Latency Trend Analysis as a Guide to Screening Malignancy Survivors for Second Primary Thyroid Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Cohort Extraction
2.3. Variables and Outcomes
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Incidence of Thyroid Cancer
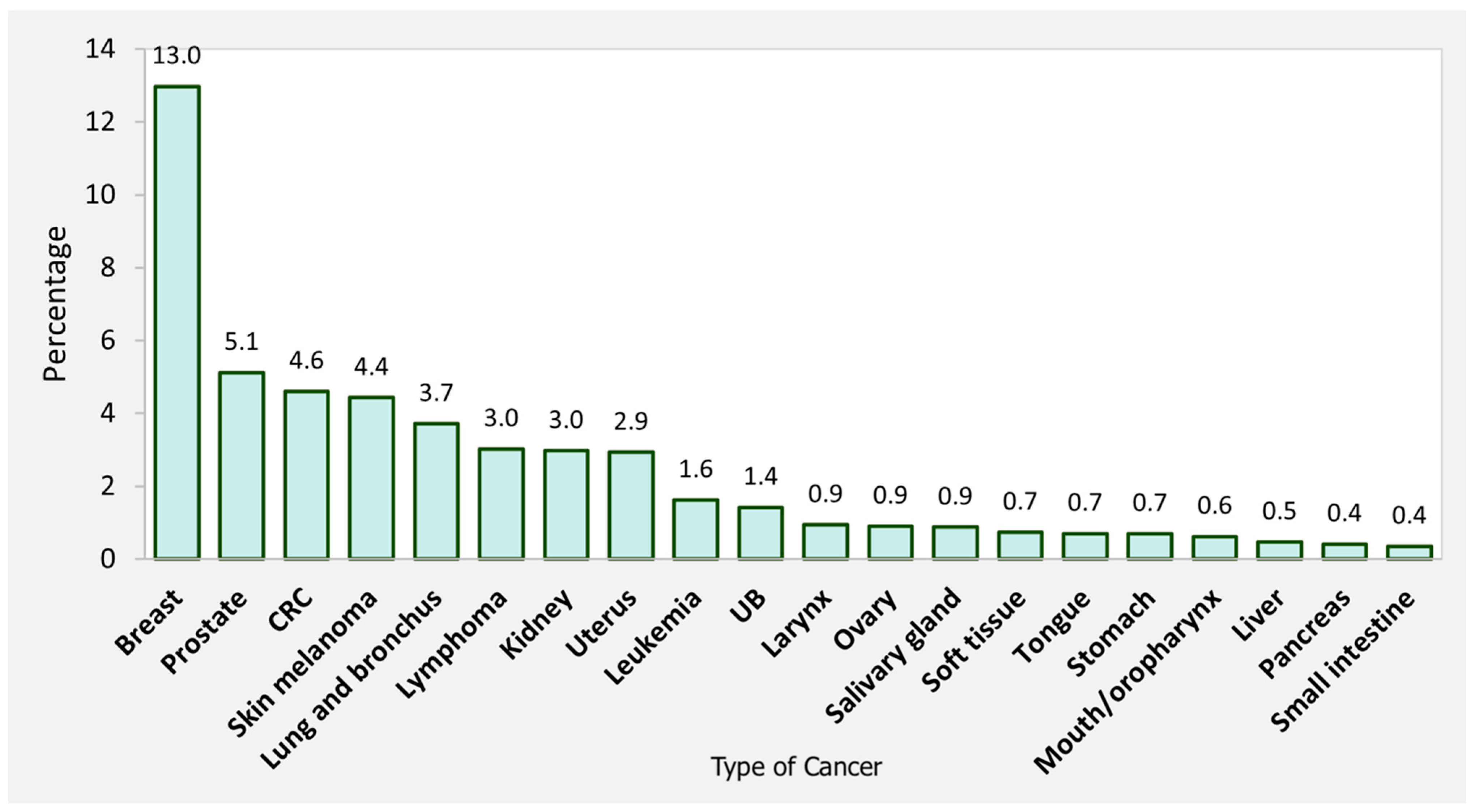
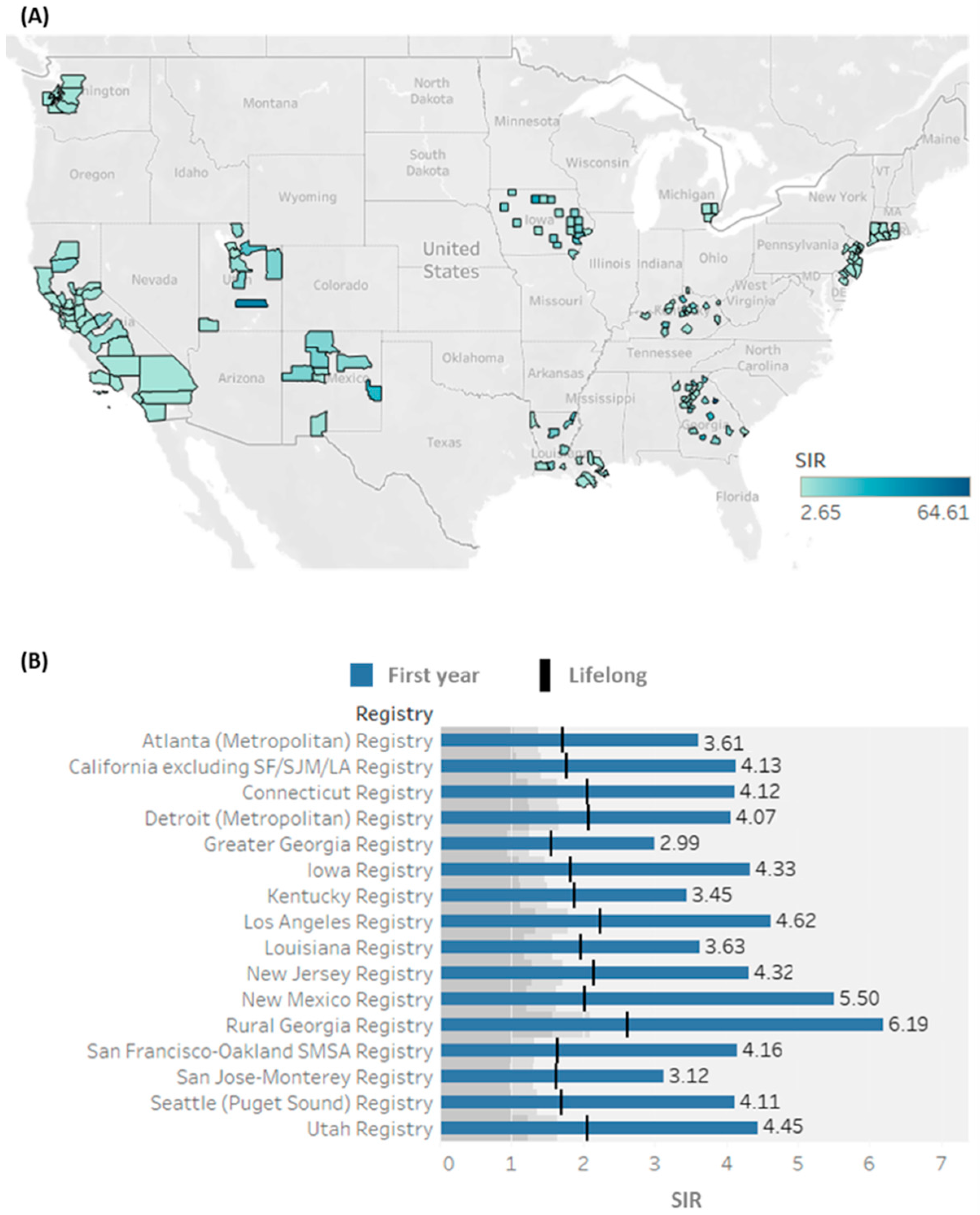
3.3. Spatial Analysis of Primary Cancer Preceding SPTC
3.4. Standardized Incidence Ratios for SPTC
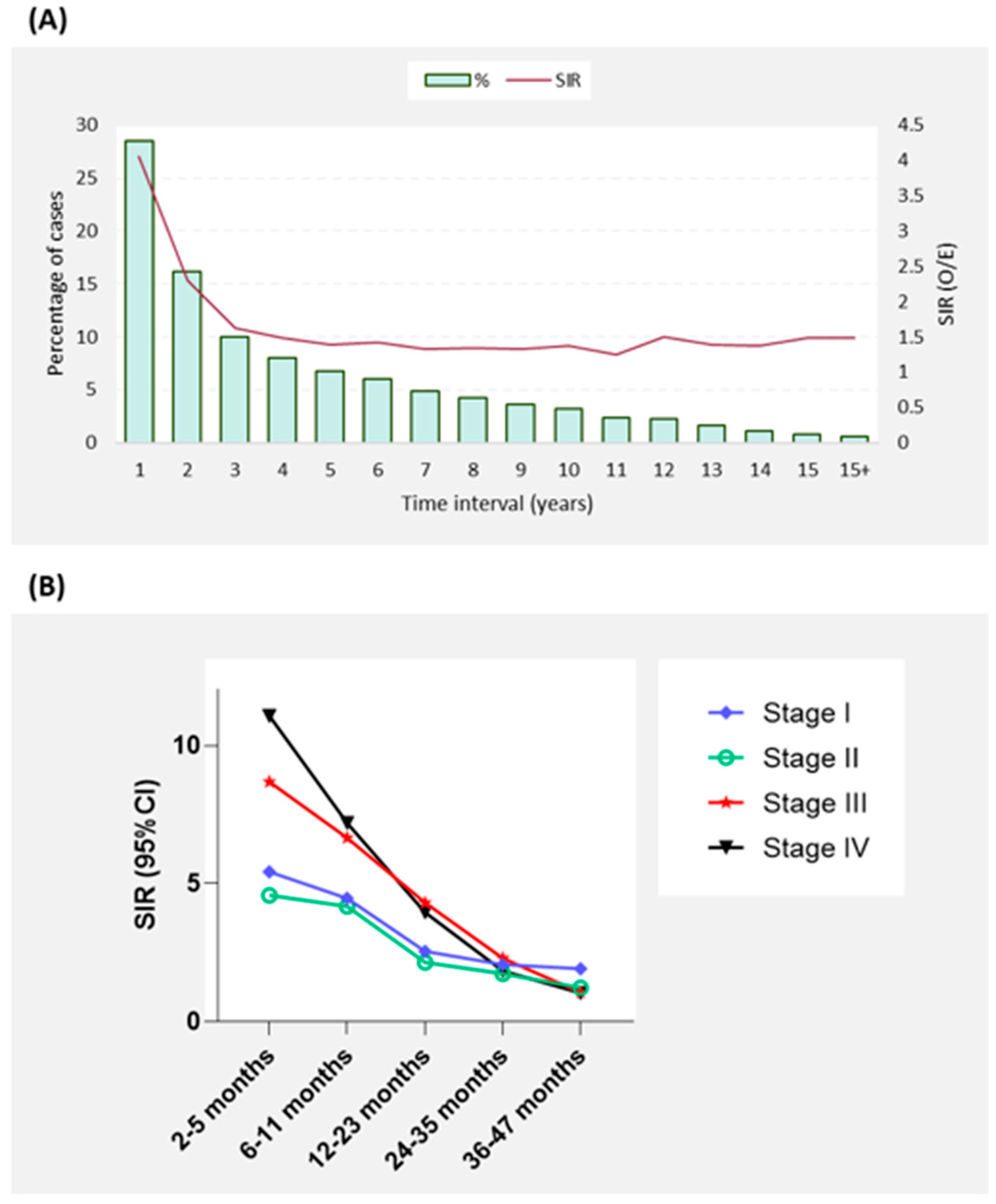
3.5. Temporal Analysis for the Latency Period to Develop SPTC
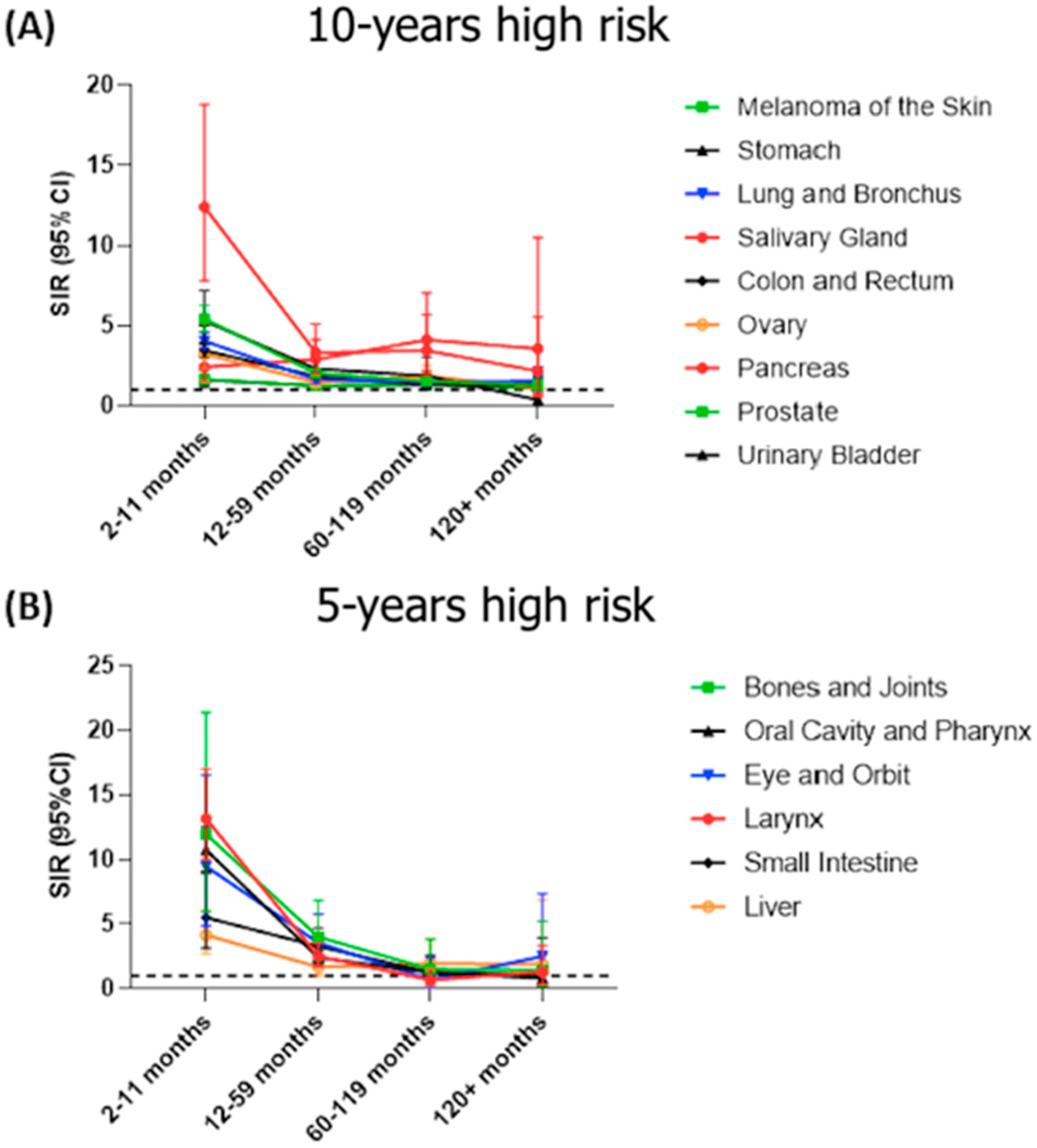
3.6. Risk of SPTC According to Patient Demographics and Primary Cancer Characteristics
3.7. The Overall Mortality of SPTC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, Q.T.; Lee, E.J.; Huang, M.G.; Park, Y.I.; Khullar, A.; Plodkowski, R.A. Diagnosis and Treatment of Patients with Thyroid Cancer. Am. Health Drug Benefits 2015, 8, 30. [Google Scholar] [PubMed]
- Davies, L.; Welch, H.G. Increasing Incidence of Thyroid Cancer in the United States, 1973–2002. JAMA 2006, 295, 2164–2167. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.; Wintheiser, G.; Wolfe, K.M.; Droessler, J.; Silberstein, P.T. Epidemiology of Thyroid Cancer: A Review of the National Cancer Database, 2000–2013. Cureus 2019, 11, e4127. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Lal, G.; Groff, M.; Howe, J.R.; Weigel, R.J.; Sugg, S.L.; Lynch, C.F. Risk of Subsequent Primary Thyroid Cancer after Another Malignancy: Latency Trends in a Population-Based Study. Ann. Surg. Oncol. 2012, 19, 1887–1896. [Google Scholar] [CrossRef]
- Taylor, A.J.; Croft, A.P.; Palace, A.M.; Winter, D.L.; Reulen, R.C.; Stiller, C.A.; Stevens, M.C.; Hawkins, M.M. Risk of Thyroid Cancer in Survivors of Childhood Cancer: Results from the British Childhood Cancer Survivor Study. Int. J. Cancer 2009, 125, 2400–2405. [Google Scholar] [CrossRef]
- Bhatti, P.; Veiga, L.H.; Ronckers, C.M.; Sigurdson, A.J.; Stovall, M.; Smith, S.A.; Weathers, R.; Leisenring, W.; Mertens, A.C.; Hammond, S. Risk of Second Primary Thyroid Cancer after Radiotherapy for a Childhood Cancer in a Large Cohort Study: An Update from the Childhood Cancer Survivor Study. Radiat. Res. 2010, 174, 741–752. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Stovall, M.; Robison, L.L. Long-Term Effects of Radiation Exposure among Adult Survivors of Childhood Cancer: Results from the Childhood Cancer Survivor Study. Radiat. Res. 2010, 174, 840–850. [Google Scholar] [CrossRef]
- Schonfeld, S.J.; Morton, L.M.; de González, A.B.; Curtis, R.E.; Kitahara, C.M. Risk of Second Primary Papillary Thyroid Cancer among Adult Cancer Survivors in the United States, 2000–2015. Cancer Epidemiol. 2020, 64, 101664. [Google Scholar] [CrossRef]
- Duggan, M.A.; Anderson, W.F.; Altekruse, S.; Penberthy, L.; Sherman, M.E. The Surveillance, Epidemiology and End Results (SEER) Program and Pathology: Towards Strengthening the Critical Relationship. Am. J. Surg. Pathol. 2016, 40, e94. [Google Scholar] [CrossRef]
- Bai, Y.; Kakudo, K.; Jung, C.K. Updates in the Pathologic Classification of Thyroid Neoplasms: A Review of the World Health Organization Classification. Endocrinol. Metab. 2020, 35, 696. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Hyun, N.; Leach, C.R.; Yabroff, K.R.; Jemal, A. Association of First Primary Cancer with Risk of Subsequent Primary Cancer among Survivors of Adult-Onset Cancers in the United States. JAMA 2020, 324, 2521–2535. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A Web Server for Cancer and Normal Gene Expression Profiling and Interactive Analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef] [PubMed]
- Canchola, A.J.; Horn-Ross, P.L.; Purdie, D.M. Risk of Second Primary Malignancies in Women with Papillary Thyroid Cancer. Am. J. Epidemiol. 2006, 163, 521–527. [Google Scholar] [CrossRef][Green Version]
- Crocetti, E.; Mattioli, V.; Buzzoni, C.; Franceschi, S.; Serraino, D.; Vaccarella, S.; Ferretti, S.; Busco, S.; Fedeli, U.; Varvarà, M. Risk of Thyroid as a First or Second Primary Cancer. A Population-based Study in Italy, 1998–2012. Cancer Med. 2021, 10, 6855–6867. [Google Scholar] [CrossRef] [PubMed]
- Sandeep, T.C.; Strachan, M.W.; Reynolds, R.M.; Brewster, D.H.; Scélo, G.; Pukkala, E.; Hemminki, K.; Anderson, A.; Tracey, E.; Friis, S. Second Primary Cancers in Thyroid Cancer Patients: A Multinational Record Linkage Study. J. Clin. Endocrinol. Metab. 2006, 91, 1819–1825. [Google Scholar] [CrossRef]
- Ronckers, C.M.; McCarron, P.; Ron, E. Thyroid Cancer and Multiple Primary Tumors in the SEER Cancer Registries 1. Int. J. Cancer 2005, 117, 281–288. [Google Scholar] [CrossRef]
- Polednik, K.M.; Simpson, M.C.; Adjei Boakye, E.; Mohammed, K.A.; Dombrowski, J.; Varvares, M.A.; Osazuwa-Peters, N. Radiation and Second Primary Thyroid Cancer Following Index Head and Neck Cancer. Laryngoscope 2019, 129, 1014–1020. [Google Scholar] [CrossRef]
- Kikuchi, S.; Perrier, N.D.; Ituarte, P.; Siperstein, A.E.; Duh, Q.-Y.; Clark, O.H. Latency Period of Thyroid Neoplasia after Radiation Exposure. Ann. Surg. 2004, 239, 536. [Google Scholar] [CrossRef]
- Chowdhry, A.K.; Fung, C.; Chowdhry, V.K.; Bergsma, D.; Dhakal, S.; Constine, L.S.; Milano, M.T. A Population-Based Study of Prognosis and Survival in Patients with Second Primary Thyroid Cancer after Hodgkin Lymphoma. Leuk. Lymphoma 2018, 59, 1180–1187. [Google Scholar] [CrossRef]
- Friedman, D.L.; Whitton, J.; Leisenring, W.; Mertens, A.C.; Hammond, S.; Stovall, M.; Donaldson, S.S.; Meadows, A.T.; Robison, L.L.; Neglia, J.P. Subsequent Neoplasms in 5-Year Survivors of Childhood Cancer: The Childhood Cancer Survivor Study. JNCI J. Natl. Cancer Inst. 2010, 102, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Kamran, S.C.; Berrington de Gonzalez, A.; Ng, A.; Haas-Kogan, D.; Viswanathan, A.N. Therapeutic Radiation and the Potential Risk of Second Malignancies. Cancer 2016, 122, 1809–1821. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.Y.; Lim, J.; Oh, C.; Ryu, J.; Jung, K.; Chung, J.H.; Won, Y.; Kim, S.W. Elevated Risks of Subsequent Primary Malignancies in Patients with Thyroid Cancer: A Nationwide, Population-based Study in Korea. Cancer 2015, 121, 259–268. [Google Scholar] [CrossRef]
- Lebel, M.; Monnat Jr, R.J. Werner Syndrome (WRN) Gene Variants and Their Association with Altered Function and Age-Associated Diseases. Ageing Res. Rev. 2018, 41, 82–97. [Google Scholar] [CrossRef]
- Gustafson, S.; Zbuk, K.M.; Scacheri, C.; Eng, C. Cowden Syndrome; Elsevier: Amsterdam, The Netherlands, 2007; Volume 34, pp. 428–434. [Google Scholar]
- Kirova, Y.; De Rycke, Y.; Gambotti, L.; Pierga, J.; Asselain, B.; Fourquet, A. Second Malignancies after Breast Cancer: The Impact of Different Treatment Modalities. Br. J. Cancer 2008, 98, 870–874. [Google Scholar] [CrossRef]
- Nielsen, S.M.; White, M.G.; Hong, S.; Aschebrook-Kilfoy, B.; Kaplan, E.L.; Angelos, P.; Kulkarni, S.A.; Olopade, O.I.; Grogan, R.H. The Breast–Thyroid Cancer Link: A Systematic Review and Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2016, 25, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Trinh, L.N.; Crawford, A.R.; Hussein, M.H.; Zerfaoui, M.; Toraih, E.A.; Randolph, G.W.; Kandil, E. Deciphering the Risk of Developing Second Primary Thyroid Cancer Following a Primary Malignancy—Who Is at the Greatest Risk? Cancers 2021, 13, 1402. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.; He, X.; Yu, L.; Ni, C.; Chen, H.; Guan, D.; Sun, K.; Zou, H. Breast Cancer Prognosis Is Better in Patients Who Develop Subsequent Metachronous Thyroid Cancer. PLoS ONE 2019, 14, e0215948. [Google Scholar] [CrossRef]
- Bolf, E.L.; Sprague, B.L.; Carr, F.E. A Linkage between Thyroid and Breast Cancer: A Common Etiology? Metachronous Thyroid and Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 643–649. [Google Scholar] [CrossRef]
- Li, C.I.; Rossing, M.A.; Voigt, L.F.; Daling, J.R. Multiple Primary Breast and Thyroid Cancers: Role of Age at Diagnosis and Cancer Treatments (United States). Cancer Causes Control 2000, 11, 805–811. [Google Scholar] [CrossRef]
- Qin, X.-J.; Lin, X.; Xue, G.; Fan, H.-L.; Wang, H.-Y.; Wu, J.-F.; Pei, D. CXCL10 Is a Potential Biomarker and Associated with Immune Infiltration in Human Papillary Thyroid Cancer. Biosci. Rep. 2021, 41, BSR20203459. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Ferrari, S.M.; Fallahi, P.; Frascerra, S.; Piaggi, S.; Gelmini, S.; Lupi, C.; Minuto, M.; Berti, P.; Benvenga, S. Dysregulation of Secretion of CXC α-Chemokine CXCL10 in Papillary Thyroid Cancer: Modulation by Peroxisome Proliferator-Activated Receptor-γ Agonists. Endocr.-Relat. Cancer 2009, 16, 1299–1311. [Google Scholar] [CrossRef]
- Lee, J.-J.; Au, A.Y.; Foukakis, T.; Barbaro, M.; Kiss, N.; Clifton-Bligh, R.; Staaf, J.; Borg, Å.; Delbridge, L.; Robinson, B.G. Array-CGH Identifies Cyclin D1 and UBCH10 Amplicons in Anaplastic Thyroid Carcinoma. Endocr.-Relat. Cancer 2008, 15, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Fluge, Φ.; Bruland, O.; Akslen, L.A.; Lillehaug, J.R.; Varhaug, J.E. Gene Expression in Poorly Differentiated Papillary Thyroid Carcinomas. Thyroid 2006, 16, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Erickson, L.A.; Yousef, O.M.; Jin, L.; Lohse, C.M.; Pankratz, V.S.; Lloyd, R.V. P27kip1 Expression Distinguishes Papillary Hyperplasia in Graves’ Disease from Papillary Thyroid Carcinoma. Mod. Pathol. 2000, 13, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.-N.; Yang, P.-S.; Lee, J.-J.; Wang, T.-Y.; Hsu, Y.-C.; Cheng, S.-P. Recurrence-Associated Genes in Papillary Thyroid Cancer: An Analysis of Data from The Cancer Genome Atlas. Surgery 2017, 161, 1642–1650. [Google Scholar] [CrossRef]
- Pan, Z.; Li, L.; Fang, Q.; Qian, Y.; Zhang, Y.; Zhu, J.; Ge, M.; Huang, P. Integrated Bioinformatics Analysis of Master Regulators in Anaplastic Thyroid Carcinoma. BioMed Res. Int. 2019, 2019, 9734576. [Google Scholar] [CrossRef]
- Mizutani, K.; Onda, M.; Asaka, S.; Akaishi, J.; Miyamoto, S.; Yoshida, A.; Nagahama, M.; Ito, K.; Emi, M. Overexpressed in Anaplastic Thyroid Carcinoma-1 (OEATC-1) as a Novel Gene Responsible for Anaplastic Thyroid Carcinoma. Cancer 2005, 103, 1785–1790. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Barry, M.J.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; Krist, A.H.; Kurth, A.E. Screening for Thyroid Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2017, 317, 1882–1887. [Google Scholar]


| Observed | SIR (95%CI) | AER | Mean Age at SPTC | |
|---|---|---|---|---|
| Oral Cavity and Pharynx | 318 | 3.1 (2.77–3.46) | 3.81 | 59.39 |
| Lip | 11 | 1.3 (0.65–2.32) | 0.5 | 63.32 |
| Tongue | 113 | 3.8 (3.13–4.56) | 5.23 | 59.37 |
| Salivary Gland | 61 | 4.36 (3.34–5.6) | 6.55 | 55.23 |
| Floor of Mouth | 15 | 3.08 (1.72–5.08) | 3.83 | 61.21 |
| Gum and Other Mouth | 40 | 3.05 (2.18–4.16) | 3.93 | 64.19 |
| Nasopharynx | 15 | 2.48 (1.39–4.09) | 2.36 | 59.78 |
| Tonsil | 38 | 1.99 (1.4–2.72) | 1.69 | 58.87 |
| Oropharynx | 8 | 3.21 (1.39–6.33) | 3.86 | 57.91 |
| Hypopharynx | 11 | 3.35 (1.67–6.0) | 3.94 | 61.56 |
| Digestive System | 1243 | 2.01 (1.9–2.12) | 1.96 | 63.13 |
| Esophagus | 32 | 2.2 (1.51–3.11) | 2.06 | 61.16 |
| Stomach | 97 | 2.72 (2.2–3.31) | 3.18 | 61.74 |
| Small Intestine | 56 | 2.83 (2.14–3.68) | 3.76 | 63.57 |
| Colon and Rectum | 849 | 1.85 (1.73–1.98) | 1.66 | 63.85 |
| Colon excluding Rectum | 558 | 1.8 (1.65–1.95) | 1.53 | 64.89 |
| Cecum | 111 | 1.73 (1.43–2.09) | 1.38 | 67.22 |
| Appendix | 20 | 2.24 (1.37–3.46) | 2.68 | 62.87 |
| Ascending Colon | 87 | 1.59 (1.27–1.96) | 1.1 | 65.93 |
| Hepatic Flexure | 18 | 1.26 (0.75–1.99) | 0.47 | 66.08 |
| Transverse Colon | 54 | 2.11 (1.59–2.76) | 2.1 | 64.48 |
| Splenic Flexure | 16 | 1.56 (0.89–2.54) | 1.06 | 65.14 |
| Descending Colon | 37 | 1.93 (1.36–2.66) | 1.78 | 59.96 |
| Sigmoid Colon | 203 | 1.92 (1.67–2.21) | 1.83 | 64.72 |
| Rectum and Rectosigmoid Junction | 291 | 1.98 (1.76–2.22) | 1.93 | 61.86 |
| Anal Canal and Anorectum | 34 | 1.57 (1.09–2.19) | 1.35 | 62.85 |
| Liver and Intrahepatic Bile Duct | 58 | 2.37 (1.8–3.06) | 2.39 | 63.27 |
| Liver | 55 | 2.42 (1.82–3.15) | 2.45 | 63.34 |
| Intrahepatic Bile Duct | 3 | 1.66 (0.34–4.86) | 1.44 | 61.92 |
| Gallbladder | 7 | 1.55 (0.62–3.19) | 1.22 | 63.37 |
| Other Biliary | 17 | 2.74 (1.59–4.38) | 3.35 | 58.8 |
| Pancreas | 69 | 2.89 (2.25–3.65) | 3.87 | 59.57 |
| Retroperitoneum | 11 | 3.19 (1.59–5.7) | 4.05 | 64.35 |
| Peritoneum and Mesentery | 11 | 2.04 (1.02–3.65) | 2.77 | 52.62 |
| Respiratory System | 613 | 2.44 (2.25–2.64) | 2.89 | 66.24 |
| Nose, Nasal Cavity and Middle Ear | 25 | 4.12 (2.66–6.08) | 5.79 | 61.82 |
| Larynx | 103 | 3.4 (2.77–4.12) | 4.06 | 63.26 |
| Lung and Bronchus | 483 | 2.26 (2.06–2.47) | 2.61 | 67.19 |
| Bones and Joints | 30 | 3.65 (2.46–5.21) | 3.59 | 45.85 |
| Soft Tissue including Heart | 138 | 4.51 (3.79–5.33) | 5.94 | 53.86 |
| Skin excluding Basal and Squamous | 695 | 2.19 (2.03–2.36) | 2.52 | 58.19 |
| Melanoma of the Skin | 639 | 2.15 (1.99–2.32) | 2.45 | 58.09 |
| Other Non-Epithelial Skin | 56 | 2.82 (2.13–3.66) | 3.35 | 59.37 |
| Breast | 2305 | 1.58 (1.51–1.64) | 1.65 | 60.56 |
| Female Genital System | 834 | 1.74 (1.62–1.86) | 2.15 | 58.46 |
| Cervix Uteri | 141 | 1.63 (1.37–1.92) | 1.87 | 50.86 |
| Corpus and Uterus | 478 | 1.73 (1.58–1.89) | 2.14 | 61.43 |
| Ovary | 161 | 1.82 (1.55–2.12) | 2.34 | 56.03 |
| Vagina | 13 | 3.68 (1.96–6.3) | 7.01 | 59.57 |
| Vulva | 30 | 1.67 (1.13–2.39) | 1.81 | 61.23 |
| Male Genital System | 1167 | 1.34 (1.26–1.42) | 0.5 | 68.61 |
| Prostate | 1105 | 1.31 (1.23–1.38) | 0.46 | 69.98 |
| Testis | 58 | 2.69 (2.05–3.48) | 1.36 | 42.44 |
| Penis | 3 | 1.14 (0.24–3.33) | 0.2 | 70.45 |
| Urinary System | 826 | 2.43 (2.26–2.6) | 2.57 | 63.62 |
| Urinary Bladder | 247 | 1.34 (1.18–1.52) | 0.59 | 68.27 |
| Kidney and Renal Pelvis | 573 | 3.77 (3.47–4.1) | 5.31 | 61.48 |
| Ureter | 4 | 1.41 (0.38–3.6) | 0.74 | 70.4 |
| Eye and Orbit | 33 | 3.32 (2.29–4.67) | 3.94 | 62.11 |
| Brain and Other Nervous System | 74 | 2.24 (1.76–2.82) | 1.75 | 45.16 |
| Brain | 62 | 2.2 (1.69–2.83) | 1.68 | 42.5 |
| Cranial Nerves | 12 | 2.48 (1.28–4.33) | 2.24 | 58.86 |
| Lymphoma | 716 | 2.93 (2.72–3.15) | 3.64 | 58.16 |
| Hodgkin Lymphoma | 115 | 3.18 (2.62–3.82) | 3.4 | 41.41 |
| Hodgkin—Nodal | 111 | 3.15 (2.59–3.79) | 3.35 | 41.07 |
| Hodgkin—Extranodal | 4 | 4.25 (1.16–10.8) | 5.5 | 50.81 |
| Non-Hodgkin Lymphoma | 601 | 2.89 (2.66–3.13) | 3.69 | 61.36 |
| NHL—Nodal | 382 | 2.77 (2.5–3.06) | 3.46 | 60.52 |
| NHL—Extranodal | 219 | 3.12 (2.72–3.56) | 4.12 | 62.83 |
| Myeloma | 89 | 1.91 (1.54–2.35) | 1.79 | 63.16 |
| Leukemia | 187 | 1.91 (1.64–2.2) | 1.44 | 59.29 |
| Lymphocytic Leukemia | 125 | 1.96 (1.63–2.33) | 1.45 | 62.23 |
| Acute Lymphocytic Leukemia | 13 | 2.47 (1.32–4.23) | 0.68 | 32.57 |
| Chronic Lymphocytic Leukemia | 105 | 1.95 (1.6–2.36) | 1.83 | 65.89 |
| Myeloid and Monocytic Leukemia | 60 | 1.89 (1.44–2.43) | 1.57 | 53.92 |
| Chronic Myeloid Leukemia | 31 | 1.93 (1.31–2.74) | 1.7 | 58.69 |
| Mesothelioma | 9 | 3.9 (1.78–7.4) | 5.33 | 65.78 |
| Kaposi Sarcoma | 6 | 1.58 (0.58–3.45) | 0.61 | 71.07 |
| Characteristics | Levels | SIR | 95%CI | AER | Z Score |
|---|---|---|---|---|---|
| Gender | Male | 2.01 | 1.94–2.08 | 1.42 | Reference |
| Female | 1.83 | 1.79–1.88 | 2.30 | −4.24 *** | |
| Age, years | <5 | 18.03 | 5.10–46.4 | 0.95 | Reference |
| 5–24 | 4.90 | 4.08–12.0 | 8.31 | −1.22 | |
| 25–44 | 2.26 | 2.04–2.65 | 11.1 | −1.50 | |
| 45–64 | 1.95 | 1.87–2.09 | 9.29 | −1.53 | |
| 65–84 | 1.67 | 1.51–1.79 | 4.46 | −1.55 | |
| 85+ | 1.22 | 0.97–1.53 | 0.23 | −1.60 | |
| Race | White | 1.84 | 1.80–1.88 | 1.80 | Reference |
| Black | 1.87 | 1.73–2.02 | 1.22 | 0.39 | |
| American Indian/Alaska Native | 2.34 | 1.70–3.13 | 2.69 | 1.37 | |
| Asian or Pacific Islander | 2.67 | 2.49–2.86 | 3.46 | 8.59 *** | |
| Grade | Well differentiated | 1.77 | 1.67–1.88 | 1.90 | Reference |
| Moderately differentiated | 1.78 | 1.71–1.84 | 1.57 | 0.16 | |
| Poorly differentiated | 1.70 | 1.63–1.78 | 1.47 | −1.06 | |
| Undifferentiated | 1.82 | 1.59–2.08 | 1.65 | 0.37 | |
| Laterality | Bilateral | 2.07 | 1.62–2.61 | 2.76 | Reference |
| Right-sided | 2.01 | 1.93–2.09 | 2.46 | −0.23 | |
| Left-sided | 1.93 | 1.85–2.01 | 2.29 | −0.55 | |
| Not a paired organ | 1.82 | 1.77–1.87 | 1.48 | Reference | |
| Midline tumor | 5.21 | 3.09–8.24 | 8.90 | 2.58 ** | |
| Tumor stage | T0 | 3.72 | 1.60–7.32 | 6.42 | Reference |
| T1 | 2.34 | 1.58–4.92 | 5.05 | −0.82 | |
| T2 | 2.64 | 1.12–35.1 | 2.37 | −0.12 | |
| T3 | 3.09 | 1.56–77.9 | 2.66 | −0.03 | |
| T4 | 3.48 | 1.23–52.0 | 3.99 | −0.02 | |
| LN infiltration | N0 | 2.34 | 1.63–6.25 | 6.36 | Reference |
| N1 | 2.92 | 1.90–4.23 | 4.61 | 0.44 | |
| N2 | 3.21 | 2.10–5.22 | 5.30 | 0.61 | |
| N3 | 3.45 | 1.46–8.39 | 7.80 | 0.52 | |
| Distal metastasis | M0 | 2.50 | 2.40–2.61 | 3.52 | Reference |
| M1 | 2.34 | 1.41–4.34 | 3.20 | −0.21 | |
| LVI | No | 5.89 | 3.22–9.88 | 4.04 | Reference |
| Yes | 4.85 | 1.57–11.3 | 2.89 | −0.35 | |
| Clinical stage | I | 2.40 | 1.28–14.19 | 14.19 | Reference |
| II | 2.36 | 1.64–14.24 | 14.24 | −0.01 | |
| III | 3.49 | 1.62–15.76 | 15.76 | 0.22 | |
| IV | 2.98 | 1.84–5.44 | 5.44 | 0.17 | |
| Management | Primary site surgery | 1.90 | 1.86–1.93 | 1.85 | Reference |
| Radiotherapy | 1.78 | 1.55–2.73 | 2.15 | −0.40 | |
| Chemotherapy | 2.10 | 2.03–2.18 | 2.58 | 4.74 *** | |
| Surgery and radiation | 1.86 | 1.08–5.70 | 2.23 | −0.03 |
| Site | Primary Site Surgery | Radiotherapy | Chemotherapy |
|---|---|---|---|
| All Sites | 1.90 (1.86–1.854) | 1.78 (1.55–2.73) | 2.10 (2.03–2.18) |
| Oral Cavity and Pharynx | 3.10 (2.77–3.46) | 3.08 (2.65–3.57) | 3.17 (2.62–3.81) |
| Salivary Gland | 4.36 (3.34–5.60) | 4.11 (2.77–5.87) | 3.74 (1.02–9.57) |
| Mouth | 3.08 (1.72–5.08) | 4.55 (1.96–8.96) | 7.46 (2.42–17.4) |
| Tonsil | 1.99 (1.40–2.72) | 1.59 (1.04–2.32) | 1.85 (1.16–2.81) |
| Oropharynx | 3.21 (1.39–6.33) | 3.51 (1.41–7.22) | 4.41 (1.77–9.08) |
| Nasopharynx | 2.48 (1.39–4.09) | 2.86 (1.60–4.72) | 2.76 (1.47–4.72) |
| Hypopharynx | 3.35 (1.67–6.00) | 3.61 (1.73–6.64) | 3.68 (1.59–7.25) |
| Digestive System | 2.01 (1.90–2.12) | 2.31 (2.03–2.62) | 2.30 (2.12–2.50) |
| Esophagus | 2.20 (1.51–3.11) | 2.97 (1.92–4.39) | 2.78 (1.82–4.08) |
| Stomach | 2.72 (2.20–3.31) | 3.10 (2.03–4.54) | 3.01 (2.18–4.05) |
| Small Intestine | 2.83 (2.14–3.68) | NA | 3.09 (1.48–5.69) |
| Colon and Rectum | 1.85 (1.73–1.98) | 2.23 (1.88–2.64) | 2.30 (2.08–2.54) |
| Liver and Bile Duct | 2.37 (1.80–3.06) | 4.49 (1.22–11.5) | 2.03 (1.27–3.07) |
| Liver | 2.42 (1.82–3.15) | 3.46 (0.42–12.5) | 2.15 (1.33–3.28) |
| Intrahepatic Bile Duct | 1.66 (0.34–4.86) | 6.39 (0.77–23.1) | 0.96 (0.02–5.33) |
| Gallbladder | 1.55 (0.62–3.19) | 4.40 (1.2–11.27) | 3.32 (1.08–7.75) |
| Pancreas | 2.89 (2.25–3.65) | 1.55 (0.71–2.93) | 2.05 (1.35–2.98) |
| Respiratory System | 2.44 (2.25–2.64) | 2.35 (2.05–2.68) | 2.16 (1.88–2.47) |
| Nasal Cavity & Middle Ear | 4.12 (2.66–6.08) | 4.45 (2.55–7.23) | 3.94 (1.45–8.58) |
| Larynx | 3.40 (2.77–4.12) | 3.18 (2.50–3.98) | 2.89 (1.83–4.34) |
| Lung and Bronchus | 2.26 (2.06–2.47) | 1.92 (1.60–2.29) | 2.05 (1.77–2.38) |
| Bones and Joints | 3.65 (2.46–5.21) | 5.60 (2.42–11.0) | 7.33 (4.1–12.09) |
| Soft Tissue | 4.51 (3.79–5.33) | 4.48 (3.37–5.83) | 6.36 (4.38–8.93) |
| Skin | 2.19 (2.03–2.36) | 3.09 (1.77–5.02) | 3.57 (1.84–6.23) |
| Melanoma of the Skin | 2.15 (1.99–2.32) | 3.76 (1.62–7.40) | 3.81 (1.83–7.01) |
| Other Non-Epithelial Skin | 2.82 (2.13–3.66) | 2.63 (1.13–5.17) | 2.70 (0.33–9.77) |
| Breast | 1.58 (1.51–1.64) | 1.52 (1.43–1.61) | 1.71 (1.62–1.82) |
| Female Genital System | 1.74 (1.62–1.86) | 2.04 (1.66–2.47) | 2.05 (1.81–2.32) |
| Cervix Uteri | 1.63 (1.37–1.92) | 1.95 (1.34–2.74) | 2.20 (1.71–2.78) |
| Corpus and Uterus | 1.72 (1.57–1.89) | 1.97 (1.48–2.58) | 2.73 (2.16–3.39) |
| Ovary | 1.82 (1.55–2.12) | 2.87 (0.59–8.38) | 1.63 (1.32–2.00) |
| Vagina | 3.68 (1.96–6.30) | 5.18 (1.9–11.27) | 4.12 (1.66–8.49) |
| Vulva | 1.67 (1.13–2.39) | 2.11 (0.77–4.58) | 3.26 (1.19–7.09) |
| Male Genital System | 1.34 (1.26–1.42) | 1.35 (1.19–1.52) | 2.76 (1.80–4.05) |
| Prostate | 1.31 (1.23–1.38) | 1.30 (1.14–1.48) | 2.68 (1.28–4.93) |
| Testis | 2.69 (2.05–3.48) | 2.46 (1.48–3.84) | 2.92 (1.67–4.75) |
| Urinary System | 2.43 (2.26–2.60) | 1.65 (0.75–3.13) | 1.73 (1.29–2.28) |
| Urinary Bladder | 1.34 (1.18–1.52) | 0.96 (0.20–2.82) | 1.46 (1.02–2.03) |
| Kidney and Renal Pelvis | 3.77 (3.47–4.10) | 2.53 (0.82–5.89) | 3.27 (1.83–5.39) |
| Eye and Orbit | 3.32 (2.29–4.67) | 2.05 (0.56–5.25) | 8.27 (1.7–24.16) |
| Brain and Nervous System | 2.24 (1.76–2.82) | 2.26 (1.60–3.10) | 2.27 (1.52–3.26) |
| Lymphoma | 2.93 (2.72–3.15) | 2.83 (2.40–3.31) | 3.04 (2.77–3.33) |
| Leukemia | 1.91 (1.64–2.20) | 2.35 (0.95–4.85) | 1.94 (1.55–2.41) |
| Recommended Follow-Up | Primary Site of Cancer |
|---|---|
| 5 Years | Liver |
| 10 Years | Colon & Rectum, Lung & Bronchus, Prostate, Melanoma |
| >10 Years | Breast, Lymphoma, Leukemia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, M.; Mueller, L.; Issa, P.P.; Haidari, M.; Trinh, L.; Toraih, E.; Kandil, E. Latency Trend Analysis as a Guide to Screening Malignancy Survivors for Second Primary Thyroid Cancer. Biomedicines 2022, 10, 1984. https://doi.org/10.3390/biomedicines10081984
Hussein M, Mueller L, Issa PP, Haidari M, Trinh L, Toraih E, Kandil E. Latency Trend Analysis as a Guide to Screening Malignancy Survivors for Second Primary Thyroid Cancer. Biomedicines. 2022; 10(8):1984. https://doi.org/10.3390/biomedicines10081984
Chicago/Turabian StyleHussein, Mohammad, Lauren Mueller, Peter P. Issa, Muhib Haidari, Lily Trinh, Eman Toraih, and Emad Kandil. 2022. "Latency Trend Analysis as a Guide to Screening Malignancy Survivors for Second Primary Thyroid Cancer" Biomedicines 10, no. 8: 1984. https://doi.org/10.3390/biomedicines10081984
APA StyleHussein, M., Mueller, L., Issa, P. P., Haidari, M., Trinh, L., Toraih, E., & Kandil, E. (2022). Latency Trend Analysis as a Guide to Screening Malignancy Survivors for Second Primary Thyroid Cancer. Biomedicines, 10(8), 1984. https://doi.org/10.3390/biomedicines10081984







