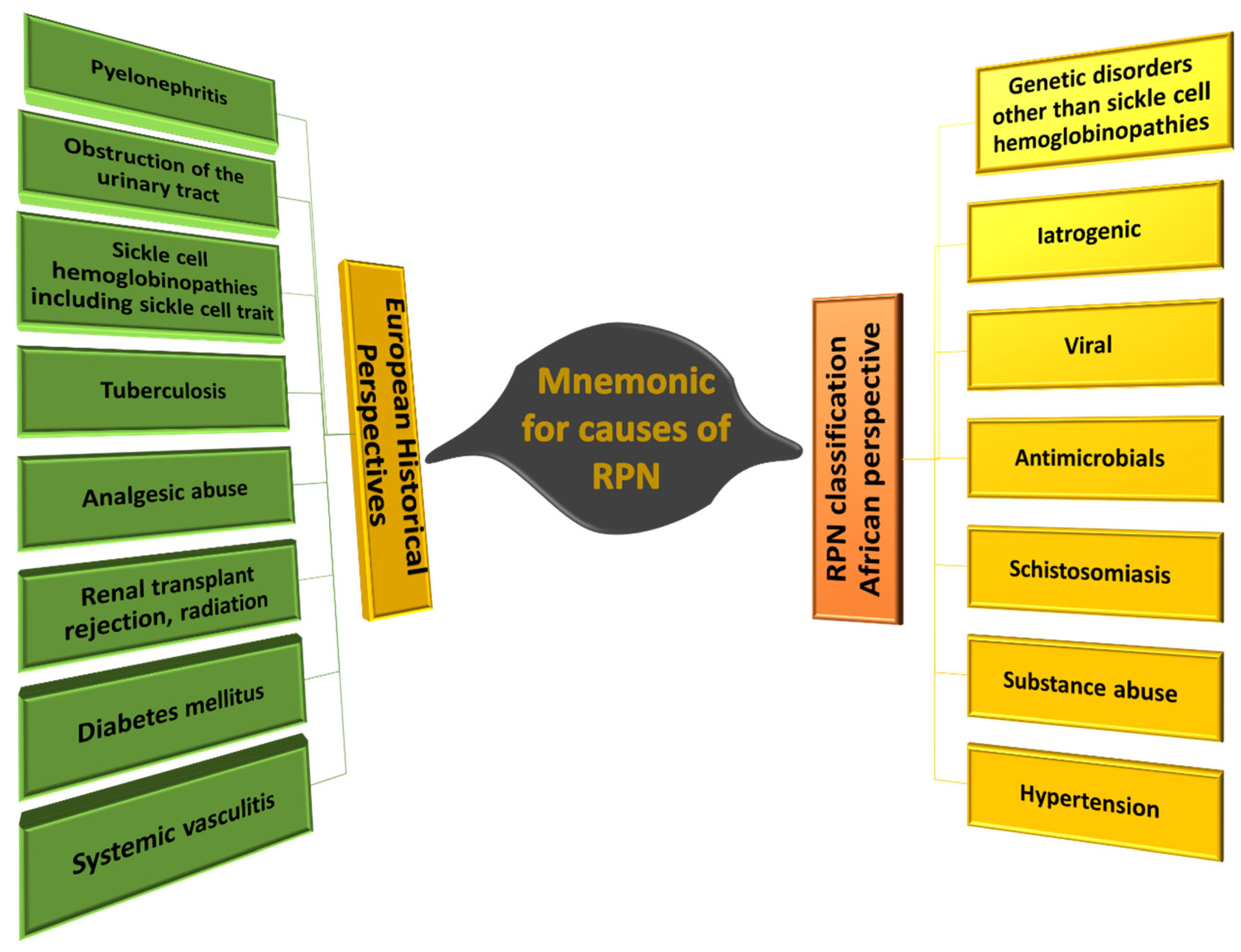
| P | Pyelonephritis |
| O | Obstruction of the urinary tract (e.g., Benign prostatic hyperplasia, [47]) |
| S | Sickle cell hemoglobinopathies, including sickle cell trait |
| T | Tuberculosis |
| C | Cirrhosis of the liver, chronic alcoholism |
| A | Analgesic abuse |
| R | Renal transplant rejection, radiation |
| D | Diabetes mellitus |
| S | Systemic vasculitis |
| G | Genetic disorders, other than sickle cell hemoglobinopathies |
| I | Iatrogenic |
| V | Viral |
| A | Antimicrobials |
| S | Schistosomiasis |
| S | Substance abuse |
| H | Hypertension |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaudji, G.R.; Bida, M.; Conradie, M.; Damane, B.P.; Bester, M.J. Renal Papillary Necrosis (RPN) in an African Population: Disease Patterns, Relevant Pathways, and Management. Biomedicines 2023, 11, 93. https://doi.org/10.3390/biomedicines11010093
Gaudji GR, Bida M, Conradie M, Damane BP, Bester MJ. Renal Papillary Necrosis (RPN) in an African Population: Disease Patterns, Relevant Pathways, and Management. Biomedicines. 2023; 11(1):93. https://doi.org/10.3390/biomedicines11010093
Chicago/Turabian StyleGaudji, Guy Roger, Meshack Bida, Marius Conradie, Botle Precious Damane, and Megan Jean Bester. 2023. "Renal Papillary Necrosis (RPN) in an African Population: Disease Patterns, Relevant Pathways, and Management" Biomedicines 11, no. 1: 93. https://doi.org/10.3390/biomedicines11010093
APA StyleGaudji, G. R., Bida, M., Conradie, M., Damane, B. P., & Bester, M. J. (2023). Renal Papillary Necrosis (RPN) in an African Population: Disease Patterns, Relevant Pathways, and Management. Biomedicines, 11(1), 93. https://doi.org/10.3390/biomedicines11010093






