Abstract
More than 100 protostane triterpenoids have been isolated from the dried rhizomes of Alisma species, designated Alismatis rhizoma (AR), commonly used in Asian traditional medicine to treat inflammatory and vascular diseases. The main products are the alisols, with the lead compounds alisol-A/-B and their acetate derivatives being the most abundant products in the plant and the best-known bioactive products. The pharmacological effects of Ali-A, Ali-A 24-acetate, Ali-B, Ali-B 23-acetate, and derivatives have been analyzed to provide an overview of the medicinal properties, signaling pathways, and molecular targets at the origin of those activities. Diverse protein targets have been proposed for these natural products, including the farnesoid X receptor, soluble epoxide hydrolase, and other enzymes (AMPK, HCE-2) and functional proteins (YAP, LXR) at the origin of the anti-atherosclerosis, anti-inflammatory, antioxidant, anti-fibrotic, and anti-proliferative activities. Activities were classified in two groups. The lipid-lowering and anti-atherosclerosis effects benefit from robust in vitro and in vivo data (group 1). The anticancer effects of alisols have been largely reported, but, essentially, studies using tumor cell lines and solid in vivo data are lacking (group 2). The survey shed light on the pharmacological properties of alisol triterpenoids frequently found in traditional phytomedicines.
1. Introduction
The dried rhizomes of the plants Alisma orientale Juzepczu and Alisma plantago-aquatica (Alismataceae), commonly referred to as Alismatis rhizoma, have long been used in folk medicine to treat a variety of diseases, including hyperlipidemia, diabetes, bacterial infection, edema, oliguria, diarrhea, dizziness, and certain inflammatory diseases. The phytomedicine is known as Takusha in Japanese, and Taeksa in Korean and Zexie in Chinese (Figure 1). In China, Alismatis rhizoma (Zexie) can be further separated into “Chuan Zexie” (Sichuan and Hubei provinces), “Jian Zexie” (Fujian and Jiangxi provinces), and “Guang Zexie” (Guangxi province) according to the producing areas [1].
Figure 1.
Medicinal uses of Alismatis rhizoma. The dried rhizomes of the plant Alisma plantago-aquatica are used to prepare the traditional medicinal products in Asian countries and the medicine is used to treat different pathologies, as indicated.
Alismatis rhizoma is known to contain a large diversity of bioactive components, mainly terpenoids endowed with anti-inflammatory, antioxidant, and antimicrobial activities. A recent phytochemical survey has identified over 220 bioactive molecules, including di-, tri-, and sesqui-terpenoids, alkaloids, phenylpropanoids, flavonoids, steroids, and polysaccharides [2]. The main subgroup of triterpenes found in Alismatis rhizoma are the alisols, a family of 22 compounds designated alisol A to alisol V [3,4]. These compounds are tetracyclic protostane-type triterpenoids, with a variable side chain at position 17 (Figure 2). Hydroxyl groups can be present at positions 11, 23, and 24, eventually acetylated or implicated in an epoxide function. The protostane triterpene skeleton is relatively rare in nature [5]. In A. orientale, its biosynthesis is regulated by methyl jasmonate [6]. The leading compounds in the series are alisol A and its epoxide derivative alisol B (Figure 2) which have been largely studied for their diverse pharmacological effects. Both Ali-A and Ali-B display marked anti-inflammatory and anti-proliferative properties, useful for the treatment of diverse human pathologies, including metabolic disorders and cancers. In the present review, I have analyzed the main pharmacological properties of these bioactive natural products, and their known or proposed molecular targets. The pharmacology of these interesting natural products is discussed.
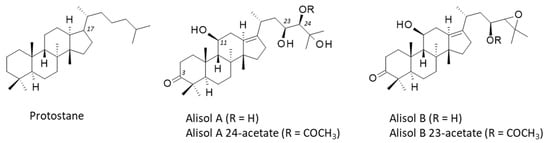
Figure 2.
Structures of the protostane terpenoid skeleton and the main alisol compounds, Ali-A/-B and their acetate derivatives. The numbering of specific positions (discussed in the text) is indicated.
2. TCM Made from Alismatis Rhizoma
Alismatis rhizoma (AR) is commonly used in traditional Chinese medicine (TCM). The two Alisma species which correspond to AR are largely cultivated, mainly in Fujian, Jiangxi, and Sichuan provinces (China). The rhizosphere microbiomes for the two plants are slightly distinct, with specific bacteria (such as Steroidobacter, Pseudolabrys, Nevskia, and Nitrospira) which are considered important for the biosynthesis and accumulation of protostane triterpenes in the plant tubers [7]. Alismatis rhizoma is listed in the 2020 edition of the Chinese Pharmacopoeia, with specific recommendations on quality standards [8]. Those rhizoma are included in several TCMs, such as Ye Tianshi used for the treatment of dampness and heat [9], Zexie Tang used to treat metabolic diseases and liver injuries [10], Liuwei Dihuang decoction used to improve kidney yin deficiency [11], Hugan Qingzhi formula which is used to treat chronic liver diseases [12], Gandou decoction used to treat Wilson’s disease [13,14], and Dangguishaoyao-san (also called Toki-shakuyaku-san or TJ-23) used to reduce the symptoms of Alzheimer’s disease [15,16]. Alismatis rhizoma is also present in different TCM prescriptions, such as Qi-Li-Qiang-Xin capsule used for the treatment of chronic heart failure [17,18] and Qing-Fei-Pai-Du which is a lung cleansing and detoxifying decoction used historically for the treatment of exogenous fever, and recently proposed to reduce symptoms associated with coronavirus disease 2019 (COVID-19) [19]. The product is also used in other traditional medicines, notably in traditional Japanese medicine (Kampo) with the preparation called Hachimijiogan which is used to alleviate anxiety disorders in patients with dementia [20], and in Korean medicine with the herbal medicine prescription called Oreongsan (also known as Goreisan in Japanese and Wulingsan in Chinese), containing AR, and used to treat symptoms such as edema, hangover, and diarrhea [21,22]. Another Korean prescription called Geumgwesingihwan is a traditional herbal product composed of 8 medicinal herbs, including Alisma plantago-aquatica, and contains Ali-B acetate [23]. I can also mention the Japanese herbal medicine Sairei-to (TJ-114, or Chai-Ling-Tang in Chinese) used to reduce lower urinary tract symptoms. Sairei-to is made from 12 medicinal herbs including AR [24,25]. There are also composite products based in part on AR, such as Yirui capsules, sold in health food stores, to improve the blood lipid state in patients with hyperlipidemia and to reduce the risk of atherosclerosis [26,27]. It is certain, AR is an essential component of Asian traditional medicine.
3. Alismatis Rhizoma Extracts: Phytoconstituents and Pharmacological Effects
Analytical methods have been developed to define the composition of Alismatis rhizoma (AR) extracts and to provide a quality control assessment for the preparations [28]. The triterpene composition can vary according to the geographic origin of the plant tubers, but the main compounds are always Ali-A and Ali-B [29]. A monitoring of the botanical source of AR is important, as it defines the phytochemical content of the extracts [2,30]. Crude AR extracts contain a multitude of triterpenoids and their composition also varies with the processing method. In a recent study, up to 114 triterpenes were identified from salt-processed and bran-processed AR extracts using state-of-the-art ultra-high performance liquid chromatography coupled with mass spectrometry [31]. Another study pointed out over 220 compounds, including di-/tri-/sesqui-terpenoids, alkaloids, phenylpropanoids, flavones, steroids, and polysaccharides from AR extracts [2]. New compounds are regularly identified from AR extracts, notably novel anti-inflammatory agents [32]. Once administered, these triterpenes are metabolized, thus producing additional new compounds and derivatives. More than 230 transformed constituents have been mentioned recently from orally administered AR extracts [33]. However, in all cases, the main bioactive compounds are the alisols, in particular Ali-A/-B/-C and their acetate derivatives [34]. Of course, other products can be found in AR extracts depending on the process used. For example, an essential oil from tubers of A. orientale can be specifically prepared, rich in aroma-active volatile compounds [35].
Extracts of Alismatis rhizoma are commonly used in China to lower cholesterol and as an anti-inflammatory agent. The TCM product is recommended for excreting dampness caused by a spleen deficiency, which relies on a diuretic effect. This effect has been well characterized when using an ethanol extract of AR, but not with an aqueous extract. In fact, the ethanolic extract displays a dual effect on renal function, promoting the diuretic activity at lower doses and inhibiting diuretic activity at higher doses [36]. The diuretic effect is largely supported by the alisol compounds [37]. RA extracts display a range of pharmacological effects, not limited to the induction of diuresis. They also present hepatoprotective, immunomodulatory, anti-osteoporotic, anti-inflammatory, antitumor, antibacterial, and antiviral activities, as described in previous comprehensive reviews [2,38,39]. AR extracts are considered to be safe and well tolerated in humans. A toxicology assessment of AR aqueous extracts in rats revealed no specific target organs and the oral no-observed-adverse-effect level (NOAEL) was >2 g/kg/day [40]. However, an evaluation with an ethanol extract pointed out a risk of chronic toxicity in kidneys when using a high dosage of the AR ethanol extract for an extended period [41]. The nephrotoxicity effect has been ascribed to the terpenoids found in those extracts and capable of inducing cell death (apoptosis) of kidney proximal tubular cells [42]. This is a paradoxical effect because the Chinese medicine Wuling San, which is made from AR and four other plants, is used to promote kidney function and diuresis. Wuling San has demonstrated a renal protective effect by modulating renal organic ion transporters in hyperuricemic mice [43]. In addition, a combination of the two Chinese herb decoctions, AR and Smilacis glabrae rhizoma, has been shown to reduce hyperuricemia and kidney damage in a rat model [44]. The nephrotoxicity reported in some studies with AR extracts may be attributed to specific compounds, such as alisol C, 16, 23-oxido-alisol B, and alisol O, which are considered more toxic than Ali-A and Ali-B [45].
4. Alisols and Related Terpenoids
In the plant, protostanes are biosynthesized from squalene with the support of squalene epoxidases regulated by methyl jasmonate [6,46]. There are 22 alisol compounds, designated alisol A (Ali-A) to alisol V (Ali-V), and a multitude of related compounds bearing an acetate group at position 23 or 24 of the side chain appended to the tetracyclic protostane unit. There are also derivatives with a modified side chain at C-17, equipped with an open or epoxy aliphatic side chain, with a fused ring at C-16/C-17 or with a modified protostane unit (nor- or seco-protostane). The series includes numerous derivatives, such as the alismanols, alismanins, alisolides, and others. In 2020, Wang and co-workers had identified more than 115 protostane-type terpenoids from Alisma species [47]. Here, we will essentially refer to Ali-A, -B, and -C, and occasionally to a few other compounds mentioned in Figure 2 and Figure 3. Most of them preserve the C-3 carbonyl group of characteristics of Ali-A and a highly oxygenated C-17 side chain. New protostane terpenoids are regularly identified and isolated from AR, taking advantage of novel, highly efficient, and precise analytical methods. For example, the two new compounds, called Alisma A and Alisma B, have been recently discovered using a new analytical methodology called ‘force iteration molecular designing’. With this approach, the authors identified 473 protostane terpenoids from the two plant species of AR [4]. Here, we will mainly focus on Ali-A and Ali-B to evoke their pharmacological effects and molecular targets. The different properties will be evocated in turn. The first three alisols, -A, -B, and -C, and their monoacetate derivatives were isolated in 1970 by researchers from Takeda Chemical Industries Ltd. (Osaka, Japan), and they also rapidly characterized the hypocholesterolemic activity of the products [48,49]. More than 50 years later, alisol A remains an interesting natural product mainly investigated for its multiple properties, and the knowledge gained from it has been utilized for hundreds of analogous natural products.
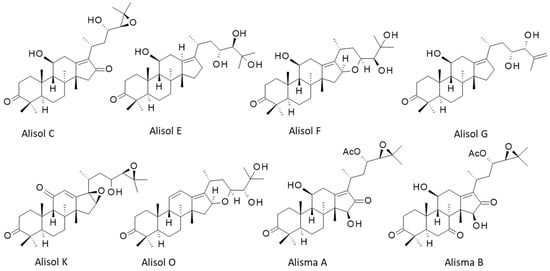
Figure 3.
Structures of 8 selected alisol derivatives. The series includes 22 compounds (Ali-A to Ali-V) and 100 derivatives.
5. Pharmacological Properties of Alisols
5.1. Lipid-Lowering Effects and Hepatoprotection
Historically, in Asia, AR has been known for its diuretic action. It was traditionally used to treat diabetes and swelling. A search of AR extracts for compounds with a hypocholesterolemic action was initiated in the late 1960s, leading to the identification of active fractions and then pure compounds. Ali-A/-B/-C, along with their monoacetates, were discovered to be capable of reducing plasma and liver cholesterol levels in rats after oral administration [49]. The mode of action for these compounds has been investigated much more deeply now, leading to a better understanding of the pathways implicated in the capacity of Ali-A/-B to reduce lipid accumulation and lipotoxicity. A recent work demonstrated how Ali-B regulates hepatic gene expression via the RARα-HNF4α-PPARγ cascade in a murine model of non-alcoholic steatohepatitis (NASH). Specifically, Ali-B enhances RARα gene expression, thereby reducing expression of HNF4α and PPARγ, leading to a suppressed expression of translocase fatty acid CD36 [50]. The lipid-lowering components of AR includes Ali-B, Ali-B 23-acetate, and Ali-C 23-acetate, which are believed to bind to the farnesoid X receptor (FXR) protein and to behave as agonists, according to a molecular docking study [51]. The hypothesis is entirely plausible because Ali-B 23-acetate has been shown to increase the expression of FXR in cultured hepatocyte L02 cells and to activate hepatic BSEP (bile salt export pump) signaling [52]. The capacity of Ali-B 23-acetate to function as a FXR agonist has been demonstrated in a model of renal ischemia-reperfusion injury. The compound was found to activate renal FXR and to induce FXR downstream gene expression in mouse kidneys [53], and also in a model of non-alcoholic steatohepatitis (NASH) [54]. It is a potent agonist of several pregnane X receptors (PXRs), including FXR, but also LXR and PPARα/δ/γ [55]. This compound displays marked hepato-protective functions, due to FXR-mediated gene regulation [56,57,58,59]. The related compound, Ali-A 24-acetate, is also able to reduce liver lipid deposition in hyperlipidemic mice via a blockade of AMPK activation to promote glucose metabolism and by promoting expression of ATP binding cassette transporters ABCG1 and ABCA1 [60,61]. The compound stimulates lipolysis of adipocytes by activating protein kinase A (PKA)-mediated phosphorylation of hormone-sensitive lipase [62]. These observations make Ali-A 24-acetate and Ali-B 23-acetate good candidates for the treatment of nonalcoholic fatty liver disease and its comorbidities [63]. They are hepatoprotective agents that are also useful to prevent or treat hepatic damages induced by chemicals [59].
5.2. Treatment of Atherosclerosis
Anti-atherosclerotic actions have been reported with Ali-A, its 24-acetate derivative and Ali-B 23-acetate. For the latter compound, the cholesterol-lowering effect is associated with an increase in FXR-BSEP signaling which leads to a reduction in liver cholesterol, hepatic lipolysis, and bile acids efflux. These cumulated effects concur in reducing damages of atherosclerosis [52]. In addition, the compound exerts anti-inflammatory effects, notably an inhibition of the production of inflammatory cytokines IL-12 (interleukin-12) and IFN-γ (interferon-γ), also contributing to the anti-atherosclerotic effect [64]. The capacity of Ali-B 23-acetate to markedly reduce the atherosclerotic plaque area and lipid accumulation is most likely the resultant of a cumulative effect implicating FXR agonism, anti-inflammatory effects and regulation of cholesterol synthesis and export (Figure 4). Indeed, Ali-B 23-acetate negatively regulates Acyl-CoA cholesterol acyltransferase 2 (ACAT2) in cultured Caco-2 cells and increases expression of ATP-binding cassette transfer proteins G5/G8 (ABCG5/G8) [65]. Multiple receptors and pathways contribute to the capacity of Ali-B 23-acetate to reduce accumulation of triglycerides and cholesterol, including the estrogen receptor α (ERα) to which the compound may bind directly, according to a molecular docking analysis [66]. However, other receptors have been proposed also. For example, the related compounds 16-hydroxy-Ali B 23-acetate and Ali-M 23-acetate have been predicted to bind, with a high affinity, to the liver X receptor β (LXRβ) on the basis of molecular docking and molecular dynamic simulations and to exert an agonist effect [67].
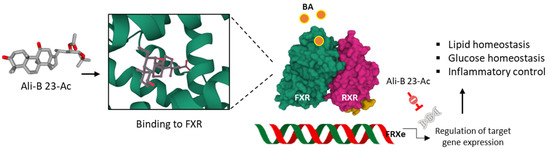
Figure 4.
Anti-atherosclerosis activity of Ali-B 23-acetate. The compound binds to the FRX protein and displays an agonist activity which leads to the regulation of the expression of genes implicated in the control of inflammation, glucose, and lipid levels. Via this mechanism, Ali-B 23-acetate reduces atherosclerotic plaque.
A distinct mechanism has been invoked to explain the anti-atherosclerotic activity of Ali-A. In this case, the compound activated the AMPK/SIRT1 signaling pathway, thereby inhibiting the formation of arterial plaques to block the progression of atherosclerosis [68]. A molecular docking study suggested that Ali-A could bind selectively to the catalytic region of AMPK, and, as such, the compound could activate the AMPK/acetyl-CoA carboxylase/SREBP-1c pathway, leading to reduced hepatic steatosis and improved liver function in a model of obese mice [69,70]. Ali-A can regulate lipid metabolism via different processes, including inhibition of inflammatory cytokine production. Its derivative Ali-A 24-acetate has been shown to inhibit the proliferation of aorta vascular smooth muscle cells induced by oxidized low-density lipoproteins, and to inhibit the expression of cyclin proteins (cyclin D1, E, p21, p27), also contributing to the reduction in atherosclerosis [71]. Ali-A 24-acetate can also inhibit activity of HMG-CoA reductase, presumably via a direct binding to HMG-CoA. This effect would also contribute to the observed reduction in total cholesterol [72]. Altogether, these different products, targets, and mechanisms contribute to the efficacy of AR to reduce hyperlipidemia and to combat atherosclerosis [73]. A recent network analysis pointed out the implications of 6 key targets regulated by AR (albumin, TNF, IL-1β, MMP9, and PPARα/γ) [74] whereas another study underlined the contribution of the FKBP/mTOR/SREBPs pathway and the direct binding of the active terpenoids to FK506-binding protein FKBP38 [10]. At least 9 triterpenes are considered to play a significant role in the lipid-lowering effect of AR (out of 87 triterpenes identified in the study) [75]. The complexity of the mechanism of action is not surprising. The natural medicine (mixture) is more potent than the individual compounds at regulating blood lipids, possibly due to synergistic effects between the compounds, with Ali-A and Ali-B being the most powerful lipid-regulating isolated products [76]. The best lipid-regulating effect has been obtained with a reconstituted mixture of Ali-A, Ali-B, and Ali-C 23-acetate in proportions of 3:1:1 [77].
5.3. Anti-Inflammatory Activity
Soluble epoxide hydrolase (sEH) is a therapeutic target considered for the treatment of inflammation-based diseases, and various pathologies, including cardiovascular diseases and neuropsychiatric disorders [78,79]. A methanol extract of AR was shown to attenuate the decrease in epoxide hydrolase induced in rats intoxicated with bromobenzene [80]. The extracts contained protostane triterpenoids acting as inhibitors of sEH, such as alismanin B, 11-deoxy-25-anhydro-Ali-E (Figure 5), and 11-deoxy-Ali-B with IC50 values of 7.15, 3.40, and 5.94 μM, respectively. The compounds were predicted to bind to the active site of she, acting as competitive inhibitors [81]. More recently, two other compounds in the series were described shesEH inhibitors: 3β-hydroxy-25-anhydro-Ali-F and 3β-hydroxy-Ali-G (2), with IC50 values of 10.06 and 30.45 μM, respectively [82]. In contrast to Ali-F, Ali-A and Ali-B have not been described as sEH inhibitors. Ali-F and 25-anhydro–Ali-F are interesting compounds because they both behave as inhibitors of lipopolysaccharide-induced NO production in macrophage RAW 264.7 macrophages [83] and regulate the production of inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, via the MAPK/STAT3/NFκB pathway [84]. sEH contributes to multiple human pathologies, chiefly inflammatory diseases but also cancer. Compounds such as Ali-E and Ali-F could be further exploited to design sEH-targeted drugs for the treatment of advanced liver cancers and other liver diseases.
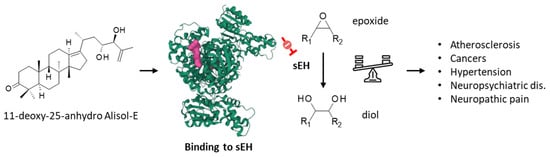
Figure 5.
Binding of 11-deoxy-25-anhydro alisol-E to soluble epoxide hydrolase (sEH), the enzyme which converts epoxide molecules into diol molecules, as represented. The modulation of the epoxide/diol ratio is a mechanism by which the compound exerts activities against multiple pathologies, as indicated.
Ali-B 23-acetate displays anti-inflammatory effects, apparently not via sEH inhibition, but through inhibition of the TLR4-NOX1/ROS signaling pathway. It also reduces production and infiltration of the same inflammatory cytokines as 25-anhydro-Ali-F. It suppressed expression of TLR4 and NOX2 and reduced the production of ROS [85]. Via these signaling pathways, the compound provides an anti-inflammatory action and maintains the integrity of the intestinal barrier as well [86,87]. Ali-B 23-acetate is an important contributor to the anti-inflammatory action of AR, but other protostane triterpenoids in the series also contribute, such as 16S,24S-dihydroxy-24-deacetyl-Ali-O and 16-oxo-11-anhydro-Ali-A, both characterized as anti-inflammatory agents [88].
5.4. Protection against Fibrosis and Kidney Injuries
Renal fibrosis is an important component of chronic kidney disease (CKD), which affects more than 10% of the world’s population [89]. Ali-B 23-acetate can attenuate the progression of CKD, at least in a rat model. An oral treatment with the compound (5–10 mg/kg) has been shown to inhibit expression of mRNA and proteins implicated in CKD pathogenesis, such as collagen I, fibronectin, vimentin, α-smooth muscle actin, and fibroblast-specific protein-1. Interestingly, the compound protected against renal fibrosis in part by re-establishing dysbiosis of the gut microbiome and regulating blood pressure (reducing hypertension). It did so by repressing the Wnt/β-catenin signaling pathway implicated in the activation and proliferation of renal fibroblasts [90]. The renin–angiotensin system and the Wnt/β-catenin axis play a role in kidney inflammation and fibrosis, and thus contribute to CKD. A treatment with Ali-B 23-acetate could reduce disease progression [91]. The activity is not specific to Ali-B 23-acetate, as the related product Ali-F 25-O-methyl has been shown also to attenuate tubulo-interstitial fibrosis, even if a distinct biochemical pathway (inhibition of TGF-β-mediated Smad3 phosphorylation) was invoked in that case [92].
The capacity of Ali-B 23-acetate to regulate the gut microbiome is beneficial not only for kidneys, but also for the gastro-intestinal tract and for immune regulation in general. The gut microbiota plays a role in metabolic diseases, cancers, and other pathologies. The probiotic regulation with Ali-B 23-acetate can reduce organ fibrosis (such as hepatic and renal fibrosis) but also colitis (associated with colon cancer) [86,93,94]. Ali-B 23-acetate and the other protostane triterpenoids of AR behave as regulators of the gut microecology. They can equilibrate the gut microflora, notably in cases of metabolic diseases and atherosclerosis [95,96].
5.5. Antioxidant Activity and Neuro-Protection
In the alisol series, the derivative Ali-A 24-acetate is an interesting cell-protective compound that is useful to limit brain damages. Both Ali-A and -B can cross the blood–brain barrier. Their acetate derivatives can also distribute in the brain tissue, although they preferentially accumulate in the intestine, stomach, liver, and kidneys, after oral administration [34]. Nevertheless, Ali-A 24-acetate has been shown to protect brain microvascular endothelial cells (BMEC) from damages induced by oxygen-glucose deprivation [97]. It exerts anti-apoptotic effects in this cell system [98] and also reduces apoptosis of neurons, via up-regulation of the expression of phosphorylated PI3K and AKT [99]. The blockade of thee PI3K/Akt/mTOR signaling pathway is important to the mechanism of action of AR and specifically to Ali-A 24-acetate, not only providing a protection against neuronal damages but also essential to the anticancer action of the extract and the product, as discussed thereafter [100].
5.6. Bone Preservation and Prevention of Osteoporosis
The acetate derivatives of Ali-A and Ali-B have been shown to protect against a decrease in bone mass. As a matter of fact, AR and different TCM preparations containing Alisma species (A. orientale, A. plantago-aquatica, A. canaliculatum) are used to regulate bone resorption, such as Yukmi-jihang-tang-Jahage [101] and Liuwei Dihuang [102]. Both Ali-A 24-acetate and Ali-C 23-acetate display anti-osteoporotic effects [103]. The latter compound is a potent inhibitor of osteoclastogenesis, acting via a blockade of RANKL-induced osteoclast differentiation and function (Figure 6) [104].
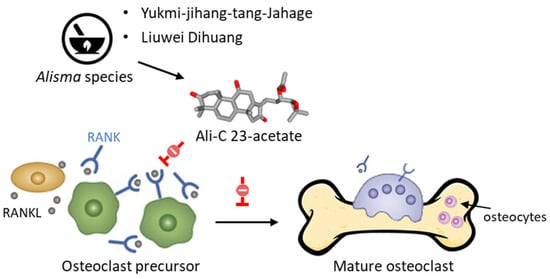
Figure 6.
Inhibition of RANKL-induced osteoclast differentiation and function by Ali-C 23-acetate (AC23A). The compound would act selectively on osteoblast precursors, rather than mature osteoblasts, by inhibiting RANKL-induced osteoclastogenesis. The compound blocks phosphorylation of JNK and reduces expression of osteoclastogenic mediators, such as TRAP, c-Fos, MMP9, NFATc1, and cathepsin K [104]. AC23A can be found in phytomedicines made from Alisma rhizoma, such as Liuwei Dihuang and Yukmi-jihang-tang-Jahage, both used to treat osteoporosis.
Ali-A 24-acetate also functions as an inhibitor of osteoclastogenesis. It inhibits RANKL-mediated osteoclast differentiation by downregulating the expression of nuclear factor of activated T cell 1 (NFATc1), which is an important regulator of osteolysis [105]. In an animal model of postmenopausal osteoporosis (ovariectomized mice), a daily administration of Ali-A 24-acetate was shown to prevent bone loss, inhibiting activity of the bone resorption marker TRAP and restoring the proportion of Treg and Th17 immune cells [106]. RANKL-mediated effects have been also reported with Ali-B, e.g., inhibition of osteoclast formation and suppression of hypercalcemia [107].
5.7. Anticancer Activity
There is ample experimental evidence to support the activity of alisol derivatives against cancer cell proliferation and tumor progression (Table 1). The main compounds Ali-A/-B and their acetate derivatives have been used in different tumor models, the other alisol derivatives have been used less frequently. However, Ali-F 24-acetate was shown to reverse multidrug resistance, thereby promoting nuclear accumulation of the cytotoxic drug doxorubicin and apoptosis of MCF-7 breast cancer cells [108]. A comparable reversal of P-glycoprotein-mediated multidrug resistance was evidenced with Ali-B 23-acetate [109]. There are not many studies comparing the anticancer efficacy of the different products, and the cell models used vary significantly from one study to another. Ali-A has revealed a marked capacity to reduce proliferation and invasion/migration of breast cancer cells [110,111], whereas Ali-B has shown a potent activity against hepatocellular carcinoma cells [112,113] and other models (Table 1). It is not easy to compare the potency of the products. However, there are common characteristics between the compounds, such as their capacity to induce G0/G1 cell cycle arrest and to trigger apoptosis via the mitochondrial pathway (Table 1).

Table 1.
Anticancer activity of alisol derivatives.
The PI3K/Akt/mTOR pathway is central to the mechanism of action of Ali-A/-B [100] but it is not the sole pathway implicated in the anti-proliferative and antimetastatic action of the natural products. A recent study pointed out the major implication of the Wnt/β-catenin axis in the mechanism of action of Ali-B 23-acetate in liver cancer [123]. The compound functioned as an inhibitor in SMMC-7721 and MHCC97 cancer cells to promote the anticancer action of the cardiac glycoside bufalin (an active ingredient of the traditional Chinese medicine Chansu) [123]. The same pathway has been invoked to explain the anti-fibrotic action of the product [91]. The complexity of the signaling pathways involved in the mechanism of action of natural products is not surprising, it is frequently the case with terpenoids [124].
At the molecular level, several protein targets have been evoked to account for the anticancer activity of Ali-A/-B, essentially on the basis of molecular modeling studies, such as the transcriptional coactivator YAP which could interact with Ali-A [115], the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase which can bind Ali-B [125], the soluble epoxide hydrolase [81], the farnesoid X receptor protein [51], human liver carboxylesterase 1 (HCE-2) [126], and hormone receptors [127] (Figure 7). Occasionally, non-protein targets have been mentioned as well, such as DNA. Both Ali-A and Ali-B have been docked onto DNA and shown to compete with DNA intercalation of the fluorescent dye ethidium bromide [128]. The c-myc DNA sequence has been considered as a potential target for Ali-A/-B acetates and the compounds’ mixture [129]. The hypothesis has not been validated experimentally at present. These terpenoids are multi-targeted products.
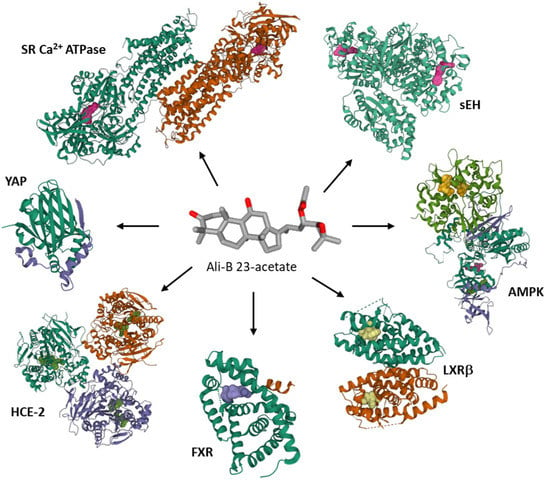
Figure 7.
Proposed protein targets for Ali-B 23-acetate. Molecular modeling studies have evaluated binding of the compound to soluble epoxide hydrolase (sEH) [81,82], 5’-adenosine monophosphate-activated protein kinase (AMPK) [70], liver X receptor β (LXRβ) [67], farnesoid X receptor (FXR) [51], human liver carboxylesterase 1 (HCE-2) [126], Yes-associated protein (YAP) [115], sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SR Ca2+-ATPase) [125]. The protein models shown correspond to the PDB structures 4HAI for sEH, 7MYJ for AMPK, 6K9H for LXRβ, 6HL1 for FXR, YA4 for HCE-1, 3KYS for YAP, 1VFP for SR Ca2+-ATPase.
There are many published in vitro studies with Ali-A/-B using different cell lines, but relatively little evidence of antitumor activity in vivo. A study reported the capacity of Ali-B 23-acetate to reduce growth of a subcutaneously implanted hepatocellular carcinoma tumor in mice, but it was a tumorigenesis experiments with tumor cells treated with the compound (at 30 μM for 24 h) prior to grafting the tumor cells to mice. The compound delayed the growth of the tumor in mice [117]. It is not an anticancer treatment sensu stricto. There is a lack of robust evidence of an antitumor action of alisols in xenograft models.
5.8. Anti-Allergic Activity
In traditional medicine, the dried rhizomes of Alisma orientale are used to treat allergic reactions. The capacity of AR extracts to inhibit antibody-mediated allergic reactions has been recognized more than 25 years ago and, at that time, the anti-allergic contribution of compounds such as Ali-A and Ali-B was already mentioned [130]. Ali-B 23-acetate has been characterized as a major inhibitor of 5-lipoxygenase-catalyzed leukotriene C4 production in rat basophilic leukemia RBL-1 cells [131]. This compound reduces IgE/antigen-induced degranulation of mast cells and ameliorates allergic reaction [132].
5.9. Antibacterial and Antiviral Effects
Ali-A/-B are not highly potent antibacterial agents per se. They showed little, if any, antimicrobial effects against pathogenic bacteria, such as Bacillus subtilis, Escherichia coli, and Staphylococcus aureus. Only minor effects have been reported with the derivatives such as Ali-G and 25-anhydro-Ali-F [83]. Both Ali-A 24-acetate and Ali-B 23-acetate have been found to be active against antibiotic-resistant strains of S. aureus and E. coli [133] but their antibacterial effects have not been further investigated. However, Ali-B 23-acetate is a useful regulator of gut microbiota, capable of reducing the expansion of pathogenic bacteria (such as Klebsiella, and Citrobacter) and increasing growth of beneficial bacteria (such as Bacteroides, and Lactobacillus) [94].
Similarly, Ali-A/-B are not highly effective antiviral compounds, although there are occasional reports of alisol derivatives being applied effectively against specific viruses, notably such as limiting the secretion of hepatitis B virus (HBV) surface antigen with a tri-acetate derivative of Ali-A and some derivatives [134,135]. Based on this observation, acylated derivatives of Ali-A have been designed, leading to the identification of tetra-acetyl Ali-A as an orally bioavailable derivative with satisfactory pharmacokinetic properties, such as being active against HBV in cultured HepG 2.2.15 cells [136]. However, no further development has been reported for this derivative.
6. Discussion and Perspectives
Ali-A/-B and their acetate derivatives are the most abundant triterpenes present in AR. They can serve as control markers to evaluate the quality of Zexie phyto-prepaprations of the Chinese Pharmacopoeia [75,137]. Ali-B and Ali-B 23-acetate are abundant in the plant roots, but also in the leaves, scapes, and inflorescences [138]. Ali-A is one of the quality markers which can be used to assess the quality of the TCM prescription Qiliqiangxin capsule (QLQX), used to treat chronic heart failure [18], whereas Ali-B 23-acetate is a quality marker for the Hugan Qingzhi formula used to treat nonalcoholic fatty liver disease [12]. However, the bioactivity not only depends on the quality of the phyto-preparation and the alisol content, but also on the respective proportions of the bioactive molecules, notably the ratio of Ali-A 24-acetate, Ali-B, and Ali-B 23-acetate [139]. There are robust methods to precisely quantified these compounds in AR extracts and methods to optimize their extraction also from AR, notably for Ali-B 23-acetate which is arguably the most potent and most abundant compound in the tubers [140].
As presented above, the main four alisol derivatives displays a large range of pharmacological effects. They have been tested in multiple models, either alone or within AR extracts, and occasionally using reconstituted mixtures. Evidence of activity has been reported in most cases, but the degree of efficacy is variable. On the one hand, the lipid-lowering effects, anti-inflammatory, anti-atherosclerosis, and antidiabetic activities have been firmly established, using both in vitro and robust in vivo models. Nobody can dispute the fact that the four compounds function as regulators of lipid levels in blood [64,95]. The capacity of both Ali-B and Ali-B 23-acetate to attenuate and to protect from non-alcoholic steatohepatitis (NASH) has been well-established in mice models with convincing data [50,54]. In contrast, we can challenge the anticancer activity reported with the same compounds, despite the multiplicity of in vitro studies with experimental cell lines. Evidence of a real anticancer effect, with xenograft models of tumors, are lacking and the potency of the compounds appear limited, at least when they were tested alone. There is no need to deny the anti-proliferative effects and pro-apoptotic activities evidenced in a variety of cell models (Table 1), but one must admit that at present there is no solid evidence of an antitumor effect in vivo with Ali-A or Ali-B in a xenograft model. There is a need to better characterize the anticancer effects of the compounds in xenografts and to consider further the combination of these lipid modulators with chemo- and immunotherapeutic drugs. Another aspect, perhaps also insufficiently exploited at present, is the capacity of AR and its constituents to regulate the microbiota. The probiotic effects of Ali-A 23-acetate evidenced recently could be useful to combat various pathologies affecting the liver and gastro-intestinal tract [86,94,95]. Other properties are secondary, such as the mild antibacterial and antiviral effects, not sufficiently robust to be exploited in terms of drug development.
The toxicological profile of the alisols needs to be properly defined. There is no published study formally evaluating the toxicity of these natural products, but there are reports on the toxicity of AR in a clinical setting and about the renal toxicity of Alisma orientalis [141]. In a rat model, renal damages have been observed after a long treatment with AR (on day 180) [142]. The dosing of AR is known to be important to control the diuretic and anti-diuretic effects observed with the products [36,143]. The molecular mechanism whereby Ali-A 24-acetate and Ali-B 23-acetate induce cell death (autophagy and then apoptosis) of human renal proximal tubular HK-2 cells has been detailed. The central point was the engagement of the PI3K/Akt/mTOR signaling pathway [144]. The two compounds contribute to the nephrotoxicity of AR, together with other terpenoids present in the extract [42]. A careful analysis of their toxicity profile is needed, all the more than the compounds bind strongly to blood proteins, such as hemoglobin [145] and they also distribute well in fat tissues [34], benefiting from an enterohepatic circulation [146].
7. Conclusions
Altogether, the pharmacological effects of Ali-A/-B and their acetate derivatives can be separated in two groups: (1) those with robust in vitro and in vivo data obtained with animal models representative of the studied human pathology; (2) those benefiting essentially, if not exclusively, from in vitro data, and for which additional supportive data are needed (Figure 8). The lipid-lowering, cell protective, and anti-inflammatory effects of Ali-A/-B have been fully characterized (group 1). In contrast, the anti-proliferative and pro-apoptotic activities of the compounds have been well evidenced at the cellular level, but further evidence to support an antitumoral effect in vivo are absolutely needed (group 2). The diverse TCM and phytomedicines containing AR and used to treat cardiovascular and inflammatory diseases can benefit from the pharmacological effects of the alisol compounds. The use of these natural medicines to prevent atherosclerosis and fibrosis can be encouraged.
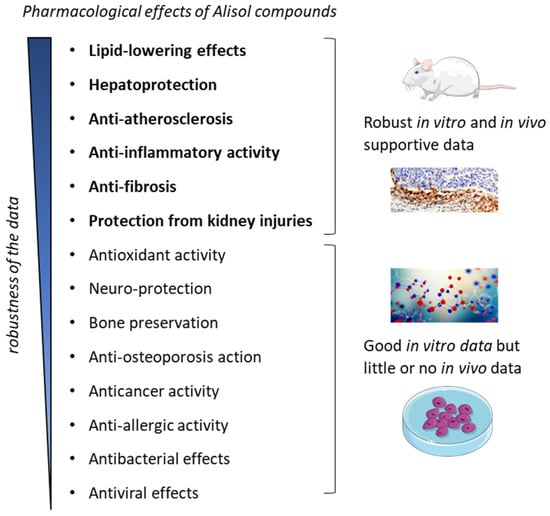
Figure 8.
Pharmacological effects reported with alisol derivatives. The effects can be separated into two categories: the effects benefiting from robust in vitro and in vivo data obtained with animal models representative of the pathology (indicated in bold) and the effects evidenced essentially using in vitro data, with purified molecular systems and/or laboratory cell lines.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Liu, S.S.; Wei, S.H.; Zhu, J.J.; Zhang, W.W.; Jiao, S.; Guo, J.; Yan, L.H.; Wang, Z.M. Herbal textural research, morphologic characteristics and DNA barcoding of botanical origins of Alismatis Rhizoma. Zhongguo Zhong Yao Za Zhi 2020, 45, 1536–1544. [Google Scholar] [PubMed]
- Liu, S.S.; Guo, J.; Li, Z.A.; Tian, S.S.; Zhu, J.J.; Yan, L.H.; Wang, Z.M.; Gao, L. Advances in studies on chemical compositions of Alismatis Rhizoma and their biological activities. Zhongguo Zhong Yao Za Zhi 2020, 45, 1578–1595. [Google Scholar] [PubMed]
- Feng, L.; Liu, T.T.; Huo, X.K.; Tian, X.G.; Wang, C.; Lv, X.; Ning, J.; Zhao, W.Y.; Zhang, B.J.; Sun, C.P.; et al. Alisma genus: Phytochemical constituents, biosynthesis, and biological activities. Phytother. Res. 2021, 35, 1872–1886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jin, Q.; Wu, W.; Jin, X.; An, Y.; Liu, C.; Wei, W.; Li, Z.; Yao, C.; Yao, S.; et al. “Force iteration molecular designing” strategy for the systematic characterization and discovery of new protostane triterpenoids from Alisma Rhizoma by UHPLC/LTQ-Orbitrap-MS. Anal. Bioanal. Chem. 2021, 413, 1749–1764. [Google Scholar] [CrossRef]
- Zhao, M.; Gödecke, T.; Gunn, J.; Duan, J.A.; Che, C.T. Protostane and fusidane triterpenes: A mini-review. Molecules 2013, 18, 4054–4080. [Google Scholar] [CrossRef] [PubMed]
- Rong, T.; Chunchun, Z.; Wei, G.; Yuchen, G.; Fei, X.; Tao, L.; Yuanyuan, J.; Chenbin, W.; Wenda, X.; Wenqing, W. Proteomic insights into protostane triterpene biosynthesis regulatory mechanism after MeJA treatment in Alisma orientale (Sam.) Juz. Biochim. Biophys. Acta Proteins Proteom. 2021, 1869, 140671. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Gu, W.; Tian, R.; Xu, F.; Han, Y.; Ji, Y.; Li, T.; Zhu, Y.; Lang, P.; Wu, W. Comparative analysis of the structure and function of rhizosphere microbiome of the Chinese medicinal herb Alisma in different regions. Arch. Microbiol. 2022, 204, 448. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.M.; Tian, S.S.; Liu, S.S.; Qu, Q.; Zhao, J.Y.; Yan, L.H.; Wang, Z.M. Recommendations on quality standards of Alismatis Rhizoma in Chinese Pharmacopoeia (2020 edition). Zhongguo Zhong Yao Za Zhi 2020, 45, 1566–1577. [Google Scholar] [PubMed]
- Liu, X.Y.; Li, Y.J.; Jiang, H.W.; Li, H.P.; Xiao, L.Y.; Zhao, Y.S. Comparison between Ye Tianshi and Xue Shengbai in treatment of diarrfea with damp-heat. Zhongguo Zhong Yao Za Zhi 2018, 43, 1720–1725. [Google Scholar] [PubMed]
- Xie, Z.; Li, E.W.; Gao, G.; Du, Y.; Wang, M.; Wang, H.; Wang, P.; Qiao, Y.; Su, Y.; Xu, J.; et al. Zexie Tang targeting FKBP38/mTOR/SREBPs pathway improves hyperlipidemia. J. Ethnopharmacol. 2022, 290, 115101. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, D.; Cheng, B.; Liu, G.; Zhang, Y.X.; Zhou, W.X. Active fraction combination from Liuwei Dihuang decoction (LW-AFC) ameliorates corticosterone-induced long-term potentiation (LTP) impairment in mice in vivo. J. Ethnopharmacol. 2019, 236, 147–154. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Xiao, C.X.; Wang, C.J.; Liang, J.; Cheng, Q.Q.; Zhang, L.; Zhou, B.J.; Zhou, H. Discovery of Quality Markers in Hugan Qingzhi Formula by Integrating a Lipid-Lowering Bioassay with UHPLC-QQQ-MS/MS. Evid.-Based Complement. Alternat. Med. 2020, 2020, 1594350. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Wu, H.; Wu, H.; Liu, X.; Zhou, A. Rapid identification of chemical profile in Gandou decoction by UPLC-Q-TOF-MSE coupled with novel informatics UNIFI platform. J. Pharm. Anal. 2020, 10, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Geng, H.; Wu, P.; Dong, J.; Li, H.; Xu, C.; Li, B.; Han, Y.; Cheng, N. Gandou decoction, a Chinese medicinal formula, in the treatment of hepatic injury by Wnt/β-catenin pathway regulation in models of Wilson disease. Ann. Palliat. Med. 2020, 9, 2872–2885. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, G.; Zhou, Y.; Yin, D.; Tao, C.; Han, L.; Yue, X.; Pan, Y.; Yao, Y.; Peng, D.; et al. The difference between blood-associated and water-associated herbs of Danggui-Shaoyao San in theory of TCM, based on serum pharmacochemistry. Biomed. Chromatogr. 2016, 30, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.R.; Chen, W.; Guo, C.Y.; Liao, W.T.; Yang, X.; Liao, F.E.; Lin, J.M.; Mei, H.F.; Zeng, Y. Dangguishaoyao-San attenuates LPS-induced neuroinflammation via the TLRs/NF-κB signaling pathway. Biomed. Pharmacother. 2018, 105, 187–194. [Google Scholar] [CrossRef]
- Yun, W.J.; Yao, Z.H.; Fan, C.L.; Qin, Z.F.; Tang, X.Y.; Gao, M.X.; Dai, Y.; Yao, X.S. Systematic screening and characterization of Qi-Li-Qiang-Xin capsule-related xenobiotics in rats by ultra-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1090, 56–64. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, Y.; Yang, K.; Zou, Z.; Fan, C.; Yao, Z.; Dai, Y.; Li, K.; Chen, J.; Yao, X. The discovery of Q-markers of Qiliqiangxin Capsule, a traditional Chinese medicine prescription in the treatment of chronic heart failure, based on a novel strategy of multi-dimensional “radar chart” mode evaluation. Phytomedicine 2021, 82, 153443. [Google Scholar] [CrossRef]
- Xu, F.; Hou, T.; Shen, A.; Jin, H.; Xiao, Y.; Yu, W.; Li, X.; Wang, J.; Liu, Y.; Liang, X. Mechanism deconvolution of Qing Fei Pai Du decoction for treatment of Coronavirus Disease 2019 (COVID-19) by label-free integrative pharmacology assays. J. Ethnopharmacol. 2021, 280, 114488. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Fukue, H.; Sato, H.; Hashimoto, K.; Fujikane, A.; Moriyama, H.; Watanabe, T.; Katsurabayashi, S.; Kainuma, M.; Iwasaki, K. The Traditional Japanese Herbal Medicine Hachimijiogan Elicits Neurite Outgrowth Effects in PC12 Cells and Improves Cognitive in AD Model Rats via Phosphorylation of CREB. Front. Pharmacol. 2017, 8, 850. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Kwon, S.; Jung, W.S.; Moon, S.K. Herbal Medicine, Oreongsan for Recurrent Chronic Subdural Hematoma: A Case Report. Explore 2017, 13, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Jin, C.; Cho, K.H. Oreongsan, an herbal medicine prescription developed as a new alternative treatment in patients with chronic subdural hematoma: A narrative review. Integr. Med. Res. 2019, 8, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.S.; Lee, M.Y. Simultaneous Determination of Fourteen Marker Compounds in the Traditional Herbal Prescription, Geumgwesingihwan, Using Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry. Molecules 2022, 27, 3890. [Google Scholar] [CrossRef]
- Hattori, T.; Nishimura, H.; Makino, B.; Shindo, S.; Kawamura, H. Sairei-to inhibits the production of endothelin-1 by nephritic glomeruli(2): Alisols, possible candidates as active compounds. Nihon Jinzo Gakkai Shi 1998, 40, 33–41. [Google Scholar] [PubMed]
- Wang, Z.; Zhu, L.; Huang, Y.; Peng, L. Successful treatment of immune-related cystitis by Chai-Ling-Tang (Sairei-To) in a gastric carcinoma patient: Case report and literature review. Explore 2022, S1550–S8307. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Zhong, L.L.; Cao, Y.; Chen, W.; Cheng, Y.; Lin, X.F.; Bian, Z.X.; Lu, A.P. Efficacy and safety of Yirui capsule in patients with hyperlipidemia: Study protocol for a multicenter, randomized, double-blind, placebo-controlled trial. Trials 2016, 17, 291. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xia, Z.; Rong, S.; Gao, H.; Yang, W.; Li, J.; Ma, C.; Deng, Q.; Huang, Q.; Xiao, L.; et al. Yirui Capsules Alleviate Atherosclerosis by Improving the Lipid Profile and Reducing Inflammation in Apolipoprotein E-Deficient Mice. Nutrients 2018, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.N.; Weng, Y.H.; Zhang, S.P.; Xu, W.; Li, X.Y.; Lin, Q.Q.; Chu, K.D.; Wu, S.S. Determination of seven ingredients of different grades Alismatis Rhizoma by QAMS method. Zhongguo Zhong Yao Za Zhi 2019, 44, 2292–2307. [Google Scholar] [PubMed]
- Wu, J.; Yang, W.; Pan, H.; Yao, S.; Wu, W.; Guo, D. Geographic impact evaluation of the quality of Alismatis Rhizoma by untargeted metabolomics and quantitative assay. J. Sep. Sci. 2018, 41, 839–846. [Google Scholar] [CrossRef]
- Yang, N.; Dong, Y.Q.; Wu, M.F.; Li, S.Z.; Yu, H.X.; Yang, S.S. Establishing a rapid classification and identification method for the major triterpenoids of Alisma orientale. Phytochem. Anal. 2020, 31, 384–394. [Google Scholar] [CrossRef]
- Dai, M.; Li, S.; Shi, Q.; Xiang, X.; Jin, Y.; Wei, S.; Zhang, L.; Yang, M.; Song, C.; Huang, R.; et al. Changes in Triterpenes in Alismatis rhizoma after Processing Based on Targeted Metabolomics Using UHPLC-QTOF-MS/MS. Molecules 2021, 27, 185. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Sheng, W.L.; Li, Y.; Zhang, S.S.; Zhu, J.J.; Gao, H.M.; Yan, L.H.; Wang, Z.M.; Gao, L.; Zhang, M. Chemical constituents from Alismatis Rhizoma and their anti-inflammatory activities in vitro and in vivo. Bioorg. Chem. 2019, 92, 103226. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, S.; Li, J.; Cheng, Z.; Feng, Y.; Ouyang, H.; Du, Z.; Jiang, H. Comprehensive metabolic profiling of Alismatis Rhizoma triterpenes in rats based on characteristic ions and a triterpene database. J. Pharm. Anal. 2021, 11, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, X.; Lin, N.; Zhang, X.; Huang, X.; Wu, T.; Tai, Y.; Chen, S.; Wu, C.H.; Huang, M.; et al. Pharmacokinetics and tissue distribution of five major triterpenoids after oral administration of Rhizoma Alismatis extract to rats using ultra high-performance liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2017, 146, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Yoshinaga, S.; Kashima, Y.; Nakahashi, H.; Hara, N.; Nakagawa, H.; Usami, A. Chemical Composition and Characteristic Odor Compounds in Essential Oil from Alismatis Rhizoma (Tubers of Alisma orientale). J. Oleo. Sci. 2016, 65, 91–97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feng, Y.L.; Chen, H.; Tian, T.; Chen, D.Q.; Zhao, Y.Y.; Lin, R.C. Diuretic and anti-diuretic activities of the ethanol and aqueous extracts of Alismatis rhizoma. J. Ethnopharmacol. 2014, 154, 386–390. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.Y.; Lin, N.; Zhao, W.L.; Huang, X.Q.; Chen, Y.; Huang, M.Q.; Xu, W.; Wu, S.S. Diuretic Activity of Compatible Triterpene Components of Alismatis rhizoma. Molecules 2017, 22, 1459. [Google Scholar] [CrossRef]
- Tian, T.; Chen, H.; Zhao, Y.Y. Traditional uses, phytochemistry, pharmacology, toxicology and quality control of Alisma orientale (Sam.) Juzep: A review. J. Ethnopharmacol. 2014, 158, 373–387. [Google Scholar] [CrossRef]
- Zhang, L.L.; Xu, W.; Xu, Y.L.; Chen, X.; Huang, M.; Lu, J.J. Therapeutic potential of Rhizoma Alismatis: A review on ethnomedicinal application, phytochemistry, pharmacology, and toxicology. Ann. N. Y. Acad. Sci. 2017, 1401, 90–101. [Google Scholar] [CrossRef]
- Lee, M.J.; Jung, H.K.; Lee, K.H.; Jang, J.H.; Sim, M.O.; Seong, T.G.; Ahn, B.K.; Shon, J.H.; Ham, S.H.; Cho, H.W.; et al. A 90-Day Repeated Oral Dose Toxicity Study of Alismatis Rhizoma Aqueous Extract in Rats. Toxicol. Res. 2019, 35, 191–200. [Google Scholar] [CrossRef]
- Wang, C.F.; Ma, L.; Hou, X.F.; Chen, H.F.; Feng, L.; Jia, X.B. Study on nephrotoxicity of ethanol extract from Alismatis Rhizoma in rats and its molecular mechanism. Zhongguo Zhong Yao Za Zhi 2016, 41, 3432–3438. [Google Scholar] [PubMed]
- Wang, C.F.; Ma, L.; Feng, L.; Yin, M.R.; Gu, J.F.; Jia, X.B. Evaluation of nephrotoxicity induced by total terpenoids from Alismatis Rhizoma on HK-2 cells in vitro and its induction of apoptosis. Zhongguo Zhong Yao Za Zhi 2016, 41, 490–497. [Google Scholar] [PubMed]
- Yang, Y.; Zhang, D.M.; Liu, J.H.; Hu, L.S.; Xue, Q.C.; Ding, X.Q.; Kong, L.D. Wuling San protects kidney dysfunction by inhibiting renal TLR4/MyD88 signaling and NLRP3 inflammasome activation in high fructose-induced hyperuricemic mice. J. Ethnopharmacol. 2015, 169, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Sun, H.; Li, X.; Yan, J.; Peng, Z.; You, Y.; Zhang, L.; Chen, J. Effects of Alismatis Rhizoma and Rhizoma Smilacis Glabrae Decoction on Hyperuricemia in Rats. Evid.-Based Complement. Alternat. Med. 2019, 2019, 4541609. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lu, L.; Zhang, Y.; Zhang, B. Study on discriminating nephrotoxic components in Zexie. Zhongguo Zhong Yao Za Zhi 2011, 36, 758–761. [Google Scholar] [PubMed]
- Tian, R.; Gu, W.; Gu, Y.; Geng, C.; Xu, F.; Wu, Q.; Chao, J.; Xue, W.; Zhou, C.; Wang, F. Methyl jasmonate promote protostane triterpenes accumulation by up-regulating the expression of squalene epoxidases in Alisma orientale. Sci. Rep. 2019, 9, 18139. [Google Scholar] [CrossRef]
- Wang, P.; Song, T.; Shi, R.; He, M.; Wang, R.; Lv, J.; Jiang, M. Triterpenoids From Alisma Species: Phytochemistry, Structure Modification, and Bioactivities. Front. Chem. 2020, 8, 363. [Google Scholar] [CrossRef]
- Murata, T.; Imai, Y.; Hirata, T.; Miyamoto, M. Biological-active trieterpenes of Alismatis rhizoma. I. Isolation of the alisols. Chem. Pharm. Bull. 1970, 18, 1347–1353. [Google Scholar] [CrossRef]
- Imai, Y.; Matsumura, H.; Aramaki, Y. Hypocholesterolemic effect of alisol A-24-monoacetate and its related compounds in rats. Jpn. J. Pharmacol. 1970, 20, 222–228. [Google Scholar] [CrossRef][Green Version]
- Zhao, Z.; Deng, Z.T.; Huang, S.; Ning, M.; Feng, Y.; Shen, Y.; Zhao, Q.S.; Leng, Y. Alisol B Alleviates Hepatocyte Lipid Accumulation and Lipotoxicity via Regulating RARα-PPARγ-CD36 Cascade and Attenuates Non-Alcoholic Steatohepatitis in Mice. Nutrients 2022, 14, 2411. [Google Scholar] [CrossRef]
- Chang, X.Y.; Wu, J.S.; Zhang, F.Q.; Li, Z.Z.; Jin, W.Y.; Wang, J.X.; Wang, W.H.; Shi, Y. A Strategy for Screening the Lipid-Lowering Components in Alismatis Rhizoma Decoction Based on Spectrum-Effect Analysis. J. Anal. Methods Chem. 2022, 2022, 2363242. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Feng, H.; Ding, X.; Meng, Q.H.; Zhang, S.R.; Li, J.; Chao, Y.; Ji, T.T.; Bi, Y.H.; Zhang, W.W.; et al. Alisol B 23-acetate adjusts bile acid metabolisim via hepatic FXR-BSEP signaling activation to alleviate atherosclerosis. Phytomedicine 2022, 101, 154120. [Google Scholar] [CrossRef] [PubMed]
- Luan, Z.L.; Ming, W.H.; Sun, X.W.; Zhang, C.; Zhou, Y.; Zheng, F.; Yang, Y.L.; Guan, Y.F.; Zhang, X.Y. A naturally occurring FXR agonist, alisol B 23-acetate, protects against renal ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 2021, 321, F617–F628. [Google Scholar] [CrossRef]
- Meng, Q.; Duan, X.P.; Wang, C.Y.; Liu, Z.H.; Sun, P.Y.; Huo, X.K.; Sun, H.J.; Peng, J.Y.; Liu, K.X. Alisol B 23-acetate protects against non-alcoholic steatohepatitis in mice via farnesoid X receptor activation. Acta Pharmacol. Sin. 2017, 38, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y.; Yatsu, T.; Yamashita, N.; Zhao, S.; Li, W.; Imai, M.; Kashima, M.; Inouye, Y.; Nemoto, K.; Koike, K. Alisol B 23-acetate from the rhizomes of Alisma orientale is a natural agonist of the human pregnane X receptor. Phytomedicine 2017, 26, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Chen, X.; Wang, C.; Liu, Q.; Sun, H.; Sun, P.; Peng, J.; Liu, K. Alisol B 23-acetate promotes liver regeneration in mice after partial hepatectomy via activating farnesoid X receptor. Biochem. Pharmacol. 2014, 92, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Chen, X.; Wang, C.; Liu, Q.; Sun, H.; Sun, P.; Huo, X.; Liu, Z.; Yao, J.; Liu, K. Protective Effects of Alisol B 23-Acetate Via Farnesoid X Receptor-Mediated Regulation of Transporters and Enzymes in Estrogen-Induced Cholestatic Liver Injury in Mice. Pharm. Res. 2015, 32, 3688–3698. [Google Scholar] [CrossRef]
- Meng, Q.; Chen, X.L.; Wang, C.Y.; Liu, Q.; Sun, H.J.; Sun, P.Y.; Huo, X.K.; Liu, Z.H.; Yao, J.H.; Liu, K.X. Alisol B 23-acetate protects against ANIT-induced hepatotoxity and cholestasis, due to FXR-mediated regulation of transporters and enzymes involved in bile acid homeostasis. Toxicol. Appl. Pharmacol. 2015, 283, 178–186. [Google Scholar] [CrossRef]
- Meng, Q.; Chen, X.; Wang, C.; Liu, Q.; Sun, H.; Sun, P.; Huo, X.; Liu, Z.; Liu, K. Protective effects of alisol B 23-acetate from edible botanical Rhizoma alismatis against carbon tetrachloride-induced hepatotoxicity in mice. Food Funct. 2015, 6, 1241–1250. [Google Scholar] [CrossRef]
- Zhou, X.; Ren, Q.; Wang, B.; Fang, G.; Ling, Y.; Li, X. Alisol A 24-Acetate Isolated from the Alismatis Rhizoma Improves Hepatic Lipid Deposition in Hyperlipidemic Mice by ABCA1/ABCG1 Pathway. J. Nanosci. Nanotechnol. 2019, 19, 5496–5502. [Google Scholar] [CrossRef]
- Chen, J.X.; Li, H.Y.; Li, T.T.; Fu, W.C.; Du, X.; Liu, C.H.; Zhang, W. Alisol A-24-acetate promotes glucose uptake via activation of AMPK in C2C12 myotubes. BMC Complement. Med. Ther. 2020, 20, 22. [Google Scholar] [CrossRef]
- Lou, H.X.; Fu, W.C.; Chen, J.X.; Li, T.T.; Jiang, Y.Y.; Liu, C.H.; Zhang, W. Alisol A 24-acetate stimulates lipolysis in 3 T3-L1 adipocytes. BMC Complement. Med. Ther. 2021, 21, 128. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Jang, E.; Lee, J.H. Pharmacological Activities of Alisma orientale against Nonalcoholic Fatty Liver Disease and Metabolic Syndrome: Literature Review. Evid. Based Complement. Alternat. Med. 2019, 2019, 2943162. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Long, J.; Chen, W.; Sun, Y.; Zhou, L.; Zhang, L.; Zeng, H.; Yuan, D. Alisol B 23-acetate, a new promoter for cholesterol efflux from dendritic cells, alleviates dyslipidemia and inflammation in advanced atherosclerotic mice. Int. Immunopharmacol. 2021, 99, 107956. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.C.; Fu, Y.; Bi, Y.H.; Zhang, W.W.; Li, J.; Ji, T.; Chao, Y.; Meng, Q.H.; Chen, Q.; Ma, M.H.; et al. Alisol B 23-acetate activates ABCG5/G8 in the jejunum via the LXRα/ACAT2 pathway to relieve atherosclerosis in ovariectomized ApoE-/- mice. Aging 2020, 12, 25744–25766. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chao, Y.; Zhang, W.; Zhang, Y.; Bi, Y.; Fu, Y.; Cai, D.; Meng, Q.; Li, Y.; Bian, H. Activation of estrogen receptor α (ERα) is required for Alisol B23-acetate to prevent post-menopausal atherosclerosis and reduced lipid accumulation. Life Sci. 2020, 258, 118030. [Google Scholar] [CrossRef]
- Lin, C.; Li, J.; Wu, C.; Bao, J. Identifying selective agonists targeting LXRβ from terpene compounds of alismatis rhizoma. J. Mol. Model. 2021, 27, 91. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, B.; Song, D.; Xi, J.; Hao, W.; Yuan, J.; Gao, C.; Cui, Z.; Cheng, Z. Alisol A Alleviates Arterial Plaque by Activating AMPK/SIRT1 Signaling Pathway in apoE-Deficient Mice. Front. Pharmacol. 2020, 11, 580073. [Google Scholar] [CrossRef]
- Zeng, L.; Tang, W.; Yin, J.; Feng, L.; Li, Y.; Yao, X.; Zhou, B. Alisol A 24-Acetate Prevents Hepatic Steatosis and Metabolic Disorders in HepG2 Cells. Cell. Physiol. Biochem. 2016, 40, 453–464. [Google Scholar] [CrossRef]
- Ho, C.; Gao, Y.; Zheng, D.; Liu, Y.; Shan, S.; Fang, B.; Zhao, Y.; Song, D.; Zhang, Y.; Li, Q. Alisol A attenuates high-fat-diet-induced obesity and metabolic disorders via the AMPK/ACC/SREBP-1c pathway. J. Cell. Mol. Med. 2019, 23, 5108–5118. [Google Scholar] [CrossRef]
- Wei, W.; Zhou, X.M.; Wang, Y.C.; Lin, Z.C.; Xue, J.H. Effect of alisol A 24-acetate on proliferation of aorta smooth muscle cells in rats induced by ox-LDL. Zhongguo Zhong Yao Za Zhi 2018, 43, 2147–2152. [Google Scholar]
- Xu, F.; Yu, H.; Lu, C.; Chen, J.; Gu, W. The Cholesterol-Lowering Effect of Alisol Acetates Based on HMG-CoA Reductase and Its Molecular Mechanism. Evid.-Based Complement. Alternat. Med. 2016, 2016, 4753852. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jin, S.; Song, C.; Chen, C.; Zhang, Y.; Xiang, Y.; Xu, Y.; Feng, Y.; Wan, Q.; Jiang, H. The metabolic change of serum lysophosphatidylcholines involved in the lipid lowering effect of triterpenes from Alismatis rhizoma on high-fat diet induced hyperlipidemia mice. J. Ethnopharmacol. 2016, 177, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Wei, Y.; Wang, M.; Tao, J.; Ouyang, H.; Du, Z.; Li, S.; Jiang, H. Network pharmacology combined with metabolomics and lipidomics to reveal the hypolipidemic mechanism of Alismatis rhizoma in hyperlipidemic mice. Food Funct. 2022, 13, 4714–4733. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, L.; Du, Z.; Jin, S.; Song, C.; Jia, S.; Zhang, Y.; Jiang, H. Identification of the lipid-lowering component of triterpenes from Alismatis rhizoma based on the MRM-based characteristic chemical profiles and support vector machine model. Anal. Bioanal. Chem. 2019, 411, 3257–3268. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Lu, C.; Wu, Q.; Gu, W.; Chen, J.; Fang, F.; Zhao, B.; Du, W.; You, M. Studies on the lipid-regulating mechanism of alisol-based compounds on lipoprotein lipase. Bioorg. Chem. 2018, 80, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Chen, J.; Zhang, Y.; Wu, Q.; Shen, Y.; Gu, W.; Liu, S.; Lu, C.; Liao, H.; Bao, K. Molecular insight into the mechanism of lipid regulating effect of Alisma orientalis based on ACAT. Int. J. Biol. Macromol. 2020, 158, 1141–1162. [Google Scholar] [CrossRef]
- Charles, R.; Eaton, P. Redox Regulation of Soluble Epoxide Hydrolase-Implications for Cardiovascular Health and Disease. Cells 2022, 11, 1932. [Google Scholar] [CrossRef]
- Shan, J.; Hashimoto, K. Soluble Epoxide Hydrolase as a Therapeutic Target for Neuropsychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 4951. [Google Scholar] [CrossRef]
- Hur, J.M.; Choi, J.W.; Park, J.C. Effects of methanol extract of Alisma orientale rhizome and its major component, alisol B 23-acetate, on hepatic drug metabolizing enzymes in rats treated with bromobenzene. Arch. Pharm. Res. 2007, 30, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.P.; Zhang, J.; Zhao, W.Y.; Yi, J.; Yan, J.K.; Wang, Y.L.; Morisseau, C.; Liu, Z.B.; Hammock, B.D.; Ma, X.C. Protostane-type triterpenoids as natural soluble epoxide hydrolase inhibitors: Inhibition potentials and molecular dynamics. Bioorg. Chem. 2020, 96, 103637. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Y.; Zhang, X.Y.; Zhou, M.R.; Tian, X.G.; Lv, X.; Zhang, H.L.; Deng, S.; Zhang, B.J.; Sun, C.P.; Ma, X.C. Natural soluble epoxide hydrolase inhibitors from Alisma orientale and their potential mechanism with soluble epoxide hydrolase. Int. J. Biol. Macromol. 2021, 183, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Han, L.; Bi, X.; Wang, X.; Mu, Y.; Guan, P.; Li, L.; Huang, X. Structures and biological activities of the triterpenoids and sesquiterpenoids from Alisma orientale. Phytochemistry 2016, 131, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Wang, P.; Ma, Q.; Han, L.; Wang, X.; Mu, Y.; Guan, P.; Qu, X.; Wang, Z.; Huang, X. Anti-Inflammatory Activities and Liver Protection of Alisol F and 25-Anhydroalisol F through the Inhibition of MAPK, STAT3, and NF-κB Activation In Vitro and In Vivo. Molecules 2017, 22, 951. [Google Scholar] [CrossRef]
- Wang, B.; Chen, L.; Dai, L.; Fang, W.; Wang, H. Alisol B 23-Acetate Ameliorates Lipopolysaccharide-Induced Cardiac Dysfunction by Suppressing Toll-Like Receptor 4 (TLR4)/NADPH Oxidase 2 (NOX2) Signaling Pathway. Med. Sci. Monit. 2019, 25, 8472–8481. [Google Scholar] [CrossRef]
- Xia, F.; Xiang, S.; Chen, Z.; Song, L.; Li, Y.; Liao, Z.; Ge, B.; Zhou, B. The probiotic effects of AB23A on high-fat-diet-induced non-alcoholic fatty liver disease in mice may be associated with suppressing the serum levels of lipopolysaccharides and branched-chain amino acids. Arch. Biochem. Biophys. 2021, 714, 109080. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Li, Y.; Deng, L.; Ren, R.; Ge, B.; Liao, Z.; Xiang, S.; Zhou, B. Alisol B 23-Acetate Ameliorates Lipopolysaccharide-Induced Intestinal Barrier Dysfunction by Inhibiting TLR4-NOX1/ROS Signaling Pathway in Caco-2 Cells. Front. Pharmacol. 2022, 13, 911196. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, X.C.; Meng, L.Y.; Wang, Y.; Li, Y.M. Alisol B 23-acetate inhibits the viability and induces apoptosis of non-small cell lung cancer cells via PI3K/AKT/mTOR signal pathway. Mol. Med. Rep. 2019, 20, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Moeller, M.J.; Kramann, R.; Lammers, T.; Hoppe, B.; Latz, E.; Ludwig-Portugall, I.; Boor, P.; Floege, J.; Kurts, C.; Weiskirchen, R.; et al. New Aspects of Kidney Fibrosis-From Mechanisms of Injury to Modulation of Disease. Front. Med. 2022, 8, 814497. [Google Scholar] [CrossRef]
- Chen, H.; Wang, M.C.; Chen, Y.Y.; Chen, L.; Wang, Y.N.; Vaziri, N.D.; Miao, H.; Zhao, Y.Y. Alisol B 23-acetate attenuates CKD progression by regulating the renin-angiotensin system and gut-kidney axis. Ther. Adv. Chronic Dis. 2020, 11, 2040622320920025. [Google Scholar] [CrossRef]
- Chen, L.; Chen, D.Q.; Wang, M.; Liu, D.; Chen, H.; Dou, F.; Vaziri, N.D.; Zhao, Y.Y. Role of RAS/Wnt/β-catenin axis activation in the pathogenesis of podocyte injury and tubulo-interstitial nephropathy. Chem. Biol. Interact. 2017, 273, 56–72. [Google Scholar] [CrossRef]
- Chen, H.; Yang, T.; Wang, M.C.; Chen, D.Q.; Yang, Y.; Zhao, Y.Y. Novel RAS inhibitor 25-O-methylalisol F attenuates epithelial-to-mesenchymal transition and tubulo-interstitial fibrosis by selectively inhibiting TGF-β-mediated Smad3 phosphorylation. Phytomedicine 2018, 42, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.H.; Zhou, X.M.; Wei, W.; Chen, T.; Su, Q.P.; Tao, J.; Chen, L.D. Alisol A 24-Acetate, a Triterpenoid Derived from Alisma orientale, Inhibits Ox-LDL-Induced Phenotypic Transformation and Migration of Rat Vascular Smooth Muscle Cells through Suppressing ERK1/2 Signaling. J. Vasc. Res. 2016, 53, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.C.; Jia, X.K.; Fan, Y.; Xu, S.H.; Li, X.Y.; Huang, M.Q.; Lan, M.L.; Xu, W.; Wu, S.S. Alisol B 23-Acetate Ameliorates Azoxymethane/Dextran Sodium Sulfate-Induced Male Murine Colitis-Associated Colorectal Cancer via Modulating the Composition of Gut Microbiota and Improving Intestinal Barrier. Front. Cell. Infect. Microbiol. 2021, 11, 640225. [Google Scholar] [CrossRef]
- Xu, X.; Li, L.; Zhang, Y.; Lu, X.; Lin, W.; Wu, S.; Qin, X.; Xu, R.; Lin, W. Hypolipidemic effect of Alisma orientale (Sam.) Juzep on gut microecology and liver transcriptome in diabetic rats. PLoS ONE 2020, 15, e0240616. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhai, Y.; Ji, M.; Wei, Y.; Wu, J.; Xue, W.; Tao, W.W.; Wu, H. Alisma orientalis Beverage Treats Atherosclerosis by Regulating Gut Microbiota in ApoE-/- Mice. Front. Pharmacol. 2020, 11, 570555. [Google Scholar] [CrossRef]
- Lu, L.; Lu, T.; Shen, J.; Lv, X.; Wei, W.; Wang, H.; Xue, X. Alisol A 24-acetate protects against brain microvascular endothelial cells injury through inhibiting miR-92a-3p/tight junctions axis. Aging 2021, 13, 15353–15365. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wei, W.; Shen, J.; Lu, L.; Lu, T.; Wang, H.; Xue, X. Alisol A 24-acetate protects oxygen-glucose deprivation-induced brain microvascular endothelial cells against apoptosis through miR-92a-3p inhibition by targeting the B-cell lymphoma-2 gene. Pharm. Biol. 2021, 59, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Li, H.; Zhou, Y.; Wei, W.; Ding, L.; Zhan, Z.; Liu, W.; Tao, J.; Xue, X. Neuroprotective effects of alisol A 24-acetate on cerebral ischaemia-reperfusion injury are mediated by regulating the PI3K/AKT pathway. J. Neuroinflammation 2022, 19, 37. [Google Scholar] [PubMed]
- Jang, E.; Lee, J.H. Promising Anticancer Activities of Alismatis rhizome and Its Triterpenes via p38 and PI3K/Akt/mTOR Signaling Pathways. Nutrients 2021, 13, 2455. [Google Scholar] [CrossRef]
- Jin, U.H.; Kim, D.I.; Lee, T.K.; Lee, D.N.; Kim, J.K.; Lee, I.S.; Kim, C.H. Herbal formulation, Yukmi-jihang-tang-Jahage, regulates bone resorption by inhibition of phosphorylation mediated by tyrosine kinase Src and cyclooxygenase expression. J. Ethnopharmacol. 2006, 106, 333–343. [Google Scholar] [CrossRef]
- Xia, B.; Xu, B.; Sun, Y.; Xiao, L.; Pan, J.; Jin, H.; Tong, P. The effects of Liuwei Dihuang on canonical Wnt/β-catenin signaling pathway in osteoporosis. J. Ethnopharmacol. 2014, 153, 133–141. [Google Scholar] [CrossRef]
- An, J.; Hao, D.; Zhang, Q.; Chen, B.; Zhang, R.; Wang, Y.; Yang, H. Natural products for treatment of bone erosive diseases: The effects and mechanisms on inhibiting osteoclastogenesis and bone resorption. Int. Immunopharmacol. 2016, 36, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhu, H.; Li, G.; Lan, M.; Li, X.; Huang, M.; Xu, W.; Wu, S. Anti-osteoporotic effects of alisol C 23-acetate via osteoclastogenesis inhibition. Biomed. Pharmacother. 2021, 137, 111321. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Leutou, A.S.; Yeon, J.T.; Choi, S.W.; Kim, S.H.; Yee, S.T.; Choi, K.H.; Nam, S.J.; Son, Y.J. The Inhibitory Effect of Alisol A 24-Acetate from Alisma canaliculatum on Osteoclastogenesis. Int. J. Endocrinol. 2015, 2015, 132436. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Kang, K.Y.; Lee, S.J.; Nam, S.J.; Son, Y.J.; Yee, S.T. The Protective Effects of Alisol A 24-Acetate from Alisma canaliculatum on Ovariectomy Induced Bone Loss in Vivo. Molecules 2016, 21, 74. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kobayashi, Y.; Nakamichi, Y.; Udagawa, N.; Takahashi, N.; Im, N.K.; Seo, H.J.; Jeon, W.B.; Yonezawa, T.; Cha, B.Y.; et al. Alisol-B, a novel phyto-steroid, suppresses the RANKL-induced osteoclast formation and prevents bone loss in mice. Biochem. Pharmacol. 2010, 80, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Li, T.; Zeng, Q.; Wang, X.; Zhu, Y. Alisol F 24 Acetate Enhances Chemosensitivity and Apoptosis of MCF-7/DOX Cells by Inhibiting P-Glycoprotein-Mediated Drug Efflux. Molecules 2016, 21, 183. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.X.; Shen, X.L.; Wan, C.K.; Tse, A.K.; Fong, W.F. Reversal of P-glycoprotein-mediated multidrug resistance by Alisol B 23-acetate. Biochem. Pharmacol. 2004, 68, 843–855. [Google Scholar]
- Lou, C.; Xu, X.; Chen, Y.; Zhao, H. Alisol A Suppresses Proliferation, Migration, and Invasion in Human Breast Cancer MDA-MB-231 Cells. Molecules 2019, 24, 3651. [Google Scholar]
- Shi, Y.; Wang, M.; Wang, P.; Zhang, T.; Yu, J.; Shi, L.; Li, M.; Wang, H.; Zhang, Q.; Zhao, H. Alisol A is potentially therapeutic in human breast cancer cells. Oncol. Rep. 2020, 44, 1266–1274. [Google Scholar] [CrossRef]
- Xia, J.; Luo, Q.; Huang, S.; Jiang, F.; Wang, L.; Wang, G.; Xie, J.; Liu, J.; Xu, Y. Alisol B 23-acetate-induced HepG2 hepatoma cell death through mTOR signaling-initiated G1 cell cycle arrest and apoptosis: A quantitative proteomic study. Chin. J. Cancer Res. 2019, 31, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cheng, J.; Zhu, D.; Shi, X.; Wei, Y.; Chen, S.; Wang, Z.; Yuan, D. The effects of Alisol B 23-acetate in hepatocellular carcinoma via inducing cell apoptosis and inhibiting cell migration and invasion. Gen. Physiol. Biophys. 2020, 39, 219–228. [Google Scholar] [CrossRef]
- Han, W.; Xing, W.; Wang, K.; Wang, B.; Bai, K. Alisol A attenuates malignant phenotypes of colorectal cancer cells by inactivating PI3K/Akt signaling. Oncol. Lett. 2022, 24, 249. [Google Scholar] [CrossRef]
- Chen, X.; Liu, H. Alisol A Inhibited the Proliferation, Migration, and Invasion of Nasopharyngeal Carcinoma Cells by Inhibiting the Hippo Signaling Pathway. Yonsei Med. J. 2021, 62, 895–902. [Google Scholar] [CrossRef]
- Yoshida, I.; Ito, C.; Matsuda, S.; Tsuji, A.; Yanaka, N.; Yuasa, K. Alisol B, a triterpene from Alismatis rhizoma (dried rhizome of Alisma orientale), inhibits melanin production in murine B16 melanoma cells. Biosci. Biotechnol. Biochem. 2017, 81, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sheng, Y.; Zou, M. Antiproliferative activity of Alisol B in MDA-MB-231 cells is mediated by apoptosis, dysregulation of mitochondrial functions, cell cycle arrest and generation of reactive oxygen species. Biomed. Pharmacother. 2017, 87, 110–117. [Google Scholar] [CrossRef]
- Zhang, L.L.; Xu, Y.L.; Tang, Z.H.; Xu, X.H.; Chen, X.; Li, T.; Ding, C.Y.; Huang, M.Q.; Chen, X.P.; Wang, Y.T.; et al. Effects of alisol B 23-acetate on ovarian cancer cells: G1 phase cell cycle arrest, apoptosis, migration and invasion inhibition. Phytomedicine 2016, 23, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, E.T.S.; Wang, M. Alisol B 23-acetate induces autophagic-dependent apoptosis in human colon cancer cells via ROS generation and JNK activation. Oncotarget 2017, 8, 70239–70249. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, H.; Wang, X.; Shen, T.; Wang, S.; Ren, D. Alisol B-23-acetate, a tetracyclic triterpenoid isolated from Alisma orientale, induces apoptosis in human lung cancer cells via the mitochondrial pathway. Biochem. Biophys. Res. Commun. 2018, 505, 1015–1021. [Google Scholar] [CrossRef]
- Xu, Y.H.; Zhao, L.J.; Li, Y. Alisol B acetate induces apoptosis of SGC7901 cells via mitochondrial and phosphatidylinositol 3-kinases/Akt signaling pathways. World J. Gastroenterol. 2009, 15, 2870–2877. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J.; Kim, J.N.; Lee, M.J.; Kim, W.K.; Nam, J.H.; Kim, B.J. Apoptotic effects of alisol B 23-acetate on gastric cancer cells. Mol. Med. Rep. 2021, 23, 248. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Tang, Y.; He, J.; Cao, X.; Liu, J.; Kou, S.; Yang, Y.; Xue, J.; Li, F. Alisol B 23-Acetate Increases the Antitumor Effect of Bufalin on Liver Cancer through Inactivating Wnt/β-Catenin Axis. Comput. Math. Methods Med. 2022, 2022, 6249534. [Google Scholar] [CrossRef] [PubMed]
- El-Baba, C.; Baassiri, A.; Kiriako, G.; Dia, B.; Fadlallah, S.; Moodad, S.; Darwiche, N. Terpenoids’ anti-cancer effects: Focus on autophagy. Apoptosis 2021, 26, 491–511. [Google Scholar] [CrossRef]
- Law, B.Y.; Wang, M.; Ma, D.L.; Al-Mousa, F.; Michelangeli, F.; Cheng, S.H.; Ng, M.H.; To, K.F.; Mok, A.Y.; Ko, R.Y.; et al. Alisol B, a novel inhibitor of the sarcoplasmic/endoplasmic reticulum Ca(2+) ATPase pump, induces autophagy, endoplasmic reticulum stress, and apoptosis. Mol. Cancer Ther. 2010, 9, 718–730. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhao, J.C.; Liang, J.H.; Tian, X.G.; Huo, X.K.; Feng, L.; Ning, J.; Wang, C.; Zhang, B.J.; Chen, G.; et al. A bioactive new protostane-type triterpenoid from Alisma plantago-aquatica subsp. orientale (Sam.) Sam. Nat. Prod. Res. 2019, 33, 776–781. [Google Scholar] [CrossRef]
- Lin, H.R. Triterpenes from Alisma orientalis act as androgen receptor agonists, progesterone receptor antagonists, and glucocorticoid receptor antagonists. Bioorg. Med. Chem. Lett. 2014, 24, 3626–3632. [Google Scholar] [CrossRef]
- Xu, F.; Lu, C.; Wu, Q.; Chen, J.; Gu, W.; Du, W.; You, M. Study on antitumor molecular mechanism of Alisols based on p53DNA. Int. J. Biol. Macromol. 2018, 116, 1163–1174. [Google Scholar] [CrossRef]
- Xu, F.; Chen, J.; Wu, Q.; Gu, W.; Shen, Y.; Lu, C.; Zhang, Y.; Liu, S.; Liao, H. The antitumor molecular mechanism of Alisma orientalis with c-myc DNA: Multi-spectroscopic analysis and molecular simulation. J. Biomol. Struct. Dyn. 2020, 38, 4189–4209. [Google Scholar] [CrossRef]
- Kubo, M.; Matsuda, H.; Tomohiro, N.; Yoshikawa, M. Studies on Alismatis rhizoma. I. Anti-allergic effects of methanol extract and six terpene components from Alismatis rhizoma (dried rhizome of Alisma orientale). Biol. Pharm. Bull. 1997, 20, 511–516. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwon, O.S.; Jin, H.G.; Woo, E.R.; Kim, Y.S.; Kim, H.P. The rhizomes of Alisma orientale and alisol derivatives inhibit allergic response and experimental atopic dermatitis. Biol. Pharm. Bull. 2012, 35, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Fu, B.; Ji, N.; Pan, S.; Zhao, X.; Zhang, Z.; Qiu, Y.; Wang, R.; Jin, M.; Wen, K.; et al. Alisol B 23-Acetate Inhibits IgE/Ag-Mediated Mast Cell Activation and Allergic Reaction. Int. J. Mol. Sci. 2018, 19, 4092. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.G.; Jin, Q.; Ryun Kim, A.; Choi, H.; Lee, J.H.; Kim, Y.S.; Lee, D.G.; Woo, E.R. A new triterpenoid from Alisma orientale and their antibacterial effect. Arch. Pharm. Res. 2012, 35, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jiang, Z.Y.; Luo, J.; Cheng, P.; Ma, Y.B.; Zhang, X.M.; Zhang, F.X.; Zhou, J.; Chen, J.J. Anti-HBV agents. Part 1: Synthesis of alisol A derivatives: A new class of hepatitis B virus inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 4647–4650. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, Z.Y.; Luo, J.; Liu, J.F.; Ma, Y.B.; Guo, R.H.; Zhang, X.M.; Zhou, J.; Chen, J.J. Anti-HBV agents. Part 2: Synthesis and in vitro anti-hepatitis B virus activities of alisol A derivatives. Bioorg. Med. Chem. Lett. 2009, 19, 2148–2153. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, Z.Y.; Luo, J.; Ma, Y.B.; Liu, J.F.; Guo, R.H.; Zhang, X.M.; Zhou, J.; Niu, W.; Du, F.F.; et al. Anti-HBV agents. Part 3: Preliminary structure-activity relationships of tetra-acylalisol A derivatives as potent hepatitis B virus inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 6659–6665. [Google Scholar] [CrossRef]
- Liao, M.; Shang, H.; Li, Y.; Li, T.; Wang, M.; Zheng, Y.; Hou, W.; Liu, C. An integrated approach to uncover quality marker underlying the effects of Alisma orientale on lipid metabolism, using chemical analysis and network pharmacology. Phytomedicine 2018, 45, 93–104. [Google Scholar] [CrossRef]
- Lin, W.; Sun, F.; Zhang, Y.; Xu, X.; Lu, X.; Li, L.; Xu, R. Comparative transcriptome and metabolite profiling of four tissues from Alisma orientale (Sam.) Juzep reveals its inflorescence developmental and medicinal characteristics. Sci. Rep. 2019, 9, 12310. [Google Scholar] [CrossRef]
- Li, R.; Li, Z.L.; Chen, Y.P.; Bu, W.Q.; Ding, W.B.; Yang, B.; Wang, C.F.; Ma, L.; Jia, X.B.; Feng, L. The structural composition of components contributes to the superiority of the geoherb Alisma orientale for “diuresis and diffusing dampness”. RSC Adv. 2020, 10, 39385–39395. [Google Scholar] [CrossRef]
- Wei, Y.C.; Yan, M.; Yang, J.; Liu, J.C.; Yin, H.M.; Wu, Y.; Sun, Y.C.; Xiao, W. Optimization of flash-type extraction technology of alisol B 23-acetate from Alismatis Rhizoma by response surface methodology. Zhongguo Zhong Yao Za Zhi 2016, 41, 438–442. [Google Scholar]
- Wang, C.F.; Cheng, X.D.; Gu, J.F.; Yuan, J.R.; Zhao, B.J.; Zhang, L.; Chen, J.; Feng, L.; Jia, X.B. Research development of the chemical material basis of Alisma orientalis and its toxicity. Zhongguo Zhong Yao Za Zhi 2015, 40, 840–846. [Google Scholar] [PubMed]
- Yu, Y.; Ma, C.; Bi, K.; Yang, G.; Xie, P.; Wang, J.; Chen, X.H. A metabonomic analysis of urine from rats treated with rhizoma alismatis using ultra-performance liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 2633–2640. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Q.; Feng, Y.L.; Tian, T.; Chen, H.; Yin, L.; Zhao, Y.Y.; Lin, R.C. Diuretic and anti-diuretic activities of fractions of Alismatis rhizoma. J. Ethnopharmacol. 2014, 157, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Feng, L.; Ma, L.; Chen, H.; Tan, X.; Hou, X.; Song, J.; Cui, L.; Liu, D.; Chen, J.; et al. Alisol A 24-Acetate and Alisol B 23-Acetate Induced Autophagy Mediates Apoptosis and Nephrotoxicity in Human Renal Proximal Tubular Cells. Front. Pharmacol. 2017, 8, 172. [Google Scholar] [CrossRef]
- Xu, F.; Wu, Q.N.; Chen, J.; Gu, W.; Fang, F.; Zhang, L.Q.; Zhao, B. The binding mechanisms of plasma protein to active compounds in Alismaorientale rhizomes (Alismatis Rhizoma). Bioorg. Med. Chem. Lett. 2014, 24, 4099–4105. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Huang, S.; Yan, J.; Cai, B. Pharmacokinetic study of six triterpenoids of raw and processed Alisma plantago-aquatica in rat plasma by using ultra performance liquid chromatography-tandem mass spectrometry approach. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1124, 323–330. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).