Preoperative Function Assessment of Ex Vivo Kidneys with Supervised Machine Learning Based on Blood and Urine Markers Measured during Normothermic Machine Perfusion
Abstract
1. Introduction
2. Materials and Methods
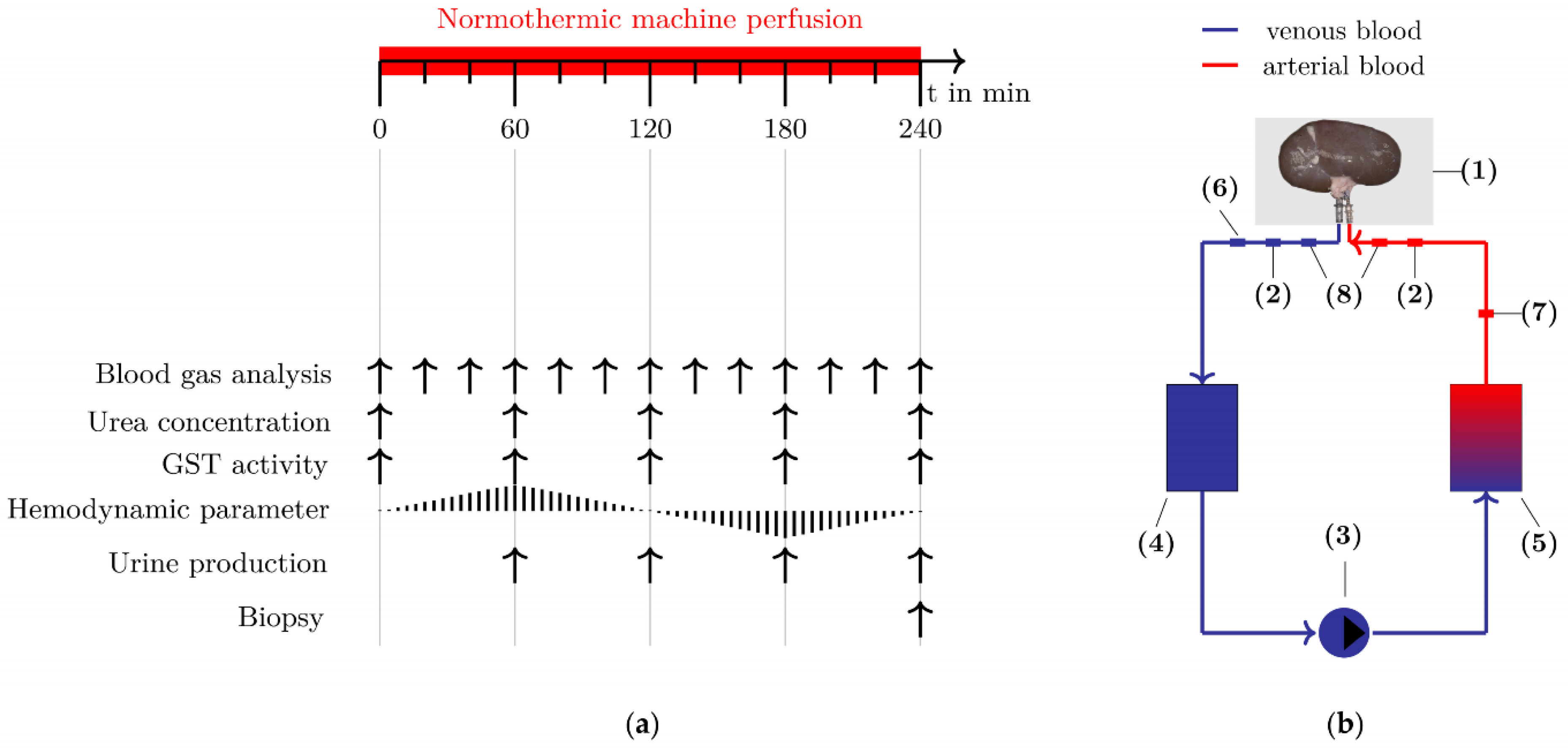
2.1. Study Design
2.2. Normothermic Machine Perfusion
2.3. Dataset
2.4. Function Markers for Metabolic Activity
2.4.1. Oxygen Delivery
2.4.2. Oxygen Consumption
2.4.3. Oxygen Extraction
2.4.4. Carbon Dioxide Production
2.5. Function Markers for Renal Hemodynamics
2.6. Function Markers for Renal Filtration
2.6.1. Urea Concentration
2.6.2. Urine Production
2.7. Injury Marker for Tubular Impairment
2.8. Supervised Learning Methods for the Functional Classification of Ex Vivo Kidneys
2.8.1. Testing for Multicollinearity
2.8.2. Supervised Learning Methods
2.8.3. Validation
2.9. Histological Assessment Using the Preoperative Remuzzi Score
2.10. Statistical Analysis
3. Results
3.1. Function Markers for Metabolic Activity
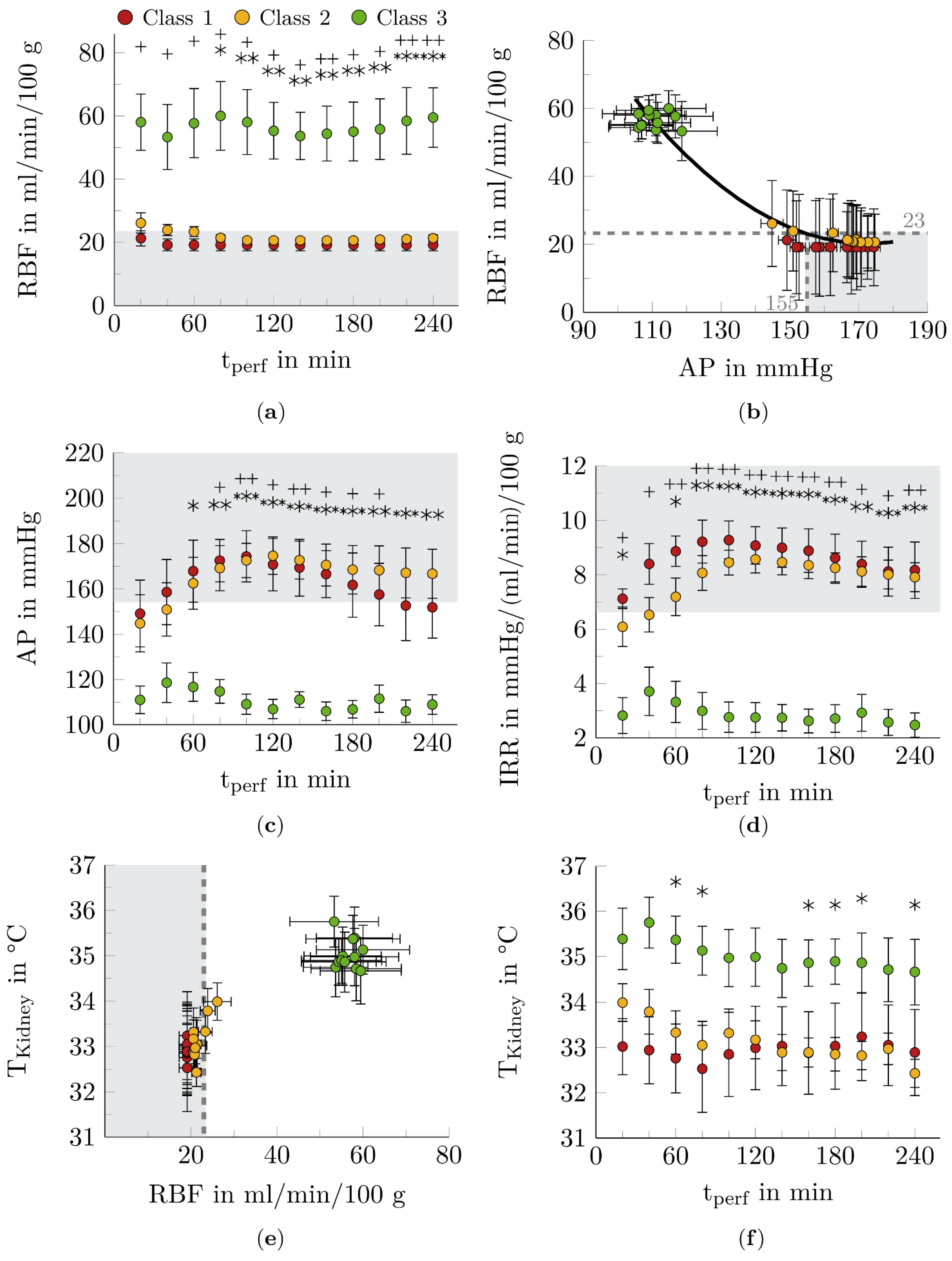
3.2. Function Markers for Renal Hemodynamics
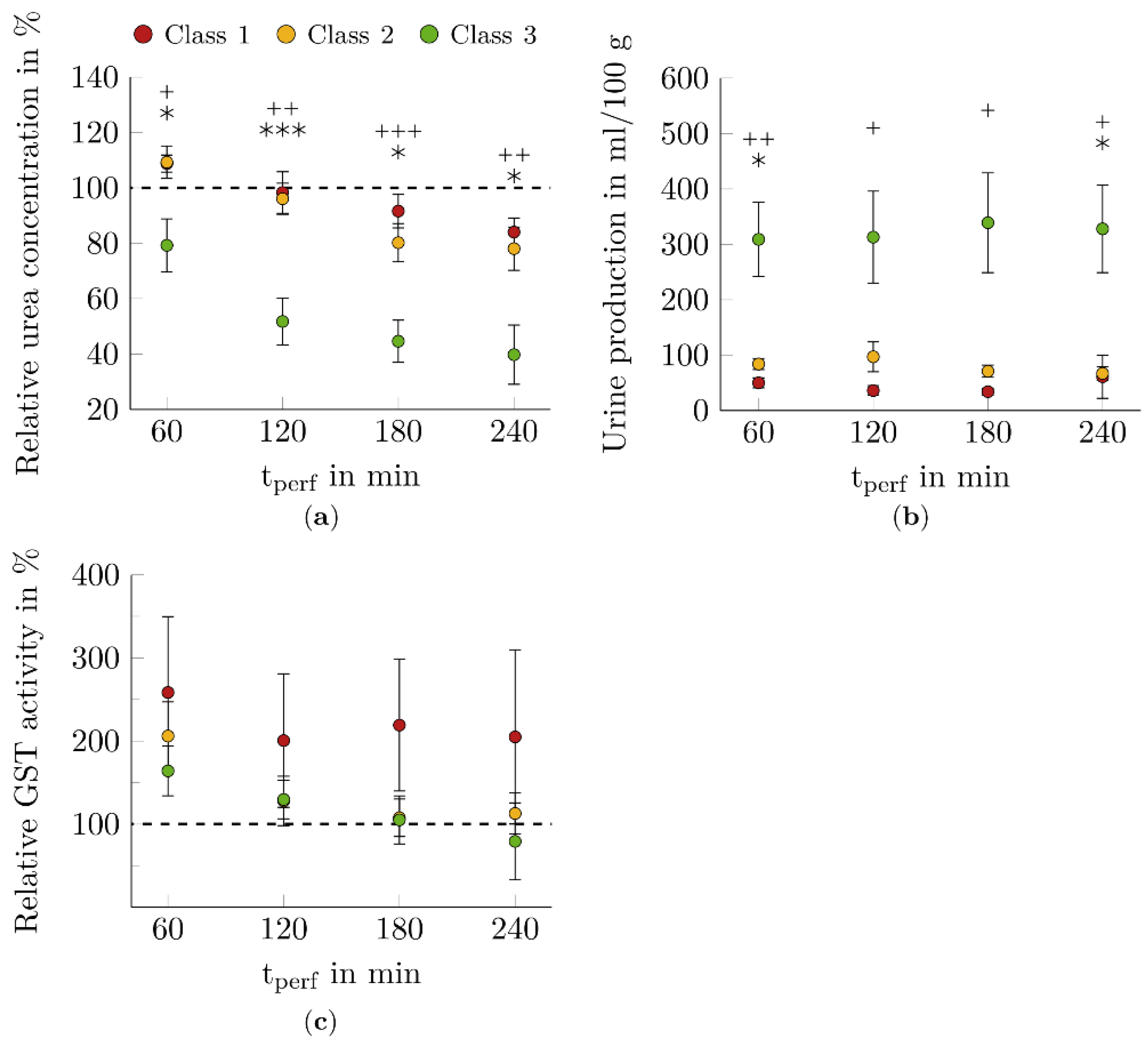
3.3. Function Markers for Renal Filtration
3.4. Injury Marker for Tubular Impairment
3.5. Detection of Multicollinearity
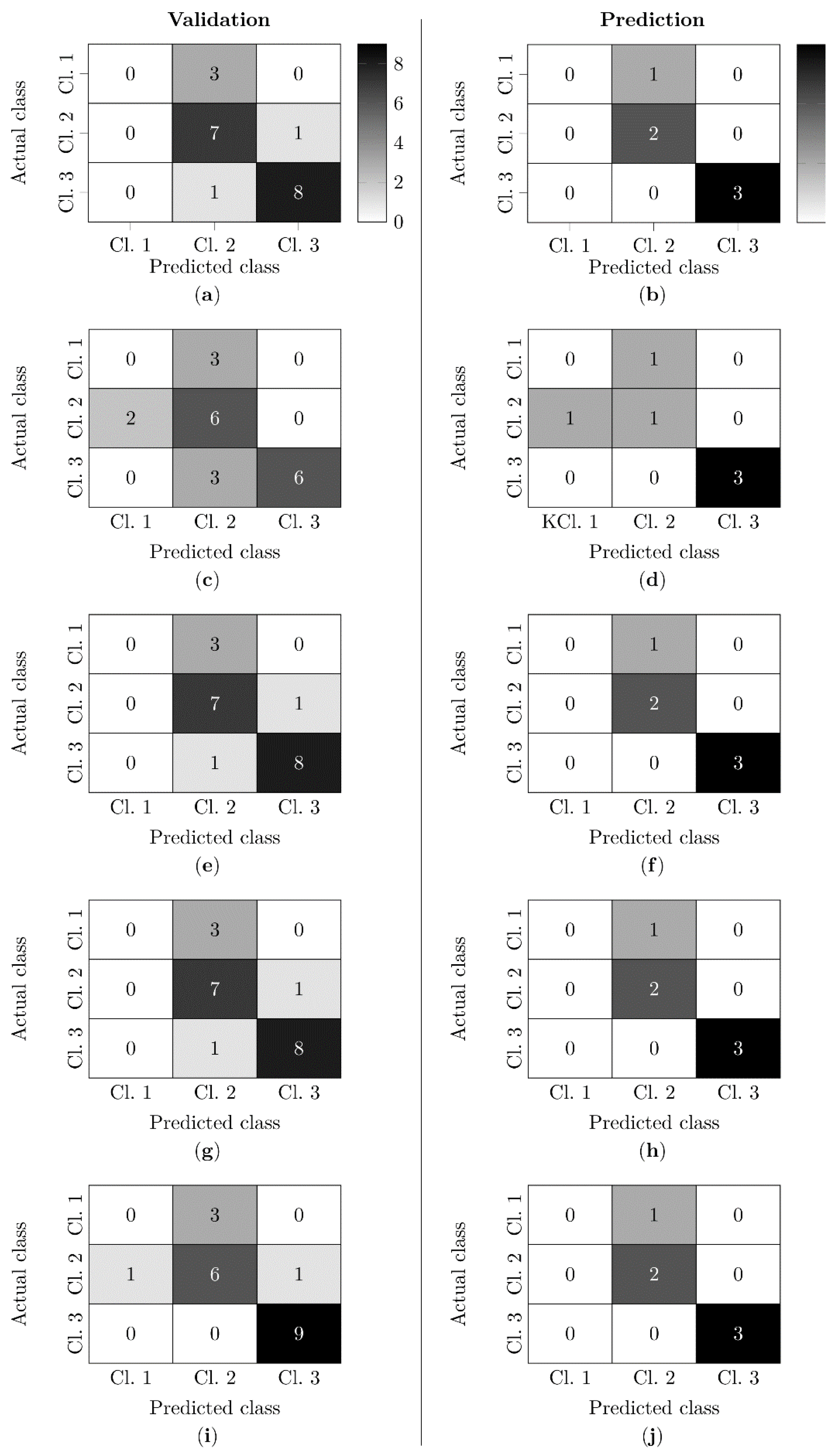
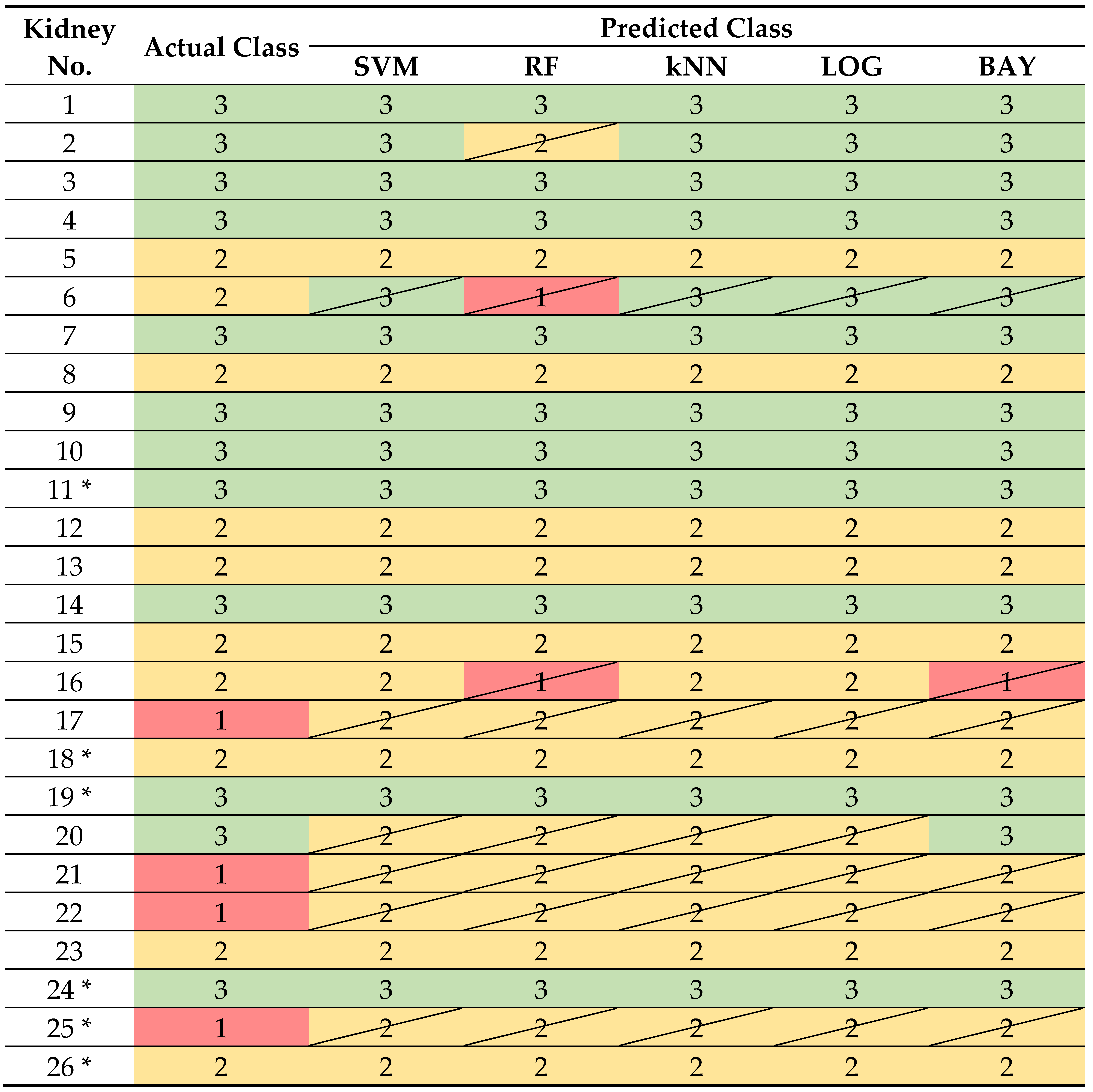
3.6. Functional Classification Based on Blood and Urine Markers
3.7. Classification Based on the Preoperative Remuzzi Score
4. Discussion
4.1. Function Markers for Metabolic Activity
4.2. Function Markers for Renal Hemodynamics
4.3. Function Markers for Renal Filtration
4.4. Injury Marker for Tubular Impairment
4.5. Functional Classification Based on Blood and Urine Markers
4.6. Classification Based on the Preoperative Remuzzi Score
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warmuzińska, N.; Łuczykowski, K.; Bojko, B. A Review of Current and Emerging Trends in Donor Graft-Quality Assessment Techniques. J. Clin. Med. 2022, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Branger, P.; Vogelaar, S. Annual Report 2020; Eurotransplant International Foundation: Leiden, The Netherlands, 2021. [Google Scholar]
- Rao, P.S.; Schaubel, D.E.; Guidinger, M.K.; Andreoni, K.A.; Wolfe, R.A.; Merion, R.M.; Port, F.K.; Sung, R.S. A Comprehensive Risk Quantification Score for Deceased Donor Kidneys: The Kidney Donor Risk Index. Transplantation 2009, 88, 231–236. [Google Scholar] [CrossRef]
- Remuzzi, G.; Grinyò, J.; Ruggenenti, P.; Beatini, M.; Cole, E.H.; Milford, E.L.; Brenner, B.M. Early Experience with Dual Kidney Transplantation in Adults using Expanded Donor Criteria. J. Am. Soc. Nephrol. 1999, 10, 2591–2598. [Google Scholar] [CrossRef]
- Stallone, G.; Grandaliano, G. To discard or not to discard: Transplantation and the art of scoring. Clin. Kidney J. 2019, 12, 564–568. [Google Scholar] [CrossRef]
- Moeckli, B.; Sun, P.; Lazeyras, F.; Morel, P.; Moll, S.; Pascual, M.; Buhler, L.H. Evaluation of donor kidneys prior to transplantation: An update of current and emerging methods. Transpl. Int. 2019, 32, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Kaths, J.M.; Paul, A.; Robinson, L.A.; Selzner, M. Ex vivo machine perfusion for renal graft preservation. Transplant. Rev. 2018, 32, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Resch, T.; Cardini, B.; Oberhuber, R.; Weissenbacher, A.; Dumfarth, J.; Krapf, C.; Boesmueller, C.; Oefner, D.; Grimm, M.; Schneeberger, S. Transplanting Marginal Organs in the Era of Modern Machine Perfusion and Advanced Organ Monitoring. Front. Immunol. 2020, 11, 631. [Google Scholar] [CrossRef] [PubMed]
- De Beule, J.; Jochmans, I. Kidney Perfusion as an Organ Quality Assessment Tool—Are We Counting Our Chickens Before They Have Hatched? J. Clin. Med. 2020, 9, 879. [Google Scholar] [CrossRef]
- Hamelink, T.L.; Ogurlu, B.; De Beule, J.; Lantinga, V.A.; Pool, M.B.F.; Venema, L.H.; Leuvenink, H.G.D.; Jochmans, I.; Moers, C. Renal Normothermic Machine Perfusion: The Road Toward Clinical Implementation of a Promising Pretransplant Organ Assessment Tool. Transplantation 2022, 106, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Markgraf, W.; Janssen, M.; Lilienthal, J.; Feistel, P.; Thiele, C.; Stöckle, M.; Malberg, H. Hyperspectral imaging for ex-vivo organ characterization during normothermic machine perfusion. Eur. Urol. Suppl. 2018, 17, e767. [Google Scholar] [CrossRef]
- Markgraf, W.; Lilienthal, J.; Feistel, P.; Thiele, C.; Malberg, H. Algorithm for Mapping Kidney Tissue Water Content during Normothermic Machine Perfusion Using Hyperspectral Imaging. Algorithms 2020, 13, 289. [Google Scholar] [CrossRef]
- Markgraf, W.; Feistel, P.; Thiele, C.; Malberg, H. Algorithms for mapping kidney tissue oxygenation during normothermic machine perfusion using hyperspectral imaging. Biomed. Eng. Biomed. Tech. 2018, 63, 557–566. [Google Scholar] [CrossRef]
- Markgraf, W.; Lilienthal, J.; Feistel, P.; Mühle, R.; Thiele, C.; Malberg, H.; Janssen, M. Hyperspectral imaging of porcine kidneys during normothermic ex vivo perfusion—An analysis of tissue-related renal ischemia injury. Transpl. Int. 2020, 33, 16–36. [Google Scholar] [CrossRef]
- Schutter, R.; Lantinga, V.A.; Hamelink, T.L.; Pool, M.B.F.; Varsseveld, O.C.; Potze, J.H.; Hillebrands, J.; Heuvel, M.C.V.D.; Dierckx, R.A.J.O.; Leuvenink, H.G.D.; et al. Magnetic resonance imaging assessment of renal flow distribution patterns during ex vivo normothermic machine perfusion in porcine and human kidneys. Transpl. Int. 2021, 34, 1643–1655. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.; Nicholson, M.L. An Assessment of Urinary Biomarkers in a Series of Declined Human Kidneys Measured During Ex Vivo Normothermic Kidney Perfusion. Transplantation 2017, 101, 2120–2125. [Google Scholar] [CrossRef]
- Kaths, J.M.; Hamar, M.; Echeverri, J.; Linares, I.; Urbanellis, P.; Cen, J.Y.; Ganesh, S.; Dingwell, L.; Yip, P.; John, R.; et al. Normothermic ex vivo kidney perfusion for graft quality assessment prior to transplantation. Am. J. Transplant. 2017, 18, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Thompson, E.; Bates, L.; Pither, T.L.; Hosgood, S.A.; Nicholson, M.L.; Watson, C.J.; Wilson, C.; Fisher, A.J.; Ali, S.; et al. Flavin Mononucleotide as a Biomarker of Organ Quality—A Pilot Study. Transplant. Direct 2020, 6, e600. [Google Scholar] [CrossRef]
- Woud, W.W.; Merino, A.; Hoogduijn, M.J.; Boer, K.; Hoogen, M.W.F.V.D.; Baan, C.C.; Minnee, R.C. Nanoparticle Release by Extended Criteria Donor Kidneys During Normothermic Machine Perfusion. Transplantation 2019, 103, e110–e111. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Barlow, A.D.; Hunter, J.P.; Nicholson, M.L. Ex vivo normothermic perfusion for quality assessment of marginal donor kidney transplants. Br. J. Surg. 2015, 102, 1433–1440. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Thompson, E.; Moore, T.; Wilson, C.H.; Nicholson, M.L. Normothermic machine perfusion for the assessment and transplantation of declined human kidneys from donation after circulatory death donors. Br. J. Surg. 2017, 105, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Saeb-Parsy, K.; Hamed, M.O.; Nicholson, M.L. Successful Transplantation of Human Kidneys Deemed Untransplantable but Resuscitated by Ex Vivo Normothermic Machine Perfusion. Am. J. Transplant. 2016, 16, 3282–3285. [Google Scholar] [CrossRef] [PubMed]
- Markgraf, W.; Mühle, R.; Lilienthal, J.; Kromnik, S.; Thiele, C.; Malberg, H.; Janssen, M.; Putz, J. Inulin Clearance During Ex vivo Normothermic Machine Perfusion as a Marker of Renal Function. ASAIO J. 2021, 68, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Sun, B.; Fischer, J.; Goldammer, M.; Thiele, C.; Malberg, H.; Markgraf, W. Hyperspectral Imaging during Normothermic Machine Perfusion—A Functional Classification of Ex Vivo Kidneys Based on Convolutional Neural Networks. Biomedicines 2022, 10, 397. [Google Scholar] [CrossRef]
- Takala, J. Oxygen Consumption and Carbon Dioxide Production: Physiological Basis and Practical Application in Intensive Care. In Anaesthesia, Pain, Intensive Care and Emergency Medicine; Gullo, A., Ed.; Springer: Milano, Italy, 1997; pp. 155–162. [Google Scholar] [CrossRef]
- Bizouarn, P.; Soulard, D.; Blanloeil, Y.; Guillet, A.; Goarin, Y. Oxygen consumption after cardiac surgery —A comparison between calculation by Fick’s principle and measurement by indirect calorimetry. Intensiv. Care Med. 1992, 18, 206–209. [Google Scholar] [CrossRef] [PubMed]
- de Boode, W.P.; Hopman, J.C.W.; Daniëls, O.; van der Hoeven, H.G.; Liem, K.D. Cardiac Output Measurement Using a Modified Carbon Dioxide Fick Method: A Validation Study in Ventilated Lambs. Pediatr. Res. 2007, 61, 279–283. [Google Scholar] [CrossRef][Green Version]
- Douglas, A.R.; Jones, N.L.; Reed, J.W. Calculation of whole blood CO2 content. J. Appl. Physiol. 1988, 65, 1–5. [Google Scholar] [CrossRef]
- Belsley, E.; Kuh, E.; Welsch, R.E. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity; John Wiley & Sons: New York, NY, USA, 1980. [Google Scholar]
- Frochte, J. Maschinelles Lernen: Grundlagen und Algorithmen in Python, 1st ed.; Carl Hanser Verlag: München, Germany, 2019. [Google Scholar]
- BenSaïda, A. Shapiro-Wilk and Shapiro-Francia Normality Tests Version 1.1.0.0 2019. Available online: https://www.mathworks.com/matlabcentral/fileexchange/13964-shapiro-wilk-and-shapiro-francia-normality-tests (accessed on 4 April 2019).
- Trujillo-Ortiz, A. Levenetest Version 1.0.0.0 2019. Available online: https://www.mathworks.com/matlabcentral/fileexchange/3375-levenetest?s%7B%5C_%7Dtid=srchtitle (accessed on 4 April 2019).
- Trujillo-Ortiz, A. Welchanova Version 1.1.0.0 2019. Available online: https://www.mathworks.com/matlabcentral/fileexchange/37121-welchanova?s%7B%5C_%7Dtid=srchtitle (accessed on 4 April 2019).
- Trujillo-Ortiz, A. GHtest Version 1.0.0.0 2019. Available online: https://www.mathworks.com/ matlabcentral/fileexchange/3676-gh est?s%7B%5C_%7Dtid=srchtitle (accessed on 4 April 2019).
- Fabry, G.; Doorschodt, B.M.; Grzanna, T.; Boor, P.; Elliott, A.; Stollenwerk, A.; Tolba, R.H.; Rossaint, R.; Bleilevens, C. Cold Preflush of Porcine Kidney Grafts Prior to Normothermic Machine Perfusion Aggravates Ischemia Reperfusion Injury. Sci. Rep. 2019, 9, 13897. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.F.; Adams, T.; Hosgood, S.A.; Nicholson, M.L. The administration of argon during ex vivo normothermic perfusion in an experimental model of kidney ischemia–reperfusion injury. J. Surg. Res. 2017, 218, 202–208. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Moore, T.; Kleverlaan, T.; Adams, T.; Nicholson, M.L. Haemoadsorption reduces the inflammatory response and improves blood flow during ex vivo renal perfusion in an experimental model. J. Transl. Med. 2017, 15, 216. [Google Scholar] [CrossRef] [PubMed]
- Maassen, H.; Hendriks, K.D.W.; Venema, L.H.; Henning, R.H.; Hofker, S.H.; Van Goor, H.; Leuvenink, H.G.D.; Coester, A.M. Hydrogen sulphide-induced hypometabolism in human-sized porcine kidneys. PLoS ONE 2019, 14, 1–12. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Moore, T.; Qurashi, M.; Adams, T.; Nicholson, M.L. Hydrogen Gas Does Not Ameliorate Renal Ischemia Reperfusion Injury in a Preclinical Model. Artif. Organs 2018, 42, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hosgood, S.A.; Meeta, P.; Long, Y.; Zhu, T.; Nicholson, M.L.; Yang, B. Cyclic Helix B Peptide in Preservation Solution and Autologous Blood Perfusate Ameliorates Ischemia-Reperfusion Injury in Isolated Porcine Kidneys. Transplant. Direct 2015, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- A Hosgood, S.; Yates, P.J.; Nicholson, M.L. 1400W reduces ischemia reperfusion injury in an ex-vivo porcine model of the donation after circulatory death kidney donor. World J. Transplant. 2014, 4, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.; Hosgood, S.A.; Nicholson, M.L. Physiological effects of altering oxygenation during kidney normothermic machine perfusion. Am. J. Physiol. Physiol. 2019, 316, F823–F829. [Google Scholar] [CrossRef] [PubMed]
- Aburawi, M.M.; Fontan, F.M.; Karimian, N.; Eymard, C.; Cronin, S.; Pendexter, C.; Nagpal, S.; Banik, P.; Ozer, S.; Mahboub, P.; et al. Synthetic hemoglobin-based oxygen carriers are an acceptable alternative for packed red blood cells in normothermic kidney perfusion. Am. J. Transplant. 2019, 19, 2814–2824. [Google Scholar] [CrossRef]
- Adams, T.; Patel, M.; Hosgood, S.A.; Nicholson, M.L. Lowering Perfusate Temperature From 37 °C to 32 °C Diminishes Function in a Porcine Model of Ex Vivo Kidney Perfusion. Transplant. Direct 2017, 3, e140. [Google Scholar] [CrossRef]
- Patel, M.; Hosgood, S.; Nicholson, M.L. The effects of arterial pressure during normothermic kidney perfusion. J. Surg. Res. 2014, 191, 463–468. [Google Scholar] [CrossRef]
- Hendriks, K.D.W.; Brüggenwirth, I.M.A.; Maassen, H.; Gerding, A.; Bakker, B.; Porte, R.J.; Henning, R.H.; Leuvenink, H.G.D. Renal temperature reduction progressively favors mitochondrial ROS production over respiration in hypothermic kidney preservation. J. Transl. Med. 2019, 17, 1–10. [Google Scholar] [CrossRef]
- Mancina, E.; Kalenski, J.; Paschenda, P.; Beckers, C.; Bleilevens, C.; Boor, P.; Doorschodt, B.M.; Tolba, R.H. Determination of the Preferred Conditions for the Isolated Perfusion of Porcine Kidneys. Eur. Surg. Res. 2015, 54, 44–54. [Google Scholar] [CrossRef]
- Blum, M.F.; Liu, Q.; Soliman, B.; Dreher, P.; Okamoto, T.; Poggio, E.D.; Goldfarb, D.A.; Baldwin, W.M.; Quintini, C. Comparison of normothermic and hypothermic perfusion in porcine kidneys donated after cardiac death. J. Surg. Res. 2017, 216, 35–45. [Google Scholar] [CrossRef]
- Georgiades, F.; Hosgood, S.A.; Butler, A.J.; Nicholson, M.L. Use of ex vivo normothermic machine perfusion after normothermic regional perfusion to salvage a poorly perfused DCD kidney. Am. J. Transplant. 2019, 19, 3415–3419. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Patel, M.; Nicholson, M.L. The conditioning effect of ex vivo normothermic perfusion in an experimental kidney model. J. Surg. Res. 2013, 182, 153–160. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Barlow, A.D.; Dormer, J.; Nicholson, M.L. The use of ex-vivo normothermic perfusion for the resuscitation and assessment of human kidneys discarded because of inadequate in situ perfusion. J. Transl. Med. 2015, 13, 1–9. [Google Scholar] [CrossRef]
- Chandak, P.; Phillips, B.; Uwechue, R.; Thompson, E.; Bates, L.; Ibrahim, I.; Sewpaul, A.; Figueiredo, R.; Olsburgh, J.; Hosgood, S.; et al. Dissemination of a novel organ perfusion technique: Ex vivo normothermic perfusion of deceased donor kidneys. Artif. Organs 2019, 43, E308–E319. [Google Scholar] [CrossRef]
- Jochmans, I.; Moers, C.; Smits, J.M.; Leuvenink, H.G.D.; Treckmann, J.; Paul, A.; Rahmel, A.; Squifflet, J.-P.; Van Heurn, E.; Monbaliu, D.; et al. The Prognostic Value of Renal Resistance During Hypothermic Machine Perfusion of Deceased Donor Kidneys. Am. J. Transplant. 2011, 11, 2214–2220. [Google Scholar] [CrossRef]
- Yang, B.; Hosgood, S.; Da, Z.; Harper, S.J.; Waller, H.L.; Kay, M.D.; Furness, P.N.; Nicholson, M.L. Biomarkers assessing warm ischemic injury using an isolated porcine kidney hemoreperfusion model. Exp. Biol. Med. 2012, 237, 1462–1473. [Google Scholar] [CrossRef]
- A Hosgood, S.; Saeb-Parsy, K.; Wilson, C.; Callaghan, C.; Collett, D.; Nicholson, M.L. Protocol of a randomised controlled, open-label trial of ex vivo normothermic perfusion versus static cold storage in donation after circulatory death renal transplantation. BMJ Open 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Kaths, J.M.; Spetzler, V.N.; Goldaracena, N.; Echeverri, J.; Louis, K.S.; Foltys, D.B.; Strempel, M.; Yip, P.; John, R.; Mucsi, I.; et al. Normothermic Ex Vivo Kidney Perfusion for the Preservation of Kidney Grafts prior to Transplantation. J. Vis. Exp. 2015, 101, e52909. [Google Scholar] [CrossRef]
- Hosgood, S.; Harper, S.; Kay, M.; Bagul, A.; Waller, H.; Nicholson, M.L. Effects of arterial pressure in an experimental isolated haemoperfused porcine kidney preservation system. Br. J. Surg. 2006, 93, 879–884. [Google Scholar] [CrossRef]
- van Essen, G.J.; Hekkert, M.T.L.; Sorop, O.; Heinonen, I.; van der Velden, J.; Merkus, D.; Duncker, D.J. Cardiovascular Function of Modern Pigs Does not Comply with Allometric Scaling Laws. Sci. Rep. 2018, 8, 792. [Google Scholar] [CrossRef]
- Waller, H.L.; Harper, S.J.F.; Hosgood, S.A.; Bagul, A.; Yang, B.; Kay, M.D.; Kaushik, M.; Nicholson, M.L. Biomarkers of oxidative damage to predict ischaemia-reperfusion injury in an isolated organ perfusion model of the transplanted kidney. Free Radic. Res. 2006, 40, 1218–1225. [Google Scholar] [CrossRef]
- Bhangoo, R.S.; Hall, I.E.; Reese, P.P.; Parikh, C.R. Deceased-donor kidney perfusate and urine biomarkers for kidney allograft outcomes: A systematic review. Nephrol. Dial. Transplant. 2012, 27, 3305–3314. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, G.; Zhu, Z.; Zhang, Z.; Yuan, X.; Han, M.; Zhao, Q.; Zheng, Y.; Tang, Y.; Huang, S.; et al. The First Case of Ischemia-Free Kidney Transplantation in Humans. Front. Med. 2019, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Weissenbacher, A.; Faro, M.L.L.; Boubriak, O.; Soares, M.F.; Roberts, I.S.; Hunter, J.P.; Voyce, D.; Mikov, N.; Cook, A.; Ploeg, R.J.; et al. Twenty-four–hour normothermic perfusion of discarded human kidneys with urine recirculation. Am. J. Transplant. 2019, 19, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Pool, M.B.F.; Hartveld, L.; Leuvenink, H.; Moers, C. Normothermic machine perfusion of ischaemically damaged porcine kidneys with autologous, allogeneic porcine and human red blood cells. PLoS ONE 2020, 15, 1–16. [Google Scholar] [CrossRef]


 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markgraf, W.; Malberg, H. Preoperative Function Assessment of Ex Vivo Kidneys with Supervised Machine Learning Based on Blood and Urine Markers Measured during Normothermic Machine Perfusion. Biomedicines 2022, 10, 3055. https://doi.org/10.3390/biomedicines10123055
Markgraf W, Malberg H. Preoperative Function Assessment of Ex Vivo Kidneys with Supervised Machine Learning Based on Blood and Urine Markers Measured during Normothermic Machine Perfusion. Biomedicines. 2022; 10(12):3055. https://doi.org/10.3390/biomedicines10123055
Chicago/Turabian StyleMarkgraf, Wenke, and Hagen Malberg. 2022. "Preoperative Function Assessment of Ex Vivo Kidneys with Supervised Machine Learning Based on Blood and Urine Markers Measured during Normothermic Machine Perfusion" Biomedicines 10, no. 12: 3055. https://doi.org/10.3390/biomedicines10123055
APA StyleMarkgraf, W., & Malberg, H. (2022). Preoperative Function Assessment of Ex Vivo Kidneys with Supervised Machine Learning Based on Blood and Urine Markers Measured during Normothermic Machine Perfusion. Biomedicines, 10(12), 3055. https://doi.org/10.3390/biomedicines10123055






