Mast Cells and Vitamin D Status: A Clinical and Biological Link in the Onset of Allergy and Bone Diseases
Abstract
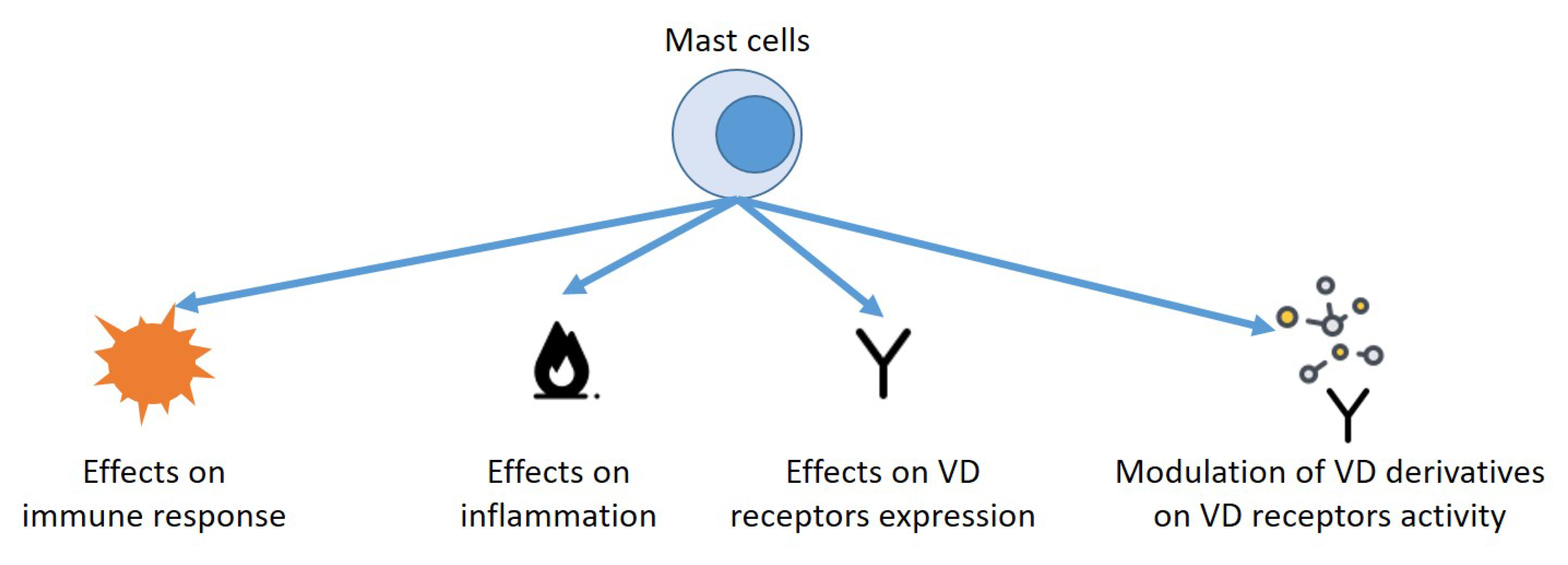
1. Introduction
2. General Considerations on Vitamin D
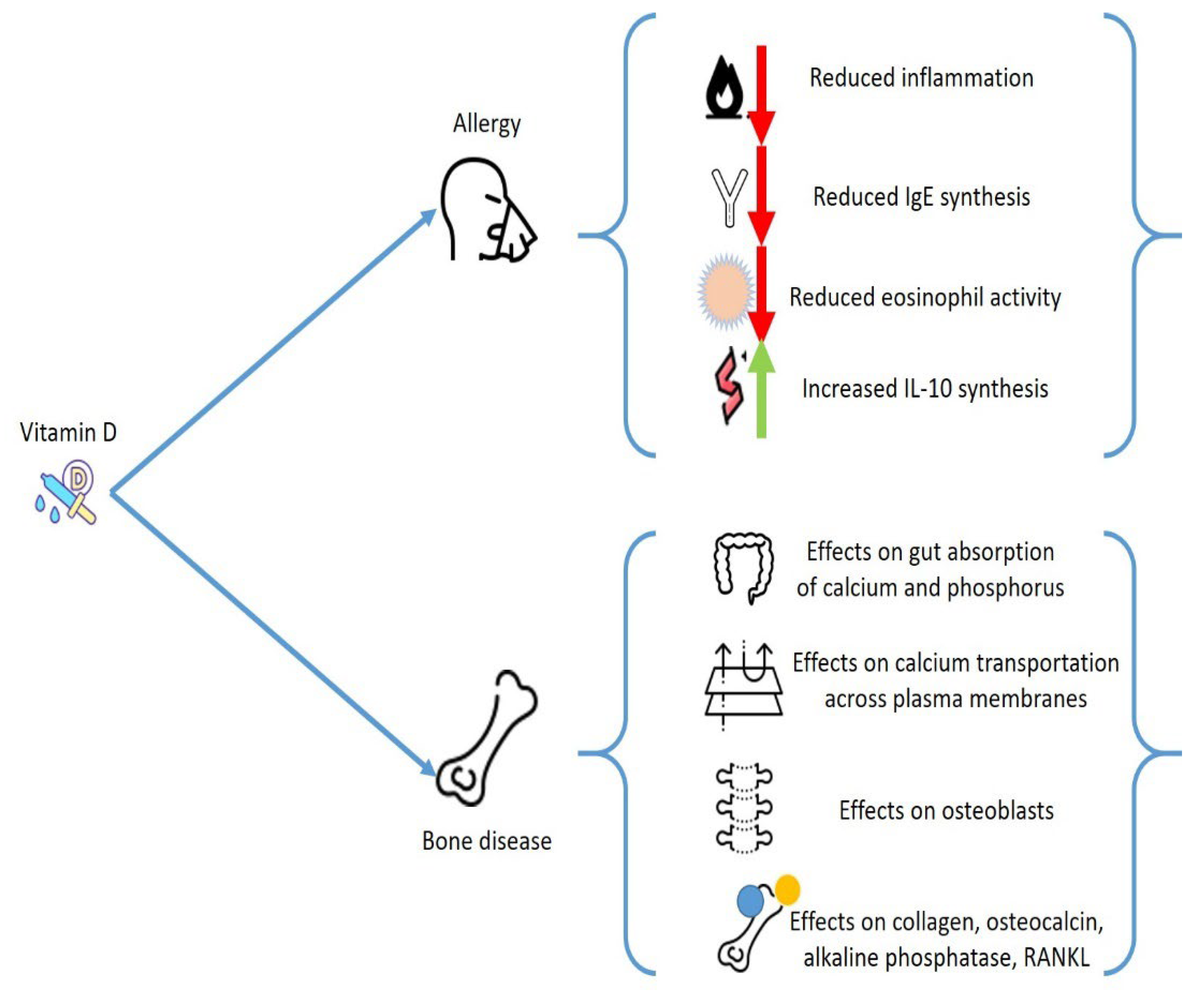
3. Vitamin D and Allergies
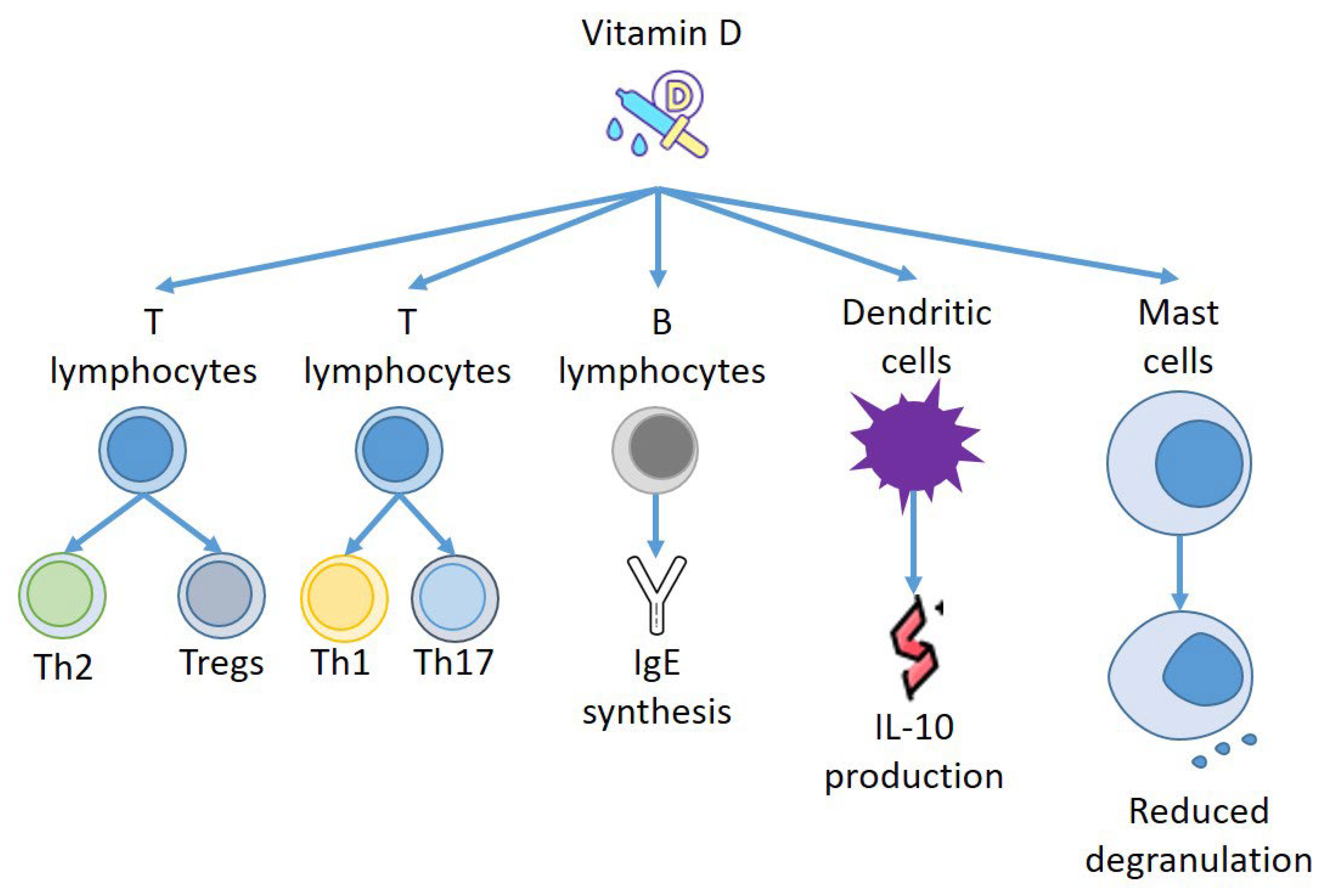
4. Vitamin D and Immune Response
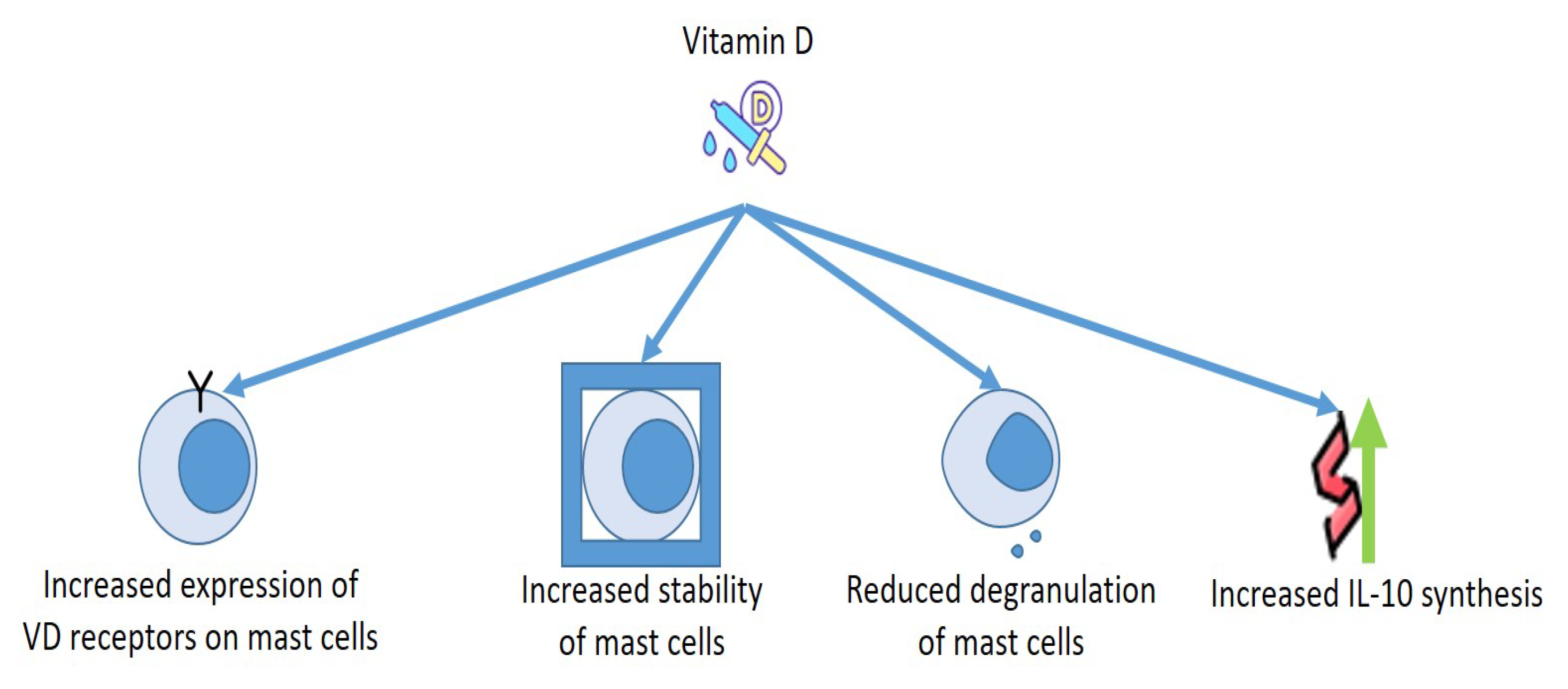
5. Vitamin D and Mast Cells: Effects on Allergies
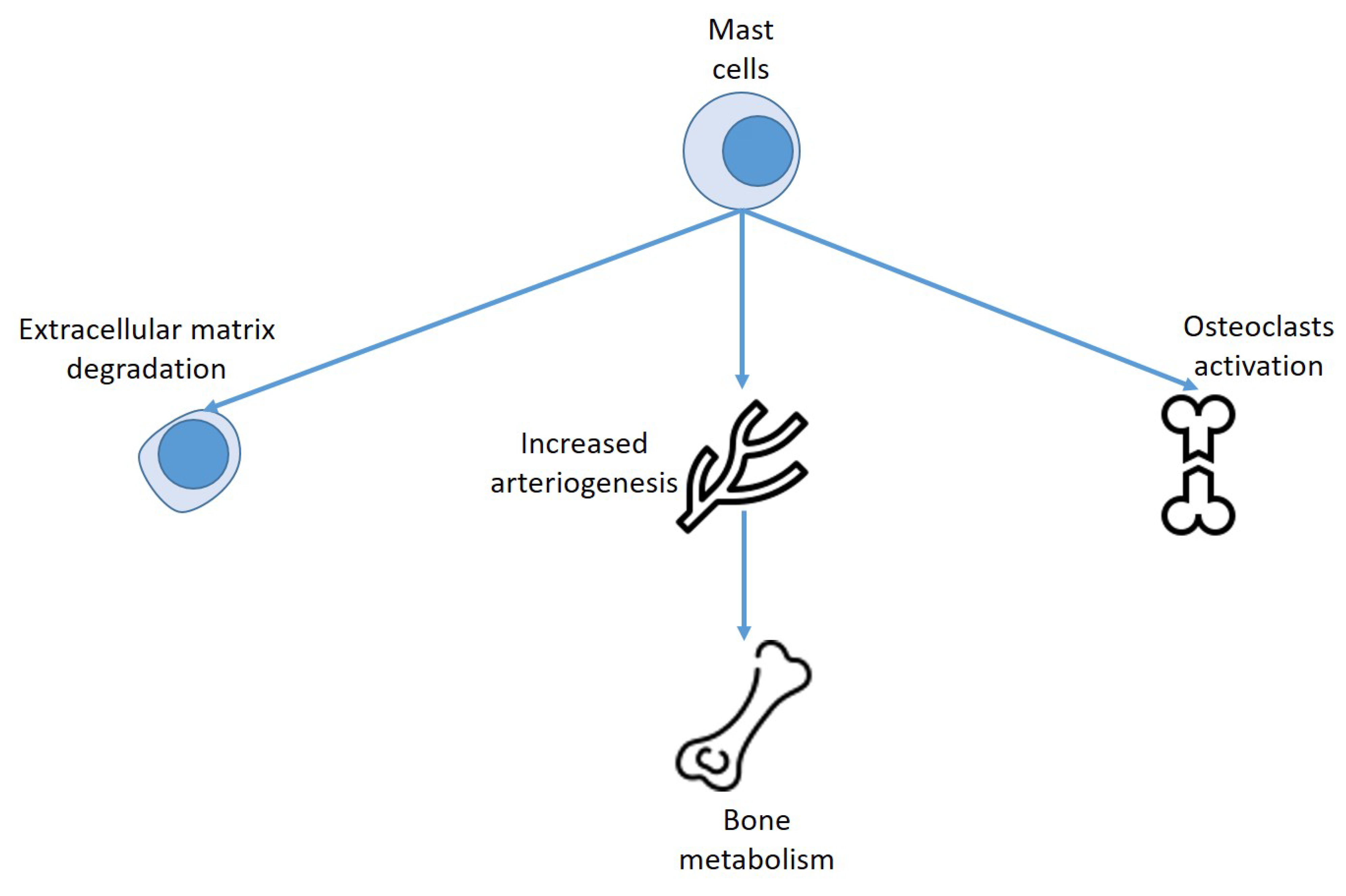
6. Mast Cells, Vitamin D, and Bone Metabolism
7. Mastocytosis, Allergy and Bone Alterations
8. Mast Cells, Vitamin D and Skin Diseases
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Martinis, M.; Sirufo, M.M.; Viscido, A.; Ginaldi, L. Food Allergy Insights: A Changing Landscape. Arch. Immunol. Ther. Exp. 2020, 68, 8. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Silverberg, J.I. Association between eczema and increased fracture and bone or joint injury in adults a us population-based study. JAMA Dermatol. 2015, 151, 33–41. [Google Scholar] [CrossRef]
- Chen, Y.W.; Ramsook, A.H.; Coxson, H.O.; Bon, J.; Reid, W.D. Prevalence and Risk Factors for Osteoporosis in Individuals with COPD: A Systematic Review and Meta-analysis. Chest 2019, 156, 1092–1110. [Google Scholar] [CrossRef]
- Barrick, B.J.; Jalan, S.; Tollefson, M.M.; Milbrandt, T.A.; Larson, A.N.; Rank, M.A.; Lohse, C.M.; Davis, D.M.R. Associations of self-reported allergic diseases and musculoskeletal problems in children: A US population-based study. Ann. Allergy Asthma Immunol. 2017, 119, 170–176. [Google Scholar] [CrossRef]
- Aljubran, S.A.; Whelan, G.J.; Glaum, M.C.; Lockey, R.F. Osteoporosis in the at-risk asthmatic. Allergy 2014, 69, 1429–1439. [Google Scholar] [CrossRef]
- Jung, J.-W.; Kang, H.-R.; Kim, J.-Y.; Lee, S.-H.; Kim, S.S.; Cho, S.H. Are asthmatic patients prone to bone loss? Ann. Allergy Asthm. Immunol. 2014, 112, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.G.; Kong, I.G. Association between chronic rhinosinusitis and osteoporosis: A case-control study using a national sample color. Int. Forum Allergy Rhinol. 2019, 9, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Zupan, J.; Jeras, M.; Marc, J. Osteoimmunology and the influence of pro-inflammatory cytokines on osteoclasts. Biochem. Med. 2013, 23, 43–63. [Google Scholar] [CrossRef]
- Ponzetti, M.; Rucci, N. Updates on Osteoimmunology: What’s New on the Cross-Talk between Bone and Immune System. Front. Endocrinol. 2019, 10, 236. [Google Scholar] [CrossRef]
- Naik, S.R.; Wala, S.M. Inflammation, allergy and asthma, complex immune origin diseases: Mechanisms and therapeutic agents. Recent Patents Inflamm. Allergy Drug Discov. 2013, 7, 62–95. [Google Scholar] [CrossRef]
- Kang, J.; Lim, H.; Lee, D.; Yim, M. Montelukast inhibits RANKL-induced osteoclast formation and bone loss via CysLTR1 and P2Y12. Mol. Med. Rep. 2018, 18, 2387–2398. [Google Scholar] [CrossRef]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W. Genome-wide principles of gene regulation by the vitamin D receptor and its activating ligand. Mol. Cell. Endocrinol. 2011, 347, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1alpha,25(OH)(2)vitamin D(3): Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Elsori, D.H.; Hammoud, M.S. Vitamin D deficiency in mothers, neonates and children. J. Steroid Biochem. Mol. Biol. 2018, 175, 195–199. [Google Scholar] [CrossRef]
- Holick, M. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, M.; Allegra, A.; Sirufo, M.M.; Tonacci, A.; Pioggia, G.; Raggiunti, M.; Ginaldi, L.; Gangemi, S. Vitamin D Deficiency, Osteoporosis and Effect on Autoimmune Diseases and Hematopoiesis: A Review. Int. J. Mol. Sci. 2021, 22, 8855. [Google Scholar] [CrossRef]
- Bivona, G.; Agnello, L.; Ciaccio, M. The immunological implication of the new vitamin D metabolism. Cent. Eur. J. Immunol. 2018, 43, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, F.; De Martinis, M.; Sirufo, M.M.; Ginaldi, L. Psoriasis Induced by Anti-Tumor Necrosis Factor Alpha Agents: A Comprehensive Review of the Literature. Acta Dermatovenerol. Croat. ADC 2016, 24, 169–174. [Google Scholar] [PubMed]
- Muehleisen, B.; Gallo, R.L. Vitamin D in allergic disease: Shedding light on a complex problem. J. Allergy Clin. Immunol. 2013, 131, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Marino, R.; Misra, M. Extra-Skeletal Effects of Vitamin D. Nutrients 2019, 11, 1460. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, C.; Passalacqua, G. Italian Vitamin D Allergy Group Vitamin D levels and allergic diseases. An Italian cross-sectional multicenter survey. Eur. Ann. Allergy Clin. Immunol. 2017, 49, 75–79. [Google Scholar]
- Souto Filho, J.T.D.; de Andrade, A.S.; Ribeiro, F.M.; Alves, P.A.S.; Simonini, V.R.F. Impact of vitamin D deficiency on increased blood eosinophil counts. Hematol. Oncol. Stem Cell Ther. 2018, 11, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Alyasin, S.; Momen, T.; Kashef, S.; Alipour, A.; Amin, R. The relationship between serum 25-hydroxyvitamin D levels and asthma in children. Allergy Asthma Immunol. Res. 2011, 3, 251–255. [Google Scholar] [CrossRef]
- Arshi, S.; Fallahpour, M.; Nabavi, M.; Bemanian, M.H.; Javad-Mousavi, S.A.; Nojomi, M.; Esmaeilzadeh, H.; Molatefi, R.; Rekabi, M.; Jalali, F.; et al. The effects of vitamin D supplementation on airway functions in mild to moderate persistent asthmal. Ann. Allergy Asthma Immunol. 2014, 113, 404–409. [Google Scholar] [CrossRef]
- Peroni, D.; Piacentini, G.; Cametti, E.; Chinellato, I.; Boner, A. Correlation between serum 25-hydroxyvitamin D levels and severity of atopic dermatitis in children. Br. J. Dermatol. 2011, 164, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Dogru, M. Is vitamin D level associated with the natural course of atopic dermatitis? Allergol. Immunopathol. 2018, 46, 546–551. [Google Scholar] [CrossRef]
- Grzanka, A.; Machura, E.; Mazur, B.; Misiolek, M.; Jochem, J.; Kasperski, J.; Kasperska-Zajac, A. Relationship between vitamin D status and the inflammatory state in patients with chronic spontaneous urticarial. J. Inflamm. 2014, 11, 24484740. [Google Scholar] [CrossRef] [PubMed]
- Quirk, S.K.; Rainwater, E.; Shure, A.K.; Agrawal, D.K. Vitamin D in atopic dermatitis, chronic urticaria and allergic contact dermatitis. Exp. Rev. Clin. Immunol. 2016, 12, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; André, F.; Church, M.K.; Maurer, M.; Metz, M. Potential blood biomarkers in chronic spontaneous urticaria. Clin. Exp. Allergy 2017, 47, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Heine, G.; Tabeling, C.; Hartmann, B.; Gonzalez Calera, C.R.; Kuhl, A.A.; Lindner, J.; Radbruch, A.; Witzenrath, M.; Worm, M. 25-hydroxvitamin D3 promotes the long-term effect of specific immunotherapy in a murine allergy model. J. Immunol. 2014, 193, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhong, Y.; Shen, X.; Yang, K.; Cai, W. Maternal and early-life vitamin D deficiency enhances allergic reaction in an ovalbumin-sensitized BALB/c mouse model. Food Nutr. Res. 2018, 62, 1401. [Google Scholar] [CrossRef] [PubMed]
- Sharief, S.; Jariwala, S.; Kumar, J.; Muntner, P.; Melamed, M.L. Vitamin D levels and food and environmental allergies in the United States: Results from the national health and nutrition examination survey 2005–2006. J Allergy Clin. Immunol. 2011, 127, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Shin, Y.H.; Chung, I.H.; Kim, H.J.; Yoo, E.G.; Yoon, J.W.; Jee, H.M.; Chang, Y.E.; Han, M.Y. The link between serum vitamin D level, sensitization to food allergens, and the severity of atopic dermatitis in infancy. J. Pediatr. 2014, 165, 849–854.e1. [Google Scholar] [CrossRef] [PubMed]
- Yepes-Nuñez, J.J.; Brożek, J.L.; Fiocchi, A.; Pawankar, R.; Cuello-García, C.; Zhang, Y.; Morgano, G.P.; Agarwal, A.; Gandhi, S.; Terracciano, L.; et al. Vitamin D supplementation in primary allergy prevention: Systematic review of randomized and non-randomized studies. Allergy 2018, 73, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Rorie, A.; Goldner, W.S.; Lyden, E.; Poole, J.A. Beneficial role for supplemental vitamin D3 treatment in chronic urticaria: A randomized study. Ann. Allergy Asthma Immunol. 2014, 112, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Nabavizadeh, S.H.; Alyasin, S.; Esmaeilzadeh, H.; Mosavat, F.; Ebrahimi, N. The effect of vitamin D add-on therapy on the improvement of quality of life and clinical symptoms of patients with chronic spontaneous urticaria. Asian Pac. J. Allergy Immunol. 2020. [Google Scholar] [CrossRef]
- Vahavihu, K.; Ala-Houhala, M.; Peric, M.; Karisola, P.; Kautiainen, H.; Hasan, T.; Snellman, E.; Alenius, H.; Schauber, J.; Reunala, T. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br. J. Dermatol. 2010, 163, 321–328. [Google Scholar] [CrossRef]
- Sidbury, R.; Sullivan, A.F.; Thadhani, R.I.; Camargo, C.A., Jr. Randomized controlled trial of vitamin D supplementation for winter-related atopic dermatitis in Boston: A pilot study. Br. J. Dermatol. 2008, 159, 245–247. [Google Scholar] [CrossRef]
- Brehm, J.M.; Celedon, J.C.; Soto-Quiros, M.E.; Avila, L.; Hunninghake, G.M.; Forno, E.; Laskey, D.; Sylvia, J.S.; Hollis, B.W.; Weiss, S.T.; et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am. J. Respir. Crit. Care Med. 2009, 179, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Goleva, E.; Searing, D.A.; Jackson, L.P.; Richers, B.N.; Leung, D.Y. Steroid requirements and immune associations with vitamin D are stronger in children than adults with asthma. J. Allergy Clin. Immunol. 2012, 129, 1243–1251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am. J. Clin. Nutr. 2010, 91, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Majak, P.; Olszowiec-Chlebna, M.; Smejda, K.; Stelmach, I. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J. Allergy Clin. Immunol. 2011, 127, 1294–1296. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.A., Jr.; Clark, S.; Kaplan, M.S.; Lieberman, P.; Wood, R.A. Regional differences in EpiPen prescriptions in the United States: The potential role of vitamin D. J. Allergy Clin. Immunol. 2007, 120, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.A., Jr.; Ingham, T.; Wickens, K.; Thadhani, R.; Silvers, K.M.; Epton, M.J.; Town, G.I.; Pattemore, P.K.; Espinola, J.A.; Crane, J. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics 2011, 127, e180–e187. [Google Scholar] [CrossRef] [PubMed]
- Heine, G.; Niesner, U.; Chang, H.D.; Steinmeyer, A.; Zugel, U.; Zuberbier, T.; Radbruch, A. Worm, M. 1,25-dihydroxyvitamin D(3) promotes IL-10 production in human B cells. Eur. J. Immunol. 2008, 38, 2210–2218. [Google Scholar] [CrossRef]
- Drozdenko, G.; Scheel, T.; Heine, G.; Baumgrass, R.; Worm, M. Impaired T cell activation and cytokine production by calcitriol-primed human B cells. Clin. Exp. Immunol. 2014, 178, 364–372. [Google Scholar] [CrossRef]
- Wittke, A.; Weaver, V.; Mahon, B.D.; August, A.; Cantorna, M.T. Vitamin D receptor deficient mice fail to develop experimental allergic asthma. J. Immunol. 2004, 173, 3432–3436. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, B.; Heine, G.; Babina, M.; Steinmeyer, A.; Zugel, U.; Radbruch, A.; Worm, M. Targeting the vitamin D receptor inhibits the B cell-dependent allergic immune response. Allergy 2011, 66, 540–548. [Google Scholar] [CrossRef]
- Széles, L.; Keresztes, G.; Töröcsik, D.; Balajthy, Z.; Krenács, L.; Póliska, S.; Steinmeyer, A.; Zuegel, U.; Pruenster, M.; Rot, A.; et al. 1,25-dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J. Immunol. 2009, 182, 2074–2083. [Google Scholar] [CrossRef] [PubMed]
- Unger, W.W.; Laban, S.; Kleijwegt, F.S.; van der Slik, A.R.; Roep, B.O. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: Differential role for PD-L1. Eur. J. Immunol. 2009, 39, 3147–3159. [Google Scholar] [CrossRef] [PubMed]
- Bakdash, G.; van Capel, T.M.; Mason, L.M.; Kapsenberg, M.L.; de Jong, E.C. Vitamin D3 metabolite calcidiol primes human dendritic cells to promote the development of immunomodulatory IL-10-producing T cells. Vaccine 2014, 32, 6294–6302. [Google Scholar] [CrossRef] [PubMed]
- Almerighi, C.; Sinistro, A.; Cavazza, A.; Ciaprini, C.; Rocchi, G.; Bergamini, A. 1α,25-dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine 2009, 45, 190–197. [Google Scholar] [CrossRef]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Walker, L.S.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef]
- Murdaca, G.; Greco, M.; Tonacci, A.; Negrini, S.; Borro, M.; Puppo, F.; Gangemi, S. IL-33/IL-31 Axis in Immune-Mediated and Allergic Diseases. Int. J. Mol. Sci. 2019, 20, 5856. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.D.; Agrawal, D.K. Hematopoietic Stem and Progenitor Cells in Inflammation and Allergy. Front. Immunol. 2013, 4, 428. [Google Scholar] [CrossRef] [PubMed]
- Uasuf, C.G.; Sano, C.D.; Gangemi, S.; Albeggiani, G.; Cigna, D.; Dino, P.; Brusca, I.; Gjomarkaj, M.; Pace, E. IL-33/s-ST2 ratio, systemic symptoms, and basophil activation in Pru p 3-sensitized allergic patients. Inflamm. Res. 2018, 67, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Ginaldi, L.; De Martinis, M.; Ciccarelli, F.; Saitta, S.; Imbesi, S.; Mannucci, C.; Gangemi, S. Increased levels of interleukin 31 (IL-31) in osteoporosis. BMC Immunol. 2015, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.; Grimbaldeston, M.; Galli, S.J. Mast cells and immunoregulation/immunomodulation. Adv. Exp. Med. Biol. 2011, 716, 186–211. [Google Scholar] [PubMed]
- Voehringer, D. Protective and pathological roles of mast cells and basophils. Nat. Rev. Immunol. 2013, 13, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Crivellato, E. The role of mast cell in tissue morphogenesis. Thymus, duodenum, and mammary gland as examples. Exp. Cell Res. 2016, 341, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Kalesnikoff, J.; Grimbaldeston, M.A.; Piliponsky, A.M.; Williams, C.M.; Tsai, M. Mast cells as “tunable” effector and immunoregulatory cells: Recent advances. Ann. Rev. Immunol. 2005, 23, 749–786. [Google Scholar] [CrossRef] [PubMed]
- Madjene, L.C.; Danelli, L.; Dahdah, A.; Vibhushan, S.; Bex-Coudrat, J.; Pacreau, E.; Vaugier, C.; Claver, J.; Rolas, L.; Pons, M.; et al. Mast cell chymase protects against acute ischemic kidney injury by limiting neutrophil hyperactivation and recruitment. Kidney Int. 2020, 97, 516–527. [Google Scholar] [CrossRef]
- Katsanos, G.S.; Anogeianaki, A.; Orso, C.; Tete, S.; Salini, V.; Antinolfi, P.L.; Sabatino, G. Mast cells and chemokines. J. Biol. Regul. Homeost. Agents 2008, 22, 145–151. [Google Scholar] [PubMed]
- Lieberman, P.; Garvey, L.H. Mast cells and anaphylaxis. Curr. Allergy Asthma Rep. 2016, 16, 20. [Google Scholar] [CrossRef]
- Finkelman, F.D.; Khodoun, M.V.; Strait, R. Human IgE-independent systemic anaphylaxis. J. Allergy Clin. Immunol. 2016, 137, 1674–1680. [Google Scholar] [CrossRef] [PubMed]
- Afrin, L.B. Mast cell activation disease and the modern epidemic of chronic inflammatory disease. Transl. Res. 2016, 174, 33–59. [Google Scholar] [CrossRef]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Li, X.X.; Qiu, S.Q.; Yu, Y.; Li, M.G.; Yang, L.T.; Li, L.J.; Wang, S.; Zheng, P.Y.; Liu, Z.G.; et al. Vitamin D contributes to mast cell stabilization. Allergy 2017, 72, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Biggs, L.; Yu, C.; Fedoric, B.; Lopez, A.F.; Galli, S.J.; Grimbaldeston, M.A. Evidence that vitamin D(3) promotes mast cell-dependent reduction of chronic UVB-induced skin pathology in mice. J. Exp. Med. 2010, 207, 455–463. [Google Scholar] [CrossRef]
- Asero, R.; Ferrucci, S.; Casazza, G.; Marzano, A.V.; Cugno, M. Total IgE and atopic status in patients with severe chronic spontaneous urticaria unresponsive to omalizumab treatment. Allergy 2019, 74, 1561–1563. [Google Scholar] [CrossRef]
- Lakin, E.; Church, M.K.; Maurer, M.; Schmetzer, O. On the Lipophilic Nature of Autoreactive IgE in Chronic Spontaneous Urticaria. Theranostics 2019, 9, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Yeverino, D.; López-García, A.I.; Caballero-López, C.G.; Ríos-López, J.J.; Papaqui-Tapia, J.S.; Ortega-Jordá Rodríguez, E.E.; Álvarez-Rivera, A.; Ruiz-Sánchez, D.M.; Flores-Gonzaga, E. Vitamin D and respiratory allergy: State of the art. Rev. Alergy Mex. 2022, 69 (Suppl. S1), s46–s54. [Google Scholar] [CrossRef]
- He, L.; Yi, W.; Huang, X.; Long, H.; Lu, Q. Chronic Urticaria: Advances in Understanding of the Disease and Clinical Management. Clin. Rev. Allergy Immunol. 2021, 61, 424–448. [Google Scholar] [CrossRef] [PubMed]
- Redegeld, F.A.; Yu, Y.; Kumari, S.; Charles, N.; Blank, U. Non-IgE mediated mast cell activation. Immunol. Rev. 2018, 282, 87–113. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.; Lammel, V.; Gibbs, B.F.; Maurer, M. Inflammatory murine skin responses to UV-B light are partially dependent on endothelin-1 and mast cells. Am. J. Pathol. 2006, 169, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Grimbaldeston, M.; Tsai, T. Immunomodulatory mast cells: Negative, as well as positive, regulators of immunity. Nat. Rev. Immunol. 2008, 8, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Townley, S.L.; Grimbaldeston, M.A.; Khalil, Z.; Finlay Jones, J.J. Mast cells, neuropeptides, histamine, and prostaglandins in UV-induced systemic immunosuppression. Methods 2002, 28, 79–89. [Google Scholar] [CrossRef]
- Khalil, Z.; Townley, S.L.; Grimbaldeston, M.A.; Finlay-Jones, J.J.; Hart, P.H. cis-Urocanic acid stimulates neuropeptide release from peripheral sensory nerves. J. Investig. Dermatol. 2002, 117, 886–891. [Google Scholar] [CrossRef]
- Yip, K.H.; Kolesnikoff, N.; Yu, C.; Hauschild, N.; Taing, H.; Biggs, L.; Goltzman, D.; Gregory, P.A.; Anderson, P.H.; Samuel, M.S.; et al. Mechanisms of vitamin D₃ metabolite repression of IgE-dependent mast cell activation. J. Allergy Clin. Immunol. 2014, 133, 1356–1364.e14. [Google Scholar] [CrossRef]
- Lindholm, R.; Lindholm, S.; Liukko, P. Fracture healing and mast cells. I. The periosteal callus in rats. Acta Orthop. Scand. 1967, 38, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Banovac, K.; Renfree, K.; Makowski, A.L.; Latta, L.L.; Altman, R.D. Fracture healing and mast cells. J. Orthop. Traum. 1995, 9, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Antebi, B.; Zhang, L.; Sheyn, D.; Pelled, G.; Zhang, X.; Gazit, Z.; Schwarz, E.M.; Gazit, D. Controlling Arteriogenesis and Mast Cells Are Central to Bioengineering Solutions for Critical Bone Defect Repair Using Allografts. Bioengineering 2016, 3, 6. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, T.; Chang, M.; Kaiser, C.; Kim, J.D.; Wu, T.; Cao, X.; Zhang, X.; Schwarz, E.M. Teriparatide Treatment Improves Bone Defect Healing Via Anabolic Effects on New Bone Formation and Non-Anabolic Effects on Inhibition of Mast Cells in a Murine Cranial Window Model. J. Bone Miner. Res. 2017, 32, 1870–1883. [Google Scholar] [CrossRef] [PubMed]
- Silveira, S.R.; Hadler, W.A. A histochemical investigation on the percutaneous absorption of vitamin D synthesized into the mammal epidermis. Acta Histochem. 1985, 77, 11–18. [Google Scholar] [CrossRef]
- Metcalfe, D.D. Mast cells and mastocytosis. Blood 2008, 112, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Arock, M.; Valent, P. Pathogenesis, classification and treatment of mastocytosis: State of the art in 2010 and future perspectives. Exp. Rev. Hematol. 2010, 3, 497–516. [Google Scholar] [CrossRef]
- Pardanani, A. Systemic mastocytosis in adults: 2019 update on diagnosis, risk stratification and management. Am. J. Hematol. 2019, 94, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Escribano, L.; Broesby-Olsen, S.; Hartmann, K.; Grattan, C.; Brockow, K.; Niedoszytko, M.; Nedoszytko, B.; Oude Elberink, J.N.; Kristensen, T.; et al. Proposed diagnostic algorithm for patients with suspected mastocytosis: A proposal of the European Competence Network on Mastocytosis. Allergy 2014, 69, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Monnier, J.; Georgin-Lavialle, S.; Canioni, D.; Lhermitte, L.; Soussan, M.; Arock, M.; Bruneau, J.; Dubreuil, P.; Bodemer, C.; Chandesris, M.O.; et al. Mast cell sarcoma: New cases and literature review. Oncotarget 2016, 7, 66299–66309. [Google Scholar] [CrossRef]
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. WHO study Group. Osteop. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Rossini, M.; Zanotti, R.; Viapiana, O.; Tripi, G.; Orsolini, G.; Idolazzi, L.; Bonadonna, P.; Schena, D.; Escribano, L.; Adami, S.; et al. Bone involvement and osteoporosis in mastocytosis. Immunol. Allergy Clin. N. Am. 2014, 34, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Rossini, M.; Zanotti, R.; Orsolini, G.; Tripi, G.; Viapiana, O.; Idolazzi, L.; Zamo, A.; Bonadonna, P.; Kunnathully, V.; Adami, S.; et al. Prevalence, pathogenesis, and treatment options for mastocytosis-related osteoporosis. Osteop. Int. 2016, 27, 2411–2421. [Google Scholar] [CrossRef] [PubMed]
- Lewiecki, E.M.; Gordon, C.M.; Baim, S.; Leonard, M.B.; Bishop, N.J.; Bianchi, M.L. International society for clinical densitometry 2007 adult and pediatric official positions. Bone 2008, 43, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Rossini, M.; Zanotti, R.; Bonadonna, P.; Artuso, A.; Caruso, B.; Schena, D.; Vecchiato, D.; Bonifacio, M.; Viapiana, O.; Gatti, D.; et al. Bone mineral density, bone turnover markers and fractures in patients with indolent systemic mastocytosis. Bone 2011, 49, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Akin, C.; Gleixner, K.V.; Sperr, W.R.; Reiter, A.; Arock, M.; Triggiani, M. Multidisciplinary Challenges in Mastocytosis and How to Address with Personalized Medicine Approaches. Int. J. Mol. Sci. 2019, 20, 2976. [Google Scholar] [CrossRef]
- Fallon, M.D.; Whyte, M.P.; Teitelbaum, S.L. Systemic mastocytosis associated with generalized osteopenia. Histopathological characterization of the skeletal lesion using undecalcified bone from two patients. Hum. Pathol. 1981, 12, 813–820. [Google Scholar] [CrossRef]
- Manara, M.; Varenna, M.; Cantoni, S.; Parafioriti, A.; Gallazzi, M.B.; Sinigaglia, L. Osteoporosis with vertebral fractures in young males, due to bone marrow mastocytosis: A report of two cases. Clin. Exp. Rheumatol. 2010, 28, 97–100. [Google Scholar]
- Orsolini, G.; Viapiana, O.; Rossini, M.; Bonifacio, M.; Zanotti, R. Bone Disease in Mastocytosis. Immunol. Allergy Clin. N. Am. 2018, 38, 443–454. [Google Scholar] [CrossRef]
- Artuso, A.; Caimmi, C.; Tripi, G.; Viapiana, O.; Bonifacio, M.; Idolazzi, L.; Gavioli, I.; Gate, D.; Zanotti, R.; Rossini, M. Longitudinal Evaluation of Bone Mineral Density and Bone Metabolism Markers in Patients with Indolent Systemic Mastocytosis Without Osteoporosis. Calcif. Tissue Int. 2017, 100, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Reif, J.S.; Brodey, R.S.; Keiser, H. Epidemiological analysis of the most prevalent sites and types of canine neoplasia observed in a veterinary hospital. Cancer Res. 1974, 34, 2859–2868. [Google Scholar] [PubMed]
- Wakshlag, J.J.; Rassnick, K.M.; Malone, E.K.; Struble, A.M.; Vachhani, P.; Trump, D.L.; Tian, L. Cross-sectional study to investigate the association between vitamin D status and cutaneous mast cell tumours in Labrador retrievers. Br. J. Nutr. 2011, 106 (Suppl. S1), S60–S63. [Google Scholar] [CrossRef] [PubMed]
- Rassnick, K.M.; Muindi, J.R.; Johnson, C.S.; Balkman, C.E.; Ramnath, N.; Yu, W.D.; Engler, K.L.; Page, R.L.; Trump, D.L. In vitro and in vivo evaluation of combined calcitriol and cisplatin in dogs with spontaneously occurring tumors. Cancer Chemother. Pharmacol. 2008, 62, 881–981. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.S.; Rassnick, K.M.; Erb, H.N.; Vaughan, M.M.; McDonough, S.P. An immunohistochemical study of vitamin D receptor expression in canine cutaneous mast cell tumours. J. Comp. Pathol. 2010, 143, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, N.; Desoutter, J.; Chandesris, O.; Merlusca, L.; Henry, I.; Georgin-Lavialle, S.; Barete, S.; Hirsch, I.; Bouredji, D.; Royer, B.; et al. Bone complications of mastocytosis: A link between clinical and biological characteristics. Am. J. Med. 2013, 126, 75. [Google Scholar] [CrossRef]
- Orsolini, G.; Gavioli, I.; Tripi, G.; Viapiana, O.; Gatti, D.; Idolazzi, L.; Zanotti, R.; Rossini, M. Denosumab for the Treatment of Mastocytosis-Related Osteoporosis: A Case Series. Calcif. Tissue Int. 2017, 100, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Roodman, G.D. Biology of osteoclast activation in cancer. J. Clin. Oncol. 2001, 19, 3562–3571. [Google Scholar] [CrossRef] [PubMed]
- King, J.J.; Crawford, E.A.; Iwenofu, O.H.; Fox, E.J. Case report: Pathologic long bone fracture in a patient with systemic mastocytosis. Clin. Orthop. Relat. Res. 2007, 459, 263–269. [Google Scholar] [CrossRef]
- Garla, V.V.; Chaudhary, K.U.Q.; Yaqub, A. Systemic mastocytosis: A rare cause of osteoporosis. Pan Afr. Med. J. 2019, 32, 169. [Google Scholar] [CrossRef]
- Rabenhorst, A.; Christopeit, B.; Leja, S.; Gerbaulet, A.; Kleiner, S.; Forster, A.; Raap, U.; Wickenhauser, C.; Hartmann, K. Serum levels of bone cytokines are increased in indolent systemic mastocytosis associated with osteopenia or osteoporosis. J. Allergy Clin. Immunol. 2013, 132, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Renke, J.; Kędzierska-Mieszkowska, S.; Lange, M.; Nedoszytko, B.; Wasilewska, E.; Liberek, A.; Renke, M.; Niedoszytko, M.; Witkowski, J.; Skórko-Glonek, J.; et al. Mast cells in mastocytosis and allergy—Important player in metabolic and immunological homeostasis. Adv. Med. Sci. 2019, 64, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Bossi, F.; Frossi, B.; Radillo, O.; Cugno, M.; Tedeschi, A.; Riboldi, P.; Asero, R.; Tedesco, F.; Pucillo, C. Mast cells are critically involved in serum-mediated vascular leakage in chronic urticaria beyond high-affinity IgE receptor stimulation. Allergy 2011, 66, 1538–1545. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.W.; Ping, J.D.; Wang, Y.F.; Liu, X.N.; Li, N.; Hu, Z.L.; Ming, L. Vitamin D suppress the production of vascular endothelial growth factor in mast cell by inhibiting PI3K/Akt/p38 MAPK/HIF-1α pathway in chronic spontaneous urticaria. Clin. Immunol. 2020, 215, 108444. [Google Scholar] [CrossRef] [PubMed]
- Bader-Meunier, B.; Livideanu, C.B.; Larroche, C.; Durieu, I.; Artru, L.; Beucher, A.; Cormier, G.; Cornec, D.; DeLarco, M.; Dubost, J.-J.; et al. Association of mastocytosis with inflammatory joint diseases: A series of 31 patients. Semin. Arthritis Rheum. 2014, 44, 362–365. [Google Scholar] [CrossRef]
- Metcalfe, D.D. Mastocytosis. In Middleton’s Allergy: Principles and Practice, 9th ed.; Wesley, B.A., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 74, pp. 1216–1227. [Google Scholar]
- Ch’ng, S.; Wallis, R.A.; Yuan, L.; Davis, P.F.; Tan, S.T. Mast cells and cutaneous malignancies. Mod. Pathol. 2006, 19, 149–159. [Google Scholar] [CrossRef]
- Harvima, I.T.; Nilsson, G. Mast cells as regulators of skin inflammation and immunity. Acta Derm. Venereol. 2011, 91, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.M. Ultraviolet radiation and immunosuppression. Br. J. Dermatol. 2009, 161, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Fedoric, B.; Anderson, P.H.; Lopez, A.F.; Grimbaldeston, M.A. Vitamin D3 signalling to mast cells: A new regulatory axis. Int. J. Biochem. Cell Biol. 2011, 43, 41–46. [Google Scholar] [CrossRef]
- Babina, M.; Krautheim, M.; Grützkau, A.; Henz, B.M. Human leukemic (HMC-1) mast cells are responsive to 1,25-dihydroxyvitamin D3: Selective promotion of ICAM-3 expression and constitutive presence of vitamin D3 receptor. Biochem. Biophys. Res. Commun. 2000, 273, 1104–1110. [Google Scholar] [CrossRef]
- Kaukinen, A.; Pelkonen, J.; Harvima, I.T. Mast cells express CYP27A1 and CYP27B1 in epithelial skin cancers and psoriasis. Eur. J. Dermatol. 2015, 25, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.R.; Molnár, F.; Peräkylä, M.; Qiao, S.; Kalueff, A.V.; St-Arnaud, R.; Carlberg, C.; Tuohimaa, P. 25-Hydroxyvitamin D(3) is an agonistic vitamin D receptor ligand. J. Steroid Biochem. Mol. Biol. 2010, 118, 162–170. [Google Scholar] [CrossRef]
- Kaukinen, A.; Siiskonen, H.; Pelkonen, J.; Harvima, I.T. Immunoreactivity to CYP24A1, but not vitamin D receptor, is increased in mast cells of keratinocyte skin cancers. Eur. J. Dermatol. 2017, 27, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Hypponen, E.; Berry, D.J.; Wjst, M.; Power, C. Serum 25-hydroxyvitamin D and IgE-a significant but nonlinear relationship. Allergy 2009, 64, 613–620. [Google Scholar] [CrossRef]
- Rothers, J.; Wright, A.L.; Stern, D.A.; Halonen, M.; Camargo, C.A., Jr. Cord blood 25-hydroxyvitamin D levels are associated with aeroallergen sensitization in children from Tucson, Arizona. J. Allergy Clin. Immunol. 2011, 128, 1093–1099.e5. [Google Scholar] [CrossRef] [PubMed]
- Cairncross, C.; Grant, C.; Stonehouse, W.; Conlon, C.; McDonald, B.; Houghton, L.; Eyles, D.; Camargo, C.A.; Coad, J.; von Hurst, P. The Relationship between Vitamin D Status and Allergic Diseases in New Zealand Preschool Children. Nutrients 2016, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hener, P.; Zhang, Z.; Kato, S.; Metzger, D.; Chambon, P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc. Natl. Acad. Sci. USA 2006, 103, 11736–11741. [Google Scholar] [CrossRef] [PubMed]
| Allergic Disease | Correlation with Vitamin D | Ref. |
|---|---|---|
| Atopic disease | Higher values of 25OH-D3 in patients with mild disease compared with patients with moderate or severe disease | [27] |
| Reduced VD levels in Atopic Disease patients | [39] | |
| Vitamin D supplementation during winter months had favorable effects on Atopic Disease symptoms | [40] | |
| Food Allergy | Presence of 25OH-D3 serum values of less than 15 ng/mL | [34] |
| Chronic Urticaria | Reduced Urticaria Symptoms Severity scores after D3 supplementation | [37] |
| Chronic Spontaneous urticaria | Reduced total urticaria score after vitamin D3 administration | [38] |
| Asthma | Presence of 25OH-D3 serum values of less than 20 ng/mL (3.4%), and of 20–30 ng/mL (24.6%) | [41] |
| Inverse correlation between maternal 25OH-D3 values and inhaled steroids | [42] | |
| Vitamin D supplementation during winter months reduced the frequency of asthma attacks | [43,44] | |
| Childhood wheezing | Correlation between high maternal 25OH-D3 values with reduced childhood wheezing | [45,46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murdaca, G.; Allegra, A.; Tonacci, A.; Musolino, C.; Ricciardi, L.; Gangemi, S. Mast Cells and Vitamin D Status: A Clinical and Biological Link in the Onset of Allergy and Bone Diseases. Biomedicines 2022, 10, 1877. https://doi.org/10.3390/biomedicines10081877
Murdaca G, Allegra A, Tonacci A, Musolino C, Ricciardi L, Gangemi S. Mast Cells and Vitamin D Status: A Clinical and Biological Link in the Onset of Allergy and Bone Diseases. Biomedicines. 2022; 10(8):1877. https://doi.org/10.3390/biomedicines10081877
Chicago/Turabian StyleMurdaca, Giuseppe, Alessandro Allegra, Alessandro Tonacci, Caterina Musolino, Luisa Ricciardi, and Sebastiano Gangemi. 2022. "Mast Cells and Vitamin D Status: A Clinical and Biological Link in the Onset of Allergy and Bone Diseases" Biomedicines 10, no. 8: 1877. https://doi.org/10.3390/biomedicines10081877
APA StyleMurdaca, G., Allegra, A., Tonacci, A., Musolino, C., Ricciardi, L., & Gangemi, S. (2022). Mast Cells and Vitamin D Status: A Clinical and Biological Link in the Onset of Allergy and Bone Diseases. Biomedicines, 10(8), 1877. https://doi.org/10.3390/biomedicines10081877









