Protective Immunity Induced by Immunization with Baculovirus, Virus-like Particle, and Vaccinia Virus Expressing the AMA1 of Plasmodium berghei
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals, Parasites, Cells, and Antibodies
2.3. Plasmodium Berghei Antigen Preparation
2.4. Generation of VLPs
2.5. Generation of Recombinant Vaccinia Virus
2.6. Recombinant Vaccinia Virus Plaque Assay
2.7. Recombinant Baculovirus Plaque Assay
2.8. Immunization and Challenge Infection
2.9. Antibody Responses in Sera
2.10. Flow Cytometry
2.11. Parasitemia
2.12. Statistics
3. Results
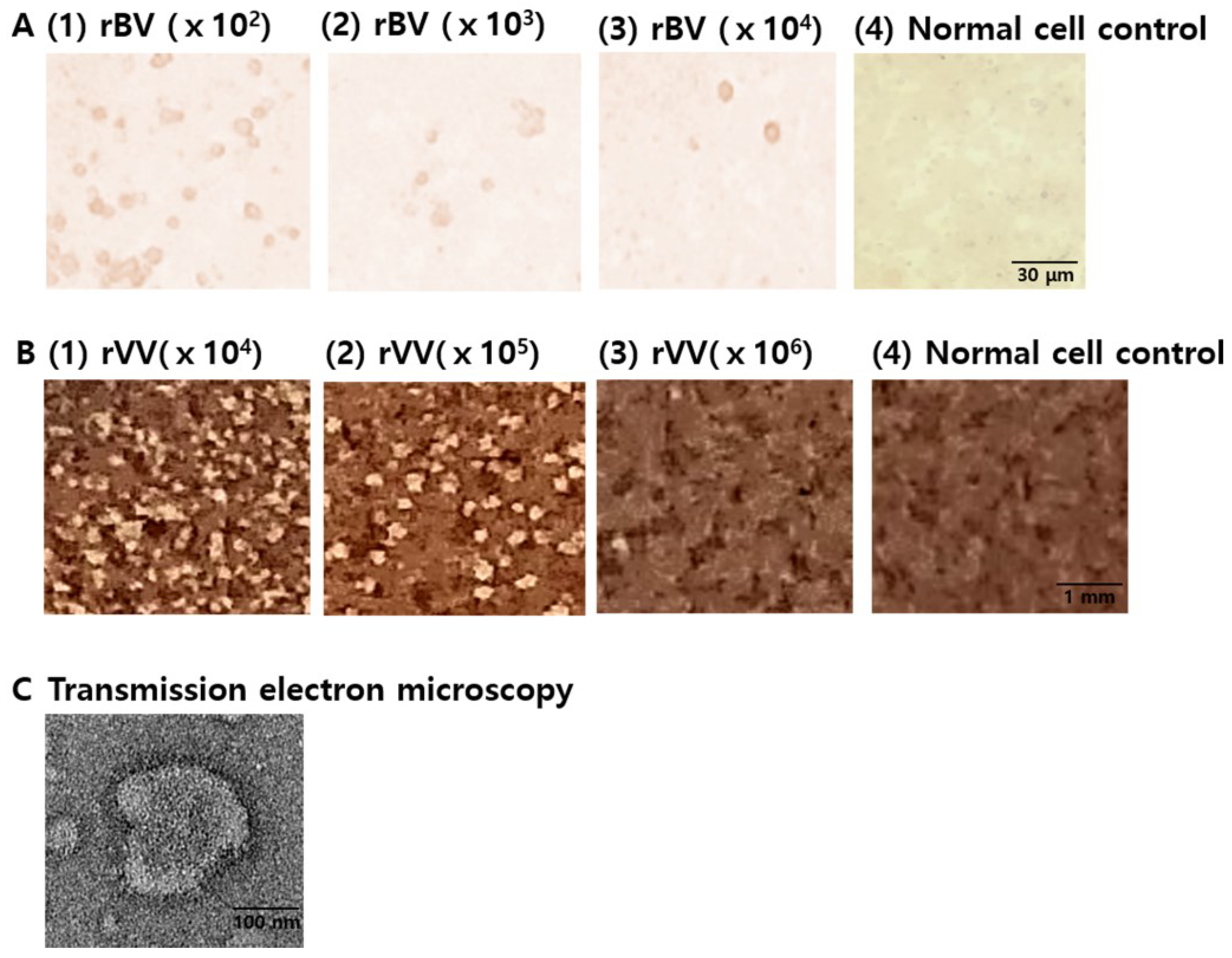
3.1. Characterization of Vaccines
3.2. IgG Antibody Response against VLP Antigens in Sera
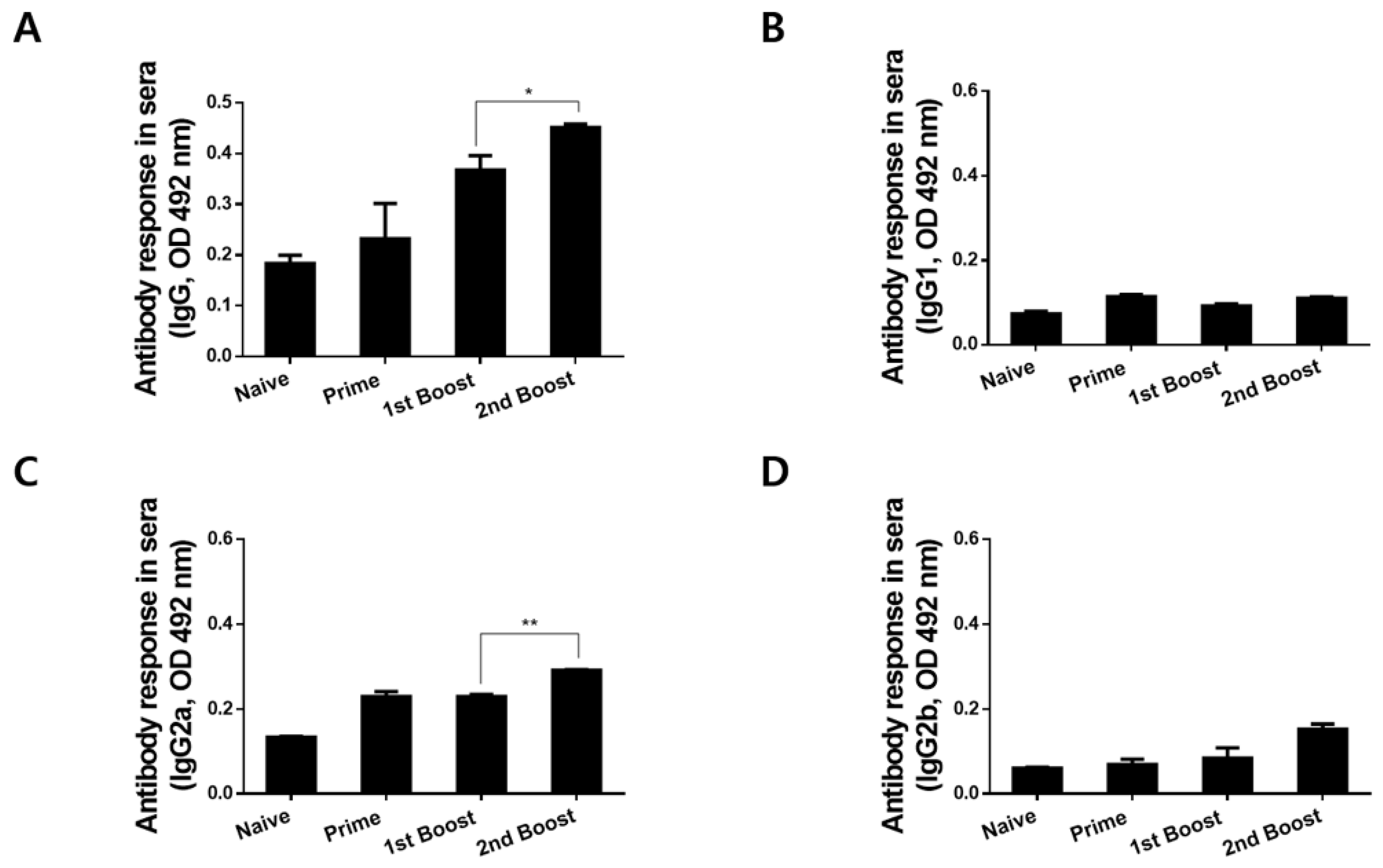
3.3. Immunizations with rBV_V_rVV Elicited Parasite-Specific IgG and IgG Subclass Responses in Sera
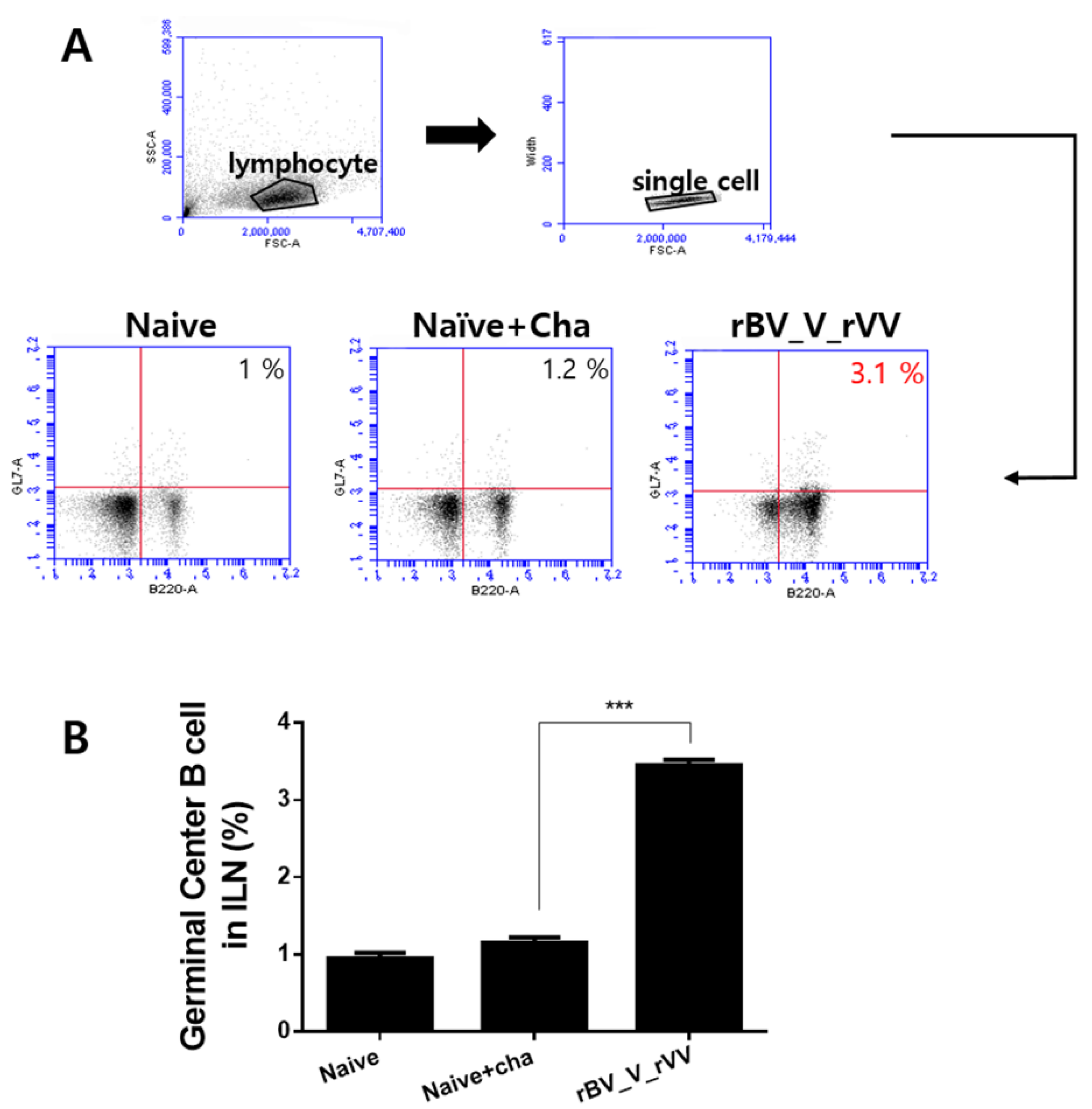
3.4. Potent Germinal Center B Cell Responses Are Induced in Inguinal Lymph Node
3.5. Immunizations with rBV_V_rVV Induced Potent CD8+, but Not CD4+ T Cell Responses in the Blood
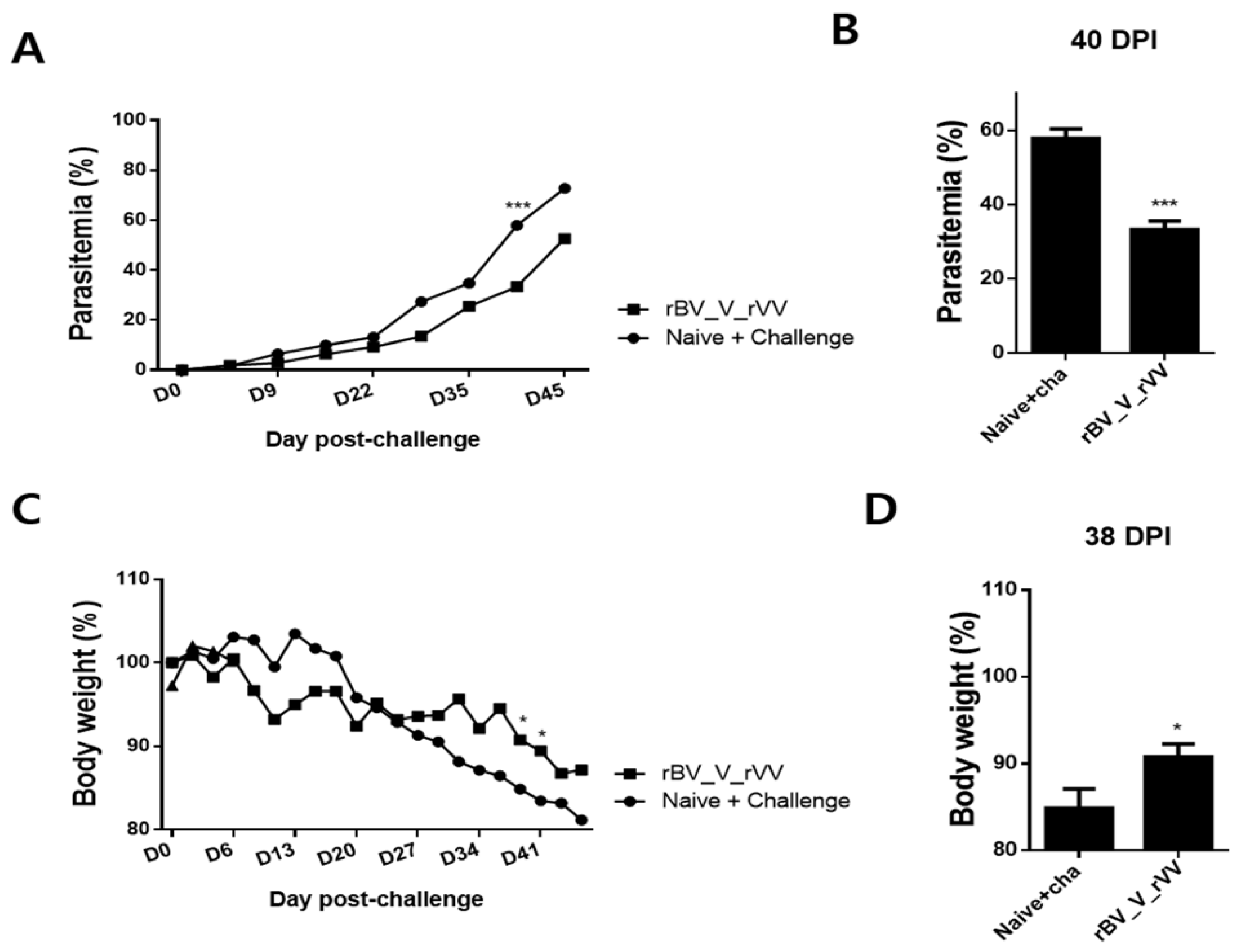
3.6. Immunizations with rBV_V_rVV Lessened Parasitemia and Bodyweight Reduction in Immunized Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. World Malaria Report 2019. Available online: https://www.who.int/publications/i/item/9789241565721 (accessed on 6 July 2021).
- RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet 2015, 386, 31–45. [Google Scholar] [CrossRef]
- Agnandji, S.T.; Lell, B.; Soulanoudjingar, S.S.; Fernandes, J.F.; Abossolo, B.P.; Conzelmann, C.; Methogo, B.G.; Doucka, Y.; Flamen, A.; Mordmüller, B.; et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N. Engl. J. Med. 2011, 365, 1863–1875. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, S.; Gilbert, S.C. Recombinant modified vaccinia virus Ankara-based malaria vaccines. Expert Rev. Vaccines 2016, 15, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Malkin, E.; Hu, J.; Li, Z.; Chen, Z.; Bi, X.; Reed, Z.; Dubovsky, F.; Liu, J.; Wang, Q.; Pan, X.; et al. A phase 1 trial of PfCP2.9: An AMA1/MSP1 chimeric recombinant protein vaccine for Plasmodium falciparum malaria. Vaccine 2008, 26, 6864–6873. [Google Scholar] [CrossRef]
- Biswas, S.; Dicks, M.D.; Long, C.A.; Remarque, E.J.; Siani, L.; Colloca, S.; Cottingham, M.G.; Holder, A.A.; Gilbert, S.C.; Hill, A.V.; et al. Transgene optimization, immunogenicity and in vitro efficacy of viral vectored vaccines expressing two alleles of Plasmodium falciparum AMA1. PLoS ONE 2011, 6, e20977. [Google Scholar] [CrossRef]
- Sheehy, S.H.; Duncan, C.J.; Elias, S.C.; Biswas, S.; Collins, K.A.; O’Hara, G.A.; Halstead, F.D.; Ewer, K.J.; Mahungu, T.; Spencer, A.J.; et al. Phase Ia clinical evaluation of the safety and immunogenicity of the Plasmodium falciparum blood-stage antigen AMA1 in ChAd63 and MVA vaccine vectors. PLoS ONE 2012, 7, e31208. [Google Scholar] [CrossRef]
- Biswas, S.; Spencer, A.J.; Forbes, E.K.; Gilbert, S.C.; Holder, A.A.; Hill, A.V.; Draper, S.J. Recombinant viral-vectored vaccines expressing Plasmodium chabaudi AS apical membrane antigen 1: Mechanisms of vaccine-induced blood-stage protection. J. Immunol. 2012, 188, 5041–5053. [Google Scholar] [CrossRef]
- Bouillet, L.; Dias, M.O.; Dorigo, N.A.; Moura, A.D.; Russell, B.; Nosten, F.; Renia, L.; Braga, E.M.; Gazzinelli, R.T.; Rodrigues, M.M.; et al. Long-term humoral and cellular immune responses elicited by a heterologous Plasmodium vivax apical membrane antigen 1 protein prime/adenovirus boost immunization protocol. Infect. Immun. 2011, 79, 3642–3652. [Google Scholar] [CrossRef]
- Jiang, G.; Shi, M.; Conteh, S.; Richie, N.; Banania, G.; Geneshan, H.; Valencia, A.; Singh, P.; Aguiar, J.; Limbach, K.; et al. Sterile protection against Plasmodium knowlesi in rhesus monkeys from a malaria vaccine: Comparison of heterologous prime boost strategies. PLoS ONE 2009, 4, e6559. [Google Scholar] [CrossRef]
- McElrath, M.J.; Corey, L.; Berger, D.; Hoffman, M.C.; Klucking, S.; Dragavon, J.; Peterson, E.; Greenberg, P.D. Immune responses elicited by recombinant vaccinia-human immunodeficiency virus (HIV) envelope and HIV envelope protein: Analysis of the durability of responses and effect of repeated boosting. J. Infect. Dis. 1994, 169, 41–47. [Google Scholar] [CrossRef]
- Graham, B.S.; Matthews, T.J.; Belshe, R.B.; Clements, M.L.; Dolin, R.; Wright, P.F.; Gorse, G.J.; Schwartz, D.H.; Keefer, M.C.; Bolognesi, D.P.; et al. Augmentation of human immunodeficiency virus type 1 neutralizing antibody by priming with gp160 recombinant vaccinia and boosting with rgp160 in vaccinia-naive adults. J. Infect. Dis. 1993, 167, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Gorse, G.J.; Frey, S.E.; Patel, G.; Newman, F.K.; Belshe, R.B. Vaccine-induced antibodies to native and recombinant human immunodeficiency virus type 1 envelope glycoproteins. Vaccine 1994, 12, 912–918. [Google Scholar] [CrossRef]
- Lee, D.H.; Chu, K.B.; Kang, H.J.; Lee, S.H.; Chopra, M.; Choi, H.J.; Moon, E.K.; Inn, K.S.; Quan, F.S. Protection induced by malaria virus-like particles containing codon-optimized AMA-1 of Plasmodium berghei. Malar. J. 2019, 18, 394. [Google Scholar] [CrossRef]
- Fonager, J.; Pasini, E.M.; Braks, J.A.; Klop, O.; Ramesar, J.; Remarque, E.J.; Vroegrijk, I.O.; van Duinen, S.G.; Thomas, A.W.; Khan, S.M.; et al. Reduced CD36-dependent tissue sequestration of Plasmodium-infected erythrocytes is detrimental to malaria parasite growth in vivo. J. Exp. Med. 2012, 209, 93–107. [Google Scholar] [CrossRef]
- Goodman, A.L.; Forbes, E.K.; Williams, A.R.; Douglas, A.D.; de Cassan, S.C.; Bauza, K.; Biswas, S.; Dicks, M.D.; Llewellyn, D.; Moore, A.C.; et al. The utility of Plasmodium berghei as a rodent model for anti-merozoite malaria vaccine assessment. Sci. Rep. 2013, 3, 1706. [Google Scholar] [CrossRef]
- Fairlie-Clarke, K.J.; Lamb, T.J.; Langhorne, J.; Graham, A.L.; Allen, J.E. Antibody isotype analysis of malaria-nematode co-infection: Problems and solutions associated with cross-reactivity. BMC Immunol. 2010, 11, 6. [Google Scholar] [CrossRef]
- Basak, S.; Kang, H.J.; Lee, S.H.; Chu, K.B.; Moon, E.K.; Quan, F.S. Influenza vaccine efficacy induced by orally administered recombinant baculoviruses. PLoS ONE 2020, 15, e0233520. [Google Scholar] [CrossRef]
- Kang, H.J.; Chu, K.B.; Kim, M.J.; Lee, S.H.; Park, H.; Jin, H.; Moon, E.K.; Quan, F.S. Protective immunity induced by CpG ODN-adjuvanted virus-like particles containing Toxoplasma gondii proteins. Parasite Immunol. 2021, 43, e12799. [Google Scholar] [CrossRef]
- Somsak, V.; Srichairatanakool, S.; Yuthavong, Y.; Kamchonwongpaisan, S.; Uthaipibull, C. Flow cytometric enumeration of Plasmodium berghei-infected red blood cells stained with SYBR Green I. Acta Trop. 2012, 122, 113–118. [Google Scholar] [CrossRef]
- Pattinson, D.J.; Apte, S.H.; Wibowo, N.; Chuan, Y.P.; Rivera-Hernandez, T.; Groves, P.L.; Lua, L.H.; Middelberg, A.P.J.; Doolan, D.L. Chimeric Murine Polyomavirus Virus-Like Particles Induce Plasmodium Antigen-Specific CD8(+) T Cell and Antibody Responses. Front. Cell. Infect. Microbiol. 2019, 9, 215. [Google Scholar] [CrossRef]
- Tiono, A.B.; Nébié, I.; Anagnostou, N.; Coulibaly, A.S.; Bowyer, G.; Lam, E.; Bougouma, E.C.; Ouedraogo, A.; Yaro, J.B.B.; Barry, A.; et al. First field efficacy trial of the ChAd63 MVA ME-TRAP vectored malaria vaccine candidate in 5–17 months old infants and children. PLoS ONE 2018, 13, e0208328. [Google Scholar] [CrossRef]
- Alves, E.; Salman, A.M.; Leoratti, F.; Lopez-Camacho, C.; Viveros-Sandoval, M.E.; Lall, A.; El-Turabi, A.; Bachmann, M.F.; Hill, A.V.; Janse, C.J.; et al. Evaluation of Plasmodium vivax Cell-Traversal Protein for Ookinetes and Sporozoites as a Preerythrocytic P. vivax Vaccine. Clin. Vaccine Immunol. 2017, 24, e00501-16. [Google Scholar] [CrossRef]
- Salman, A.M.; Montoya-Díaz, E.; West, H.; Lall, A.; Atcheson, E.; Lopez-Camacho, C.; Ramesar, J.; Bauza, K.; Collins, K.A.; Brod, F.; et al. Rational development of a protective P. vivax vaccine evaluated with transgenic rodent parasite challenge models. Sci. Rep. 2017, 7, 46482. [Google Scholar] [CrossRef]
- Dontfraid, F.; Cochran, M.A.; Pombo, D.; Knell, J.D.; Quakyi, I.A.; Kumar, S.; Houghten, R.A.; Berzofsky, J.A.; Miller, L.H.; Good, M.F. Human and murine CD4 T cell epitopes map to the same region of the malaria circumsporozoite protein: Limited immunogenicity of sporozoites and circumsporozoite protein. Mol. Biol. Med. 1988, 5, 185–196. [Google Scholar]
- Anders, R.F.; Crewther, P.E.; Edwards, S.; Margetts, M.; Matthew, M.L.; Pollock, B.; Pye, D. Immunisation with recombinant AMA-1 protects mice against infection with Plasmodium chabaudi. Vaccine 1998, 16, 240–247. [Google Scholar] [CrossRef]
- Strauss, R.; Hüser, A.; Ni, S.; Tuve, S.; Kiviat, N.; Sow, P.S.; Hofmann, C.; Lieber, A. Baculovirus-based vaccination vectors allow for efficient induction of immune responses against plasmodium falciparum circumsporozoite protein. Mol. Ther. 2007, 15, 193–202. [Google Scholar] [CrossRef]
- Yoshida, S.; Kondoh, D.; Arai, E.; Matsuoka, H.; Seki, C.; Tanaka, T.; Okada, M.; Ishii, A. Baculovirus virions displaying Plasmodium berghei circumsporozoite protein protect mice against malaria sporozoite infection. Virology 2003, 316, 161–170. [Google Scholar] [CrossRef][Green Version]
- Mizutani, M.; Iyori, M.; Blagborough, A.M.; Fukumoto, S.; Funatsu, T.; Sinden, R.E.; Yoshida, S. Baculovirus-vectored multistage Plasmodium vivax vaccine induces both protective and transmission-blocking immunities against transgenic rodent malaria parasites. Infect. Immun. 2014, 82, 4348–4357. [Google Scholar] [CrossRef]
- Iyori, M.; Yamamoto, D.S.; Sakaguchi, M.; Mizutani, M.; Ogata, S.; Nishiura, H.; Tamura, T.; Matsuoka, H.; Yoshida, S. DAF-shielded baculovirus-vectored vaccine enhances protection against malaria sporozoite challenge in mice. Malar. J. 2017, 16, 390. [Google Scholar] [CrossRef]
- Iyori, M.; Blagborough, A.M.; Sala, K.A.; Nishiura, H.; Takagi, K.; Yoshida, S. Protective efficacy of an IL-12-expressing baculoviral malaria vaccine. Parasite Immunol. 2017, 39, e12498. [Google Scholar] [CrossRef]
- Yoshida, K.; Iyori, M.; Blagborough, A.M.; Salman, A.M.; Dulal, P.; Sala, K.A.; Yamamoto, D.S.; Khan, S.M.; Janse, C.J.; Biswas, S.; et al. Adenovirus-prime and baculovirus-boost heterologous immunization achieves sterile protection against malaria sporozoite challenge in a murine model. Sci. Rep. 2018, 8, 3896. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.Y.; Lin, S.Y.; Lo, K.W.; Lu, C.H.; Hung, C.L.; Chen, C.Y.; Chang, C.C.; Hu, Y.C. Adaptive immune responses elicited by baculovirus and impacts on subsequent transgene expression in vivo. J. Virol. 2013, 87, 4965–4973. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, A.K.; Lipińska, A.D.; Rohde, J.; Szewczyk, B.; Bienkowska-Szewczyk, K.; Rziha, H.J. New baculovirus recombinants expressing Pseudorabies virus (PRV) glycoproteins protect mice against lethal challenge infection. Vaccine 2009, 27, 3584–3591. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Liu, H.J.; Tsai, C.P.; Chung, C.Y.; Shih, Y.S.; Chang, P.C.; Chiu, Y.T.; Hu, Y.C. Baculovirus as an avian influenza vaccine vector: Differential immune responses elicited by different vector forms. Vaccine 2010, 28, 7644–7651. [Google Scholar] [CrossRef]
- Kurtovic, L.; Boyle, M.J.; Opi, D.H.; Kennedy, A.T.; Tham, W.H.; Reiling, L.; Chan, J.A.; Beeson, J.G. Complement in malaria immunity and vaccines. Immunol. Rev. 2020, 293, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Ong, G.L.; Mattes, M.J. Mouse strains with typical mammalian levels of complement activity. J. Immunol. Methods 1989, 125, 147–158. [Google Scholar] [CrossRef]
- Schwarz, K.; Meijerink, E.; Speiser, D.E.; Tissot, A.C.; Cielens, I.; Renhof, R.; Dishlers, A.; Pumpens, P.; Bachmann, M.F. Efficient homologous prime-boost strategies for T cell vaccination based on virus-like particles. Eur. J. Immunol. 2005, 35, 816–821. [Google Scholar] [CrossRef]
- Lee, S.H.; Chu, K.B.; Kang, H.J.; Basak, S.; Kim, M.J.; Park, H.; Jin, H.; Moon, E.K.; Quan, F.S. Virus-like particles expressing Plasmodium berghei MSP-8 induce protection against P. berghei infection. Parasite Immunol. 2020, 42, e12781. [Google Scholar] [CrossRef]
- Lee, S.H.; Kang, H.J.; Chu, K.B.; Basak, S.; Lee, D.H.; Moon, E.K.; Quan, F.S. Protective Immunity Induced by Virus-Like Particle Containing Merozoite Surface Protein 9 of Plasmodium berghei. Vaccines 2020, 8, 428. [Google Scholar] [CrossRef]
- Todryk, S.M.; Walther, M. Building better T-cell-inducing malaria vaccines. Immunology 2005, 115, 163–169. [Google Scholar] [CrossRef]
- Iacono-Connors, L.C.; Welkos, S.L.; Ivins, B.E.; Dalrymple, J.M. Protection against anthrax with recombinant virus-expressed protective antigen in experimental animals. Infect. Immun. 1991, 59, 1961–1965. [Google Scholar] [CrossRef]
- de Freitas, L.F.D.; Oliveira, R.P.; Miranda, M.C.G.; Rocha, R.P.; Barbosa-Stancioli, E.F.; Faria, A.M.C.; da Fonseca, F.G. The Virulence of Different Vaccinia Virus Strains Is Directly Proportional to Their Ability To Downmodulate Specific Cell-Mediated Immune Compartments In Vivo. J. Virol. 2019, 93, e02191-18. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-J.; Chu, K.-B.; Kang, H.-J.; Yoon, K.-W.; Eom, G.-D.; Mao, J.; Lee, S.-H.; Subbiah, J.; Kang, S.-M.; Moon, E.-K.; et al. Protective Immunity Induced by Immunization with Baculovirus, Virus-like Particle, and Vaccinia Virus Expressing the AMA1 of Plasmodium berghei. Biomedicines 2022, 10, 2289. https://doi.org/10.3390/biomedicines10092289
Kim M-J, Chu K-B, Kang H-J, Yoon K-W, Eom G-D, Mao J, Lee S-H, Subbiah J, Kang S-M, Moon E-K, et al. Protective Immunity Induced by Immunization with Baculovirus, Virus-like Particle, and Vaccinia Virus Expressing the AMA1 of Plasmodium berghei. Biomedicines. 2022; 10(9):2289. https://doi.org/10.3390/biomedicines10092289
Chicago/Turabian StyleKim, Min-Ju, Ki-Back Chu, Hae-Ji Kang, Keon-Woong Yoon, Gi-Deok Eom, Jie Mao, Su-Hwa Lee, Jeeva Subbiah, Sang-Moo Kang, Eun-Kyung Moon, and et al. 2022. "Protective Immunity Induced by Immunization with Baculovirus, Virus-like Particle, and Vaccinia Virus Expressing the AMA1 of Plasmodium berghei" Biomedicines 10, no. 9: 2289. https://doi.org/10.3390/biomedicines10092289
APA StyleKim, M.-J., Chu, K.-B., Kang, H.-J., Yoon, K.-W., Eom, G.-D., Mao, J., Lee, S.-H., Subbiah, J., Kang, S.-M., Moon, E.-K., & Quan, F.-S. (2022). Protective Immunity Induced by Immunization with Baculovirus, Virus-like Particle, and Vaccinia Virus Expressing the AMA1 of Plasmodium berghei. Biomedicines, 10(9), 2289. https://doi.org/10.3390/biomedicines10092289





