Atherosclerosis Specific Features in Chronic Kidney Disease (CKD)
Abstract
1. Introduction
2. Chronic Kidney Disease (CKD)
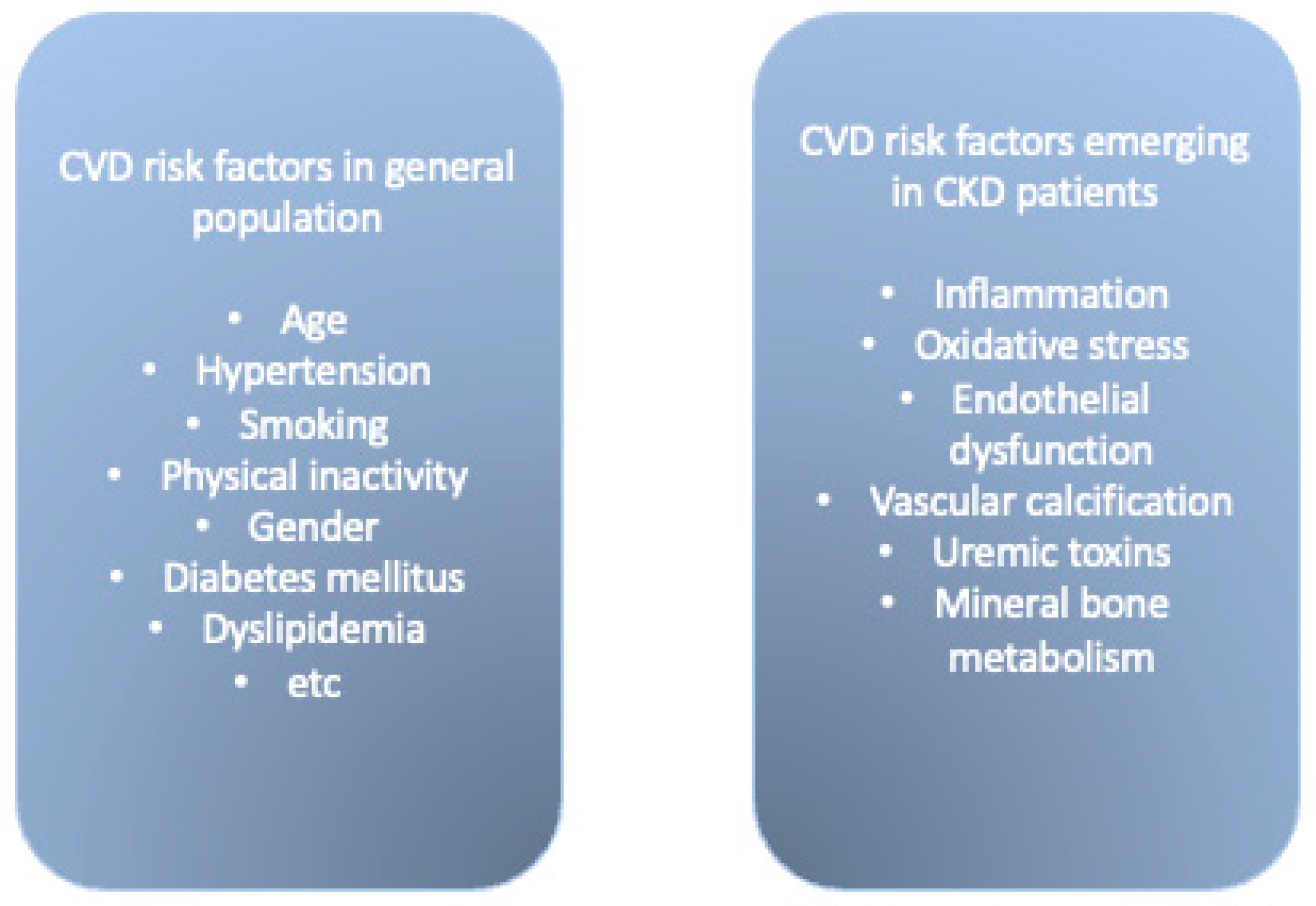
3. Cardiovascular Risk in CKD
3.1. Arterial Hypertension
3.2. Dyslipidaemia
3.3. Mineral Bone Metabolism
3.4. Inflammation
3.5. Proteinuria
4. Cardiovascular Diseases
5. Determining Atherosclerosis in CKD
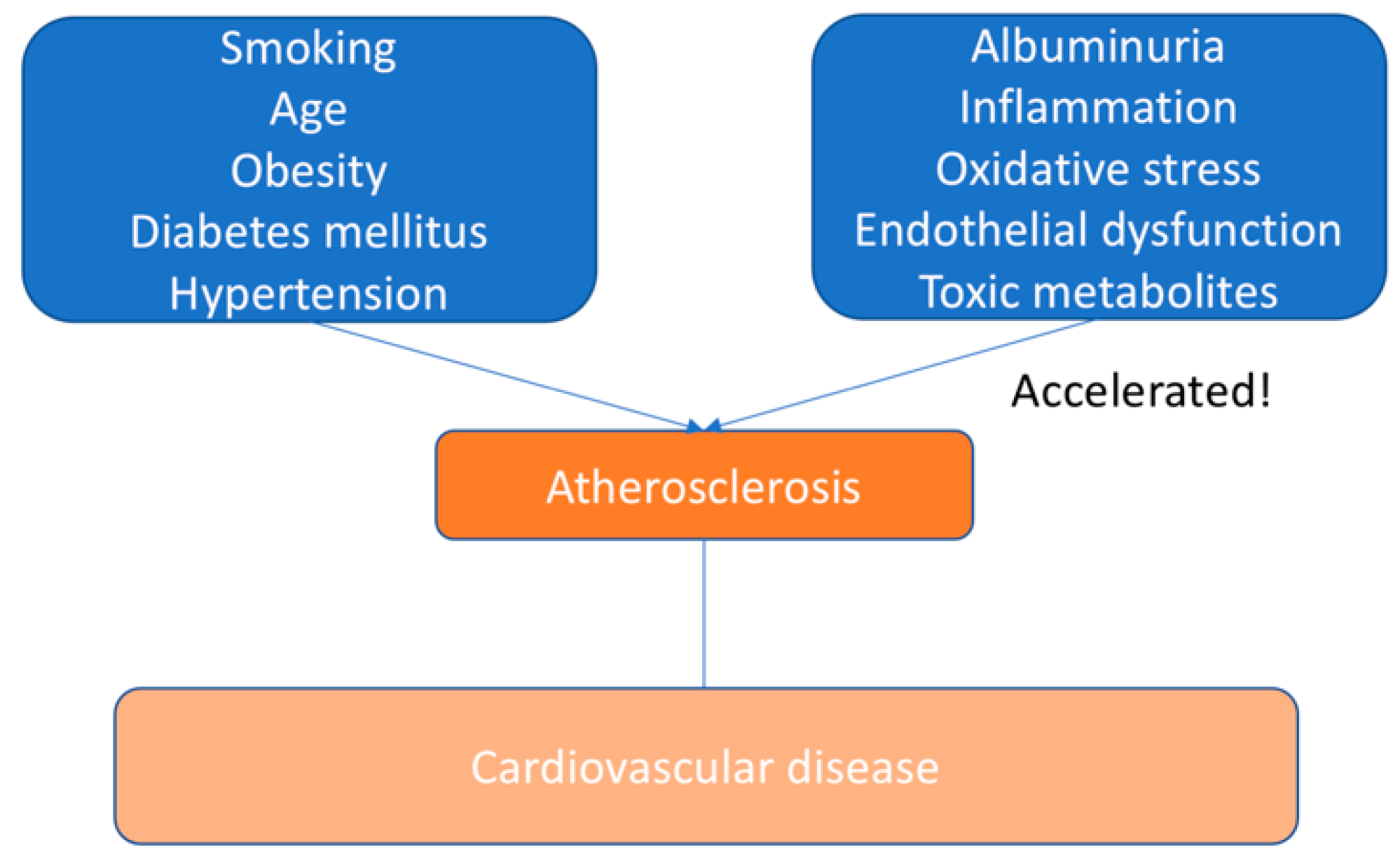
6. Pathogenesis of Atherosclerosis
7. Managing ASCVD in CKD Populations
Medical Management of Stable Ischemic Heart Disease in CKD
8. Pathophysiologic Considerations of ASCVD in CKD
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pálsson, R.; Patel, U.D. Cardiovascular Complications of Diabetic Kidney Disease. Adv. Chronic Kidney Dis. 2014, 21, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.P.; Liu, S.; Song, W.; Xu, E.Y.; Nabity, M.B. Small RNA sequencing evaluation of renal microRNA biomarkers in dogs with X-linked hereditary nephropathy. Sci. Rep. 2021, 11, 17437. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Mitesh, S.; Gkogkou, A.; Geladari, E. Chronic Kidney Disease and Cardiovascular Disease: Is there Any Relationship? Curr. Cardiol. Rev. 2019, 15, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, S.R.; Aeddula, N.R. Chronic Renal Failure. [Updated 2021 Jul 16]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK535404/ (accessed on 25 June 2022).
- Menn-Josephy, H.; Lee, C.S.; Nolin, A.; Christov, M.; Rybin, D.V.; Weinberg, J.M.; Henderson, J.; Bonegio, R.; Havasi, A. Renal Interstitial Fibrosis: An Imperfect Predictor of Kidney Disease Progression in Some Patient Cohorts. Am. J. Nephrol. 2016, 44, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Ketteler, M.; Block, G.A.; Evenepoel, P.; Fukagawa, M.; Herzog, C.A.; McCann, L.; Moe, S.M.; Shroff, R.; Tonelli, M.A.; Toussaint, N.D.; et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD) Guideline Update: What’s changed and why it matters. Kidney Int. 2017, 92, 26–36. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Romagnani, P.; Remuzzi, G.; Glassock, R.; Levin, A.; Jager, K.J.; Tonelli, M.; Massy, Z.; Wanner, C.; Anders, H.-J. Chronic kidney disease. Nat. Rev. Dis. Primers 2017, 3, 17088. [Google Scholar] [CrossRef]
- Bianchi, S.; Aucella, F.; De Nicola, L.; Genovesi, S.; Paoletti, E.; Regolisti, G. Management of hyperkalemia in patients with kidney disease: A position paper endorsed by the Italian Society of Nephrology. J. Nephrol. 2019, 32, 499–516. [Google Scholar] [CrossRef]
- Levin, A.S.; Bilous, R.W.; Coresh, J. Chapter 1: Definition and classification of CKD. Kidney Int. Suppl. 2013, 3, 19–62. [Google Scholar] [CrossRef]
- Thomas, R.; Kanso, A.; Sedor, J.R. Chronic Kidney Disease and Its Complications. Prim. Care 2008, 35, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mucino, M.J.; Ronco, C. Renal Functional Reserve and Renal Recovery after Acute Kidney Injury. Nephron Clin. Pract. 2014, 127, 94–100. [Google Scholar] [CrossRef] [PubMed]
- De Moor, B.; Vanwalleghem, J.F.; Swennen, Q.; Stas, K.J.; I Meijers, B.K. Haemodynamic or metabolic stimulation tests to reveal the renal functional response: Requiem or revival? Clin. Kidney J. 2018, 11, 623–654. [Google Scholar] [CrossRef] [PubMed]
- Lees, J.S.; Welsh, C.; Celis-Morales, C.A.; Mackay, D.; Lewsey, J.; Gray, S.R.; Lyall, D.M.; Cleland, J.G.; Gill, J.M.R.; Jhund, P.S.; et al. Glomerular filtration rate by differing measures, albuminuria and prediction of cardiovascular disease, mortality and end-stage kidney disease. Nat. Med. 2019, 25, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Chazot, R.; Botelho-Nevers, E.; Mariat, C.; Frésard, A.; Cavalier, E.; Lucht, F.; Delanaye, P.; Maillard, N.; Gagneux-Brunon, A. Cystatin C and Urine Albumin to Creatinine Ratio Predict 5-Year Mortality and Cardiovascular Events in People Living With HIV. J. Infect. Dis. 2021, 223, 885–892. [Google Scholar] [CrossRef]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.-M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef] [PubMed]
- Ranganna, K.; Yousefipour, Z.; Yatsu, F.M.; Milton, S.G.; Hayes, B.E. Gene expression profile of butyrate-inhibited vascular smooth muscle cell proliferation. Mol. Cell. Biochem. 2003, 254, 21–36. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Sobenin, I.; Orekhov, A. Strategies to deliver microRNAs as potential therapeutics in the treatment of cardiovascular pathology. Drug Deliv. 2012, 19, 392–405. [Google Scholar] [CrossRef][Green Version]
- Pugh, D.; Gallacher, P.J.; Dhaun, N. Management of Hypertension in Chronic Kidney Disease. Drugs 2019, 79, 365–379, Erratum in Drugs 2020, 80, 1381. [Google Scholar] [CrossRef]
- Mitsnefes, M.M. Cardiovascular Disease in Children with Chronic Kidney Disease. J. Am. Soc. Nephrol. 2012, 23, 578–585. [Google Scholar] [CrossRef]
- Hamrahian, S.M.; Falkner, B. Hypertension in Chronic Kidney Disease. Adv. Exp. Med. Biol. 2017, 956, 307–325. [Google Scholar] [CrossRef]
- Keane, W.F.; E Tomassini, J.; Neff, D.R. Lipid Abnormalities in Patients with Chronic Kidney Disease: Implications for the Pathophysiology of Atherosclerosis. J. Atheroscler. Thromb. 2013, 20, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Summerhill, V.I.; Grechko, A.V.; Yet, S.-F.; Sobenin, I.A.; Orekhov, A.N. The Atherogenic Role of Circulating Modified Lipids in Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 3561. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Voloshyna, I.; De Leon, J.; Miyawaki, N.; Mattana, J. Cholesterol Metabolism in CKD. Am. J. Kidney Dis. 2015, 66, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, C.; Cefalù, A.; Giammanco, A.; Noto, D.; Caldarella, R.; Ciaccio, M.; Averna, M.; Nardi, E. Lipoprotein Abnormalities in Chronic Kidney Disease and Renal Transplantation. Life 2021, 11, 315. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Araújo, N.; Marreiros, C.; Simes, D. The interplay between mineral metabolism, vascular calcification and inflammation in Chronic Kidney Disease (CKD): Challenging old concepts with new facts. Aging 2019, 11, 4274–4299. [Google Scholar] [CrossRef]
- Yamada, S.; Giachelli, C.M. Vascular calcification in CKD-MBD: Roles for phosphate, FGF23, and Klotho. Bone 2017, 100, 87–93. [Google Scholar] [CrossRef]
- Campos-Obando, N.; Lahousse, L.; Brusselle, G.; Stricker, B.H.; Hofman, A.; Franco, O.H.; Uitterlinden, A.G.; Zillikens, M.C. Serum phosphate levels are related to all-cause, cardiovascular and COPD mortality in men. Eur. J. Epidemiology 2018, 33, 859–871. [Google Scholar] [CrossRef]
- Dube, P.; DeRiso, A.; Patel, M.; Battepati, D.; Khatib-Shahidi, B.; Sharma, H.; Gupta, R.; Malhotra, D.; Dworkin, L.; Haller, S.; et al. Vascular Calcification in Chronic Kidney Disease: Diversity in the Vessel Wall. Biomedicines 2021, 9, 404. [Google Scholar] [CrossRef]
- Podkowińska, A.; Formanowicz, D. Chronic Kidney Disease as Oxidative Stress- and Inflammatory-Mediated Cardiovascular Disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, D.H.; Kim, Y.-H.; Roh, Y.K.; Ju, S.Y.; Nam, H.-Y.; Nam, G.-E.; Choi, J.-S.; Lee, J.-E.; Sang, J.-E.; et al. Relationship Between Dyslipidemia and Albuminuria in Hypertensive Adults: A Nationwide Population-Based Study. Medicine 2016, 95, e3224. [Google Scholar] [CrossRef]
- Stewart, D.J.; Langlois, V.; Noone, D. Hyperuricemia and Hypertension: Links and Risks. Integr. Blood Press. Control 2019, 12, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Alani, H.; Tamimi, A.; Tamimi, N. Cardiovascular co-morbidity in chronic kidney disease: Current knowledge and future research needs. World J. Nephrol. 2014, 3, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Paloian, N.J.; Giachelli, C.M. A current understanding of vascular calcification in CKD. Am. J. Physiol. Renal. Physiol. 2014, 307, F891–F900. [Google Scholar] [CrossRef] [PubMed]
- Vervloet, M.; Cozzolino, M. Vascular calcification in chronic kidney disease: Different bricks in the wall? Kidney Int. 2017, 91, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Demer, L.L.; Tintut, Y. Inflammatory, Metabolic, and Genetic Mechanisms of Vascular Calcification. Arter. Thromb. Vasc. Biol. 2014, 34, 715–723. [Google Scholar] [CrossRef]
- Petrie, J.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef]
- Ahmadmehrabi, S.; Tang, W.H.W. Hemodialysis-induced cardiovascular disease. Semin. Dial. 2018, 31, 258–267. [Google Scholar] [CrossRef]
- Kon, V.; Linton, M.F.; Fazio, S. Atherosclerosis in chronic kidney disease: The role of macrophages. Nat. Rev. Nephrol. 2010, 7, 45–54. [Google Scholar] [CrossRef]
- Mavrakanas, T.; Charytan, D.M. Cardiovascular complications in chronic dialysis patients. Curr. Opin. Nephrol. Hypertens. 2016, 25, 536–544. [Google Scholar] [CrossRef]
- Gulizia, M.M.; Colivicchi, F.; Ricciardi, G.; Giampaoli, S.; Maggioni, A.P.; Averna, M.; Graziani, M.S.; Ceriotti, F.; Mugelli, A.; Rossi, F.; et al. ANMCO/ISS/AMD/ANCE/ARCA/FADOI/GICR-IACPR/SICI-GISE/SIBioC/SIC/SICOA/SID/SIF/SIMEU/SIMG/SIMI/SISA Joint Consensus Document on cholesterol and cardiovascular risk: Diagnostic–therapeutic pathway in Italy. Eur. Heart J. Suppl. 2017, 19, D3–D54. [Google Scholar] [CrossRef]
- Linton, M.R.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; and Vickers, C.K. The Role of Lipids and Lipoproteins in Atherosclerosis. [Updated 2019 Jan 3]. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK343489/ (accessed on 25 June 2022).
- Sobenin, I.A.; Salonen, J.T.; Zhelankin, A.V.; Melnichenko, A.A.; Kaikkonen, J.; Bobryshev, Y.V.; Orekhov, A.N. Low Density Lipoprotein-Containing Circulating Immune Complexes: Role in Atherosclerosis and Diagnostic Value. BioMed Res. Int. 2014, 2014, 205697. [Google Scholar] [CrossRef] [PubMed]
- Chawla, L.S.; Bellomo, R.; Bihorac, A.; Goldstein, S.L.; Siew, E.D.; Bagshaw, S.M.; Bittleman, D.; Cruz, D.; Endre, Z.; Fitzgerald, R.L.; et al. Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat. Rev. Nephrol. 2017, 13, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Barbey, F.; Brakch, N.; Linhart, A.; Jeanrenaud, X.; Palecek, T.; Bultas, J.; Burnier, M.; Hayoz, D. Increased carotid intima–media thickness in the absence of atherosclerotic plaques in an adult population with Fabry disease. Acta Paediatr. Suppl. 2006, 95, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Disthabanchong, S. Vascular calcification in chronic kidney disease: Pathogenesis and clinical implication. World J. Nephrol. 2012, 1, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Memarian, E.; Nilsson, P.M.; Zia, I.; Christensson, A.; Engström, G. The risk of chronic kidney disease in relation to anthropometric measures of obesity: A Swedish cohort study. BMC Nephrol. 2021, 22, 330. [Google Scholar] [CrossRef] [PubMed]
- Valdivielso, J.M.; Rodríguez-Puyol, D.; Pascual, J.; Barrios, C.; Bermúdez-López, M.; Sánchez-Niño, M.D.; Pérez-Fernández, M.; Ortiz, A. Atherosclerosis in Chronic Kidney Disease: More, Less, or Just Different? Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1938–1966. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The Vascular Endothelium and Human Diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef]
- Chistiakov, D.; Revin, V.; Sobenin, I.; Orekhov, A.; Bobryshev, Y. Vascular Endothelium: Functioning in Norm, Changes in Atherosclerosis and Current Dietary Approaches to Improve Endothelial Function. Mini-Reviews Med. Chem. 2015, 15, 338–350. [Google Scholar] [CrossRef]
- Granger, D.N.; Senchenkova, E. Chapter 7, Leukocyte–Endothelial Cell Adhesion. In Inflammation and the Microcirculation; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. Available online: https://www.ncbi.nlm.nih.gov/books/NBK53380/ (accessed on 25 June 2022).
- Palomino, D.C.T.; Marti, L.C. Chemokines and immunity. Einstein 2015, 13, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Puchenkova, O.A.; Nadezhdin, S.V.; Soldatov, V.O.; Zhuchenko, M.A.; Korshunova, D.S.; Kubekina, M.V.; Korshunov, E.N.; Korokina, L.V.; Golubinskaya, P.A.; Kulikov, A.L.; et al. Study of antiatherosclerotic and endothelioprotective activity of peptide agonists of EPOR/CD131 heteroreceptor. Pharm. Pharmacol. 2020, 8, 100–111. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Nikiforov, N.G.; Starodubova, A.V.; Popkova, T.V.; Orekhov, A.N. Macrophages and Foam Cells: Brief Overview of Their Role, Linkage, and Targeting Potential in Atherosclerosis. Biomedicines 2021, 9, 1221. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef] [PubMed]
- I Miller, Y. Toll-like receptors and atherosclerosis: Oxidized LDL as an endogenous Toll-like receptor ligand. Futur. Cardiol. 2005, 1, 785–792. [Google Scholar] [CrossRef]
- Getz, G.S. T Cells in Atherosclerosis in Ldlr-/- and Apoe-/- Mice. J. Immunol. Sci. 2018, 2, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Nusair, M.B.; Rajpurohit, N.; Alpert, M.A. Chronic Inflammation and Coronary Atherosclerosis in Patients with End-Stage Renal Disease. Cardiorenal Med. 2012, 2, 117–124. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Sobenin, I.; Bobryshev, Y.V. Plasmacytoid dendritic cells: Development, functions, and role in atherosclerotic inflammation. Front. Physiol. 2014, 5, 279. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Sobenin, I.A.; Orekhov, A.N.; Bobryshev, Y.V. Myeloid dendritic cells: Development, functions, and role in atherosclerotic inflammation. Immunobiology 2015, 220, 833–844. [Google Scholar] [CrossRef]
- Subbiah, A.K.; Chhabra, Y.K.; Mahajan, S. Cardiovascular disease in patients with chronic kidney disease: A neglected subgroup. Heart Asia 2016, 8, 56–61. [Google Scholar] [CrossRef]
- Samad, F.; Agarwal, A.; Samad, Z. Stable ischemic heart disease in women: Current perspectives. Int. J. Women’s Health 2017, 9, 701–709. [Google Scholar] [CrossRef]
- Obialo, C.I.; Ofili, E.O.; Norris, K.C. Statins and Cardiovascular Disease Outcomes in Chronic Kidney Disease: Reaffirmation vs. Repudiation. Int. J. Environ. Res. Public Health 2018, 15, 2733. [Google Scholar] [CrossRef] [PubMed]
- Krane, V.; Schmidt, K.-R.; Gutjahr-Lengsfeld, L.J.; Mann, J.F.; März, W.; Swoboda, F.; Wanner, C. 4D Study Investigators (the German Diabetes and Dialysis Study Investigators). Long-term effects following 4 years of randomized treatment with atorvastatin in patients with type 2 diabetes mellitus on hemodialysis. Kidney Int. 2016, 89, 1380–1387. [Google Scholar] [CrossRef][Green Version]
- Herrera-Gómez, F.; Chimeno, M.M.; Martín-García, D.; Lizaraso-Soto, F.; Maurtua-Briseño-Meiggs, Á.; Grande-Villoria, J.; Bustamante-Munguira, J.; Alamartine, E.; Vilardell, M.; Ochoa-Sangrador, C.; et al. Cholesterol-Lowering Treatment in Chronic Kidney Disease: Multistage Pairwise and Network Meta-Analyses. Sci. Rep. 2019, 9, 8951. [Google Scholar] [CrossRef]
- Barbieri, L.; Pergolini, P.; Verdoia, M.; Rolla, R.; Nardin, M.; Marino, P.; Bellomo, G.; Suryapranata, H.; De Luca, G. Novara Atherosclerosis Study Group (NAS) Platelet reactivity in patients with impaired renal function receiving dual antiplatelet therapy with clopidogrel or ticagrelor. Vasc. Pharmacol. 2016, 79, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Dababneh, E.; Goldstein, S. Chronic Ischemic Heart Disease Selection of Treatment Modality. [Updated 2021 Jul 29]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507703/ (accessed on 25 June 2022).
- Park, S.; Choi, Y.; Kang, J.; Kim, M.; Geum, M.J.; Kim, S.; Rhie, S. P2Y12 Antiplatelet Choice for Patients with Chronic Kidney Disease and Acute Coronary Syndrome: A Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Libby, P. Acute Coronary Syndromes: The Way Forward From Mechanisms to Precision Treatments. Circulation 2017, 136, 1155–1166. [Google Scholar] [CrossRef]
- Palit, S. Vascular Calcification in Chronic Kidney Disease: Role of Disordered Mineral Metabolism. Curr. Pharm. Des. 2014, 20, 5829–5833. [Google Scholar] [CrossRef]
- Shang, D.; Xie, Q.; Ge, X.; Yan, H.; Tian, J.; Kuang, D.; Hao, C.-M.; Zhu, T. Hyperphosphatemia as an independent risk factor for coronary artery calcification progression in peritoneal dialysis patients. BMC Nephrol. 2015, 16, 107. [Google Scholar] [CrossRef]
- Kono, K.; Fujii, H.; Nakai, K.; Goto, S.; Shite, J.; Hirata, K.-I.; Fukagawa, M.; Nishi, S. Composition and plaque patterns of coronary culprit lesions and clinical characteristics of patients with chronic kidney disease. Kidney Int. 2012, 82, 344–351. [Google Scholar] [CrossRef]
- Ganesh, S.K.; Stack, A.G.; Levin, N.W.; Hulbert-Shearon, T.; Port, F.K. Association of Elevated Serum PO4, Ca × PO4Product, and Parathyroid Hormone with Cardiac Mortality Risk in Chronic Hemodialysis Patients. J. Am. Soc. Nephrol. 2001, 12, 2131–2138. [Google Scholar] [CrossRef]
- Drüeke, T.B. Hyperparathyroidism in Chronic Kidney Disease. [Updated 2018 Apr 28]. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Levy, M., Korbonits, M., Dungan, K., Dhatariya, K., Wilson, D.P., McGee, E.A., Kuohung, W., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK278975/ (accessed on 25 June 2022).
- Li, Y.; Sun, Z.; Zhang, L.; Yan, J.; Shao, C.; Jing, L.; Li, L.; Wang, Z. Role of Macrophages in the Progression and Regression of Vascular Calcification. Front. Pharmacol. 2020, 11, 661. [Google Scholar] [CrossRef] [PubMed]
- Takx, R.A.; Blomberg, B.A.; El Aidi, H.; Habets, J.; de Jong, P.A.; Nagel, E.; Hoffmann, U.; Leiner, T. Diagnostic Accuracy of Stress Myocardial Perfusion Imaging Compared to Invasive Coronary Angiography With Fractional Flow Reserve Meta-Analysis. Circ. Cardiovasc. Imaging 2015, 8, e002666. [Google Scholar] [CrossRef] [PubMed]
- Jansz, T.T.; Go, M.H.; Hartkamp, N.S.; Stöger, J.L.; Celeng, C.; Leiner, T.; de Jong, P.A.; Visseren, F.J.; Verhaar, M.C.; van Jaarsveld, B.C. Coronary Artery Calcification as a Marker for Coronary Artery Stenosis: Comparing Kidney Failure to the General Population. Kidney Med. 2021, 3, 386–394.e1. [Google Scholar] [CrossRef] [PubMed]
- Nadra, I.; Mason, J.C.; Philippidis, P.; Florey, O.; Smythe, C.D.; McCarthy, G.M.; Landis, R.C.; Haskard, D.O. Proinflammatory Activation of Macrophages by Basic Calcium Phosphate Crystals via Protein Kinase C and MAP Kinase Pathways: A vicious cycle of inflammation and arterial calcification? Circ. Res. 2005, 96, 1248–1256. [Google Scholar] [CrossRef]
- Ewence, A.E.; Bootman, M.; Roderick, H.; Skepper, J.N.; McCarthy, G.; Epple, M.; Neumann, M.; Shanahan, C.; Proudfoot, D. Calcium Phosphate Crystals Induce Cell Death in Human Vascular Smooth Muscle Cells: A potential mechanism in atherosclerotic plaque destabilization. Circ. Res. 2008, 103, e28–e34. [Google Scholar] [CrossRef]
- Schlieper, G.; Floege, J. Calcimimetics in CKD—results from recent clinical studies. Pediatr. Nephrol. 2008, 23, 1721–1728. [Google Scholar] [CrossRef]
- Goodwill, A.G.; Dick, G.M.; Kiel, A.M.; Tune, J.D. Regulation of Coronary Blood Flow. Compr. Physiol. 2017, 7, 321–382. [Google Scholar] [CrossRef]
- Lee, J.M.; Jung, J.-H.; Hwang, D.; Park, J.; Fan, Y.; Na, S.-H.; Doh, J.-H.; Nam, C.-W.; Shin, E.-S.; Koo, B.-K. Coronary Flow Reserve and Microcirculatory Resistance in Patients With Intermediate Coronary Stenosis. J. Am. Coll. Cardiol. 2016, 67, 1158–1169. [Google Scholar] [CrossRef]
- Bajaj, N.; Singh, A.; Zhou, W.; Gupta, A.; Fujikura, K.; Byrne, C.; Harms, H.J.; Osborne, M.T.; Bravo, P.; Andrikopoulou, E.; et al. Coronary Microvascular Dysfunction, Left Ventricular Remodeling, and Clinical Outcomes in Patients With Chronic Kidney Impairment. Circulation 2020, 141, 21–33. [Google Scholar] [CrossRef]
- Vijayan, S.; Barmby, D.S.; Pearson, I.R.; Davies, A.G.; Wheatcroft, S.B.; Sivananthan, M. Assessing Coronary Blood Flow Physiology in the Cardiac Catheterisation Laboratory. Curr. Cardiol. Rev. 2017, 13, 232–243. [Google Scholar] [CrossRef]
- Flammer, A.J.; Anderson, T.; Celermajer, D.S.; Creager, M.A.; Deanfield, J.; Ganz, P.; Hamburg, N.M.; Lüscher, T.F.; Shechter, M.; Taddei, S.; et al. The Assessment of Endothelial Function: From research into clinical practice. Circulation 2012, 126, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Soldatov, V.O.; Malorodova, T.N.; Balamutova, T.I.; Ksenofontov, A.O.; Dovgan, A.P.; Urozhevskaya, Z.S. Endothelial dysfunction: Comparative evaluation of ultrasound dopplerography, laser dopplerflowmetry and direct monitoring of arterial pressure for conducting pharmacological tests in rats. Res. Results Pharmacol. 2018, 4, 73–80. [Google Scholar] [CrossRef]
- Soldatov, V.O.; Malorodova, T.N.; Pokrovskaya, T.G.; Pokrovskii, M.V.; Kulchenkova, T.I.; Ksenofontov, A.O.; Filippova, O.V. Ultrasonic dopplerography for the evaluation of endothelial function in the conduct of pharmacological vascular samples in an experiment. Int. J. Res. Pharm. Sci. 2018, 9, 735–740. [Google Scholar] [CrossRef]
- Rein, P.; Vonbank, A.; Saely, C.H.; Beer, S.; Jankovic, V.; Boehnel, C.; Breuss, J.; Risch, L.; Fraunberger, P.; Drexel, H. Relation of Albuminuria to Angiographically Determined Coronary Arterial Narrowing in Patients With and Without Type 2 Diabetes Mellitus and Stable or Suspected Coronary Artery Disease. Am. J. Cardiol. 2011, 107, 1144–1148. [Google Scholar] [CrossRef]
- Rein, P.; Saely, C.H.; Vonbank, A.; Fraunberger, P.; Drexel, H. Is albuminuria a myocardial infarction risk equivalent for atherothrombotic events? Atherosclerosis 2015, 240, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Gluba-Brzózka, A.; Rysz-Górzyńska, M.; Franczyk, B. The Role and Function of HDL in Patients with Chronic Kidney Disease and the Risk of Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 601. [Google Scholar] [CrossRef]
- Lekawanvijit, S.; Kompa, A.R.; Manabe, M.; Wang, B.H.; Langham, R.G.; Nishijima, F.; Kelly, D.J.; Krum, H. Chronic Kidney Disease-Induced Cardiac Fibrosis Is Ameliorated by Reducing Circulating Levels of a Non-Dialysable Uremic Toxin, Indoxyl Sulfate. PLoS ONE 2012, 7, e41281. [Google Scholar] [CrossRef] [PubMed]
- Kaesler, N.; Babler, A.; Floege, J.; Kramann, R. Cardiac Remodeling in Chronic Kidney Disease. Toxins 2020, 12, 161. [Google Scholar] [CrossRef]
- Bhatti, N.K.; Galougahi, K.K.; Paz, Y.; Nazif, T.; Moses, J.W.; Leon, M.B.; Stone, G.W.; Kirtane, A.J.; Karmpaliotis, D.; Bokhari, S.; et al. Diagnosis and Management of Cardiovascular Disease in Advanced and End-Stage Renal Disease. J. Am. Heart Assoc. 2016, 5, e003648. [Google Scholar] [CrossRef]
- Wojtaszek, E.; Oldakowska-Jedynak, U.; Kwiatkowska, M.; Glogowski, T.; Malyszko, J. Uremic Toxins, Oxidative Stress, Atherosclerosis in Chronic Kidney Disease, and Kidney Transplantation. Oxidative Med. Cell. Longev. 2021, 2021, 6651367. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poznyak, A.V.; Sadykhov, N.K.; Kartuesov, A.G.; Borisov, E.E.; Sukhorukov, V.N.; Orekhov, A.N. Atherosclerosis Specific Features in Chronic Kidney Disease (CKD). Biomedicines 2022, 10, 2094. https://doi.org/10.3390/biomedicines10092094
Poznyak AV, Sadykhov NK, Kartuesov AG, Borisov EE, Sukhorukov VN, Orekhov AN. Atherosclerosis Specific Features in Chronic Kidney Disease (CKD). Biomedicines. 2022; 10(9):2094. https://doi.org/10.3390/biomedicines10092094
Chicago/Turabian StylePoznyak, Anastasia V., Nikolay K. Sadykhov, Andrey G. Kartuesov, Evgeny E. Borisov, Vasily N. Sukhorukov, and Alexander N. Orekhov. 2022. "Atherosclerosis Specific Features in Chronic Kidney Disease (CKD)" Biomedicines 10, no. 9: 2094. https://doi.org/10.3390/biomedicines10092094
APA StylePoznyak, A. V., Sadykhov, N. K., Kartuesov, A. G., Borisov, E. E., Sukhorukov, V. N., & Orekhov, A. N. (2022). Atherosclerosis Specific Features in Chronic Kidney Disease (CKD). Biomedicines, 10(9), 2094. https://doi.org/10.3390/biomedicines10092094







