Saturated Fatty Acid Blood Levels and Cardiometabolic Phenotype in Patients with HFpEF: A Secondary Analysis of the Aldo-DHF Trial
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Aldo-DHF Trial
2.2.1. Laboratory Measurements
2.2.2. Echocardiography and Other Variables
2.3. Ethics
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Long Chain Fatty Acids
3.2.1. Myristic Acid (MA, C14:0)
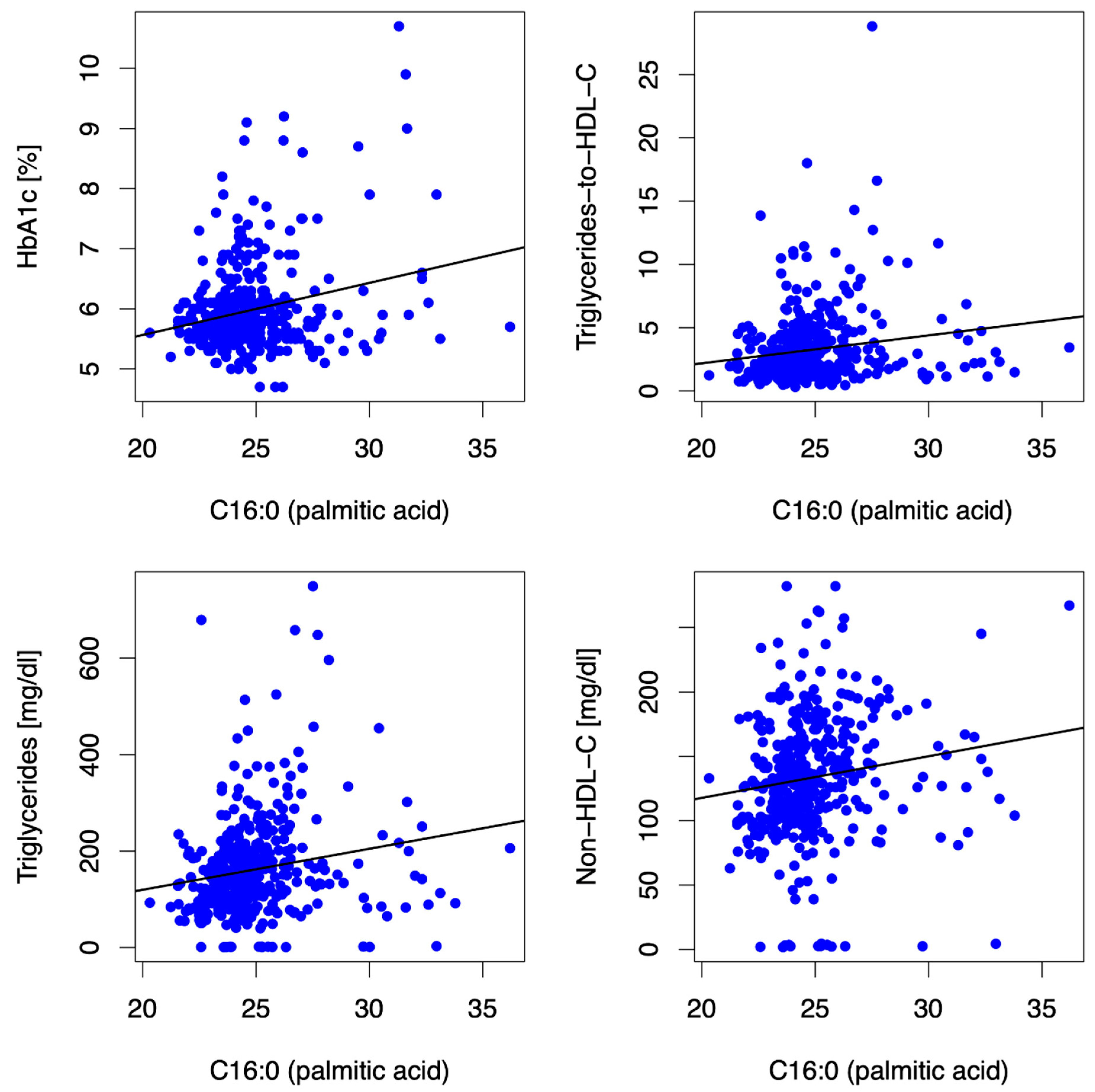
3.2.2. Palmitic Acid (PA, C16:0)
3.2.3. Stearic Acid (SA, C18:0)
3.3. Very Long Chain Fatty Acids
3.3.1. Arachidic Acid (AA, C20:0)
3.3.2. Behenic Acid (C22:0)
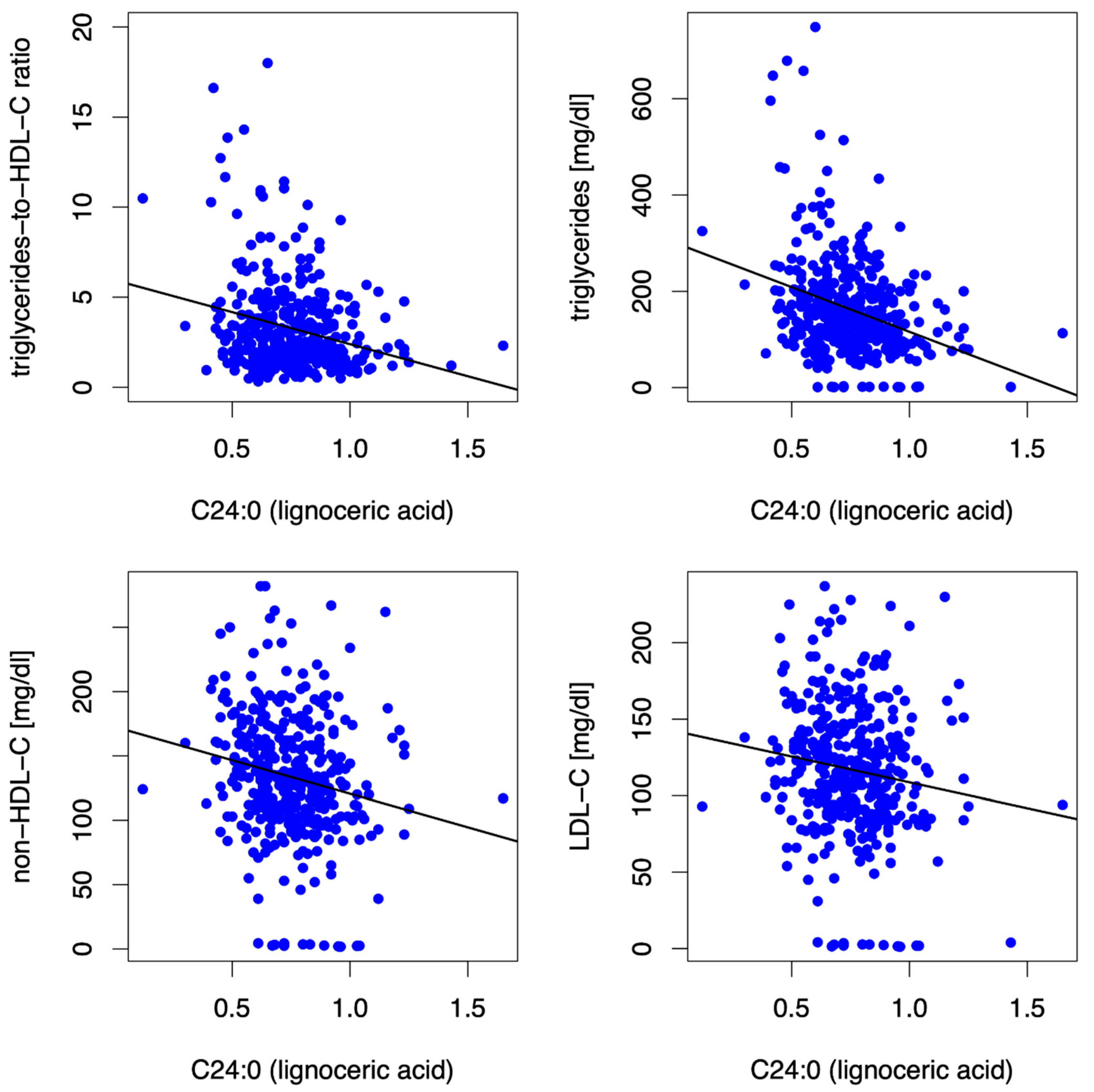
3.3.3. Lignoceric Acid (C24:0)
3.4. Sensitivity Analyses
3.5. Sex-Specific Analyses
- HbA1c: In women, but neither in men nor in the entire cohort, the long-chain SFA C14:0 (MA, C14:0) was consistently directly associated with HbA1c at baseline (β = 0.48, p = 0.004) and at 12 mFU (β = 0.41, p = 0.009).
- Lipid phenotype: In women (and in the entire cohort, as described above), but not in men, the long-chain SFA stearic acid (SA, C18:0) was inversely associated with non-HDL-C (β = −9.11, p = 0.001) and LDL-C (β = −8.52, p = <0.001) at baseline and at 12 mFU (non-HDL-C (β = −9.33, p = 0.001), LDL-C (β = −7.66, p = 0.001)).
- Submaximal aerobic capacity: The very long-chain SFA lignoceric acid (C24:0) was predictive of higher distance covered in 6MWT at 12 mFU in the entire cohort (β = 64.86, p = 0.019) and in men (β = 91.55, p = 0.014) but not in women.
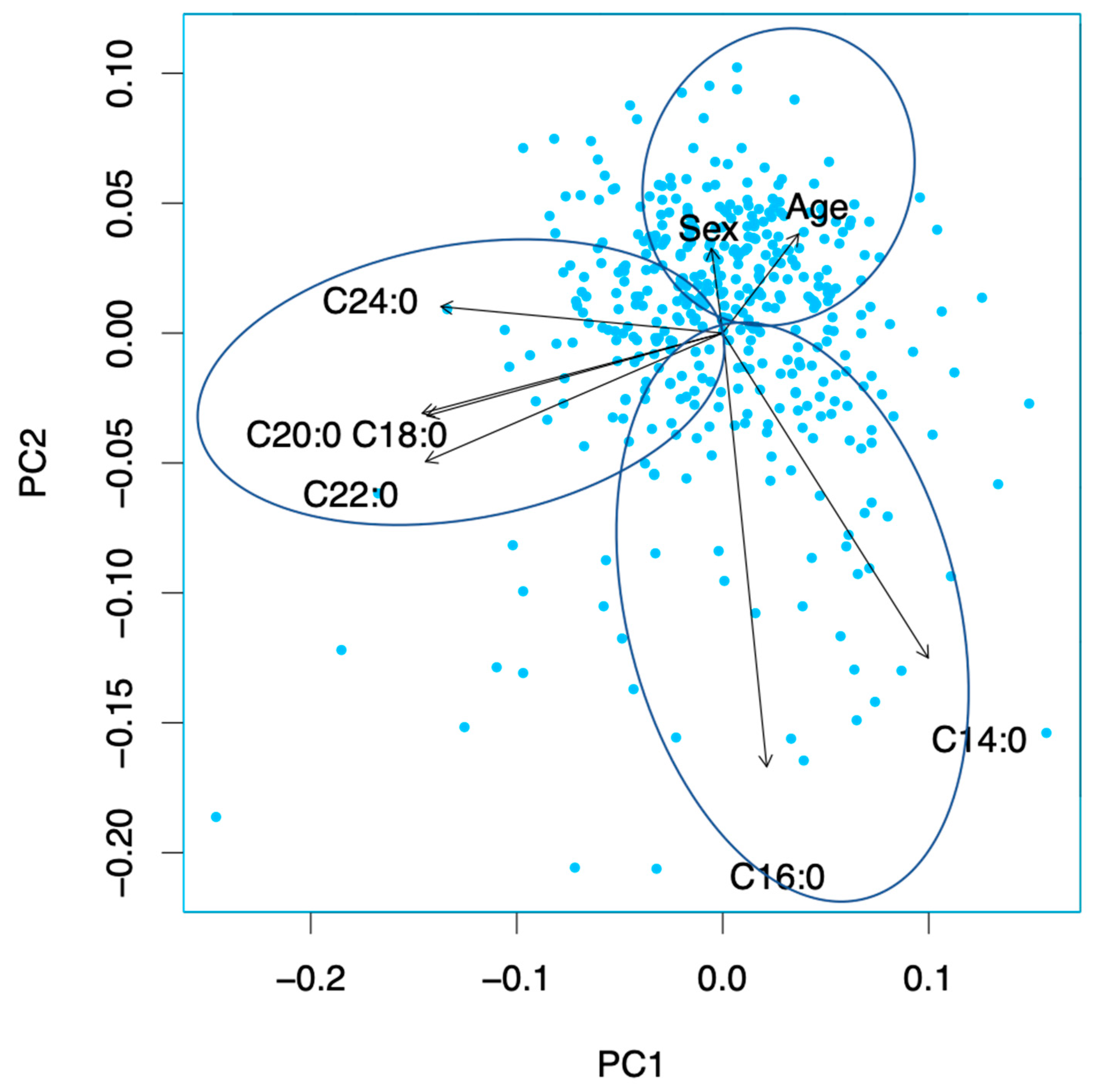
3.6. Principal Component Analysis
4. Discussion
4.1. Main Findings
4.2. Individual SFAs and Patient Characteristics
4.2.1. SFA Conglomeration 1 (C14:0 and C16:0)
4.2.2. SFA Conglomeration 2 (C18:0 and Very Long-Chain Saturated Fatty Acids)
5. Strengths and Limitations
6. Translational Outlook
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA–PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Kitzman, D.W.; Borlaug, B.A.; van Heerebeek, L.; Zile, M.R.; Kass, D.A.; Paulus, W.J. Phenotype-Specific Treatment of Heart Failure with Preserved Ejection Fraction. Circulation 2016, 134, 73. [Google Scholar] [CrossRef] [PubMed]
- Seferović, P.M.; Petrie, M.C.; Filippatos, G.S.; Anker, S.D.; Rosano, G.; Bauersachs, J.; Paulus, W.J.; Komajda, M.; Cosentino, F.; de Boer, R.A.; et al. Type 2 diabetes mellitus and heart failure: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 853–872. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Gheorghiade, M. Heart failure with preserved ejection fraction: Treat now by treating comorbidities. JAMA 2008, 300, 431–433. [Google Scholar]
- Astrup, A.; Magkos, F.; Bier, D.M.; Brenna, J.T.; de Oliveira Otto, M.C.; Hill, J.O.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; et al. Saturated Fats and Health: A Reassessment and Proposal for Food-Based Recommendations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 844–857. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. Flavonoids, Dairy Foods, and Cardiovascular and Metabolic Health: A Review of Emerging Biologic Pathways. Circ. Res. 2018, 122, 369–384. [Google Scholar] [CrossRef]
- Lechner, K.; McKenzie, A.L.; Kränkel, N.; Von Schacky, C.; Worm, N.; Nixdorff, U.; Lechner, B.; Scherr, J.; Weingärtner, O.; Krauss, R.M. High-Risk Atherosclerosis and Metabolic Phenotype: The Roles of Ectopic Adiposity, Atherogenic Dyslipidemia, and Inflammation. Metab. Syndr. Relat. Disord. 2020, 18, 176–185. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef]
- Lechner, K.; von Schacky, C.; McKenzie, A.L.; Worm, N.; Nixdorff, U.; Lechner, B.; Kränkel, N.; Halle, M.; Krauss, R.M.; Scherr, J. Lifestyle factors and high-risk atherosclerosis: Pathways and mechanisms beyond traditional risk factors. Eur. J. Prev. Cardiol. 2020, 27, 394–406. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; McKnight, B.; Sotoodehnia, N.; Fretts, A.M.; Qureshi, W.T.; Song, X.; King, I.B.; Sitlani, C.M.; Siscovick, D.S.; Psaty, B.M.; et al. Circulating Very Long-Chain Saturated Fatty Acids and Heart Failure: The Cardiovascular Health Study. J. Am. Heart Assoc. 2018, 7, e010019. [Google Scholar] [CrossRef]
- Matsumoto, C.; Hanson, N.Q.; Tsai, M.Y.; Glynn, R.J.; Gaziano, J.M.; Djoussé, L. Plasma phospholipid saturated fatty acids and heart failure risk in the Physicians’ Health Study. Clin. Nutr. 2013, 32, 819–823. [Google Scholar] [CrossRef]
- Yamagishi, K.; Nettleton, J.A.; Folsom, A.R. Plasma fatty acid composition and incident heart failure in middle-aged adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am. Heart J. 2008, 156, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, R.N.; King, I.B. Very long-chain saturated fatty acids and diabetes and cardiovascular disease. Curr. Opin. Lipidol. 2022, 33, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vázquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef]
- Kleber, M.E.; Delgado, G.E.; Dawczynski, C.; Lorkowski, S.; März, W.; von Schacky, C. Saturated fatty acids and mortality in patients referred for coronary angiography-The Ludwigshafen Risk and Cardiovascular Health study. J. Clin. Lipidol. 2018, 12, 455–463.e3. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, W.; Santaren, I.D.; Hanley, A.J.; Watkins, S.M.; Lorenzo, C.; Wagenknecht, L.E. Risk of diabetes associated with fatty acids in the de novo lipogenesis pathway is independent of insulin sensitivity and response: The Insulin Resistance Atherosclerosis Study (IRAS). BMJ Open Diabetes Res. Care 2019, 7, e000691. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lai, H.T.M.; de Oliveira Otto, M.C.; Lemaitre, R.N.; McKnight, B.; King, I.B.; Song, X.; Huggins, G.S.; Vest, A.R.; Siscovick, D.S.; et al. Serial Biomarkers of De Novo Lipogenesis Fatty Acids and Incident Heart Failure in Older Adults: The Cardiovascular Health Study. J. Am. Heart Assoc. 2020, 9, e014119. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Sands, S.A.; Windsor, S.L.; Ali, H.A.; Stevens, T.L.; Magalski, A.; Porter, C.B.; Borkon, A.M. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: Correlation with erythrocytes and response to supplementation. Circulation 2004, 110, 1645–1649. [Google Scholar] [CrossRef]
- Harris, W.S.; Thomas, R.M. Biological variability of blood omega-3 biomarkers. Clin. Biochem. 2010, 43, 338–340. [Google Scholar] [CrossRef]
- Archer, E.; Hand, G.A.; Blair, S.N. Validity of U.S. nutritional surveillance: National Health and Nutrition Examination Survey caloric energy intake data, 1971–2010. PLoS ONE 2013, 8, e76632. [Google Scholar] [CrossRef]
- Edelmann, F.; Wachter, R.; Schmidt, A.G.; Kraigher-Krainer, E.; Colantonio, C.; Kamke, W.; Duvinage, A.; Stahrenberg, R.; Durstewitz, K.; Löffler, M.; et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: The Aldo-DHF randomized controlled trial. JAMA 2013, 309, 781–791. [Google Scholar] [CrossRef] [PubMed]
- von Schacky, C. The Omega-3 Index as a risk factor for cardiovascular diseases. Prostaglandins Other Lipid Mediat. 2011, 96, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Lechner, K.; Scherr, J.; Lorenz, E.; Lechner, B.; Haller, B.; Krannich, A.; Halle, M.; Wachter, R.; Duvinage, A.; Edelmann, F. Omega-3 fatty acid blood levels are inversely associated with cardiometabolic risk factors in HFpEF patients: The Aldo-DHF randomized controlled trial. Clin. Res. Cardiol. 2021, 111, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Cheitlin, M.D.; Armstrong, W.F.; Aurigemma, G.P.; Beller, G.A.; Bierman, F.Z.; Davis, J.L.; Douglas, P.S.; Faxon, D.P.; Gillam, L.D.; Kimball, T.R.; et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: Summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation 2003, 108, 1146–1162. [Google Scholar] [PubMed]
- Berliner, D.; Mattern, S.; Wellige, M.; Malsch, C.; Güder, G.; Brenner, S.; Morbach, C.; Deubner, N.; Breunig, M.; Kiefl, R.; et al. The omega-3 index in patients with heart failure: A prospective cohort study. Prostaglandins Leukot. Essent. Fat. Acids 2019, 140, 34–41. [Google Scholar]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2019, 41, 111–188. [Google Scholar] [CrossRef]
- Lechner, K.; Halle, M. Are Atherogenic Lipoprotein Phenotype and Inflammation Indicative of Plaque Phenotype and Clinical Stability in Coronary Artery Disease? JAMA Cardiol. 2019, 4, 950–951. [Google Scholar] [CrossRef]
- Vergallo, R.; Porto, I.; Crea, F. Are Atherogenic Lipoprotein Phenotype and Inflammation Indicative of Plaque Phenotype and Clinical Stability in Coronary Artery Disease?-Reply. JAMA Cardiol. 2019, 4, 951–952. [Google Scholar] [CrossRef]
- Vergallo, R.; Porto, I.; D’Amario, D.; Annibali, G.; Galli, M.; Benenati, S.; Bendandi, F.; Migliaro, S.; Fracassi, F.; Aurigemma, C.; et al. Coronary Atherosclerotic Phenotype and Plaque Healing in Patients With Recurrent Acute Coronary Syndromes Compared With Patients With Long-term Clinical Stability: An In Vivo Optical Coherence Tomography Study. JAMA Cardiol. 2019, 4, 321–329. [Google Scholar]
- Lechner, K.; Lechner, B.; Crispin, A.; Schwarz, P.E.H.; von Bibra, H. Waist-to-height ratio and metabolic phenotype compared to the Matsuda index for the prediction of insulin resistance. Sci. Rep. 2021, 11, 8224. [Google Scholar] [CrossRef]
- Banderas, D.Z.; Escobedo, J.; Gonzalez, E.; Liceaga, M.G.; Ramírez, J.C.; Castro, M.G. γ-Glutamyl transferase: A marker of nonalcoholic fatty liver disease in patients with the metabolic syndrome. Eur. J. Gastroenterol. Hepatol. 2012, 24, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Jang, C.; Liu, J.; Uehara, K.; Gilbert, M.; Izzo, L.; Zeng, X.; Trefely, S.; Fernandez, S.; Carrer, A.; et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature 2020, 579, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.E.; Xanthakis, V.; Moore, L.L.; Vasan, R.S.; Jacques, P.F. Cumulative sugar-sweetened beverage consumption is associated with higher concentrations of circulating ceramides in the Framingham Offspring Cohort. Am. J. Clin. Nutr. 2020, 111, 420–428. [Google Scholar] [CrossRef]
- Al-Mrabeh, A.; Zhyzhneuskaya, S.V.; Peters, C.; Barnes, A.C.; Melhem, S.; Jesuthasan, A.; Aribisala, B.; Hollingsworth, K.G.; Lietz, G.; Mathers, J.C.; et al. Hepatic Lipoprotein Export and Remission of Human Type 2 Diabetes after Weight Loss. Cell Metab. 2020, 31, 233–249.e4. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.H.; Kim, J.A.; Lee, J.Y. Mechanisms for the activation of Toll-like receptor 2/4 by saturated fatty acids and inhibition by docosahexaenoic acid. Eur. J. Pharm. 2016, 785, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Zewinger, S.; Reiser, J.; Jankowski, V.; Alansary, D.; Hahm, E.; Triem, S.; Klug, M.; Schunk, S.J.; Schmit, D.; Kramann, R.; et al. Apolipoprotein C3 induces inflammation and organ damage by alternative inflammasome activation. Nat. Immunol. 2020, 21, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, O.; Speziali, G.; Castagna, A.; Pattini, P.; Udali, S.; Pizzolo, F.; Liesinger, L.; Gindlhuber, J.; Tomin, T.; Schittmayer, M.; et al. The Positive Association between Plasma Myristic Acid and ApoCIII Concentrations in Cardiovascular Disease Patients Is Supported by the Effects of Myristic Acid in HepG2 Cells. J. Nutr. 2020, 150, 2707–2715. [Google Scholar] [CrossRef]
- Mayneris-Perxachs, J.; Sala-Vila, A.; Chisaguano, M.; Castellote, A.I.; Estruch, R.; Covas, M.I.; Fitó, M.; Salas-Salvadó, J.; Martínez-González, M.A.; Lamuela-Raventós, R.; et al. Effects of 1-year intervention with a Mediterranean diet on plasma fatty acid composition and metabolic syndrome in a population at high cardiovascular risk. PLoS ONE 2014, 9, e85202. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X.; Li, X.; Chu, Q.; Zhou, Y.; Li, Z.; Zhang, H.; Brenna, T.J.; Song, Y.; Gao, Y. Associations of plasma very-long-chain SFA and the metabolic syndrome in adults. Br. J. Nutr. 2018, 120, 855–862. [Google Scholar] [CrossRef]
- Prada, M.; Wittenbecher, C.; Eichelmann, F.; Wernitz, A.; Drouin-Chartier, J.P.; Schulze, M.B. Association of the odd-chain fatty acid content in lipid groups with type 2 diabetes risk: A targeted analysis of lipidomics data in the EPIC-Potsdam cohort. Clin. Nutr. 2021, 40, 4988–4999. [Google Scholar] [CrossRef]

| Characteristics a | Total (n = 404) |
|---|---|
| Demographics | |
| Age, mean (SD), y | 67 (8) |
| Female | 212 (53) |
| Laboratory measures | |
| HbA1c (%) | 6.0 (0.8) |
| LDL-C (mg/dl) | 117 (42) |
| HDL-C (mg/dl) | 56 (18) |
| Triglycerides (mg/dl) | 161 (103) |
| Non-HDL-C (mg/dl) | 133 (47) |
| TG/HDL-C ratio | 3.3 (2.8) |
| NT-proBNP, median (IQR), ng/L | 158 (82–298) |
| C14:0 (myristic acid) (%) | 0.69 (0.26) |
| C16:0 (palmitic acid) (%) | 24.89 (2.17) |
| C18:0 (stearic acid) (%) | 13.87 (1.35) |
| C20:0 (arachidic acid) (%) | 0.18 (0.03) |
| C22:0 (behenic acid) (%) | 0.5 (0.12) |
| C24:0 (lignoceric acid) (%) | 0.75 (0.18) |
| Medical history | |
| Hospitalization for heart failure in past 12 months | 149 (37) |
| Hypertension | 370 (92) |
| Diabetes mellitus | 66 (16) |
| Atrial fibrillation | 65 (16) |
| Physical examination, mean (SD) | |
| Body mass index b | 28.9 (3.6) |
| Waist circumference, (cm) | 98.1 (11.0) |
| In men | 103.7 (9.0) |
| In women | 93.1 (10.3) |
| Waist-to-height ratio | 0.49 (0.1) |
| Systolic blood pressure, mm Hg | 135 (18) |
| Diastolic blood pressure, mm Hg | 79 (11) |
| Heart rate, /min | 66 (11) |
| Signs and symptoms | |
| NYHA functional class | |
| II | 350 (87) |
| III | 54 (13) |
| Peripheral edema | 160 (40) |
| Nocturia | 325 (80) |
| Paroxysmal nocturnal dyspnea | 66 (16) |
| Nocturnal cough | 61 (15) |
| Fatigue | 241 (60) |
| Current medications | |
| ACE inhibitors/angiotensin receptor antagonists | 310 (77) |
| Betablockers | 290 (72) |
| Diuretics | 213 (53) |
| Calcium antagonists | 97 (24) |
| Lipid-lowering drugs | 221 (55) |
| Echocardiography, mean (SD) | |
| LV ejection fraction, % | 68 (8) |
| LV diameter (end diastolic), mm | 46.5 (6.2) |
| LV diameter (end systolic), mm | 25.3 (6.4) |
| LV mass index, g/m2 | 114.15 (45.53) |
| Left atrial volume index, mL/m2 | 43.1 (41.6) |
| E-wave velocity, cm/s | 73 (20) |
| Medial e’ wave velocity, cm/s | 5.9 (1.3) |
| E/e’ | 7.1 (1.5) |
| E/A velocity ratio | 0.91 (0.33) |
| Isovolumic relaxation time, ms | 88 (26) |
| Deceleration time, ms | 243 (63) |
| Grade of diastolic dysfunction, No. (%) | |
| I | 295 (73) |
| II | 81 (20) |
| III | 4 (1) |
| IV | 3 (1) |
| C14:0 | C16:0 | C18:0 | C20:0 | C22:0 | C24:0 | ||
|---|---|---|---|---|---|---|---|
| LDL-C | r § | 0.145 | 0.148 | −0.178 | −0.153 | −0.059 | −0.12 |
| p-value * | 0.005 | 0.004 | 0.001 | 0.003 | 0.255 | 0.021 | |
| non-HDL-C | r § | 0.234 | 0.257 | −0.212 | −0.23 | −0.107 | −0.227 |
| p-value * | <0.001 | <0.001 | <0.001 | <0.001 | 0.039 | <0.001 | |
| triglycerides | r § | 0.428 | 0.299 | −0.155 | −0.407 | −0.232 | −0.283 |
| p-value * | <0.001 | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 | |
| triglycerides-to-HDL-C ratio | r § | 0.393 | 0.239 | −0.097 | −0.373 | −0.19 | −0.15 |
| p-value * | <0.001 | <0.001 | 0.061 | <0.001 | <0.001 | 0.004 | |
| HbA1c | r § | 0.089 | 0.083 | −0.053 | 0.036 | 0.016 | −0.035 |
| p-value * | 0.087 | 0.112 | 0.306 | 0.489 | 0.76 | 0.501 | |
| ASAT | r § | 0.137 | 0.107 | 0.038 | −0.092 | −0.029 | −0.084 |
| p-value * | 0.008 | 0.04 | 0.467 | 0.078 | 0.576 | 0.105 | |
| ALAT | r § | 0.122 | 0.166 | 0.129 | −0.143 | −0.023 | −0.047 |
| p-value * | 0.019 | 0.001 | 0.013 | 0.006 | 0.656 | 0.371 | |
| GGT | r § | 0.095 | 0.163 | −0.027 | −0.206 | −0.091 | −0.102 |
| p-value * | 0.069 | 0.002 | 0.6 | <0.001 | 0.081 | 0.049 | |
| BMI | r § | 0.109 | 0.085 | 0.081 | −0.125 | −0.024 | −0.041 |
| p-value * | 0.036 | 0.101 | 0.119 | 0.016 | 0.651 | 0.435 | |
| waist circumference | r § | 0.08 | 0.036 | 0.024 | −0.159 | −0.098 | 0.078 |
| p-value * | 0.124 | 0.493 | 0.645 | 0.002 | 0.059 | 0.135 | |
| waist-to-height ratio | r § | 0.068 | 0.06 | 0.076 | −0.151 | −0.044 | 0.038 |
| p-value * | 0.191 | 0.246 | 0.146 | 0.004 | 0.394 | 0.462 | |
| distance covered 6MWT | r § | −0.099 | −0.14 | 0.008 | 0.011 | −0.009 | 0.105 |
| p-value * | 0.058 | 0.007 | 0.885 | 0.83 | 0.864 | 0.044 | |
| VO2peak | r § | −0.032 | 0.042 | 0.027 | −0.002 | 0.112 | 0.14 |
| p-value * | 0.538 | 0.422 | 0.608 | 0.968 | 0.031 | 0.007 | |
| E/e‘ | r § | −0.045 | −0.109 | 0.036 | 0.026 | 0.035 | 0.052 |
| p-value * | 0.384 | 0.037 | 0.488 | 0.617 | 0.504 | 0.321 | |
| NT-proBNP | r § | −0.138 | −0.147 | −0.058 | 0.075 | −0.009 | −0.027 |
| p-value * | 0.008 | 0.005 | 0.262 | 0.151 | 0.867 | 0.607 |
| C14:0 | C16:0 | C18:0 | C20:0 | C22:0 | C24:0 | ||
|---|---|---|---|---|---|---|---|
| LDL-C | r § | 0.102 | 0.151 | −0.085 | −0.126 | −0.044 | −0.085 |
| p-value * | 0.059 | 0.005 | 0.116 | 0.02 | 0.421 | 0.118 | |
| non-HDL-C | r § | 0.185 | 0.265 | −0.122 | −0.196 | −0.073 | −0.154 |
| p-value * | 0.001 | <0.001 | 0.024 | <0.001 | 0.181 | 0.004 | |
| triglycerides | r § | 0.291 | 0.306 | −0.172 | −0.32 | −0.153 | −0.196 |
| p-value * | <0.001 | <0.001 | 0.001 | <0.001 | 0.005 | <0.001 | |
| triglycerides-to-HDL-C ratio | r § | 0.285 | 0.269 | −0.115 | −0.292 | −0.109 | −0.106 |
| p-value * | <0.001 | <0.001 | 0.033 | <0.001 | 0.045 | 0.051 | |
| HbA1c | r § | 0.146 | 0.116 | −0.114 | −0.048 | −0.048 | −0.062 |
| p-value* | 0.007 | 0.032 | 0.036 | 0.373 | 0.373 | 0.255 | |
| ASAT | r § | 0.148 | 0.102 | −0.046 | −0.119 | 0.024 | −0.061 |
| p-value * | 0.006 | 0.061 | 0.393 | 0.028 | 0.653 | 0.263 | |
| ALAT | r § | 0.11 | 0.112 | 0.074 | −0.139 | 0.026 | 0.039 |
| p-value * | 0.042 | 0.038 | 0.174 | 0.01 | 0.636 | 0.475 | |
| GGT | r § | 0.095 | 0.161 | 0.033 | −0.133 | −0.025 | −0.014 |
| p-value * | 0.078 | 0.003 | 0.544 | 0.014 | 0.651 | 0.803 | |
| BMI | r § | 0.095 | 0.094 | 0.041 | −0.126 | −0.039 | −0.028 |
| p-value * | 0.081 | 0.084 | 0.451 | 0.02 | 0.469 | 0.603 | |
| waist circumference | r § | 0.061 | 0.1 | 0.046 | −0.114 | −0.07 | 0.103 |
| p-value * | 0.262 | 0.064 | 0.399 | 0.036 | 0.199 | 0.058 | |
| waist-to-height ratio | r § | 0.117 | 0.149 | 0.032 | −0.093 | −0.053 | −0.009 |
| p-value * | 0.031 | 0.006 | 0.557 | 0.086 | 0.327 | 0.866 | |
| distance covered 6MWT | r § | −0.076 | −0.209 | 0.036 | 0.022 | 0.018 | 0.176 |
| p-value * | 0.159 | <0.001 | 0.508 | 0.687 | 0.738 | 0.001 | |
| VO2peak | r § | −0.037 | −0.111 | −0.018 | 0.157 | 0.046 | 0.214 |
| p-value * | 0.494 | 0.041 | 0.735 | 0.004 | 0.396 | <0.001 | |
| E/e‘ | r § | −0.028 | −0.101 | 0.064 | 0.093 | 0.087 | 0.123 |
| p-value * | 0.608 | 0.062 | 0.24 | 0.087 | 0.111 | 0.023 | |
| NT-proBNP | r § | −0.071 | −0.098 | −0.111 | 0.077 | −0.021 | −0.067 |
| p-value * | 0.192 | 0.072 | 0.041 | 0.155 | 0.701 | 0.219 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lechner, K.; von Schacky, C.; Scherr, J.; Lorenz, E.; Bock, M.; Lechner, B.; Haller, B.; Krannich, A.; Halle, M.; Wachter, R.; et al. Saturated Fatty Acid Blood Levels and Cardiometabolic Phenotype in Patients with HFpEF: A Secondary Analysis of the Aldo-DHF Trial. Biomedicines 2022, 10, 2296. https://doi.org/10.3390/biomedicines10092296
Lechner K, von Schacky C, Scherr J, Lorenz E, Bock M, Lechner B, Haller B, Krannich A, Halle M, Wachter R, et al. Saturated Fatty Acid Blood Levels and Cardiometabolic Phenotype in Patients with HFpEF: A Secondary Analysis of the Aldo-DHF Trial. Biomedicines. 2022; 10(9):2296. https://doi.org/10.3390/biomedicines10092296
Chicago/Turabian StyleLechner, Katharina, Clemens von Schacky, Johannes Scherr, Elke Lorenz, Matthias Bock, Benjamin Lechner, Bernhard Haller, Alexander Krannich, Martin Halle, Rolf Wachter, and et al. 2022. "Saturated Fatty Acid Blood Levels and Cardiometabolic Phenotype in Patients with HFpEF: A Secondary Analysis of the Aldo-DHF Trial" Biomedicines 10, no. 9: 2296. https://doi.org/10.3390/biomedicines10092296
APA StyleLechner, K., von Schacky, C., Scherr, J., Lorenz, E., Bock, M., Lechner, B., Haller, B., Krannich, A., Halle, M., Wachter, R., Duvinage, A., & Edelmann, F. (2022). Saturated Fatty Acid Blood Levels and Cardiometabolic Phenotype in Patients with HFpEF: A Secondary Analysis of the Aldo-DHF Trial. Biomedicines, 10(9), 2296. https://doi.org/10.3390/biomedicines10092296






