The Grasping Test Revisited: A Systematic Review of Functional Recovery in Rat Models of Median Nerve Injury
Abstract
1. Introduction
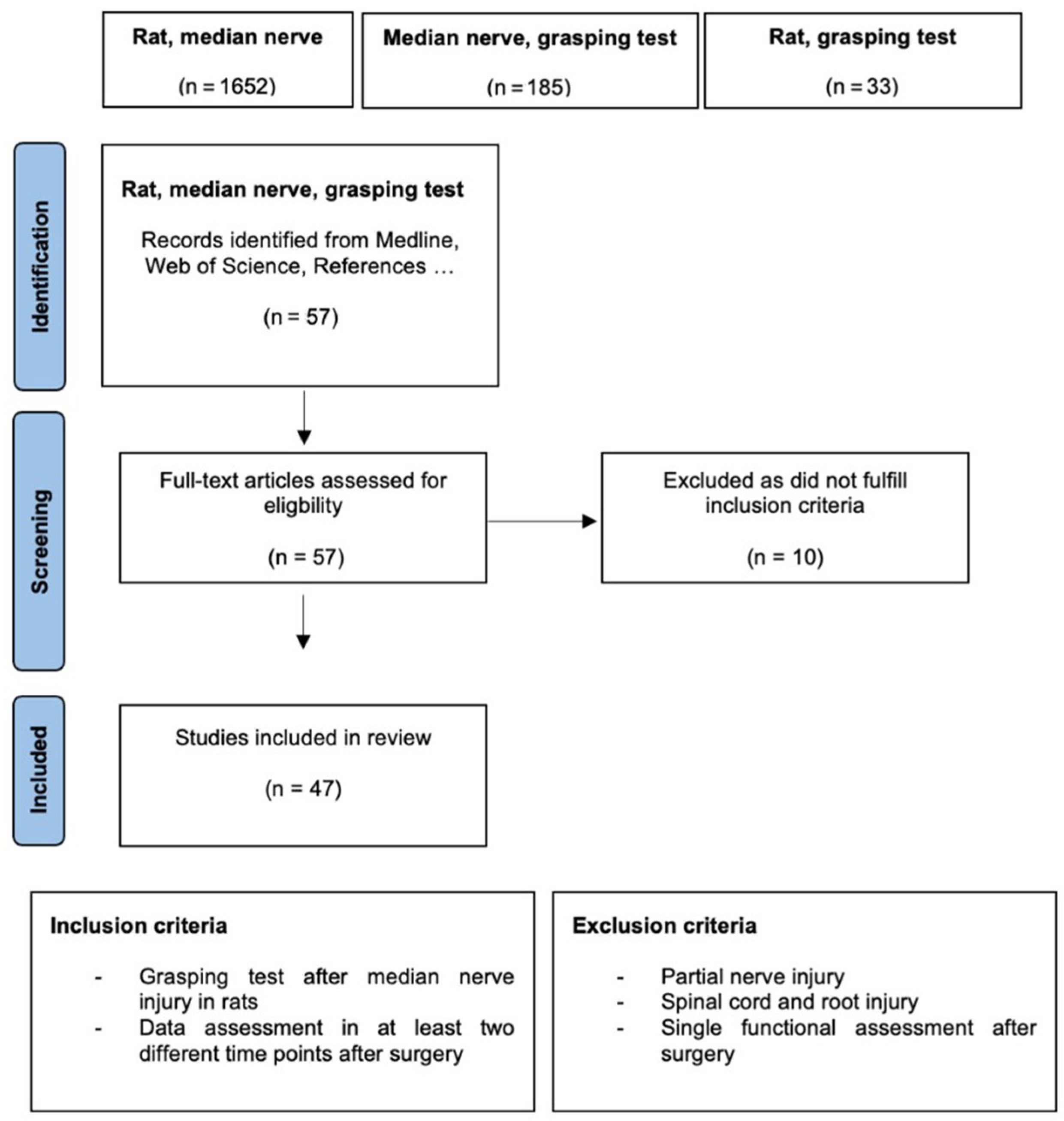
2. Materials and Methods
Data Analysis
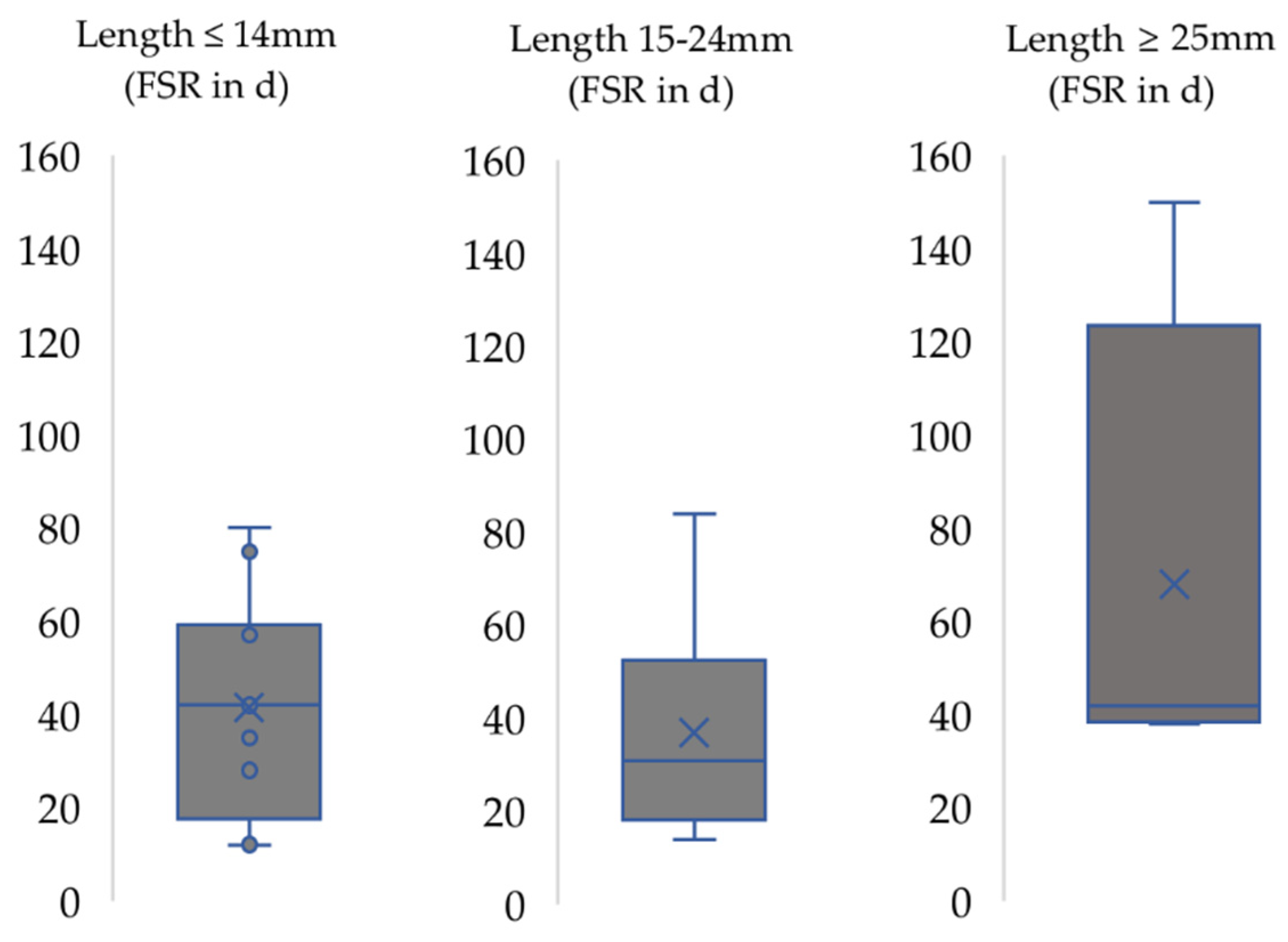
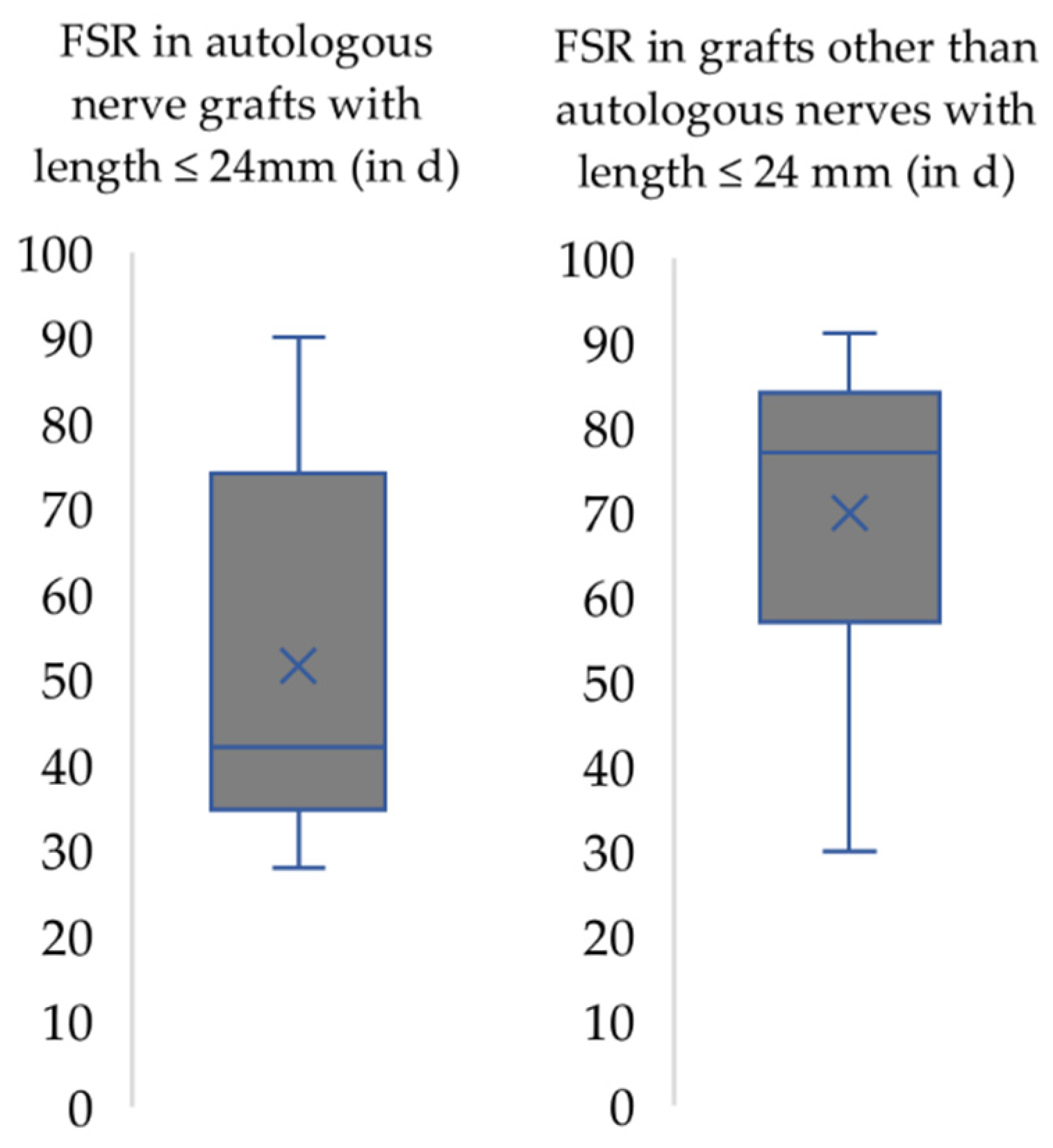
3. Results
4. Discussion
4.1. Use of the Grasping Test to Assess Functional Recovery in the Rat Median Nerve Model
4.2. Functional Recovery in the Context of the Respective Nerve Injury Model Used
4.3. Limitations of This Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Study | Content | Rat Strain | n | Type of Injury | Defect Length | Graft Length | Observation Interval | FSR | MRGS |
|---|---|---|---|---|---|---|---|---|---|
| Accioli-De-Vaconcellos et al., 1999 [69] | Comparison of fresh or frozen allografts or autografts or allografts repopulated by autologous Schwann cells | Sprague Dawley | 64 | Segmental neurotmesis | 10 mm | 20 mm | Every 3 months for 12 months | 3 months for fresh contralateral autograft | 12 months |
| Beck-Broichsitter et al., 2014 [67] | Babysitter procedure | Wistar | 20 | Nerve transection | / | / | Weekly for 15 months | Two weeks | 13 weeks |
| Beck-Broichsitter et al., 2014 [70] | Pulse magnetic field therapy | Wistar | 24 | Nerve transection | / | / | Weekly for 15 months | Two weeks | 13 weeks |
| J. A. Bertelli & Mira, 1995 [10] | Grasping test | Sprague Dawley | 65 | Crush injury | / | / | Daily | 8 days | 32 days |
| J. A. Bertelli et al., 1996 [71] | Vascularised ulnaris vs. Conventional ulnaris transfer | Sprague Dawley | 84 | Nerve transection | ? | 20 mm | Daily | 19 days | 360 days |
| J. A. Bertelli et al., 1998 [38] | Effect of neurolysis | Wistar | 30 | Crush injury | / | / | Daily for 14 days + after 21, 42 and 84 days | 10 days | 21 days |
| J. A. Bertelli et al., 2004 [55] | 40 mm graft | Wistar | 33 | Nerve transection | ? | 40 mm | Daily for 60 days + after 90, 180, 240, 360 and 510 days | 44 days | ? |
| J. A. Bertelli et al., 2005 [72] | Muscle graft | Sprague Dawley | 124 | Nerve transection | 8 mm, 18 mm | 10 mm, 20 mm | Daily | 8 mm: 57-65 days 10 mm: 68-73 days | ? |
| J. A. Bertelli et al., 2005 [54] | Graft length | Sprague Dawley | 84 | Nerve transection | 8 mm, 13 mm, 20 mm, 25 mm | 10 mm, 15 mm, 20 mm, 25 mm | Daily + after 90, 180, 270 and 360 days following onset of recovery | 10 mm: 12–15 days, | 360 days |
| 15 mm: 14–16 days, | |||||||||
| 20 mm: 32–40 days, | |||||||||
| 25 mm: 38–46 days | |||||||||
| J. A. Bertelli et al., 2006 [73] | Predegenerated graft vs. normal graft | Sprague Dawley | 68 | Nerve transection | 18 mm | 20 mm | Daily + after 90, 180, 270 and 360 days following onset of recovery | 18–22 days | 360 days |
| Casal et al., 2018 [28] | Autologous conduits with different patterns of blood supply | Wistar | 120 | Nerve transection | 10 mm | 10 mm | Every 15 days for 100 days after reconstruction | 30 days | 90 days for conventional nerve flap and arterialized neurovenous flap |
| Casal et al., 2020 [11] | Evaluation methods of recovery | Wistar | 34 | Nerve transection | 10 mm | 10 mm? | Weekly for 14 weeks | 60 days | 100 days |
| Colonna et al., 2019 [74] | Wrapping with collagen conduit | Wistar | 16 | Nerve transection | / | / | After 30, 90, 150 and 210 days | 30 days | 210 days |
| Daeschler et al., 2018 [31] | Low intensity ultrasound for axonal regeneration | Sprague Dawley | 60 | Nerve transection | / | / | Weekly until 7 weeks post-surgery | Three weeks | 8 weeks |
| Dietzmeyer et al., 2019 [68] | Chitosan nerve guides | Lewis | 16 | Nerve transection | 10 mm | 14 mm | Every two weeks for 16 weeks | Six weeks | ? |
| Ferreira et al., 2019 [75] | Effect of treadmill exercise after crush injury | Wistar | 21 | Crush injury | / | / | At day 11 and day 21 post-surgery | 11 days | 21 days |
| Fregnan et al., 2016 [76] | Chitosan conduits | Wistar | 12 | Nerve transection | 5 mm | 10 mm | Every three weeks for 12 weeks | Six weeks | ? |
| Fregnan et al., 2020 [77] | Silk-based nerve conduit | Wistar | 36 | Nerve transection | 10 mm | 12 mm | Every 3 weeks for 24 weeks | 6 weeks | 12 weeks |
| Fornazari et al., 2011 [78] | Effect of neurotrophic factor | Wistar | 40 | Nerve transection | / | / | Weekly for 12 weeks | 42 days | 84 days |
| Galtrey & Fawcett, 2007 [19] | Characterization of functional tests | Lewis + Lister Hooded | 24 | Nerve transection + Crush injury | / | / | Weekly in week 1–4 postoperatively + 6 and 14 weeks postoperatively | crush injury: 10 days nerve transection: 21 days for | 4 weeks for crush injury, 14 weeks for nerve transection |
| Gambarotta et al., 2015 [79] | Local delivery of Neuregulin 1 receptor | Wistar | 15 | Nerve transection | ? | 10 mm | Every 3 weeks for 12 weeks | 6 weeks | 12 weeks |
| Ghizoni et al., 2013 [23] | Nandrolen therapy | Sprague Dawley | 60 | Nerve transection | ? | 40 mm | Daily up to first signs of recovery + 90 and 180 days postoperatively | 40 days | ? |
| Gigo-Benato et al., 2004 [80] | Low-power laser biostimulation after end-to-side neurorrhaphy | Wistar | 16 | Nerve transection | / | / | Biweekly for 16 weeks | 10 weeks | 16 weeks |
| Hanwright et al., 2021 [81] | Porcine extracellular matrix nerve wrap | Lewis | 40 | Nerve transection | / | / | Weekly for 15 weeks | 4 weeks | ? |
| Heinzel et al., 2021 [7] | Gait analysis | Lewis | 10 | Nerve transection | 7 mm | 7 mm | Weekly for 12 weeks | 4 weeks | 12 weeks |
| Jung et al., 2009 [82] | End-to-side neurorrhaphy | Sprague Dawley | 45 | Nerve transection | / | / | Preoperatively, 10 and 20 weeks after operation | 10 weeks | 20 weeks |
| Kechele et al., 2011 [60] | Suture under tension | Wistar | 40 | Nerve transection | / | / | Daily until recovery + 30, 60, 90, 120, 150 and 180 days postoperatively | 14 days | 180 days |
| Lutz et al., 1999 [83] | Effects of systemically applied IGF-1 | Sprague Dawley | 32 | Nerve transection | / | / | Every 3–4 weeks | 2 weeks | 15 weeks |
| Lutz, Wei, et al., 1999 [84] | Effects of IGF-1 after nerve transection and repair vs. Nerve crushing injury | Sprague Dawley | 32 | Nerve transection | / | / | Every two weeks for 15 weeks | 2 weeks | ? |
| Lutz et al., 2000 [40] | Selection of donor sites for end-to-side neurorrhaphy | Sprague Dawley | 21 | Nerve transection | / | / | After first signs of recovery, 12 and 16 weeks | 4 weeks | 16 weeks |
| Machado et al., 2013 [85] | Stretch-induced nerve injury | Wistar | 36 | Crush and stretch injury | / | / | Daily for 30 days | 12 days | 30 days |
| Marchesini et al., 2018 [86] | Amnion muscle combined graft conduits | Wistar | 14 | Nerve transection | 15 mm | 15 mm | After 30, 60 and 90 days | 30 days | ? |
| Papalia, Geuna, et al., 2003 [87] | Terminolateral neurorrhaphy of the ulnar nerve | Wistar | 20 | Nerve transection | / | / | Preoperatively, 2, 8, 22 and 28 weeks | 22 weeks | ? |
| Papalia, Tos, et al., 2003 [13] | Modified device of the grasping test | Wistar | 6 | Nerve transection | / | / | Biweekly | 10 weeks | 16 weeks |
| Papalia et al., 2007 [42] | End-to-side neurorrhaphy (radialis) | Wistar | 14 | Nerve transection | / | / | 4, 10, 18, 24 and 30 weeks | 70 days | 210 days |
| Papalia et al., 2013 [88] | Vein conduits filled with lipoaspirate-derived entire adipose tissue | Wistar | 20 | Nerve transection | 10 mm | 10 mm (?) | Monthly | 2 months | ? |
| Papalia et al., 2016 [89] | Epineural window while end-to-side neurorraphy | Wistar | 19 | Nerve transection | / | / | Preoperatively, 15, 25 and 36 weeks after surgery | 15 weeks | ? |
| Ronchi et al., 2009 [36] | Assessment of crush injury | Wistar | 20 | Crush injury | / | / | Preoperatively, every 5 days postoperatively | 15 days | 30 days |
| Ronchi et al., 2017 [90] | Delayed nerve repair | Wistar | 36 | Nerve transection | / | / | Every 3 weeks for 6 months | 6 weeks | ? |
| Santos et al., 2012 [91] | Early and delayed use of phototherapies in crushed median nerves | Wistar | 24 | Crush injury | / | / | 10 and 21 days postoperatively | 10 days | 21 days |
| Sinis et al., 2005 [21] | Conduits filled with Schwann cells | Lewis | 76 | Nerve transection | 20 mm | 20 mm | Weekly after 12 weeks postoperatively for 33 weeks | 8 weeks | 24 weeks |
| Sinis et al., 2006 [53] | Cross chest median nerve transfer | Lewis | 12 | Nerve transection | ? | 40 mm | Monthly for 12 months | 5 months | 12 months |
| Sinis et al., 2006 [92] | Experiences and results with different synthetically developed materials, cellular and acellular tubes and venous conduits | Lewis | 76 | Nerve transection | ? | 20 mm | ? | Six weeks | ? |
| Sinis et al., 2009 [93] | Administration of Deferoxamine | Wistar | 48 | Nerve transection | / | / | Weekly | 4 weeks | 12 weeks |
| Sinis et al., 2011 [94] | Hemostatic procedures during nerve reconstruction | Wistar | 36 | Nerve transection | / | / | Weekly | 4 weeks | 12 weeks |
| Stößel et al., 2017 [22] | Reflex-based grasping, skilled forelimb reaching and electrodiagnostic evaluation in comparison | Lewis | 16 | Nerve transection | 7 mm | 7 mm | Every 4 weeks | 5 weeks | 12 weeks |
| Werdin et al., 2009 [30] | Electrophysical testing | Wistar | 54 | Nerve transection | 20 mm | 20 mm | Weekly after 4 weeks postoperatively for 20 weeks | Neurorrhaphy: 5 weeks Autograft: 12 weeks | 16 weeks |
References
- DeLeonibus, A.; Rezaei, M.; Fahradyan, V.; Silver, J.; Rampazzo, A.; Bassiri Gharb, B. A meta-analysis of functional outcomes in rat sciatic nerve injury models. Microsurgery 2021, 41, 286–295. [Google Scholar] [CrossRef]
- Heinzel, J.C.; Dadun, L.F.; Prahm, C.; Winter, N.; Bressler, M.; Lauer, H.; Ritter, J.; Daigeler, A.; Kolbenschlag, J. Beyond the Knife—Reviewing the Interplay of Psychosocial Factors and Peripheral Nerve Lesions. J. Pers. Med. 2021, 11, 1200. [Google Scholar] [CrossRef] [PubMed]
- Bergmeister, K.D.; Grosse-Hartlage, L.; Daeschler, S.C.; Rhodius, P.; Bocker, A.; Beyersdorff, M.; Kern, A.O.; Kneser, U.; Harhaus, L. Acute and long-term costs of 268 peripheral nerve injuries in the upper extremity. PLoS ONE 2020, 15, e0229530. [Google Scholar] [CrossRef] [PubMed]
- Varejao, A.S.; Melo-Pinto, P.; Meek, M.F.; Filipe, V.M.; Bulas-Cruz, J. Methods for the experimental functional assessment of rat sciatic nerve regeneration. Neurol Res. 2004, 26, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, A.; Scheffel, J.; Brook, G.A.; Joosten, E.A.; Suschek, C.V.; O’Dey, D.M.; Pallua, N.; Deumens, R. Aspects of static and dynamic motor function in peripheral nerve regeneration: SSI and CatWalk gait analysis. Behav Brain Res. 2011, 219, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Irintchev, A. Potentials and limitations of peripheral nerve injury models in rodents with particular reference to the femoral nerve. Ann. Anat. 2011, 193, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Heinzel, J.C.; Oberhauser, V.; Keibl, C.; Swiadek, N.; Längle, G.; Frick, H.; Kolbenschlag, J.; Prahm, C.; Grillari, J.; Hercher, D. Evaluation of Functional Recovery in Rats After Median Nerve Resection and Autograft Repair Using Computerized Gait Analysis. Front. Neurosci. 2021, 14, 593545. [Google Scholar] [CrossRef] [PubMed]
- Dellon, A.L.; Mackinnon, S.E. Sciatic nerve regeneration in the rat. Validity of walking track assessment in the presence of chronic contractures. Microsurgery 1989, 10, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, G.; Morano, M.; Fregnan, F.; Pugliese, P.; Crosio, A.; Tos, P.; Geuna, S.; Haastert-Talini, K.; Gambarotta, G. The Median Nerve Injury Model in Pre-clinical Research – A Critical Review on Benefits and Limitations. Front. Cell. Neurosci. 2019, 13, 288. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, J.A.; Mira, J.C. The grasping test: A simple behavioral method for objective quantitative assessment of peripheral nerve regeneration in the rat. J. Neurosci Methods 1995, 58, 151–155. [Google Scholar] [CrossRef]
- Casal, D.; Mota-Silva, E.; Iria, I.; Pais, D.; Farinho, A.; Alves, S.; Pen, C.; Mascarenhas-Lemos, L.; Ferreira-Silva, J.; Ferraz-Oliveira, M.; et al. Functional and Physiological Methods of Evaluating Median Nerve Regeneration in the Rat. JoVE 2020, 158, e59767. [Google Scholar] [CrossRef]
- Jackson, C.M. Anatomy of the rat. By Eunice Chace Greene. With Foreword by Henry H. Donaldson. Transactions of the American Philosophical Society, Philadelphia, New Series, Volume XXVII, 1935, 370 pp., 339 figures. Anat. Rec. 1936, 65, 127–129. [Google Scholar] [CrossRef]
- Papalia, I.; Tos, P.; Stagno d’Alcontres, F.; Battiston, B.; Geuna, S. On the use of the grasping test in the rat median nerve model: A re-appraisal of its efficacy for quantitative assessment of motor function recovery. J. Neurosci. Methods 2003, 127, 43–47. [Google Scholar] [CrossRef]
- Foster, C.H.; Karsy, M.; Jensen, M.R.; Guan, J.; Eli, I.; Mahan, M.A. Trends and Cost-Analysis of Lower Extremity Nerve Injury Using the National Inpatient Sample. Neurosurgery 2019, 85, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Karsy, M.; Watkins, R.; Jensen, M.R.; Guan, J.; Brock, A.A.; Mahan, M.A. Trends and Cost Analysis of Upper Extremity Nerve Injury Using the National (Nationwide) Inpatient Sample. World Neurosurg 2019, 123, e488–e500. [Google Scholar] [CrossRef]
- Bertelli, J.A.; Mira, J.C.; Gilbert, A.; Michot, G.A.; Legagneux, J. Anatomical basis of rat brachial plexus reconstruction. Surg. Radiol. Anat. 1992, 14, 85–86. [Google Scholar] [CrossRef]
- Montoya, C.P.; Campbell-Hope, L.J.; Pemberton, K.D.; Dunnett, S.B. The “staircase test”: A measure of independent forelimb reaching and grasping abilities in rats. J. Neurosci. Methods 1991, 36, 219–228. [Google Scholar] [CrossRef]
- Metz, G.A.; Whishaw, I.Q. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: A new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J. Neurosci. Methods 2002, 115, 169–179. [Google Scholar] [CrossRef]
- Galtrey, C.M.; Fawcett, J.W. Characterization of tests of functional recovery after median and ulnar nerve injury and repair in the rat forelimb. J. Peripher. Nerv. Syst. 2007, 12, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Tos, P.; Ronchi, G.; Papalia, I.; Sallen, V.; Legagneux, J.; Geuna, S.; Giacobini-Robecchi, M.G. Chapter 4: Methods and protocols in peripheral nerve regeneration experimental research: Part I-experimental models. Int. Rev. Neurobiol. 2009, 87, 47–79. [Google Scholar] [CrossRef] [PubMed]
- Sinis, N.; Schaller, H.E.; Schulte-Eversum, C.; Schlosshauer, B.; Doser, M.; Dietz, K.; Rosner, H.; Muller, H.W.; Haerle, M. Nerve regeneration across a 2-cm gap in the rat median nerve using a resorbable nerve conduit filled with Schwann cells. J. Neurosurg. 2005, 103, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Stößel, M.; Rehra, L.; Haastert-Talini, K. Reflex-based grasping, skilled forelimb reaching, and electrodiagnostic evaluation for comprehensive analysis of functional recovery-The 7-mm rat median nerve gap repair model revisited. Brain Behav. 2017, 7, e00813. [Google Scholar] [CrossRef] [PubMed]
- Ghizoni, M.F.; Bertelli, J.A.; Grala, C.G.; da Silva, R.M. The anabolic steroid nandrolone enhances motor and sensory functional recovery in rat median nerve repair with long interpositional nerve grafts. Neurorehabilit. Neural Repair 2013, 27, 269–276. [Google Scholar] [CrossRef]
- Hanwright, P.J.; Rath, J.L.; von Guionneau, N.; Harris, T.G.W.; Sarhane, K.A.; Kemp, S.W.P.; Hoke, A.; Cederna, P.S.; Tuffaha, S.H. Stimulated grip strength measurement: Validation of a novel method for functional assessment. Muscle Nerve 2019, 60, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Heinzel, J.; Langle, G.; Oberhauser, V.; Hausner, T.; Kolbenschlag, J.; Prahm, C.; Grillari, J.; Hercher, D. Use of the CatWalk gait analysis system to assess functional recovery in rodent models of peripheral nerve injury—A systematic review. J. Neurosci. Methods 2020, 345, 108889. [Google Scholar] [CrossRef] [PubMed]
- Haastert-Talini, K. Appropriate Animal Models for Translational Nerve Research. In Peripheral Nerve Tissue Engineering and Regeneration; Phillips, J., Hercher, D., Hausner, T., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–17. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Casal, D.; Mota-Silva, E.; Iria, I.; Alves, S.; Farinho, A.; Pen, C.; Lourenco-Silva, N.; Mascarenhas-Lemos, L.; Silva-Ferreira, J.; Ferraz-Oliveira, M.; et al. Reconstruction of a 10-mm-long median nerve gap in an ischemic environment using autologous conduits with different patterns of blood supply: A comparative study in the rat. PLoS ONE 2018, 13, e0195692. [Google Scholar] [CrossRef]
- de Medinaceli, L.; Freed, W.J.; Wyatt, R.J. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp. Neurol. 1982, 77, 634–643. [Google Scholar] [CrossRef]
- Werdin, F.; Grussinger, H.; Jaminet, P.; Kraus, A.; Manoli, T.; Danker, T.; Guenther, E.; Haerlec, M.; Schaller, H.E.; Sinis, N. An improved electrophysiological method to study peripheral nerve regeneration in rats. J. Neurosci. Methods 2009, 182, 71–77. [Google Scholar] [CrossRef]
- Daeschler, S.C.; Harhaus, L.; Bergmeister, K.D.; Boecker, A.; Hoener, B.; Kneser, U.; Schoenle, P. Clinically Available Low Intensity Ultrasound Devices do not Promote Axonal Regeneration After Peripheral Nerve Surgery-A Preclinical Investigation of an FDA-Approved Device. Front. Neurol. 2018, 9, 1057. [Google Scholar] [CrossRef] [PubMed]
- Navarro, X. Functional evaluation of peripheral nerve regeneration and target reinnervation in animal models: A critical overview. Eur. J. Neurosci. 2016, 43, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, N.M.; Lee, J.B.; Halavi, S.; Hartman, R.E.; Obenaus, A. Repeated isoflurane in adult male mice leads to acute and persistent motor decrements with long-term modifications in corpus callosum microstructural integrity. J. Neurosci. Res. 2019, 97, 332–345. [Google Scholar] [CrossRef]
- Albrecht, M.; Henke, J.; Tacke, S.; Markert, M.; Guth, B. Influence of repeated anaesthesia on physiological parameters in male Wistar rats: A telemetric study about isoflurane, ketamine-xylazine and a combination of medetomidine, midazolam and fentanyl. BMC Vet. Res. 2014, 10, 310. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, S. A classification of peripheral nerve injuries producing loss of function. Brain 1951, 74, 491–516. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, G.; Nicolino, S.; Raimondo, S.; Tos, P.; Battiston, B.; Papalia, I.; Varejao, A.S.; Giacobini-Robecchi, M.G.; Perroteau, I.; Geuna, S. Functional and morphological assessment of a standardized crush injury of the rat median nerve. J. Neurosci. Methods 2009, 179, 51–57. [Google Scholar] [CrossRef]
- Nichols, C.M.; Myckatyn, T.M.; Rickman, S.R.; Fox, I.K.; Hadlock, T.; Mackinnon, S.E. Choosing the correct functional assay: A comprehensive assessment of functional tests in the rat. Behav. Brain Res. 2005, 163, 143–158. [Google Scholar] [CrossRef]
- Bertelli, J.A.; Soares dos Santos, A.R.; Calixto, J.B. Effects of neurolysis during nerve regeneration: A behavioral and electrophysiologic study. J. Reconstr. Microsurg. 1998, 14, 165–170. [Google Scholar] [CrossRef]
- Heinzel, J.C.; Hercher, D.; Redl, H. The course of recovery of locomotor function over a 10-week observation period in a rat model of femoral nerve resection and autograft repair. Brain Behav. 2020, 10, e01580. [Google Scholar] [CrossRef] [PubMed]
- Lutz, B.S.; Chuang, D.C.; Hsu, J.C.; Ma, S.F.; Wei, F.C. Selection of donor nerves—An important factor in end-to-side neurorrhaphy. Br. J. Plast. Surg. 2000, 53, 149–154. [Google Scholar] [CrossRef]
- Houschyar, K.S.; Momeni, A.; Pyles, M.N.; Cha, J.Y.; Maan, Z.N.; Duscher, D.; Jew, O.S.; Siemers, F.; van Schoonhoven, J. The Role of Current Techniques and Concepts in Peripheral Nerve Repair. Plast. Surg. Int. 2016, 2016, 4175293. [Google Scholar] [CrossRef]
- Papalia, I.; Cardaci, A.; d’Alcontres, F.S.; Lee, J.M.; Tos, P.; Geuna, S. Selection of the donor nerve for end-to-side neurorrhaphy. J. Neurosurg. 2007, 107, 378–382. [Google Scholar] [CrossRef]
- Domeshek, L.F.; Novak, C.B.; Patterson, J.M.M.; Hasak, J.M.; Yee, A.; Kahn, L.C.; Mackinnon, S.E. Nerve Transfers-A Paradigm Shift in the Reconstructive Ladder. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2290. [Google Scholar] [CrossRef]
- Gyori, E.; Radtke, C.; Gordon, T.; Borschel, G.H. [“Pathway protection”—Enhanced motoneuron regeneration by end-to-side coaptation of sensory axons]. Handchir. Mikrochir. Plast. Chir. 2018, 50, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Terzis, J.K.; Tzafetta, K. “Babysitter” procedure with concomitant muscle transfer in facial paralysis. Plast. Reconstr. Surg. 2009, 124, 1142–1156. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.; Patel, G.; Mallu, S.; Ugwu-Oju, O.; Desai, A.; Borschel, G.; David, D.; Protzuk, O.; Shah, S.; Semus, R. Effect of Reverse End-to-Side (Supercharging) Neurotization in Long Processed Acellular Nerve Allograft in a Rat Model. J. Hand Surg. Am. 2019, 44, 419.e1–419.e10. [Google Scholar] [CrossRef]
- Luft, M.; Klepetko, J.; Muceli, S.; Ibáñez, J.; Tereshenko, V.; Festin, C.; Laengle, G.; Politikou, O.; Maierhofer, U.; Farina, D.; et al. Proof of concept for multiple nerve transfers to a single target muscle. Elife 2021, 10, e71312. [Google Scholar] [CrossRef]
- Bergmeister, K.D.; Aman, M.; Muceli, S.; Vujaklija, I.; Manzano-Szalai, K.; Unger, E.; Byrne, R.A.; Scheinecker, C.; Riedl, O.; Salminger, S.; et al. Peripheral nerve transfers change target muscle structure and function. Sci. Adv. 2019, 5, eaau2956. [Google Scholar] [CrossRef]
- Sinis, N.; Schaller, H.E.; Becker, S.T.; Schlosshauer, B.; Doser, M.; Roesner, H.; Oberhoffner, S.; Muller, H.W.; Haerle, M. Long nerve gaps limit the regenerative potential of bioartificial nerve conduits filled with Schwann cells. Restor. Neurol. Neurosci. 2007, 25, 131–141. [Google Scholar]
- Beris, A.; Gkiatas, I.; Gelalis, I.; Papadopoulos, D.; Kostas-Agnantis, I. Current concepts in peripheral nerve surgery. Eur. J. Orthop. Surg. Traumatol. 2019, 29, 263–269. [Google Scholar] [CrossRef]
- Schmidhammer, R.; Rosenauer, R.; Hausner, T. Surgical Techniques in Nerve Repair. In Peripheral Nerve Tissue Engineering and Regeneration; Phillips, J.B., Hercher, D., Hausner, T., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 467–490. [Google Scholar]
- Kaplan, H.M.; Mishra, P.; Kohn, J. The overwhelming use of rat models in nerve regeneration research may compromise designs of nerve guidance conduits for humans. J. Mater. Sci. Mater. Med. 2015, 26, 226. [Google Scholar] [CrossRef]
- Sinis, N.; Schaller, H.E.; Becker, S.T.; Lanaras, T.; Schulte-Eversum, C.; Muller, H.W.; Vonthein, R.; Rosner, H.; Haerle, M. Cross-chest median nerve transfer: A new model for the evaluation of nerve regeneration across a 40 mm gap in the rat. J. Neurosci. Methods 2006, 156, 166–172. [Google Scholar] [CrossRef]
- Bertelli, J.A.; Taleb, M.; Mira, J.C.; Ghizoni, M.F. Variation in nerve autograft length increases fibre misdirection and decreases pruning effectiveness: An experimental study in the rat median nerve. Neurol. Res. 2005, 27, 657–665. [Google Scholar] [PubMed]
- Bertelli, J.A.; dos Santos, A.R.; Taleb, M.; Calixto, J.B.; Mira, J.C.; Ghizoni, M.F. Long interpositional nerve graft consistently induces incomplete motor and sensory recovery in the rat. An experimental model to test nerve repair. J. Neurosci. Methods 2004, 134, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Kornfeld, T.; Vogt, P.M.; Radtke, C. Nerve grafting for peripheral nerve injuries with extended defect sizes. Wien. Med. Wochenschr. 2019, 169, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Hoben, G.M.; Ee, X.; Schellhardt, L.; Yan, Y.; Hunter, D.A.; Moore, A.M.; Snyder-Warwick, A.K.; Stewart, S.; Mackinnon, S.E.; Wood, M.D. Increasing Nerve Autograft Length Increases Senescence and Reduces Regeneration. Plast. Reconstr. Surg. 2018, 142, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Saheb-Al-Zamani, M.; Yan, Y.; Farber, S.J.; Hunter, D.A.; Newton, P.; Wood, M.D.; Stewart, S.A.; Johnson, P.J.; Mackinnon, S.E. Limited regeneration in long acellular nerve allografts is associated with increased Schwann cell senescence. Exp. Neurol. 2013, 247, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Hunter, D.A.; Schellhardt, L.; Jo, S.; Santosa, K.B.; Larson, E.L.; Fuchs, A.G.; Snyder-Warwick, A.K.; Mackinnon, S.E.; Wood, M.D. The accumulation of T cells within acellular nerve allografts is length-dependent and critical for nerve regeneration. Exp. Neurol. 2019, 318, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Kechele, P.R.; Bertelli, J.A.; Dalmarco, E.M.; Frode, T.S. The mesh repair: Tension free alternative on dealing with nerve gaps-experimental results. Microsurgery 2011, 31, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Krarup, C.; Archibald, S.J.; Madison, R.D. Factors that influence peripheral nerve regeneration: An electrophysiological study of the monkey median nerve. Ann. Neurol. 2002, 51, 69–81. [Google Scholar] [CrossRef]
- Mahan, M.A. Nerve stretching: A history of tension. J. Neurosurg. 2019, 132, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Haq, A.; Tiwari, M.; Saxena, A.K. Approach to management of nerve gaps in peripheral nerve injuries. Injury 2022, 53, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Fujino, K.; Yokota, A.; Ohno, K.; Hirofuji, S.; Neo, M. Impairment and restoration of the blood-nerve barrier and its correlation with pain following gradual nerve elongation of the rat sciatic nerve. Int. J. Neurosci. 2021, 131, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Prahm, C.; Heinzel, J.; Kolbenschlag, J. Blood Supply and Microcirculation of the Peripheral Nerve. In Peripheral Nerve Tissue Engineering and Regeneration; Phillips, J., Hercher, D., Hausner, T., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–46. [Google Scholar]
- Millesi, H. Peripheral nerve injuries. Nerve sutures and nerve grafting. Scand. J. Plast Reconstr. Surg. Suppl. 1982, 19, 25–37. [Google Scholar]
- Beck-Broichsitter, B.E.; Becker, S.T.; Lamia, A.; Fregnan, F.; Geuna, S.; Sinis, N. Sensoric protection after median nerve injury: Babysitter-procedure prevents muscular atrophy and improves neuronal recovery. Biomed. Res. Int. 2014, 2014, 724197. [Google Scholar] [CrossRef] [PubMed]
- Dietzmeyer, N.; Forthmann, M.; Leonhard, J.; Helmecke, O.; Brandenberger, C.; Freier, T.; Haastert-Talini, K. Two-Chambered Chitosan Nerve Guides With Increased Bendability Support Recovery of Skilled Forelimb Reaching Similar to Autologous Nerve Grafts in the Rat 10 mm Median Nerve Injury and Repair Model. Front. Cell. Neurosci. 2019, 13, 149. [Google Scholar] [CrossRef]
- Accioli-De-Vaconcellos, Z.A.; Kassar-Duchossoy, L.; Mira, J.C. Long term evaluation of experimental median nerve repair by frozen and fresh nerve autografts, allografts and allografts repopulated by autologous Schwann cells. Restor. Neurol. Neurosci. 1999, 15, 17–24. [Google Scholar]
- Beck-Broichsitter, B.E.; Lamia, A.; Geuna, S.; Fregnan, F.; Smeets, R.; Becker, S.T.; Sinis, N. Does pulsed magnetic field therapy influence nerve regeneration in the median nerve model of the rat? Biomed. Res. Int. 2014, 2014, 401760. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, J.A.; Taleb, M.; Mira, J.C.; Calixto, J.B. Muscle fiber type reorganization and behavioral functional recovery of rat median nerve repair with vascularized or conventional nerve grafts. Restor. Neurol. Neurosci. 1996, 10, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, J.A.; Taleb, M.; Mira, J.C.; Ghizoni, M.F. The course of aberrant reinnervation following nerve repair with fresh or denatured muscle autografts. J. Peripher. Nerv. Syst. 2005, 10, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, J.A.; Taleb, M.; Mira, J.C.; Ghizoni, M.F. Functional recovery improvement is related to aberrant reinnervation trimming. A comparative study using fresh or predegenerated nerve grafts. Acta Neuropathol. 2006, 111, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.R.; Fazio, A.; Costa, A.L.; Galletti, F.; Lo Giudice, R.; Galletti, B.; Galletti, C.; Lo Giudice, G.; Dell’Aversana Orabona, G.; Papalia, I.; et al. The Use of a Hypoallergenic Dermal Matrix for Wrapping in Peripheral Nerve Lesions Regeneration: Functional and Quantitative Morphological Analysis in an Experimental Animal Model. Biomed. Res. Int. 2019, 2019, 4750624. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Oliveira, M.X.; Souza, J.I.; Souza, R.A.; Machado, T.P.G.; Santos, A.P. Effects of two intensities of treadmill exercise on neuromuscular recovery after median nerve crush injury in Wistar rats. J. Exerc. Rehabil. 2019, 15, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Fregnan, F.; Ciglieri, E.; Tos, P.; Crosio, A.; Ciardelli, G.; Ruini, F.; Tonda-Turo, C.; Geuna, S.; Raimondo, S. Chitosan crosslinked flat scaffolds for peripheral nerve regeneration. Biomed. Mater. 2016, 11, 045010. [Google Scholar] [CrossRef]
- Fregnan, F.; Muratori, L.; Bassani, G.A.; Crosio, A.; Biagiotti, M.; Vincoli, V.; Carta, G.; Pierimarchi, P.; Geuna, S.; Alessandrino, A.; et al. Preclinical Validation of SilkBridge(TM) for Peripheral Nerve Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 835. [Google Scholar] [CrossRef] [PubMed]
- Fornazari, A.A.; Rezende, M.R.; Mattar, R., Jr.; Taira, R.I.; Santos, G.B.; Paulos, R.G. Effect of neurotrophic factor, MDP, on rats’ nerve regeneration. Braz. J. Med. Biol. Res. 2011, 44, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Gambarotta, G.; Pascal, D.; Ronchi, G.; Morano, M.; Jager, S.B.; Moimas, S.; Zentilin, L.; Giacca, M.; Perroteau, I.; Tos, P.; et al. Local delivery of the Neuregulin1 receptor ecto-domain (ecto-ErbB4) has a positive effect on regenerated nerve fiber maturation. Gene 2015, 22, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Gigo-Benato, D.; Geuna, S.; de Castro Rodrigues, A.; Tos, P.; Fornaro, M.; Boux, E.; Battiston, B.; Giacobini-Robecchi, M.G. Low-power laser biostimulation enhances nerve repair after end-to-side neurorrhaphy: A double-blind randomized study in the rat median nerve model. Lasers Med. Sci. 2004, 19, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Hanwright, P.J.; Rath, J.B.; von Guionneau, N.; Slavin, B.; Pinni, S.; Zlotolow, D.; Shores, J.; Dellon, A.L.; Tuffaha, S.H. The Effects of a Porcine Extracellular Matrix Nerve Wrap as an Adjunct to Primary Epineurial Repair. J. Hand Surg. Am. 2021. [Google Scholar] [CrossRef]
- Jung, J.M.; Chung, M.S.; Kim, M.B.; Baek, G.H. Contribution of the proximal nerve stump in end-to-side nerve repair: In a rat model. Clin. Orthop. Surg. 2009, 1, 90–95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lutz, B.S.; Ma, S.F.; Chuang, D.C.; Wei, F.C. Effects of Systemically Applied IGF-1 on Motor Nerve Recovery After Peripheral Nerve Transection and Repair in the Rat - A Functional Study. Hand Surg. 1999, 4, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Lutz, B.S.; Wei, F.C.; Ma, S.F.; Chuang, D.C. Effects of insulin-like growth factor-1 in motor nerve regeneration after nerve transection and repair vs. nerve crushing injury in the rat. Acta Neurochir. 1999, 141, 1101–1106. [Google Scholar] [CrossRef]
- Machado, J.A.; Ghizoni, M.F.; Bertelli, J.; Teske, G.C.; Teske, G.C.; Martins, D.F.; Mazzardo-Martins, L.; Cargnin-Ferreira, E.; Santos, A.R.; Piovezan, A.P. Stretch-induced nerve injury: A proposed technique for the study of nerve regeneration and evaluation of the influence of gabapentin on this model. Braz. J. Med. Biol. Res. 2013, 46, 929–935. [Google Scholar] [CrossRef]
- Marchesini, A.; Raimondo, S.; Zingaretti, N.; Riccio, V.; Battiston, B.; Provinciali, M.; Geuna, S.; Riccio, M. The amnion muscle combined graft (AMCG) conduits in nerves repair: An anatomical and experimental study on a rat model. J. Mater. Sci. Mater. Med. 2018, 29, 120. [Google Scholar] [CrossRef] [PubMed]
- Papalia, I.; Geuna, S.; Tos, P.L.; Boux, E.; Battiston, B.; Stagno D’Alcontres, F. Morphologic and functional study of rat median nerve repair by terminolateral neurorrhaphy of the ulnar nerve. J. Reconstr. Microsurg. 2003, 19, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Papalia, I.; Raimondo, S.; Ronchi, G.; Magaudda, L.; Giacobini-Robecchi, M.G.; Geuna, S. Repairing nerve gaps by vein conduits filled with lipoaspirate-derived entire adipose tissue hinders nerve regeneration. Ann. Anat. 2013, 195, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Papalia, I.; Magaudda, L.; Righi, M.; Ronchi, G.; Viano, N.; Geuna, S.; Colonna, M.R. Epineurial Window Is More Efficient in Attracting Axons than Simple Coaptation in a Sutureless (Cyanoacrylate-Bound) Model of End-to-Side Nerve Repair in the Rat Upper Limb: Functional and Morphometric Evidences and Review of the Literature. PLoS ONE 2016, 11, e0148443. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, G.; Cillino, M.; Gambarotta, G.; Fornasari, B.E.; Raimondo, S.; Pugliese, P.; Tos, P.; Cordova, A.; Moschella, F.; Geuna, S. Irreversible changes occurring in long-term denervated Schwann cells affect delayed nerve repair. J. Neurosurg. 2017, 127, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.P.; Suaid, C.A.; Xavier, M.; Yamane, F. Functional and morphometric differences between the early and delayed use of phototherapy in crushed median nerves of rats. Lasers Med. Sci. 2012, 27, 479–486. [Google Scholar] [CrossRef]
- Sinis, N.; Schaller, H.E.; Schulte-Eversum, C.; Schlosshauer, B.; Doser, M.; Dietz, K.; Rosner, H.; Muller, H.W.; Haerle, M. [Tissue engineering of peripheral nerves]. Handchir. Mikrochir. Plast. Chir. 2006, 38, 378–389. [Google Scholar] [CrossRef]
- Sinis, N.; Di Scipio, F.; Schonle, P.; Werdin, F.; Kraus, A.; Koopmanns, G.; Masanneck, C.; Hermanns, S.; Danker, T.; Guenther, E.; et al. Local administration of DFO-loaded lipid particles improves recovery after end-to-end reconstruction of rat median nerve. Restor. Neurol. Neurosci. 2009, 27, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Sinis, N.; Manoli, T.; Schiefer, J.L.; Werdin, F.; Jaminet, P.; Kraus, A.; Fornaro, M.; Raimondo, S.; Geuna, S.; Schaller, H.E. Application of two different hemostatic procedures during microsurgical median nerve reconstruction in the rat does not hinder axonal regeneration. Neurosurgery 2011, 68, 1399–1403, discussion 1403–1394. [Google Scholar] [CrossRef] [PubMed]


| Strain and Sexes * | Female | Male |
|---|---|---|
| Wistar | 23 | 4 |
| Lewis | 5 | 3 |
| Lister Hooded | 1 | 0 |
| Sprague Dawley | 12 | 0 |
| Group | |
|---|---|
| I | 7 (14.9%) |
| II | 13 (21.2%) |
| III | 8 (17%) |
| IV | 21 (44.7%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauer, H.; Prahm, C.; Thiel, J.T.; Kolbenschlag, J.; Daigeler, A.; Hercher, D.; Heinzel, J.C. The Grasping Test Revisited: A Systematic Review of Functional Recovery in Rat Models of Median Nerve Injury. Biomedicines 2022, 10, 1878. https://doi.org/10.3390/biomedicines10081878
Lauer H, Prahm C, Thiel JT, Kolbenschlag J, Daigeler A, Hercher D, Heinzel JC. The Grasping Test Revisited: A Systematic Review of Functional Recovery in Rat Models of Median Nerve Injury. Biomedicines. 2022; 10(8):1878. https://doi.org/10.3390/biomedicines10081878
Chicago/Turabian StyleLauer, Henrik, Cosima Prahm, Johannes Tobias Thiel, Jonas Kolbenschlag, Adrien Daigeler, David Hercher, and Johannes C. Heinzel. 2022. "The Grasping Test Revisited: A Systematic Review of Functional Recovery in Rat Models of Median Nerve Injury" Biomedicines 10, no. 8: 1878. https://doi.org/10.3390/biomedicines10081878
APA StyleLauer, H., Prahm, C., Thiel, J. T., Kolbenschlag, J., Daigeler, A., Hercher, D., & Heinzel, J. C. (2022). The Grasping Test Revisited: A Systematic Review of Functional Recovery in Rat Models of Median Nerve Injury. Biomedicines, 10(8), 1878. https://doi.org/10.3390/biomedicines10081878






