Immunopathogenesis of Immune Checkpoint Inhibitor Induced Myocarditis: Insights from Experimental Models and Treatment Implications
Abstract
1. Introduction
1.1. A New Era of Cancer Immunotherapy
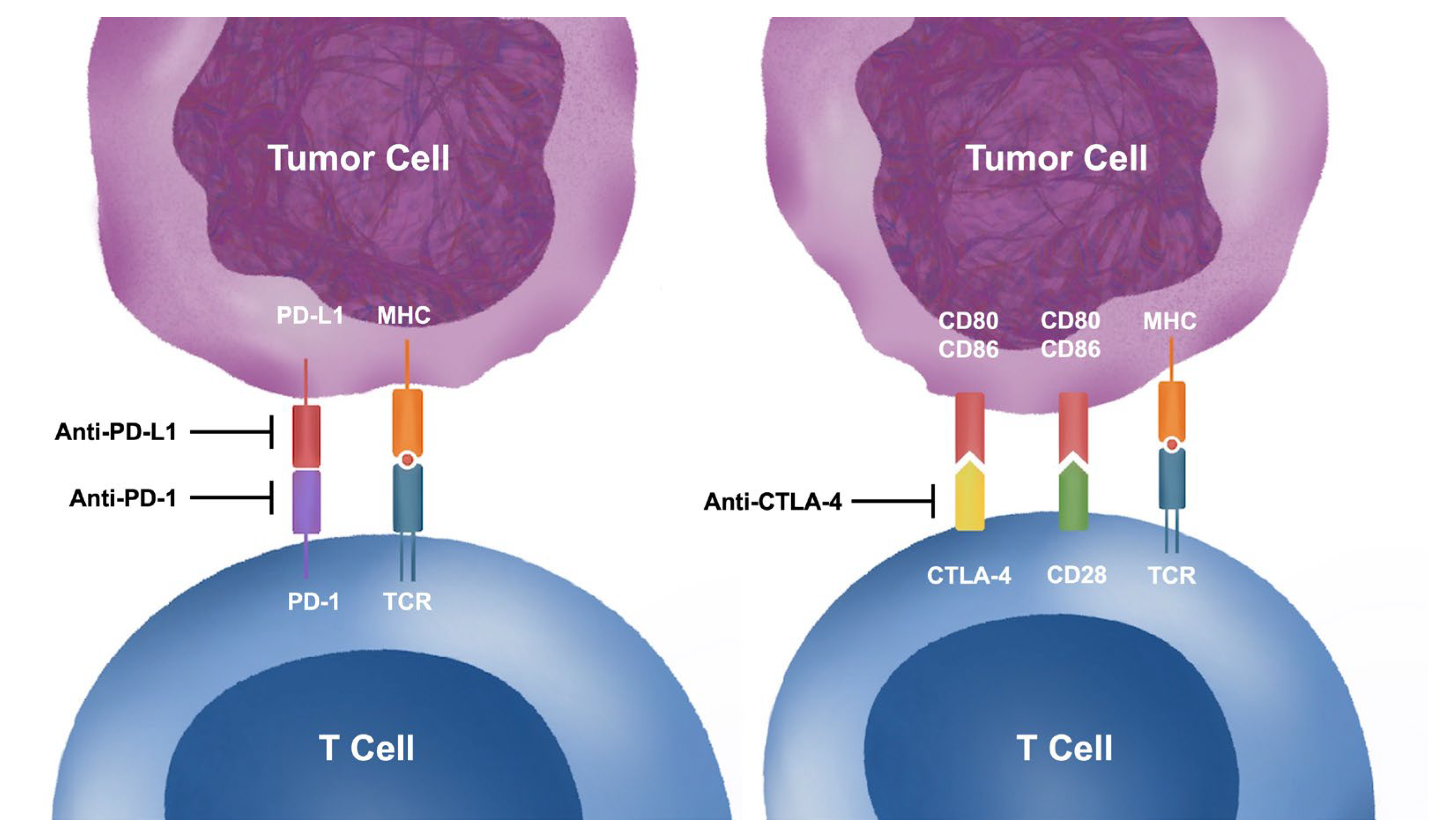
1.2. Mechanisms of Immune Checkpoint Inhibitors
1.3. Immune-Related Adverse Events
1.3.1. Diverse Presentations of Immune-Related Adverse Events
1.3.2. Epidemiology, Presentation, Diagnosis and Contemporary Treatment of Immune Checkpoint Inhibitor-Induced Myocarditis
2. Human Studies of ICI-Induced Myocarditis
2.1. Endomyocardial Histology, Immunohistochemistry and Tissue Transcriptomics
2.2. Peripheral Blood Mononuclear Cell Profile
2.3. Serum Cytokine and Biomarker Levels
3. Animal Models of ICI-Induced Myocarditis
3.1. Transgenic Mice
3.1.1. CTLA4−/−
3.1.2. PDCD1−/−
3.1.3. PDL1−/−
3.1.4. PDCD1−/− CTLA4+/−
3.2. Induction with ICI
3.2.1. Combinational ICI
3.2.2. Tumor Inoculation
3.2.3. Cardiac Sarcomere Immunization
3.2.4. Cardiac Irradiation
3.3. Comparison between Animal Models and Human Studies
3.4. Merits and Limitations of Experimental Animal Models
4. Drug Screening and Opportunities for Treatment
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.A.; Reed, K.; et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef]
- Salmaninejad, A.; Valilou, S.F.; Shabgah, A.G.; Aslani, S.; Alimardani, M.; Pasdar, A.; Sahebkar, A. PD-1/PD-L1 pathway: Basic biology and role in cancer immunotherapy. J. Cell Physiol. 2019, 234, 16824–16837. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Zhao, Y.; Yang, W.; Huang, Y.; Cui, R.; Li, X.; Li, B. Evolving Roles for Targeting CTLA-4 in Cancer Immunotherapy. Cell Physiol. Biochem. 2018, 47, 721–734. [Google Scholar] [CrossRef]
- Mokyr, M.B.; Kalinichenko, T.; Gorelik, L.; Bluestone, J.A. Realization of the therapeutic potential of CTLA-4 blockade in low-dose chemotherapy-treated tumor-bearing mice. Cancer Res. 1998, 58, 5301–5304. [Google Scholar]
- Kanda, Y.; Okazaki, T.; Katakai, T. Motility Dynamics of T Cells in Tumor-Draining Lymph Nodes: A Rational Indicator of Antitumor Response and Immune Checkpoint Blockade. Cancers 2021, 13, 4616. [Google Scholar] [CrossRef]
- Sarvaria, A.; Madrigal, J.A.; Saudemont, A. B cell regulation in cancer and anti-tumor immunity. Cell Mol. Immunol. 2017, 14, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Bueno, O.F.; Brandt, E.B.; Rothenberg, M.E.; Molkentin, J.D. Defective T cell development and function in calcineurin A beta -deficient mice. Proc. Natl. Acad. Sci. USA 2002, 99, 9398–9403. [Google Scholar] [CrossRef] [PubMed]
- Sansom, D.M. CD28, CTLA-4 and their ligands: Who does what and to whom? Immunology 2000, 101, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Curtsinger, J.M.; Mescher, M.F. Inflammatory cytokines as a third signal for T cell activation. Curr. Opin. Immunol. 2010, 22, 333–340. [Google Scholar] [CrossRef]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.F.; Testori, A.; Grob, J.J.; et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Chiarion-Sileni, V.; Grob, J.J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N. Engl. J. Med. 2016, 375, 1845–1855. [Google Scholar] [CrossRef]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Rodig, N.; Ryan, T.; Allen, J.A.; Pang, H.; Grabie, N.; Chernova, T.; Greenfield, E.A.; Liang, S.C.; Sharpe, A.H.; Lichtman, A.H.; et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur. J. Immunol. 2003, 33, 3117–3126. [Google Scholar] [CrossRef]
- Nishimura, H.; Honjo, T. PD-1: An inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol. 2001, 22, 265–268. [Google Scholar] [CrossRef]
- Yi, M.; Niu, M.; Xu, L.; Luo, S.; Wu, K. Regulation of PD-L1 expression in the tumor microenvironment. J. Hematol. Oncol. 2021, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, P.T.; Bhatia, S.; Lipson, E.J.; Kudchadkar, R.R.; Miller, N.J.; Annamalai, L.; Berry, S.; Chartash, E.K.; Daud, A.; Fling, S.P.; et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N. Engl. J. Med. 2016, 374, 2542–2552. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kummel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Andre, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Colombo, N.; Dubot, C.; Lorusso, D.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Tewari, K.S.; Salman, P.; Hoyos Usta, E.; Yanez, E.; et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021, 385, 1856–1867. [Google Scholar] [CrossRef]
- Makker, V.; Colombo, N.; Casado Herraez, A.; Santin, A.D.; Colomba, E.; Miller, D.S.; Fujiwara, K.; Pignata, S.; Baron-Hay, S.; Ray-Coquard, I.; et al. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N. Engl. J. Med. 2022, 386, 437–448. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Sonpavde, G.; Manitz, J.; Gao, C.; Tayama, D.; Kaiser, C.; Hennessy, D.; Makari, D.; Gupta, A.; Abdullah, S.E.; Niegisch, G.; et al. Five-Factor Prognostic Model for Survival of Post-Platinum Patients with Metastatic Urothelial Carcinoma Receiving PD-L1 Inhibitors. J. Urol. 2020, 204, 1173–1179. [Google Scholar] [CrossRef]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 2017, 276, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Huard, B.; Prigent, P.; Tournier, M.; Bruniquel, D.; Triebel, F. CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. Eur. J. Immunol. 1995, 25, 2718–2721. [Google Scholar] [CrossRef]
- Sobottka, B.; Moch, H.; Varga, Z. Differential PD-1/LAG-3 expression and immune phenotypes in metastatic sites of breast cancer. Breast Cancer Res. 2021, 23, 4. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutierrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Darnell, E.P.; Mooradian, M.J.; Baruch, E.N.; Yilmaz, M.; Reynolds, K.L. Immune-Related Adverse Events (irAEs): Diagnosis, Management, and Clinical Pearls. Curr. Oncol. Rep. 2020, 22, 39. [Google Scholar] [CrossRef]
- Fujii, T.; Colen, R.R.; Bilen, M.A.; Hess, K.R.; Hajjar, J.; Suarez-Almazor, M.E.; Alshawa, A.; Hong, D.S.; Tsimberidou, A.; Janku, F.; et al. Incidence of immune-related adverse events and its association with treatment outcomes: The MD Anderson Cancer Center experience. Investig. New Drugs 2018, 36, 638–646. [Google Scholar] [CrossRef]
- Chennamadhavuni, A.; Abushahin, L.; Jin, N.; Presley, C.J.; Manne, A. Risk Factors and Biomarkers for Immune-Related Adverse Events: A Practical Guide to Identifying High-Risk Patients and Rechallenging Immune Checkpoint Inhibitors. Front. Immunol. 2022, 13, 779691. [Google Scholar] [CrossRef]
- Liu, X.; Shi, Y.; Zhang, D.; Zhou, Q.; Liu, J.; Chen, M.; Xu, Y.; Zhao, J.; Zhong, W.; Wang, M. Risk factors for immune-related adverse events: What have we learned and what lies ahead? Biomark. Res. 2021, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef]
- Masuda, K.; Shoji, H.; Nagashima, K.; Yamamoto, S.; Ishikawa, M.; Imazeki, H.; Aoki, M.; Miyamoto, T.; Hirano, H.; Honma, Y.; et al. Correlation between immune-related adverse events and prognosis in patients with gastric cancer treated with nivolumab. BMC Cancer 2019, 19, 974. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, W. Cardiovascular immune-related adverse events: Evaluation, diagnosis and management. Asia Pac. J. Clin. Oncol. 2020, 16, 232–240. [Google Scholar] [CrossRef]
- Nso, N.; Antwi-Amoabeng, D.; Beutler, B.D.; Ulanja, M.B.; Ghuman, J.; Hanfy, A.; Nimo-Boampong, J.; Atanga, S.; Doshi, R.; Enoru, S.; et al. Cardiac adverse events of immune checkpoint inhibitors in oncology patients: A systematic review and meta-analysis. World J. Cardiol. 2020, 12, 584–598. [Google Scholar] [CrossRef]
- Berthod, G.; Lazor, R.; Letovanec, I.; Romano, E.; Noirez, L.; Mazza Stalder, J.; Speiser, D.E.; Peters, S.; Michielin, O. Pulmonary sarcoid-like granulomatosis induced by ipilimumab. J. Clin. Oncol. 2012, 30, e156–e159. [Google Scholar] [CrossRef] [PubMed]
- Eckert, A.; Schoeffler, A.; Dalle, S.; Phan, A.; Kiakouama, L.; Thomas, L. Anti-CTLA4 monoclonal antibody induced sarcoidosis in a metastatic melanoma patient. Dermatology 2009, 218, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; Wang, X.; Woo, K.M.; Iyriboz, T.; Halpenny, D.; Cunningham, J.; Chaft, J.E.; Segal, N.H.; Callahan, M.K.; Lesokhin, A.M.; et al. Pneumonitis in Patients Treated with Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. 2017, 35, 709–717. [Google Scholar] [CrossRef]
- Reuss, J.E.; Kunk, P.R.; Stowman, A.M.; Gru, A.A.; Slingluff, C.L., Jr.; Gaughan, E.M. Sarcoidosis in the setting of combination ipilimumab and nivolumab immunotherapy: A case report & review of the literature. J. Immunother. Cancer 2016, 4, 94. [Google Scholar] [CrossRef]
- Bot, I.; Blank, C.U.; Boogerd, W.; Brandsma, D. Neurological immune-related adverse events of ipilimumab. Pract. Neurol. 2013, 13, 278–280. [Google Scholar] [CrossRef]
- Möhn, N.; Beutel, G.; Gutzmer, R.; Ivanyi, P.; Satzger, I.; Skripuletz, T. Neurological Immune Related Adverse Events Associated with Nivolumab, Ipilimumab, and Pembrolizumab Therapy-Review of the Literature and Future Outlook. J. Clin. Med. 2019, 8, 1777. [Google Scholar] [CrossRef]
- Safa, H.; Johnson, D.H.; Trinh, V.A.; Rodgers, T.E.; Lin, H.; Suarez-Almazor, M.E.; Fa’ak, F.; Saberian, C.; Yee, C.; Davies, M.A.; et al. Immune checkpoint inhibitor related myasthenia gravis: Single center experience and systematic review of the literature. J. Immunother. Cancer 2019, 7, 319. [Google Scholar] [CrossRef]
- Bermejo, S.; Bolufer, M.; Riveiro-Barciela, M.; Soler, M.J. Immunotherapy and the Spectrum of Kidney Disease: Should We Individualize the Treatment? Front. Med. 2022, 9, 906565. [Google Scholar] [CrossRef]
- Espi, M.; Teuma, C.; Novel-Catin, E.; Maillet, D.; Souquet, P.J.; Dalle, S.; Koppe, L.; Fouque, D. Renal adverse effects of immune checkpoints inhibitors in clinical practice: ImmuNoTox study. Eur. J. Cancer 2021, 147, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Mamlouk, O.; Selamet, U.; Machado, S.; Abdelrahim, M.; Glass, W.F.; Tchakarov, A.; Gaber, L.; Lahoti, A.; Workeneh, B.; Chen, S.; et al. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: Single-center experience. J. Immunother. Cancer 2019, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Mathew, A.; Abraham, F.; Amin, R.; Kono, M.; Overman, M.; Zhao, D.; Khan, A.; Khan, M.A.; Thomas, A.S.; et al. Immune checkpoint inhibitor-related gastrointestinal toxicity in patients with malignancy involving the luminal gastrointestinal tract and its impact on cancer outcomes. Ann. Gastroenterol. 2022, 35, 514–521. [Google Scholar] [CrossRef]
- Zhang, M.L.; Neyaz, A.; Patil, D.; Chen, J.; Dougan, M.; Deshpande, V. Immune-related adverse events in the gastrointestinal tract: Diagnostic utility of upper gastrointestinal biopsies. Histopathology 2020, 76, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.J.; Powers, A.C.; Johnson, D.B. Endocrine toxicities of immune checkpoint inhibitors. Nat. Rev. Endocrinol. 2021, 17, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.; Zaremba, A.; Moreira, A.; Ugurel, S.; Johnson, D.B.; Hassel, J.C.; Salzmann, M.; Gesierich, A.; Weppler, A.; Spain, L.; et al. Hematological immune related adverse events after treatment with immune checkpoint inhibitors. Eur. J. Cancer 2021, 147, 170–181. [Google Scholar] [CrossRef]
- Petrelli, F.; Ardito, R.; Borgonovo, K.; Lonati, V.; Cabiddu, M.; Ghilardi, M.; Barni, S. Haematological toxicities with immunotherapy in patients with cancer: A systematic review and meta-analysis. Eur. J. Cancer 2018, 103, 7–16. [Google Scholar] [CrossRef]
- Cappelli, L.C.; Gutierrez, A.K.; Bingham, C.O., 3rd; Shah, A.A. Rheumatic and Musculoskeletal Immune-Related Adverse Events Due to Immune Checkpoint Inhibitors: A Systematic Review of the Literature. Arthritis Care Res. 2017, 69, 1751–1763. [Google Scholar] [CrossRef]
- Muntyanu, A.; Netchiporouk, E.; Gerstein, W.; Gniadecki, R.; Litvinov, I.V. Cutaneous Immune-Related Adverse Events (irAEs) to Immune Checkpoint Inhibitors: A Dermatology Perspective on Management [Formula: See text]. J. Cutan. Med. Surg. 2021, 25, 59–76. [Google Scholar] [CrossRef]
- Tattersall, I.W.; Leventhal, J.S. Cutaneous Toxicities of Immune Checkpoint Inhibitors: The Role of the Dermatologist. Yale J. Biol. Med. 2020, 93, 123–132. [Google Scholar]
- Papavasileiou, E.; Prasad, S.; Freitag, S.K.; Sobrin, L.; Lobo, A.M. Ipilimumab-induced Ocular and Orbital Inflammation—A Case Series and Review of the Literature. Ocul. Immunol. Inflamm. 2016, 24, 140–146. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated with Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Hu, Y.; Yang, C.; Zhao, B. Myocarditis following the use of different immune checkpoint inhibitor regimens: A real-world analysis of post-marketing surveillance data. Int. Immunopharmacol. 2019, 76, 105866. [Google Scholar] [CrossRef] [PubMed]
- Awadalla, M.; Mahmood, S.S.; Groarke, J.D.; Hassan, M.Z.O.; Nohria, A.; Rokicki, A.; Murphy, S.P.; Mercaldo, N.D.; Zhang, L.; Zlotoff, D.A.; et al. Global Longitudinal Strain and Cardiac Events in Patients with Immune Checkpoint Inhibitor-Related Myocarditis. J. Am. Coll. Cardiol. 2020, 75, 467–478. [Google Scholar] [CrossRef]
- Zhang, L.; Awadalla, M.; Mahmood, S.S.; Nohria, A.; Hassan, M.Z.O.; Thuny, F.; Zlotoff, D.A.; Murphy, S.P.; Stone, J.R.; Golden, D.L.A.; et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur. Heart J. 2020, 41, 1733–1743. [Google Scholar] [CrossRef]
- Lam, T.H.; Wong, J.S.L.; Wong, C.-K.; Kwok, G.G.-W.; Zhou, M.; So, B.Y.-F.; Leung, I.Y.-H.; Tse, H.-F. From Bad to Worse: The Clinical Spectrum of Immune Checkpoint Inhibitor Myocarditis and Associated 3M Syndrome with Concomitant Myositis and Myasthenia. J. Hong Kong Coll. Cardiol. 2022, 29, 135–142. [Google Scholar] [CrossRef]
- Moslehi, J.J.; Salem, J.E.; Sosman, J.A.; Lebrun-Vignes, B.; Johnson, D.B. Reporting of immune checkpoint inhibitor-associated myocarditis—Authors’ reply. Lancet 2018, 392, 384–385. [Google Scholar] [CrossRef]
- Waliany, S.; Neal, J.W.; Reddy, S.; Wakelee, H.; Shah, S.A.; Srinivas, S.; Padda, S.K.; Fan, A.C.; Colevas, A.D.; Wu, S.M.; et al. Myocarditis Surveillance with High-Sensitivity Troponin I During Cancer Treatment with Immune Checkpoint Inhibitors. JACC CardioOncol. 2021, 3, 137–139. [Google Scholar] [CrossRef]
- Deharo, F.; Carvelli, J.; Cautela, J.; Garcia, M.; Sarles, C.; Maues de Paula, A.; Bourenne, J.; Gainnier, M.; Bichon, A. Immune Checkpoint Inhibitor-Induced Myositis/Myocarditis with Myasthenia Gravis-like Misleading Presentation: A Case Series in Intensive Care Unit. J. Clin. Med. 2022, 11, 5611. [Google Scholar] [CrossRef]
- Longinow, J.; Zmaili, M.; Skoza, W.; Kondoleon, N.; Marquardt, R.; Calabrese, C.; Funchain, P.; Moudgil, R. Immune checkpoint inhibitor induced myocarditis, myasthenia gravis, and myositis: A single-center case series. Cancer Med. 2022. [Google Scholar] [CrossRef]
- Wai Siu, D.H.; O’Neill, R.S.; Harris, C.A.; Wang, J.; Ardolino, L.; Downton, T.; Tong, M.; Hong, J.H.; Chin, V.; Clingan, P.R.; et al. Immune checkpoint inhibitor-induced myocarditis, myositis, myasthenia gravis and transaminitis: A case series and review. Immunotherapy 2022, 14, 511–520. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef] [PubMed]
- Thuny, F.; Alexandre, J.; Salem, J.E.; Mirabel, M.; Dolladille, C.; Cohen-Solal, A.; Cohen, A.; Ederhy, S.; Cautela, J.; French Working Group of Cardio-Oncology. Management of Immune Checkpoint Inhibitor-Induced Myocarditis: The French Working Group’s Plea for a Pragmatic Approach. JACC CardioOncol. 2021, 3, 157–161. [Google Scholar] [CrossRef]
- Hazebroek, M.R.; Everaerts, K.; Heymans, S. Diagnostic approach of myocarditis: Strike the golden mean. Neth. Heart J. 2014, 22, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, M.S.; Fano, S.; Stenor, C.; Moller, A.K. A case report of immune checkpoint inhibitor-related steroid-refractory myocarditis and myasthenia gravis-like myositis treated with abatacept and mycophenolate mofetil. Eur. Heart J. Case Rep. 2021, 5, ytab342. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.W.; Alexander, M.; Dib, Y.; Lau, P.K.H.; Weppler, A.M.; Au-Yeung, G.; Lee, B.; Khoo, C.; Mooney, D.; Joshi, S.B.; et al. A closer look at immune-mediated myocarditis in the era of combined checkpoint blockade and targeted therapies. Eur. J. Cancer 2020, 124, 15–24. [Google Scholar] [CrossRef]
- Arangalage, D.; Delyon, J.; Lermuzeaux, M.; Ekpe, K.; Ederhy, S.; Pages, C.; Lebbe, C. Survival After Fulminant Myocarditis Induced by Immune-Checkpoint Inhibitors. Ann. Intern. Med. 2017, 167, 683–684. [Google Scholar] [CrossRef]
- Xing, Q.; Zhang, Z.; Zhu, B.; Lin, Q.; Shen, L.; Li, F.; Xia, Z.; Zhao, Z. Case Report: Treatment for steroid-refractory immune-related myocarditis with tofacitinib. Front. Immunol. 2022, 13, 944013. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Talamo, L.; Dillon, P.; Gentzler, R.D.; Millard, T.; Salerno, M.; Slingluff, C.L., Jr.; Gaughan, E.M. Severe combined cardiac and neuromuscular toxicity from immune checkpoint blockade: An institutional case series. Cardiooncology 2020, 6, 21. [Google Scholar] [CrossRef]
- Konstantina, T.; Konstantinos, R.; Anastasios, K.; Anastasia, M.; Eleni, L.; Ioannis, S.; Sofia, A.; Dimitris, M. Fatal adverse events in two thymoma patients treated with anti-PD-1 immune check point inhibitor and literature review. Lung Cancer 2019, 135, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Zhang, Z.W.; Lin, Q.H.; Shen, L.H.; Wang, P.M.; Zhang, S.; Fan, M.; Zhu, B. Myositis-myasthenia gravis overlap syndrome complicated with myasthenia crisis and myocarditis associated with anti-programmed cell death-1 (sintilimab) therapy for lung adenocarcinoma. Ann. Transl. Med. 2020, 8, 250. [Google Scholar] [CrossRef]
- Bozzi, A.; Sayed, N.; Matsa, E.; Sass, G.; Neofytou, E.; Clemons, K.V.; Correa-Oliveira, R.; Stevens, D.A.; Wu, J.C. Using Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes as a Model to Study Trypanosoma cruzi Infection. Stem Cell Rep. 2019, 12, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Marceau, C.; Hamaguchi, R.; Burridge, P.W.; Rajarajan, K.; Churko, J.M.; Wu, H.; Sallam, K.I.; Matsa, E.; Sturzu, A.C.; et al. Human induced pluripotent stem cell-derived cardiomyocytes as an in vitro model for coxsackievirus B3-induced myocarditis and antiviral drug screening platform. Circ. Res. 2014, 115, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Lau, Y.M.; Lai, W.H.; Zhang, R.R.; Luk, H.K.; Wong, A.C.; Woo, P.C.; Lau, S.K.; Chan, K.H.; Hung, I.F.; et al. Angiotensin converting enzyme and sodium glucose cotransporter inhibitors alleviate inflammatory effects of SARS-CoV-2 in cardiomyocytes. Cardiol. J. 2022, 29, 702–706. [Google Scholar] [CrossRef]
- Wong, C.K.; Luk, H.K.; Lai, W.H.; Lau, Y.M.; Zhang, R.R.; Wong, A.C.; Lo, G.C.; Chan, K.H.; Hung, I.F.; Tse, H.F.; et al. Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes Platform to Study SARS-CoV-2 Related Myocardial Injury. Circ. J. 2020, 84, 2027–2031. [Google Scholar] [CrossRef]
- Caforio, A.L.; Calabrese, F.; Angelini, A.; Tona, F.; Vinci, A.; Bottaro, S.; Ramondo, A.; Carturan, E.; Iliceto, S.; Thiene, G.; et al. A prospective study of biopsy-proven myocarditis: Prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur. Heart J. 2007, 28, 1326–1333. [Google Scholar] [CrossRef]
- Ghawanmeh, M.; Simon Frances, B.; Kerai, A.; Patel, P.; Du, J.; Kumar, P. Management of Recurrent Myocarditis Due to Desmoplakin Cardiomyopathy: Diagnostic and Therapeutic Challenges. JACC Case Rep. 2022, 4, 59–62. [Google Scholar] [CrossRef]
- Nguyen, L.S.; Cooper, L.T.; Kerneis, M.; Funck-Brentano, C.; Silvain, J.; Brechot, N.; Hekimian, G.; Ammirati, E.; Ben M’Barek, B.; Redheuil, A.; et al. Systematic analysis of drug-associated myocarditis reported in the World Health Organization pharmacovigilance database. Nat. Commun. 2022, 13, 25. [Google Scholar] [CrossRef]
- Thurner, L.; Kessel, C.; Fadle, N.; Regitz, E.; Seidel, F.; Kindermann, I.; Lohse, S.; Kos, I.; Tschöpe, C.; Kheiroddin, P.; et al. IL-1RA Antibodies in Myocarditis after SARS-CoV-2 Vaccination. N Engl. J. Med. 2022, 387, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Champion, S.N.; Stone, J.R. Immune checkpoint inhibitor associated myocarditis occurs in both high-grade and low-grade forms. Mod. Pathol. 2020, 33, 99–108. [Google Scholar] [CrossRef]
- Moslehi, J.; Lichtman, A.H.; Sharpe, A.H.; Galluzzi, L.; Kitsis, R.N. Immune checkpoint inhibitor-associated myocarditis: Manifestations and mechanisms. J. Clin. Investig. 2021, 131, e145186. [Google Scholar] [CrossRef]
- Palaskas, N.L.; Segura, A.; Lelenwa, L.; Siddiqui, B.A.; Subudhi, S.K.; Lopez-Mattei, J.; Durand, J.B.; Deswal, A.; Zhao, B.; Maximilian Buja, L.; et al. Immune checkpoint inhibitor myocarditis: Elucidating the spectrum of disease through endomyocardial biopsy. Eur. J. Heart Fail 2021, 23, 1725–1735. [Google Scholar] [CrossRef]
- Sobol, I.; Chen, C.L.; Mahmood, S.S.; Borczuk, A.C. Histopathologic Characterization of Myocarditis Associated With Immune Checkpoint Inhibitor Therapy. Arch. Pathol. Lab. Med. 2020, 144, 1392–1396. [Google Scholar] [CrossRef]
- Finke, D.; Heckmann, M.B.; Salatzki, J.; Riffel, J.; Herpel, E.; Heinzerling, L.M.; Meder, B.; Volkers, M.; Muller, O.J.; Frey, N.; et al. Comparative Transcriptomics of Immune Checkpoint Inhibitor Myocarditis Identifies Guanylate Binding Protein 5 and 6 Dysregulation. Cancers 2021, 13, 2498. [Google Scholar] [CrossRef]
- Xie, X.; Wang, L.; Li, Y.; Xu, Y.; Wu, J.; Lin, X.; Lin, W.; Mai, Q.; Chen, Z.; Zhang, J.; et al. Multi-organ Immune-Related Adverse Event Is a Risk Factor of Immune Checkpoint Inhibitor-Associated Myocarditis in Cancer Patients: A Multi-center Study. Front. Immunol. 2022, 13, 879900. [Google Scholar] [CrossRef] [PubMed]
- Boughdad, S.; Latifyan, S.; Fenwick, C.; Bouchaab, H.; Suffiotti, M.; Moslehi, J.J.; Salem, J.E.; Schaefer, N.; Nicod-Lalonde, M.; Costes, J.; et al. (68)Ga-DOTATOC PET/CT to detect immune checkpoint inhibitor-related myocarditis. J. Immunother. Cancer 2021, 9, e003594. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Galdos, F.X.; Lee, D.; Waliany, S.; Huang, Y.V.; Ryan, J.; Dang, K.; Neal, J.W.; Wakelee, H.A.; Reddy, S.A.; et al. Identification of Pathogenic Immune Cell Subsets Associated with Checkpoint Inhibitor-Induced Myocarditis. Circulation 2022, 146, 316–335. [Google Scholar] [CrossRef] [PubMed]
- Tsuruda, T.; Yoshikawa, N.; Kai, M.; Yamaguchi, M.; Toida, R.; Kodama, T.; Kajihara, K.; Kawabata, T.; Nakamura, T.; Sakata, K.; et al. The Cytokine Expression in Patients with Cardiac Complication after Immune Checkpoint Inhibitor Therapy. Intern. Med. 2021, 60, 423–429. [Google Scholar] [CrossRef]
- Yin, N.; Liu, X.; Ye, X.; Song, W.; Lu, J.; Chen, X. PD-1 inhibitor therapy causes multisystem immune adverse reactions: A case report and literature review. Front. Oncol. 2022, 12, 961266. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Tanaka, Y.; Nishio, R.; Mitsuiye, T.; Mizoguchi, A.; Wang, J.; Ishida, M.; Hiai, H.; Matsumori, A.; Minato, N.; et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med. 2003, 9, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001, 291, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Okazaki, I.M.; Yoshida, T.; Chikuma, S.; Kato, Y.; Nakaki, F.; Hiai, H.; Honjo, T.; Okazaki, T. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int. Immunol. 2010, 22, 443–452. [Google Scholar] [CrossRef]
- Lucas, J.A.; Menke, J.; Rabacal, W.A.; Schoen, F.J.; Sharpe, A.H.; Kelley, V.R. Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J. Immunol. 2008, 181, 2513–2521. [Google Scholar] [CrossRef]
- Tivol, E.A.; Borriello, F.; Schweitzer, A.N.; Lynch, W.P.; Bluestone, J.A.; Sharpe, A.H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995, 3, 541–547. [Google Scholar] [CrossRef]
- Klocke, K.; Sakaguchi, S.; Holmdahl, R.; Wing, K. Induction of autoimmune disease by deletion of CTLA-4 in mice in adulthood. Proc. Natl. Acad. Sci. USA 2016, 113, E2383–E2392. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, C.; Li, Y.; Qin, J.; Amancherla, K.; Jing, Y.; Hu, Q.; Liang, K.; Zhang, Z.; Ye, Y.; et al. Hormonal therapies up-regulate MANF and overcome female susceptibility to immune checkpoint inhibitor myocarditis. Sci. Transl. Med. 2022, 14, eabo1981. [Google Scholar] [CrossRef]
- Wei, S.C.; Meijers, W.C.; Axelrod, M.L.; Anang, N.A.S.; Screever, E.M.; Wescott, E.C.; Johnson, D.B.; Whitley, E.; Lehmann, L.; Courand, P.Y.; et al. A Genetic Mouse Model Recapitulates Immune Checkpoint Inhibitor-Associated Myocarditis and Supports a Mechanism-Based Therapeutic Intervention. Cancer Discov. 2021, 11, 614–625. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.K.; Kim, T.Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef]
- Liu, S.Y.; Huang, W.C.; Yeh, H.I.; Ko, C.C.; Shieh, H.R.; Hung, C.L.; Chen, T.Y.; Chen, Y.J. Sequential Blockade of PD-1 and PD-L1 Causes Fulminant Cardiotoxicity-From Case Report to Mouse Model Validation. Cancers 2019, 11, 580. [Google Scholar] [CrossRef] [PubMed]
- Tay, W.T.; Fang, Y.H.; Beh, S.T.; Liu, Y.W.; Hsu, L.W.; Yen, C.J.; Liu, P.Y. Programmed Cell Death-1: Programmed Cell Death-Ligand 1 Interaction Protects Human Cardiomyocytes Against T-Cell Mediated Inflammation and Apoptosis Response In Vitro. Int. J. Mol. Sci. 2020, 21, 2399. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, J.; Shen, Y.; Pan, J.; Wang, C.; Cheng, L. Protective Effect of Crocin on Immune Checkpoint Inhibitors-Related Myocarditis Through Inhibiting NLRP3 Mediated Pyroptosis in Cardiomyocytes via NF-kappaB Pathway. J. Inflamm. Res. 2022, 15, 1653–1666. [Google Scholar] [CrossRef]
- Tsuruoka, K.; Wakabayashi, S.; Morihara, H.; Matsunaga, N.; Fujisaka, Y.; Goto, I.; Imagawa, A.; Asahi, M. Exacerbation of autoimmune myocarditis by an immune checkpoint inhibitor is dependent on its time of administration in mice. Int. J. Cardiol. 2020, 313, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Song, Y.J.; Liu, X.H.; Xu, S.C.; Kong, C.; Chen, L.F.; Qian, H.; Wu, W. PD-1 inhibitor induces myocarditis by reducing regulatory T cells, activating inflammatory responses, promoting myocardial apoptosis and autophagy. Cytokine 2022, 157, 155932. [Google Scholar] [CrossRef]
- Du, S.; Zhou, L.; Alexander, G.S.; Park, K.; Yang, L.; Wang, N.; Zaorsky, N.G.; Ma, X.; Wang, Y.; Dicker, A.P.; et al. PD-1 Modulates Radiation-Induced Cardiac Toxicity through Cytotoxic T Lymphocytes. J. Thorac. Oncol. 2018, 13, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Vilela, E.M.; Bettencourt-Silva, R.; da Costa, J.T.; Barbosa, A.R.; Silva, M.P.; Teixeira, M.; Primo, J.; Gama Ribeiro, V.; Nunes, J.P.L. Anti-cardiac troponin antibodies in clinical human disease: A systematic review. Ann. Transl. Med. 2017, 5, 307. [Google Scholar] [CrossRef] [PubMed]
- Mocumbi, A.O.; Latif, N.; Yacoub, M.H. Presence of circulating anti-myosin antibodies in endomyocardial fibrosis. PLoS Negl. Trop. Dis. 2010, 4, e661. [Google Scholar] [CrossRef]
- von Reibnitz, D.; Chaft, J.E.; Wu, A.J.; Samstein, R.; Hellmann, M.D.; Plodkowski, A.J.; Zhang, Z.; Shi, W.; Dick-Godfrey, R.; Panchoo, K.H.; et al. Safety of combining thoracic radiation therapy with concurrent versus sequential immune checkpoint inhibition. Adv. Radiat. Oncol. 2018, 3, 391–398. [Google Scholar] [CrossRef]
- Chen, H.S.; Wang, W.; Wu, S.N.; Liu, J.P. Corticosteroids for viral myocarditis. Cochrane Database Syst. Rev. 2013, 2013, Cd004471. [Google Scholar] [CrossRef]
- Robinson, J.; Hartling, L.; Vandermeer, B.; Sebastianski, M.; Klassen, T.P. Intravenous immunoglobulin for presumed viral myocarditis in children and adults. Cochrane Database Syst. Rev. 2020, 8, Cd004370. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Beaumont, G.; Martinez Calatrava, M.J.; Castaneda, S. Abatacept mechanism of action: Concordance with its clinical profile. Reumatol. Clin. 2012, 8, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.E.; Allenbach, Y.; Vozy, A.; Brechot, N.; Johnson, D.B.; Moslehi, J.J.; Kerneis, M. Abatacept for Severe Immune Checkpoint Inhibitor-Associated Myocarditis. N. Engl. J. Med. 2019, 380, 2377–2379. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K.; Committee, E.G. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef]
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer 2020, 19, 116. [Google Scholar] [CrossRef]
- Chapman, N.M.; Chi, H. mTOR signaling, Tregs and immune modulation. Immunotherapy 2014, 6, 1295–1311. [Google Scholar] [CrossRef]
| System | Immune-Related Adverse Events |
|---|---|
| Cardiovascular | Myocarditis and heart failure, pericarditis and pericardial effusion, arrhythmia, sudden cardiac death, hypertension [41,42] |
| Respiratory | Pneumonitis, sarcoidosis [43,44,45,46] |
| Neurological | Encephalitis and encephalopathy, meningitis, transverse myelitis, Guillain Barré syndrome, posterior reversible encephalopathy syndrome, multiple sclerosis, neuropathy, myasthenia gravis [47,48,49] |
| Renal | Glomerulonephritis (including nephrotic syndrome), interstitial nephritis, acute tubular necrosis, renal failure [50,51,52] |
| Gastrointestinal | Gastritis, enteritis, colitis, gastroenteritis, hepatitis, pancreatitis [53,54] |
| Endocrine | Thyroiditis, autoimmune hyperthyroidism and hypothyroidism, hypophysitis, adrenalitis and primary adrenal insufficiency, autoimmune diabetes [55] |
| Hematological | Cytopenias (commonly thrombocytopenia and leucopenia), hemophagocytic lymphohistiocytosis, aplastic anaemia, hemolytic anemia, acquired hemophilia and other coagulopathies [56,57] |
| Rheumatic/Musculoskeletal | Arthritis, myositis, fasciitis, vasculitis, polymyalgia-like syndrome, dermatomyositis, sicca syndrome [58] |
| Skin | Morbilliform exanthem, lichenoid reactions, vitiligo-like depigmentation, psoriasis, alopecia areata, bullous pemphigoid, Stevens-Johnson syndrome/toxic epidermal necrolysis, Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) [59,60] |
| Eye | Episcleritis, conjunctivitis, uveitis, retinopathy, orbital inflammation [61] |
| Specimen | Findings |
|---|---|
| Endomyocardial biopsy | Leukocyte infiltration predominated by CD8+cytotoxic T cells, with smaller proportion of CD68+ or CD163+ macrophages and CD4+ helper T cells [92,93,94,95]. PD-L1+ staining of cardiomyocytes adjacent to site of injury and infiltrating macrophages were reported [92,94,95]. C4d staining was observed in necrotic cardiomyocyte in one study, suggesting role of antigen-antibody interaction and immune complex formation with complement fixation [92]. Transcriptomic studies demonstrated increased expression of genes involving in multiple inflammatory pathways, especially interferon responses [95]. |
| Peripheral blood mononuclear cells | Neutrophil-to-lymphocyte ratio was increased in ICI-induced myocarditis patients [97]. Increased TH1, TH17 and regulatory T cells; decreased TH2 cells [98]. Smaller proportion of naïve CD8+ cytotoxic T cells and larger proportion of terminally differentiated effector memory CD45RA re-expression CD8+ T cells (Temra) [99]. |
| Plasma | Consistently elevated in multiple studies: IL-6 and IL-10. Other cytokines that were reported to have increased levels: IL-8, CXCL9, CXCL10, CXCL13, MNCAF, GM-CSF, hepatocyte growth factor and VEGF-A [97,98,100,101]. |
| Mice Strain | Genotype | Cardiac Phenotype |
|---|---|---|
| BALB/c | PDCD1−/− | DCMP [102,103] |
| MRL/Mpj | PDCD1−/− | Myocarditis [99,104] |
| MRL/MpJ- | PDL1−/−, Fas(lpr) | Myocarditis [105] |
| BALB/c | CTLA4−/− | Myocarditis [106] |
| Cross breed C57BL/10.Q and Tg(Cd4-cre)1Cwi | CTLA4fl/fl, CD4-Cre | Myocarditis [107] |
| C57BL/6J | CTLA4+/−, PDCD1−/− | Myocarditis [108,109] |
| Mice Strain | Monoclonal Antibodies Therapy | Tumor Inoculation | Cardiac Sarcomere Immunization | Additional Therapy | Cardiac Phenotype |
|---|---|---|---|---|---|
| MRL/MpJ-Fas(lpr) | Combinational anti-PD-1 (200 μg) and anti-CTLA4 (200 μg) 2 times per week for 8 weeks | None | None | None | Myocarditis [109] |
| C57/BL7J | Combinational anti-PD-1 (25 mg/kg) and anti-CTLA4 (25 mg/kg) every 3 days for x days | None | None | None | Myocarditis [108] |
| C57/BL7J | Combinational anti-PD-1 (25 mg/kg) and anti-CTLA4 (25 mg/kg) every 3 days for x days after tumor size reaches 200–250 mm3 | Colorectal cancer (MC38), melanoma (B16F10) and breast cancer (EO771) | None | None | Myocarditis [108] |
| BALB/c | Combinational anti-PD-1 (200 μg) and PD-L1 (200 μg) on days 0, 2 and 4 after confirmation of lung metastasis | Colorectal adenocarcinoma (CT26) tail vein injection to induce lung metastasis | None | None | Myocarditis [111] |
| BALB/c | Sequential anti-PD-1 (200 μg) on days 0, 2 and 4, followed by anti-PD-L1 (200 μg) on days 6, 8 and 10 after confirmation of lung metastasis | Colorectal adenocarcinoma (CT26) tail vein injection to induce lung metastasis | None | None | Myocarditis [111] |
| BALB/c | Sequential anti-PD-L1 (200 μg) on days 0, 2 and 4, followed by anti-PD-1 (200 μg) on days 6, 8 and 10 after confirmation of lung metastasis | Colorectal adenocarcinoma (CT26) tail vein injection to induce lung metastasis | None | None | Myocarditis [111] |
| BALB/cByJNarl | Anti-PD-1 (250 μg) every 72 h for 6 doses after tumor size reaches 100 mm3 | Mouse melanoma cells (B16-F10) subcutaneous injection | None | None | Myocarditis [112] |
| BALB/c | Anti-PD-1 (5 mg/kg) on days 7, 9, 11, 13 and 15 | None | Murine troponin I peptide (250 μg) subcutaneous injection on days 0 and 7 | Freund’s complete adjuvant on days 0 and 7 | Myocarditis [113] |
| BALB/c | Anti-PD-1 (0.1 mg) on days 14, 16, 8 and 20 | None | Murine myosin heavy chain α (MHCα) fragment (amino acid 614–629: Acetyl-SLKLMATLFSTYASAD-COOH) subcutaneous injection on days 0 and 7 | Freund’s complete adjuvant on days 0 and 7; and Pertussis toxin (500 ng) on day 0 | Myocarditis [114] |
| BALB/c | Anti-PD-1 (0.1 mg) on days 0, 2, 4 and 6 | None | Murine myosin heavy chain α (MHCα) fragment (amino acid 614-629: Acetyl-SLKLMATLFSTYASAD-COOH) subcutaneous injection on days 0 and 7 | Freund’s complete adjuvant on days 0 and 7; and Pertussis toxin (500 ng) on day 0 | Myocarditis [114] |
| BALB/c | Anti-PD-1 (2 μg/kg) on weeks 5 and 6 | None | Skeletal muscle homogenate of guinea pigs (0.25 mL) once per week for 6 weeks | Freund’s complete adjuvant (0.25 mL) once per week for 6 weeks | Myocarditis [115] |
| C57BL/6 | Anti-PD-1 (10 mg/kg) 1 day before radiotherapy | None | None | Cardiac irradiation | Myocarditis [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, C.-K.; Lam, T.-H.; Liao, S.-Y.; Lau, Y.-M.; Tse, H.-F.; So, B.Y.F. Immunopathogenesis of Immune Checkpoint Inhibitor Induced Myocarditis: Insights from Experimental Models and Treatment Implications. Biomedicines 2023, 11, 107. https://doi.org/10.3390/biomedicines11010107
Wong C-K, Lam T-H, Liao S-Y, Lau Y-M, Tse H-F, So BYF. Immunopathogenesis of Immune Checkpoint Inhibitor Induced Myocarditis: Insights from Experimental Models and Treatment Implications. Biomedicines. 2023; 11(1):107. https://doi.org/10.3390/biomedicines11010107
Chicago/Turabian StyleWong, Chun-Ka, Tsun-Ho Lam, Song-Yan Liao, Yee-Man Lau, Hung-Fat Tse, and Benjamin Y. F. So. 2023. "Immunopathogenesis of Immune Checkpoint Inhibitor Induced Myocarditis: Insights from Experimental Models and Treatment Implications" Biomedicines 11, no. 1: 107. https://doi.org/10.3390/biomedicines11010107
APA StyleWong, C.-K., Lam, T.-H., Liao, S.-Y., Lau, Y.-M., Tse, H.-F., & So, B. Y. F. (2023). Immunopathogenesis of Immune Checkpoint Inhibitor Induced Myocarditis: Insights from Experimental Models and Treatment Implications. Biomedicines, 11(1), 107. https://doi.org/10.3390/biomedicines11010107






