Hashimoto’s Thyroiditis Minimizes Lymph Node Metastasis in BRAF Mutant Papillary Thyroid Carcinomas
Abstract
:1. Introduction
2. Methods
2.1. Study Design & Recruited Cohort
2.2. Determination of BRAF Mutation and Hashimoto’s Thyroiditis Status
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
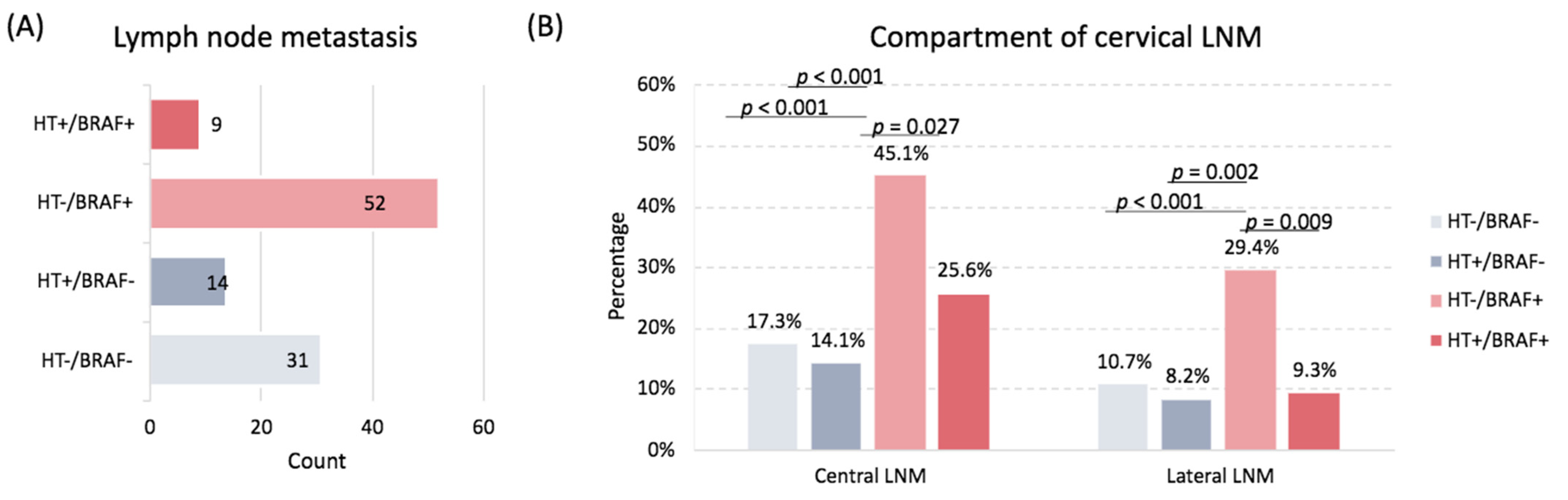
3.2. Association of HT with Lymph Node Metastasis
3.3. Independent Predictors of Lymph Node Metastasis in BRAF-Mutated Tumors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davies, L.; Welch, H.G. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006, 295, 2164–2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, L.G.; Sikora, A.G.; Tosteson, T.D.; Davies, L. The increasing incidence of thyroid cancer: The influence of access to care. Thyroid 2013, 23, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Tufano, R.P.; Noureldine, S.I.; Angelos, P. Incidental thyroid nodules and thyroid cancer: Considerations before determining management. JAMA Otolaryngol.–Head Neck Surg. 2015, 141, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Kilfoy, B.A.; Zheng, T.; Holford, T.R.; Han, X.; Ward, M.H.; Sjodin, A.; Zhang, Y.; Bai, Y.; Zhu, C.; Guo, G.L.; et al. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control. 2009, 20, 525–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, M. BRAF mutation in papillary thyroid microcarcinoma: The promise of better risk management. Ann. Surg. Oncol. 2009, 16, 801–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kebebew, E.; Weng, J.; Bauer, J.; Ranvier, G.; Clark, O.H.; Duh, Q.Y.; Shibru, D.; Bastian, B.; Griffin, A. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann. Surg. 2007, 246, 466. [Google Scholar] [CrossRef]
- Kim, T.H.; Park, Y.J.; Lim, J.A.; Ahn, H.Y.; Lee, E.K.; Lee, Y.J.; Kim, K.W.; Hahn, S.K.; Youn, Y.K.; Kim, K.H.; et al. The association of the BRAFV600E mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: A meta-analysis. Cancer 2012, 118, 1764–1773. [Google Scholar] [CrossRef]
- Elisei, R.; Ugolini, C.; Viola, D.; Lupi, C.; Biagini, A.; Giannini, R.; Romei, C.; Miccoli, P.; Pinchera, A.; Basolo, F. BRAFV600E mutation and outcome of patients with papillary thyroid carcinoma: A 15-year median follow-up study. J. Clin. Endocrinol. Metab. 2008, 93, 3943–3949. [Google Scholar] [CrossRef] [Green Version]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Viola, D.; Elisei, R.; Bendlova, B.; Yip, L.; Mian, C.; Vianello, F.; Tuttle, R.M.; et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 2013, 309, 1493–1501. [Google Scholar] [CrossRef] [Green Version]
- Antonelli, A.; Ferrari, S.M.; Corrado, A.; Di Domenicantonio, A.; Fallahi, P. Autoimmune thyroid disorders. Autoimmun. Rev. 2015, 14, 174–180. [Google Scholar] [CrossRef]
- Caturegli, A.; De Remigis, N.R.; Rose, P. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dai, J.; Wu, T.; Yang, N.; Yin, Z. The study of the coexistence of Hashimoto’s thyroiditis with papillary thyroid carcinoma. J. Cancer Res. Clin. Oncol. 2014, 140, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Jara, S.M.; Carson, K.A.; Pai, S.I.; Agrawal, N.; Richmon, J.D.; Prescott, J.D.; Dackiw, A.; Zeiger, M.A.; Bishop, J.A.; Tufano, R.P. The relationship between chronic lymphocytic thyroiditis and central neck lymph node metastasis in North American patients with papillary thyroid carcinoma. Surgery 2013, 154, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Nam, E.S.; Shin, H.S.; Cho, S.J.; Park, H.R.; Kwon, M.J. P2X7 receptor expression in coexistence of papillary thyroid carcinoma with Hashimoto’s thyroiditis. Korean J. Pathol. 2014, 48, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battistella, E.; Pomba, L.; Costantini, A.; Scapinello, A.; Toniato, A. Hashimoto’s Thyroiditis and Papillary Cancer Thyroid Coexistence Exerts a Protective Effect: A Single Centre Experience. Indian J. Surg. Oncol. 2022, 13, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mammen, J.S.; Cappola, A.R. Autoimmune thyroid disease in women. JAMA 2021, 325, 2392–2393. [Google Scholar] [CrossRef] [PubMed]
- Büyükaşık, O.; Hasdemir, A.O.; Yalçın, E.; Celep, B.; Sengül, S.; Yandakçı, K.; Tunç, G.; Küçükpınar, T.; Alkoy, S.; Cöl, C. The association between thyroid malignancy and chronic lymphocytic thyroiditis: Should it alter the surgical approach? Endokrynol. Pol. 2011, 62, 303–308. [Google Scholar]
- McLeod, D.S.; Watters, K.F.; Carpenter, A.D.; Ladenson, P.W.; Cooper, D.S.; Ding, E.L. Thyrotropin and thyroid cancer diagnosis: A systematic review and dose-response meta-analysis. J. Clin. Endocrinol. Metab. 2012, 97, 2682–2692. [Google Scholar] [CrossRef] [Green Version]
- Crile, G., Jr.; JB, H. Incidence of cancer in struma lymphomatosa. Surg. Gynecol. Obstet. 1962, 115, 101–103. [Google Scholar]
- Mukasa, K.; Noh, J.Y.; Kunii, Y.; Matsumoto, M.; Sato, S.; Yasuda, S.; Suzuki, M.; Ito, K.; Ito, K. Prevalence of malignant tumors and adenomatous lesions detected by ultrasonographic screening in patients with autoimmune thyroid diseases. Thyroid 2011, 21, 37–41. [Google Scholar] [CrossRef]
- Okayasu, I.; Fujiwara, M.; Hara, Y.; Tanaka, Y.; Rose, N.R. Association of chronic lymphocytic thyroiditis and thyroid papillary carcinoma. A study of surgical cases among Japanese, and white and African Americans. Cancer 1995, 76, 2312–2318. [Google Scholar] [CrossRef]
- Bradly, D.P.; Reddy, V.; Prinz, R.A.; Gattuso, P. Incidental papillary carcinoma in patients treated surgically for benign thyroid diseases. Surgery 2009, 146, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, B.; Le, K.T.; Hershman, J.M. Hashimoto’s thyroiditis and papillary thyroid carcinoma: Is there a correlation? J. Clin. Endocrinol. Metab. 2013, 98, 474–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamimi, D.M. The association between chronic lymphocytic thyroiditis and thyroid tumors. Int. J. Surg. Pathol. 2002, 10, 141–146. [Google Scholar] [CrossRef]
- Ahn, D.; Heo, S.J.; Park, J.H.; Kim, J.H.; Sohn, J.H.; Park, J.Y.; Park, S.K.; Park, J. Clinical relationship between Hashimoto’s thyroiditis and papillary thyroid cancer. Acta Oncol. 2011, 50, 1228–1234. [Google Scholar] [CrossRef] [Green Version]
- Dvorkin, S.; Robenshtok, E.; Hirsch, D.; Strenov, Y.; Shimon, I.; Benbassat, C.A. Differentiated thyroid cancer is associated with less aggressive disease and better outcome in patients with coexisting Hashimotos thyroiditis. J. Clin. Endocrinol. Metab. 2013, 98, 2409–2414. [Google Scholar] [CrossRef] [Green Version]
- Toniato, A.; Boschin, I.; Casara, D.; Mazzarotto, R.; Rubello, D.; Pelizzo, M. Papillary thyroid carcinoma: Factors influencing recurrence and survival. Ann. Surg. Oncol. 2008, 15, 1518–1522. [Google Scholar] [CrossRef]
- Ma, B.; Wang, Y.; Yang, S.; Ji, Q. Predictive factors for central lymph node metastasis in patients with cN0 papillary thyroid carcinoma: A systematic review and meta-analysis. Int. J. Surg. 2016, 28, 153–161. [Google Scholar] [CrossRef]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Shong, Y.K.; Kim, T.Y.; Viola, D.; Elisei, R.; Bendlová, B.; Yip, L.; Mian, C.; et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J. Clin. Oncol. 2015, 33, 42. [Google Scholar] [CrossRef] [Green Version]
- Howell, G.M.; Nikiforova, M.N.; Carty, S.E.; Armstrong, M.J.; Hodak, S.P.; Stang, M.T.; McCoy, K.L.; Nikiforov, Y.E.; Yip, L. BRAF V600E mutation independently predicts central compartment lymph node metastasis in patients with papillary thyroid cancer. Ann. Surg. Oncol. 2013, 20, 47–52. [Google Scholar] [CrossRef]
- Marotta, V.; Guerra, A.; Zatelli, M.C.; Uberti, E.D.; Di Stasi, V.; Faggiano, A.; Colao, A.; Vitale, M. BRAF mutation positive papillary thyroid carcinoma is less advanced when H ashimoto’s thyroiditis lymphocytic infiltration is present. Clin. Endocrinol. 2013, 79, 733–738. [Google Scholar]
- Xing, M. BRAF mutation in thyroid cancer. Endocr.-Relat. Cancer 2005, 12, 245–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajeev, P.; Ahmed, S.; Ezzat, T.M.; Sadler, G.P.; Mihai, R. The number of positive lymph nodes in the central compartment has prognostic impact in papillary thyroid cancer. Langenbeck’s Arch. Surg. 2013, 398, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.Y.; Park, J.Y.; Yoon, Y.H.; Choi, B.; Kim, J.M.; Jo, Y.S.; Shong, M.; Koo, B.S. Prediction of occult central lymph node metastasis in papillary thyroid carcinoma by preoperative BRAF analysis using fine-needle aspiration biopsy: A prospective study. J. Clin. Endocrinol. Metab. 2012, 97, 3996–4003. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Guo, T.; Huang, X.; Wu, Q.; Niu, D.; Ji, X.; Feng, Q.; Li, Z.; Kakudo, K. In papillary thyroid carcinoma, expression by immunohistochemistry of BRAF V600E, PD-L1, and PD-1 is closely related. Virchows Arch. 2018, 472, 779–787. [Google Scholar] [CrossRef]
- Dell’Aquila, M.; Granitto, A.; Martini, M.; Capodimonti, S.; Cocomazzi, A.; Musarra, T.; Fiorentino, V.; Pontecorvi, A.; Lombardi, C.P.; Fadda, G.; et al. PD-L1 and thyroid cytology: A possible diagnostic and prognostic marker. Cancer Cytopathol. 2020, 128, 177–189. [Google Scholar] [CrossRef]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef] [Green Version]
- An, H.J.; Ko, G.H.; Lee, J.H.; Lee, J.S.; Kim, D.C.; Yang, J.W.; Kim, M.H.; Kim, J.P.; Jung, E.J.; Song, D.H. Programmed death-ligand 1 expression and its correlation with lymph node metastasis in papillary thyroid carcinoma. J. Pathol. Transl. Med. 2018, 52, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Fu, G.; Polyakova, O.; MacMillan, C.; Ralhan, R.; Walfish, P.G. Programmed death-ligand 1 expression distinguishes invasive encapsulated follicular variant of papillary thyroid carcinoma from noninvasive follicular thyroid neoplasm with papillary-like nuclear features. EBioMedicine 2017, 18, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.; Kim, T.H.; Kim, S.W.; Ki, C.S.; Jang, H.W.; Kim, J.S.; Kim, J.H.; Choe, J.H.; Shin, J.H.; Hahn, S.Y.; et al. Comprehensive screening for PD-L1 expression in thyroid cancer. Endocr. Relat. Cancer 2017, 24, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Xing, M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Ricarte-Filho, J.C.; Ryder, M.; Chitale, D.A.; Rivera, M.; Heguy, A.; Ladanyi, M.; Janakiraman, M.; Solit, D.; Knauf, J.A.; Tuttle, R.M.; et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009, 69, 4885–4893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, V.; Light, T.J.; Adil, A.A.; Tao, M.; Chiu, A.S.; Hitchcock, M.; Arroyo, N.; Fernandes-Taylor, S.; Francis, D.O. Complication Rates of Total Thyroidectomy vs Hemithyroidectomy for Treatment of Papillary Thyroid Microcarcinoma: A Systematic Review and Meta-analysis. JAMA Otolaryngol.–Head Neck Surg. 2022, 148, 531–539. [Google Scholar] [CrossRef]

| Characteristics | Levels | Total | No Hashimoto Thyroiditis | Hashimoto Thyroiditis | p-Value |
|---|---|---|---|---|---|
| Number | 427 | 299 | 128 | ||
| Demographic data | |||||
| Age | <55 years | 248 (58.1) | 166 (55.5) | 82 (64.1) | 0.11 |
| ≥55 years | 179 (41.9) | 133 (44.5) | 46 (35.9) | ||
| Sex | Female | 334 (78.2) | 225 (75.3) | 109 (85.2) | 0.029 |
| Male | 93 (21.8) | 74 (24.7) | 19 (14.8) | ||
| Race | White | 283 (66.3) | 186 (62.2) | 97 (75.8) | 0.007 |
| African American | 144 (33.7) | 113 (37.8) | 31 (24.2) | ||
| Pathological data | |||||
| microPTC | T1a | 175 (41) | 112 (37.5) | 63 (49.2) | 0.025 |
| T stage | T1 | 309 (72.4) | 214 (71.6) | 95 (74.2) | 0.11 |
| T2 | 54 (12.6) | 34 (11.4) | 20 (15.6) | ||
| T3 | 56 (13.1) | 43 (14.4) | 13 (10.2) | ||
| T4 | 8 (1.9) | 8 (2.7) | 0 (0) | ||
| N stage | N0 | 321 (75.2) | 216 (72.2) | 105 (82) | 0.037 |
| N1 | 106 (24.8) | 83 (27.8) | 23 (18) | ||
| Compartment | Central LNM | 103 (24.1) | 80 (26.8) | 23 (18) | 0.06 |
| Lateral LNM | 62 (14.5) | 51 (17.1) | 11 (8.6) | 0.024 | |
| M stage | M0 | 413 (96.7) | 286 (95.7) | 127 (99.2) | 0.07 |
| M1 | 14 (3.3) | 13 (4.3) | 1 (0.8) | ||
| Focality | Unifocal | 250 (58.5) | 182 (60.9) | 68 (53.1) | 0.16 |
| Multifocal | 177 (41.5) | 117 (39.1) | 60 (46.9) | ||
| Laterality | Unilateral | 312 (73.1) | 224 (74.9) | 88 (68.8) | 0.19 |
| Bilateral | 115 (26.9) | 75 (25.1) | 40 (31.3) | ||
| Extrathyroidal extension | Positive | 51 (11.9) | 41 (13.7) | 10 (7.8) | 0.09 |
| Angioinvasion | Positive | 30 (7) | 24 (8) | 6 (4.7) | 0.22 |
| Perineural invasion | Positive | 5 (1.2) | 3 (1) | 2 (1.6) | 0.62 |
| Capsular invasion | Positive | 94 (22) | 66 (22.1) | 28 (21.9) | 0.96 |
| Extranodal extension | Positive | 38 (8.9) | 32 (10.7) | 6 (4.7) | 0.046 |
| Characteristics | Total | No Hashimoto’s Thyroiditis | Hashimoto’s Thyroiditis | p-Value | OR (95%CI) | p-Value | |
|---|---|---|---|---|---|---|---|
| BRAF Wild Type | |||||||
| Overall LNM | Negative | 237 (84) | 166 (84.3) | 71 (83.5) | 0.86 | Reference | |
| Positive | 45 (16) | 31 (15.7) | 14 (16.5) | 1.06 (0.53–2.1) | 0.87 | ||
| Central LNM | Negative | 236 (83.7) | 163 (82.7) | 73 (85.9) | 0.60 | Reference | |
| Positive | 46 (16.3) | 34 (17.3) | 12 (14.1) | 0.79 (0.39–1.61) | 0.51 | ||
| Lateral LNM | Negative | 254 (90.1) | 176 (89.3) | 78 (91.8) | 0.66 | Reference | |
| Positive | 28 (9.9) | 21 (10.7) | 7 (8.2) | 0.75 (0.31–1.84) | 0.53 | ||
| BRAF mutant type | |||||||
| Overall LNM | Negative | 84 (57.9) | 50 (49) | 34 (79.1) | 0.001 | Reference | |
| Positive | 61 (42.1) | 52 (51) | 9 (20.9) | 0.25 (0.11–0.58) | 0.001 | ||
| Central LNM | Negative | 88 (60.7) | 56 (54.9) | 32 (74.4) | 0.040 | Reference | |
| Positive | 57 (39.3) | 46 (45.1) | 11 (25.6) | 0.42 (0.19–0.92) | 0.030 | ||
| Lateral LNM | Negative | 111 (76.6) | 72 (70.6) | 39 (90.7) | 0.010 | Reference | |
| Positive | 34 (23.4) | 30 (29.4) | 4 (9.3) | 0.25 (0.08–0.75) | 0.014 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Issa, P.P.; Omar, M.; Buti, Y.; Issa, C.P.; Chabot, B.; Carnabatu, C.J.; Munshi, R.; Hussein, M.; Aboueisha, M.; Shama, M.; et al. Hashimoto’s Thyroiditis Minimizes Lymph Node Metastasis in BRAF Mutant Papillary Thyroid Carcinomas. Biomedicines 2022, 10, 2051. https://doi.org/10.3390/biomedicines10082051
Issa PP, Omar M, Buti Y, Issa CP, Chabot B, Carnabatu CJ, Munshi R, Hussein M, Aboueisha M, Shama M, et al. Hashimoto’s Thyroiditis Minimizes Lymph Node Metastasis in BRAF Mutant Papillary Thyroid Carcinomas. Biomedicines. 2022; 10(8):2051. https://doi.org/10.3390/biomedicines10082051
Chicago/Turabian StyleIssa, Peter P., Mahmoud Omar, Yusef Buti, Chad P. Issa, Bert Chabot, Christopher J. Carnabatu, Ruhul Munshi, Mohammad Hussein, Mohamed Aboueisha, Mohamed Shama, and et al. 2022. "Hashimoto’s Thyroiditis Minimizes Lymph Node Metastasis in BRAF Mutant Papillary Thyroid Carcinomas" Biomedicines 10, no. 8: 2051. https://doi.org/10.3390/biomedicines10082051
APA StyleIssa, P. P., Omar, M., Buti, Y., Issa, C. P., Chabot, B., Carnabatu, C. J., Munshi, R., Hussein, M., Aboueisha, M., Shama, M., Corsetti, R. L., Toraih, E., & Kandil, E. (2022). Hashimoto’s Thyroiditis Minimizes Lymph Node Metastasis in BRAF Mutant Papillary Thyroid Carcinomas. Biomedicines, 10(8), 2051. https://doi.org/10.3390/biomedicines10082051







