The Haemodynamic and Pathophysiological Mechanisms of Calcific Aortic Valve Disease
1. Introduction
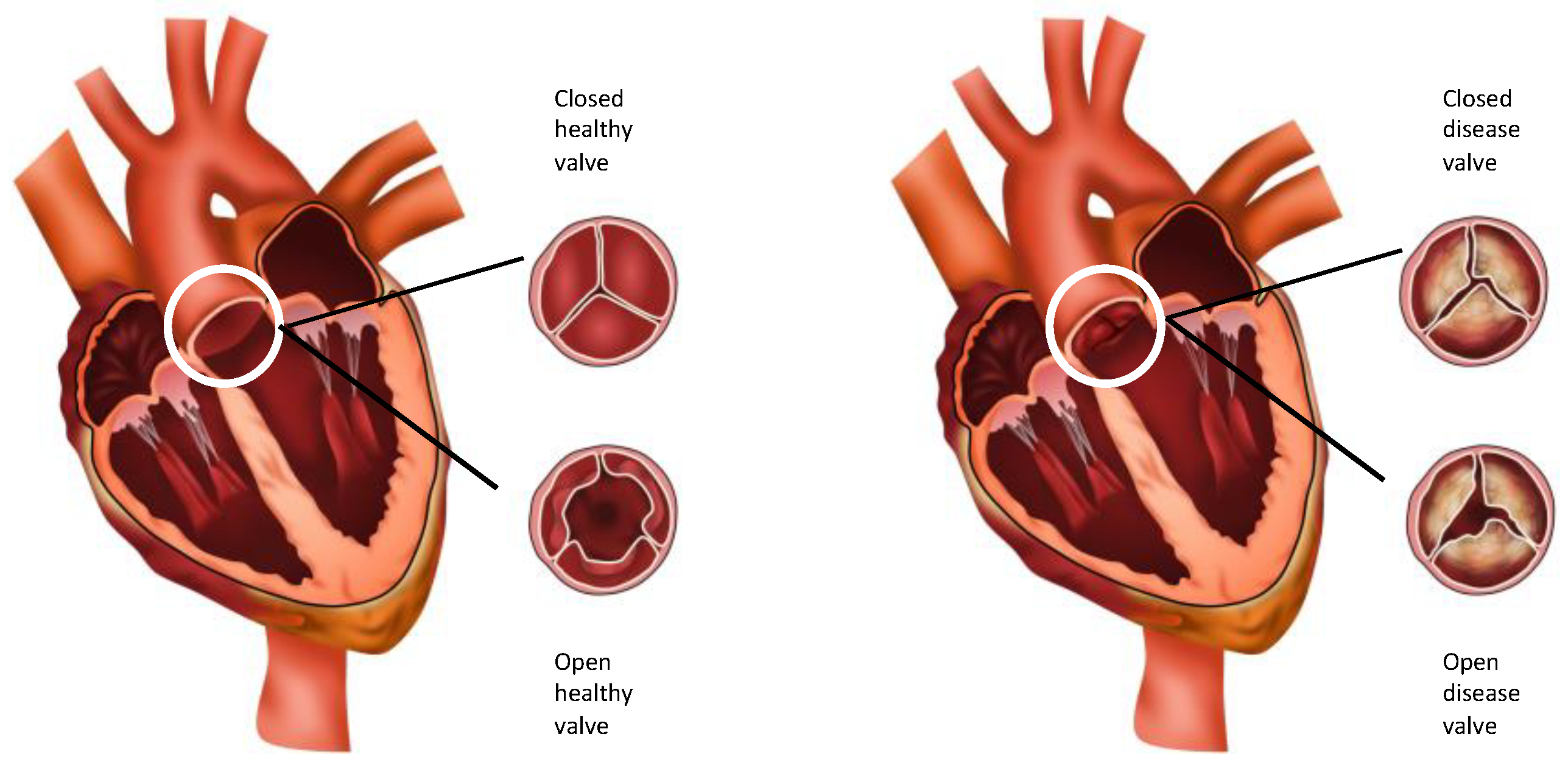
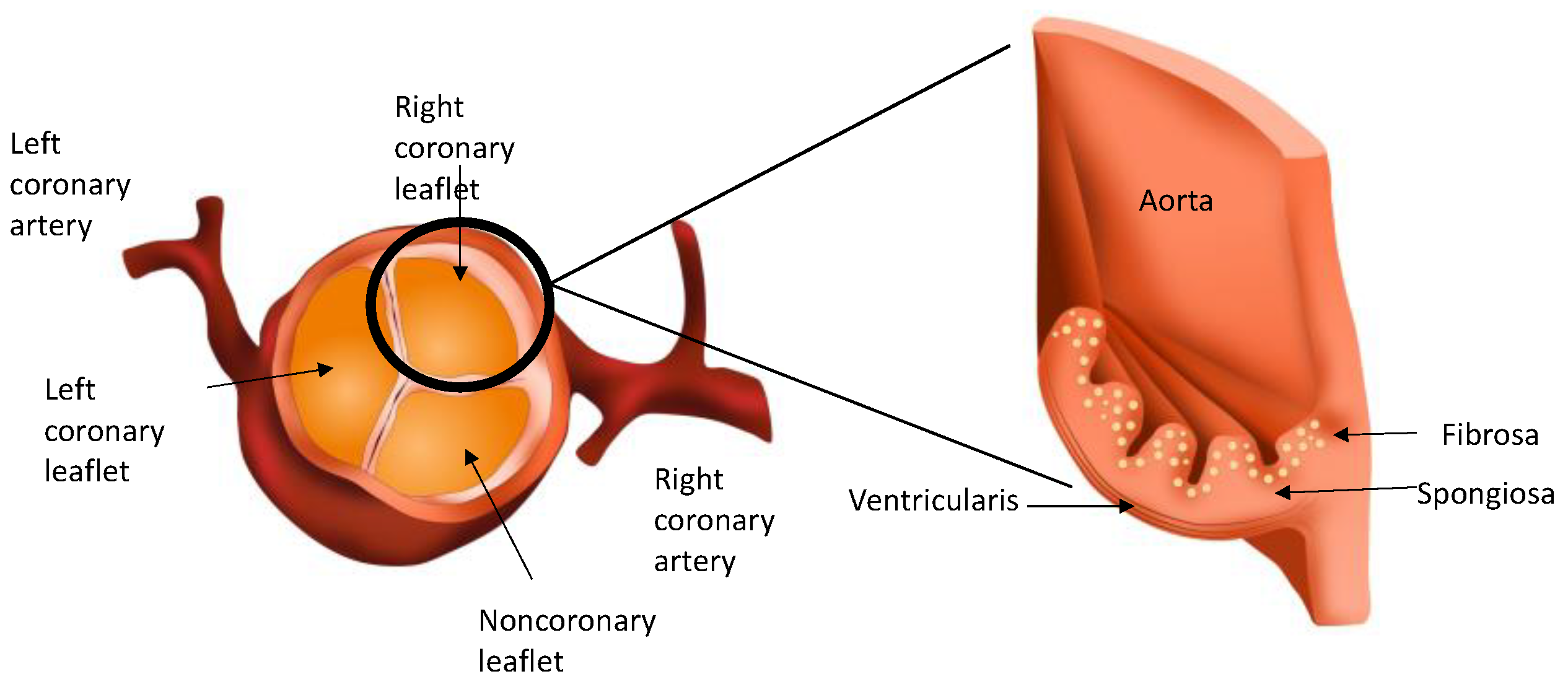
2. Anatomy and Histology of the Normal Aortic Valve
2.1. Layers of the Aortic Valve
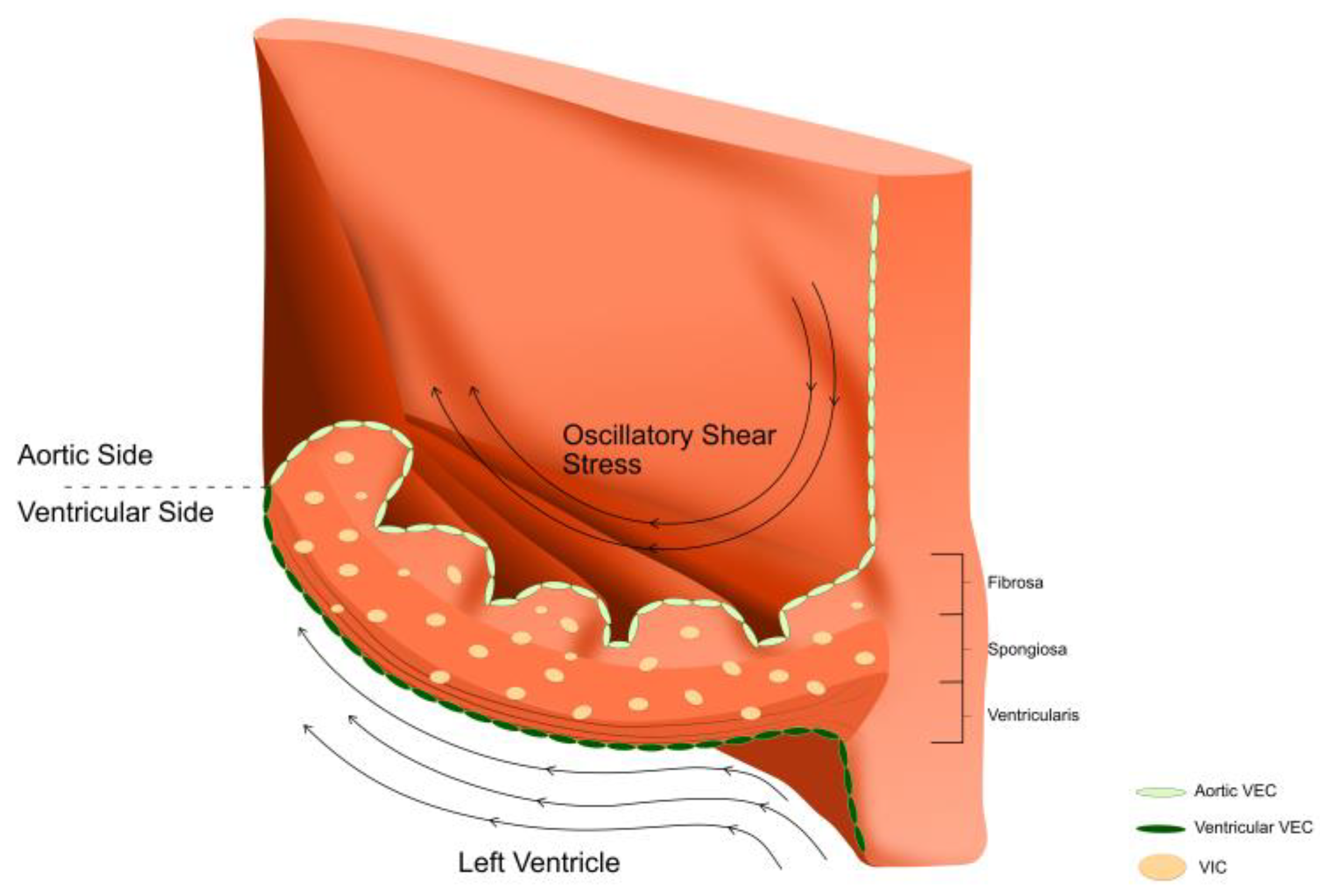
2.2. Valvular Endothelial Cells (VECs)
2.3. Valvular Interstitial Cells (VICs)
3. The Impact of Aortic Valve Haemodynamics on Molecular Pathways That Lead to Calcification of the Aortic Valve
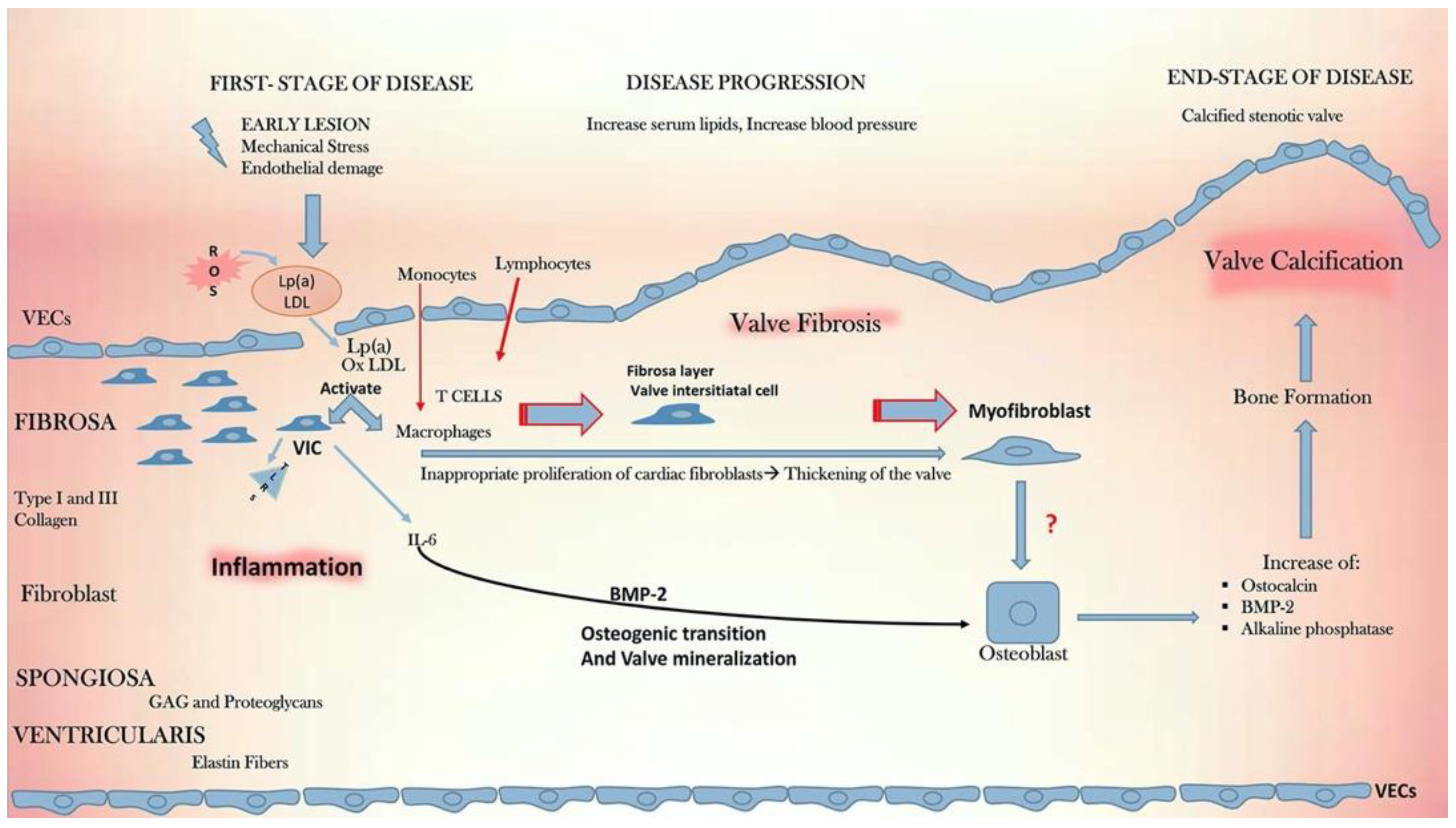
4. Downstream Molecular and Cellular Pathways Leading to Calcific Aortic Valve Disease
4.1. Lipidogenesis
4.2. Vitamin K
4.3. Inflammation
4.4. Angiotensin Converting Enzyme (ACE)
4.5. Reactive Oxygen Species
5. Valvular Endothelial Cell to Valvular Interstitial Cell Interactions in the Pathogenesis of Calcific Aortic Valve Disease
6. The Role of the Extracellular Matrix
7. Future Perspectives
7.1. Clinical Translation of Aortic Haemodynamics for the Diagnosis and Prognostication of Calcific Aortic Valve Disease
7.2. Pharmacological Clinical Trials for Calcific Aortic Disease
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Otto, C.M. Clinical practice. Evaluation and management of chronic mitral regurgitation. N. Engl. J. Med. 2001, 345, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Rader, F.; Sachdev, E.; Arsanjani, R.; Siegel, R.J. Left ventricular hypertrophy in valvular aortic stenosis: Mechanisms and clinical implications. Am. J. Med. 2015, 128, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; Gasser, T.C.; Michel, J.B.; Caligiuri, G. Biomechanical factors in the biology of aortic wall and aortic valve diseases. Cardiovasc. Res. 2013, 99, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Kuusisto, J.; Reichenbach, D.D.; Gown, A.M.; O’Brien, K.D. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation 1994, 90, 844–853. [Google Scholar] [CrossRef]
- Bonow, R.O.; Greenland, P. Population-Wide Trends in Aortic Stenosis Incidence and Outcomes. Circulation 2015, 131, 969–971. [Google Scholar] [CrossRef]
- Eveborn, G.W.; Schirmer, H.; Heggelund, G.; Lunde, P.; Rasmussen, K. The evolving epidemiology of valvular aortic stenosis. The Tromsø study. Heart 2013, 99, 396–400. [Google Scholar] [CrossRef]
- Yi, B.; Zeng, W.; Lv, L.; Hua, P. Changing epidemiology of calcific aortic valve disease: 30-year trends of incidence, prevalence, and deaths across 204 countries and territories. Aging 2021, 13, 12710–12732. [Google Scholar] [CrossRef]
- Chen, H.Y.; Engert, J.C.; Thanassoulis, G. Risk factors for valvular calcification. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 96–102. [Google Scholar] [CrossRef]
- Mohler, E.R., 3rd; Gannon, F.; Reynolds, C.; Zimmerman, R.; Keane, M.G.; Kaplan, F.S. Bone formation and inflammation in cardiac valves. Circulation 2001, 103, 1522–1528. [Google Scholar] [CrossRef]
- Cote, N.; Mahmut, A.; Bosse, Y.; Couture, C.; Pagé, S.; Trahan, S.; Boulanger, M.C.; Fournier, D.; Pibarot, P.; Mathieu, P. Inflammation is associated with the remodeling of calcific aortic valve disease. Inflammation 2013, 36, 573–581. [Google Scholar] [CrossRef]
- Vesely, I. The role of elastin in aortic valve mechanics. J. Biomech. 1998, 31, 115–123. [Google Scholar] [CrossRef]
- Xu, S.; Liu, A.C.; Gotlieb, A.I. Common pathogenic features of atherosclerosis and calcific aortic stenosis: Role of transforming growth factor-beta. Cardiovasc. Pathol. 2010, 19, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.; Grande-Allen, K.J. Elastic fibers in the aortic valve spongiosa: A fresh perspective on its structure and role in overall tissue function. Acta Biomater. 2011, 7, 2101–2108. [Google Scholar] [CrossRef] [PubMed]
- Chester, A.H.; Kershaw, J.D.; Sarathchandra, P.; Yacoub, M.H. Localisation and function of nerves in the aortic root. J. Mol. Cell Cardiol. 2008, 44, 1045–1052. [Google Scholar] [CrossRef]
- El-Hamamsy, I.; Yacoub, M.H.; Chester, A.H. Neuronal regulation of aortic valve cusps. Curr. Vasc. Pharmacol. 2009, 7, 40–46. [Google Scholar] [CrossRef]
- Deck, J.D. Endothelial cell orientation on aortic valve leaflets. Cardiovasc. Res. 1986, 20, 760–767. [Google Scholar] [CrossRef]
- Butcher, J.T.; Penrod, A.M.; García, A.J.; Nerem, R.M. Unique morphology and focal adhesion development of valvular endothelial cells in static and fluid flow environments. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1429–1434. [Google Scholar] [CrossRef]
- Malek, A.M.; Izumo, S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J. Cell Sci. 1996, 109, 713–726. [Google Scholar] [CrossRef]
- Butcher, J.T.; Nerem, R.M. Valvular endothelial cells and the mechanoregulation of valvular pathology. Philos. Trans. R Soc. Lond. B Biol. Sci. 2007, 362, 1445–1457. [Google Scholar] [CrossRef]
- Mahler, G.J.; Farrar, E.J.; Butcher, J.T. Inflammatory cytokines promote mesenchymal transformation in embryonic and adult valve endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 121–130. [Google Scholar] [CrossRef]
- Gould, S.T.; Matherly, E.E.; Smith, J.N.; Heistad, D.D.; Anseth, K.S. The role of valvular endothelial cell paracrine signaling and matrix elasticity on valvular interstitial cell activation. Biomaterials 2014, 35, 3596–3606. [Google Scholar] [CrossRef] [PubMed]
- Shapero, K.; Wylie-Sears, J.; Levine, R.A.; Mayer, J.E., Jr.; Bischoff, J. Reciprocal interactions between mitral valve endothelial and interstitial cells reduce endothelial-to-mesenchymal transition and myofibroblastic activation. J. Mol. Cell Cardiol. 2015, 80, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.L.; Merryman, W.D. Side-specific valvular endothelial-interstitial cell mechano-communication via cadherin-11. J. Biomech. 2021, 119, 110253. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.C.; Joag, V.R.; Gotlieb, A.I. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am. J. Pathol. 2007, 171, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Leinwand, L.A.; Anseth, K.S. Cardiac valve cells and their microenvironment—Insights from in vitro studies. Nat. Rev. Cardio. 2014, 11, 715–727. [Google Scholar] [CrossRef]
- Alushi, B.; Curini, L.; Christopher, M.R.; Grubitzch, H.; Landmesser, U.; Amedei, A.; Lauten, A. Calcific Aortic Valve Disease—Natural History and Future Therapeutic Strategies. Front. Pharmacol. 2020, 11, 685. [Google Scholar] [CrossRef]
- Halevi, R.; Hamdan, A.; Marom, G.; Mega, M.; Raanani, E.; Haj-Ali, R. Progressive aortic valve calcification: Three-dimensional visualization and biomechanical analysis. J. Biomech. 2015, 48, 489–497. [Google Scholar] [CrossRef]
- Balachandran, K.; Sucosky, P.; Yoganathan, A.P. Hemodynamics and mechanobiology of aortic valve inflammation and calcification. Int. J. Inflam. 2011, 2011, 263870. [Google Scholar] [CrossRef]
- Cujec, B.; Pollick, C. Isolated thickening of one aortic cusp: Preferential thickening of the noncoronary cusp. J. Am. Soc. Echocardiogr. 1988, 1, 430–432. [Google Scholar] [CrossRef]
- Weinberg, E.J.; Mack, P.J.; Schoen, F.J.; García-Cardeña, G.; Kaazempur Mofrad, M.R. Hemodynamic environments from opposing sides of human aortic valve leaflets evoke distinct endothelial phenotypes in vitro. Cardiovasc. Eng. 2010, 10, 5–11. [Google Scholar] [CrossRef]
- Holliday, C.J.; Ankeny, R.F.; Jo, H.; Nerem, R.M. Discovery of shear- and side-specific mRNAs and miRNAs in human aortic valvular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H856–H867. [Google Scholar] [CrossRef] [PubMed]
- Villar, A.V.; Cobo, M.; Llano, M.; Montalvo, C.; González-Vílchez, F.; Martín-Durán, R.; Hurlé, M.A.; Nistal, J.F. Plasma levels of transforming growth factor-beta1 reflect left ventricular remodeling in aortic stenosis. PLoS ONE 2009, 4, e8476. [Google Scholar] [CrossRef] [PubMed]
- Yetkin, E.; Tchaikovski, V.; Erdil, N.; Alan, S.; Waltenberger, J. Increased expression of cystatin C and transforming growth factor beta-1 in calcific aortic valves. Int. J. Cardiol. 2014, 176, 1252–1254. [Google Scholar] [CrossRef] [PubMed]
- Jian, B.; Narula, N.; Li, Q.Y.; Mohler, E.R., 3rd; Levy, R.J. Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann. Thorac. Surg. 2003, 75, 457–465. [Google Scholar] [CrossRef]
- Sucosky, P.; Balachandran, K.; Elhammali, A.; Jo, H.; Yoganathan, A.P. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-beta1-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 254–260. [Google Scholar] [CrossRef]
- Butcher, J.T.; Tressel, S.; Johnson, T.; Turner, D.; Sorescu, G.; Jo, H.; Nerem, R.M. Transcriptional profiles of valvular and vascular endothelial cells reveal phenotypic differences: Influence of shear stress. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 69–77. [Google Scholar] [CrossRef]
- Balachandran, K.; Sucosky, P.; Jo, H.; Yoganathan, A.P. Elevated cyclic stretch induces aortic valve calcification in a bone morphogenic protein-dependent manner. Am. J. Pathol. 2010, 177, 49–57. [Google Scholar] [CrossRef]
- White, M.P.; Theodoris, C.V.; Liu, L.; Collins, W.J.; Blue, K.W.; Lee, J.H.; Meng, X.; Robbins, R.C.; Ivey, K.N.; Srivastava, D. NOTCH1 regulates matrix gla protein and calcification gene networks in human valve endothelium. J. Mol. Cell Cardiol. 2015, 84, 13–23. [Google Scholar] [CrossRef]
- Garg, V.; Muth, A.N.; Ransom, J.F.; Schluterman, M.K.; Barnes, R.; King, I.N.; Grossfeld, P.D.; Srivastava, D. Mutations in NOTCH1 cause aortic valve disease. Nature 2005, 437, 270–274. [Google Scholar] [CrossRef]
- Driscoll, K.; Gee, T.; Butcher, J. The Cell-specific Engagement of Notch and Wnt Pathways in Calcific Aortic Valve Disease. Struct. Heart 2021, 5, 25. [Google Scholar] [CrossRef]
- Balachandran, K.; Alford, P.W.; Wylie-Sears, J.; Goss, J.A.; Grosberg, A.; Bischoff, J.; Aikawa, E.; Levine, R.A.; Parker, K.K. Cyclic strain induces dual-mode endothelial-mesenchymal transformation of the cardiac valve. Proc. Natl. Acad. Sci. USA 2011, 108, 19943–19948. [Google Scholar] [CrossRef] [PubMed]
- Albanese, I.; Yu, B.; Al-Kindi, H.; Barratt, B.; Ott, L.; Al-Refai, M.; de Varennes, B.; Shum-Tim, D.; Cerruti, M.; Gourgas, O.; et al. Role of Noncanonical Wnt Signaling Pathway in Human Aortic Valve Calcification. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Mahler, G.J.; Frendl, C.M.; Cao, Q.; Butcher, J.T. Effects of shear stress pattern and magnitude on mesenchymal transformation and invasion of aortic valve endothelial cells. Biotechnol. Bioeng. 2014, 111, 2326–2337. [Google Scholar] [CrossRef] [PubMed]
- Metzler, S.A.; Pregonero, C.A.; Butcher, J.T.; Burgess, S.C.; Warnock, J.N. Cyclic strain regulates pro-inflammatory protein expression in porcine aortic valve endothelial cells. J. Heart Valve Dis. 2008, 17, 571–577. [Google Scholar]
- Ozawa, N.; Shichiri, M.; Iwashina, M.; Fukai, N.; Yoshimoto, T.; Hirata, Y. Laminar shear stress up-regulates inducible nitric oxide synthase in the endothelium. Hypertens. Res. 2004, 27, 93–99. [Google Scholar] [CrossRef][Green Version]
- van Driel, B.O.; Schuldt, M.; Algül, S.; Levin, E.; Güclü, A.; Germans, T.; Rossum, A.C.V.; Pei, J.; Harakalova, M.; Baas, A.; et al. Metabolomics in Severe Aortic Stenosis Reveals Intermediates of Nitric Oxide Synthesis as Most Distinctive Markers. Int. J. Mol. Sci. 2021, 22, 3569. [Google Scholar] [CrossRef]
- Richards, J.; El-Hamamsy, I.; Chen, S.; Sarang, Z.; Sarathchandra, P.; Yacoub, M.H.; Chester, A.H.; Butcher, J.T. Side-specific endothelial-dependent regulation of aortic valve calcification: Interplay of hemodynamics and nitric oxide signaling. Am. J. Pathol. 2013, 182, 1922–1931. [Google Scholar] [CrossRef]
- Rathan, S.; Ankeny, C.J.; Arjunon, S.; Ferdous, Z.; Kumar, S.; Fernandez Esmerats, J.; Heath, J.M.; Nerem, R.M.; Yoganathan, A.P.; Jo, H. Identification of side- and shear-dependent microRNAs regulating porcine aortic valve pathogenesis. Sci. Rep. 2016, 6, 25397. [Google Scholar] [CrossRef]
- Heath, J.M.; Fernandez Esmerats, J.; Khambouneheuang, L.; Kumar, S.; Simmons, R.; Jo, H. Mechanosensitive microRNA-181b Regulates Aortic Valve Endothelial Matrix Degradation by Targeting TIMP3. Cardiovasc. Eng. Technol. 2018, 9, 141–150. [Google Scholar] [CrossRef]
- Fernandez Esmerats, J.; Villa-Roel, N.; Kumar, S.; Gu, L.; Salim, M.T.; Ohh, M.; Taylor, W.R.; Nerem, R.M.; Yoganathan, A.P.; Jo, H. Disturbed Flow Increases UBE2C (Ubiquitin E2 Ligase C) via Loss of miR-483-3p, Inducing Aortic Valve Calcification by the pVHL (von Hippel-Lindau Protein) and HIF-1alpha (Hypoxia-Inducible Factor-1alpha) Pathway in Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 467–481. [Google Scholar] [CrossRef]
- Toshima, T.; Watanabe, T.; Narumi, T.; Otaki, Y.; Shishido, T.; Aono, T.; Goto, J.; Watanabe, K.; Sugai, T.; Takahashi, T.; et al. Therapeutic inhibition of microRNA-34a ameliorates aortic valve calcification via modulation of Notch1-Runx2 signalling. Cardiovasc. Res. 2020, 116, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhu, X.; Ni, Y.; Wu, D.; Tian, Y.; Chen, Z.; Li, M.; Zhang, H.; Liang, D. MicroRNA-34c Inhibits Osteogenic Differentiation and Valvular Interstitial Cell Calcification via STC1-Mediated JNK Pathway in Calcific Aortic Valve Disease. Front. Physiol. 2020, 11, 829. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.D.; Reichenbach, D.D.; Marcovina, S.M.; Kuusisto, J.; Alpers, C.E.; Otto, C.M. Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of ‘degenerative’ valvular aortic stenosis. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Langsted, A. Lipoprotein (a) as a cause of cardiovascular disease: Insights from epidemiology, genetics, and biology. J. Lipid Res. 2016, 57, 1953–1975. [Google Scholar] [CrossRef]
- Mahmut, A.; Boulanger, M.C.; El Husseini, D.; Fournier, D.; Bouchareb, R.; Després, J.P.; Pibarot, P.; Bossé, Y.; Mathieu, P. Elevated expression of lipoprotein-associated phospholipase A2 in calcific aortic valve disease: Implications for valve mineralization. J. Am. Coll. Cardiol. 2014, 63, 460–469. [Google Scholar] [CrossRef]
- Berliner, J.A.; Leitinger, N.; Tsimikas, S. The role of oxidized phospholipids in atherosclerosis. J. Lipid Res. 2009, 50, S207–S212. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Ao, L.; Song, Y.; Babu, A.; Yang, X.; Wang, M.; Weyant, M.J.; Dinarello, C.A.; Cleveland, J.C., Jr.; Fullerton, D.A. Expression of functional Toll-like receptors 2 and 4 in human aortic valve interstitial cells: Potential roles in aortic valve inflammation and stenosis. Am. J. Physiol. Cell Physiol. 2008, 294, C29–C35. [Google Scholar] [CrossRef] [PubMed]
- Bouchareb, R.; Mahmut, A.; Nsaibia, M.J.; Boulanger, M.C.; Dahou, A.; Lépine, J.L.; Laflamme, M.H.; Hadji, F.; Couture, C.; Trahan, S. Autotaxin Derived from Lipoprotein (a) and Valve Interstitial Cells Promotes Inflammation and Mineralization of the Aortic Valve. Circulation 2015, 132, 677–690. [Google Scholar] [CrossRef]
- Cowell, S.J.; Newby, D.E.; Prescott, R.J.; Bloomfield, P.; Reid, J.; Northridge, D.B.; Boon, N.A. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N. Engl. J. Med. 2005, 352, 2389–2397. [Google Scholar] [CrossRef]
- Farmer, J.A. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis (the SEAS trial). Curr. Atheroscler. Rep. 2009, 11, 82–83. [Google Scholar]
- Chan, K.L.; Dumesnil, J.G.; Ni, A.; Tam, J. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: Results of the aortic stenosis progression observation: Measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 2010, 121, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Dichtl, W.; Alber, H.F.; Feuchtner, G.M.; Hintringer, F.; Reinthaler, M.; Bartel, T.; Süssenbacher, A.; Grander, W.; Ulmer, H.; Pachinger, O.; et al. Prognosis and risk factors in patients with asymptomatic aortic stenosis and their modulation by atorvastatin (20 mg). Am. J. Cardiol. 2008, 102, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Capoulade, R.; Chan, K.L.; Yeang, C.; Mathieu, P.; Bossé, Y.; Dumesnil, J.G.; Tam, J.W.; Teo, K.K.; Mahmut, A.; Yang, X.; et al. Oxidized Phospholipids, Lipoprotein (a), and Progression of Calcific Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2015, 66, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Chiyoya, M.; Seya, K.; Yu, Z.; Daitoku, K.; Motomura, S.; Imaizumi, T.; Fukuda, I.; Furukawa, K.I. Matrix Gla protein negatively regulates calcification of human aortic valve interstitial cells isolated from calcified aortic valves. J. Pharmacol. Sci. 2018, 136, 257–265. [Google Scholar] [CrossRef]
- Venardos, N.; Bennett, B.; Weyant, M.J.; Reece, T.B.; Meng, X.; Fullerton, D.A. Matrix Gla protein regulates calcification of the aortic valve. J. Surg. Res. 2015, 199, 1–6. [Google Scholar] [CrossRef]
- Brandenburg, V.M.; Reinartz, S.; Kaesler, N.; Krüger, T.; Dirrichs, T.; Kramann, R.; Peeters, F.; Floege, J.; Keszei, A.; Marx, N. Slower Progress of Aortic Valve Calcification with Vitamin K Supplementation: Results from a Prospective Interventional Proof-of-Concept Study. Circulation 2017, 135, 2081–2083. [Google Scholar] [CrossRef]
- Natorska, J.; Marek, G.; Sadowski, J.; Undas, A. Presence of B cells within aortic valves in patients with aortic stenosis: Relation to severity of the disease. J. Cardiol. 2016, 67, 80–85. [Google Scholar] [CrossRef]
- Helske, S.; Lindstedt, K.A.; Laine, M.; Mäyränpää, M.; Werkkala, K.; Lommi, J.; Turto, H.; Kupari, M.; Kovanen, P.T. Induction of local angiotensin II-producing systems in stenotic aortic valves. J. Am. Coll. Cardiol. 2004, 44, 1859–1866. [Google Scholar] [CrossRef]
- Ghaisas, N.K.; Foley, J.B.; O’Briain, D.S.; Crean, P.; Kelleher, D.; Walsh, M. Adhesion molecules in nonrheumatic aortic valve disease: Endothelial expression, serum levels and effects of valve replacement. J. Am. Coll. Cardiol. 2000, 36, 2257–2262. [Google Scholar] [CrossRef]
- Passos, L.S.A.; Lupieri, A.; Becker-Greene, D.; Aikawa, E. Innate and adaptive immunity in cardiovascular calcification. Atherosclerosis 2020, 306, 59–67. [Google Scholar] [CrossRef]
- Kaden, J.J.; Bickelhaupt, S.; Grobholz, R.; Haase, K.K.; Sarikoç, A.; Kiliç, R.; Brueckmann, M.; Lang, S.; Zahn, I.; Vahl, C.; et al. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulate aortic valve calcification. J. Mol. Cell Cardiol. 2004, 36, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Pawade, T.A.; Doris, M.K.; Bing, R.; White, A.C.; Forsyth, L.; Evans, E.; Graham, C.; Williams, M.C.; van Beek, E.J.R.; Fletcher, A.; et al. Effect of Denosumab or Alendronic Acid on the Progression of Aortic Stenosis: A Double-Blind Randomized Controlled Trial. Circulation 2021, 143, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Diehl, P.; Nagy, F.; Sossong, V.; Helbing, T.; Beyersdorf, F.; Olschewski, M.; Bode, C.; Moser, M. Increased levels of circulating microparticles in patients with severe aortic valve stenosis. Thromb. Haemost. 2008, 99, 711–719. [Google Scholar] [PubMed]
- Shroff, R.C.; McNair, R.; Skepper, J.N.; Figg, N.; Schurgers, L.J.; Deanfield, J.; Rees, L.; Shanahan, C.M. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J. Am. Soc. Nephrol. 2010, 21, 103–112. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.D.; Shavelle, D.M.; Caulfield, M.T.; McDonald, T.O.; Olin-Lewis, K.; Otto, C.M.; Probstfield, J.L. Association of angiotensin-converting enzyme with low-density lipoprotein in aortic valvular lesions and in human plasma. Circulation 2002, 106, 2224–2230. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.K.; Griendling, K.K. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2007, 292, C82–C97. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ruiz-Ortega, M.; Lorenzo, O.; Ruperez, M.; Esteban, V.; Egido, J. Inflammation and angiotensin II. Int. J. Biochem. Cell. Biol. 2003, 35, 881–900. [Google Scholar] [CrossRef]
- Arishiro, K.; Hoshiga, M.; Negoro, N.; Jin, D.; Takai, S.; Miyazaki, M.; Ishihara, T.; Hanafusa, T. Angiotensin receptor-1 blocker inhibits atherosclerotic changes and endothelial disruption of the aortic valve in hypercholesterolemic rabbits. J. Am. Coll. Cardiol. 2007, 49, 1482–1489. [Google Scholar] [CrossRef]
- O’Brien, K.D.; Probstfield, J.L.; Caulfield, M.T.; Nasir, K.; Takasu, J.; Shavelle, D.M.; Wu, A.H.; Zhao, X.Q.; Budoff, M.J. Angiotensin-converting enzyme inhibitors and change in aortic valve calcium. Arch. Intern. Med. 2005, 165, 858–862. [Google Scholar] [CrossRef]
- Bull, S.; Loudon, M.; Francis, J.M.; Joseph, J.; Gerry, S.; Karamitsos, T.D.; Prendergast, B.D.; Banning, A.P.; Neubauer, S.; Myerson, S.G. A prospective, double-blind, randomized controlled trial of the angiotensin-converting enzyme inhibitor Ramipril in Aortic Stenosis (RIAS trial). Eur. Heart J. Cardiovasc. Imaging. 2015, 16, 834–841. [Google Scholar] [CrossRef]
- Rosenhek, R.; Rader, F.; Loho, N.; Gabriel, H.; Heger, M.; Klaar, U.; Schemper, M.; Binder, T.; Maurer, G.; Baumgartner, H. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation 2004, 110, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Cueto, R.; Effi, C.; Zhang, Y.; Tan, H.; Qin, X.; Ji, Y.; Yang, X.; Wang, H. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 2019, 26, 101284. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Hwang, J.; Ing, M.H.; Salazar, A.; Lassègue, B.; Griendling, K.; Navab, M.; Sevanian, A.; Hsiai, T.K. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: Implication for native LDL oxidation. Circ. Res. 2003, 93, 1225–1232. [Google Scholar] [CrossRef]
- Miller, J.D.; Chu, Y.; Brooks, R.M.; Richenbacher, W.E.; Peña-Silva, R.; Heistad, D.D. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J. Am. Coll. Cardiol. 2008, 52, 843–850. [Google Scholar] [CrossRef]
- Branchetti, E.; Sainger, R.; Poggio, P.; Grau, J.B.; Patterson-Fortin, J.; Bavaria, J.E.; Chorny, M.; Lai, E.; Gorman, R.C.; Levy, R.J.; et al. Antioxidant enzymes reduce DNA damage and early activation of valvular interstitial cells in aortic valve sclerosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, e66–e74. [Google Scholar] [CrossRef]
- Liberman, M.; Bassi, E.; Martinatti, M.K.; Lario, F.C.; Wosniak, J., Jr.; Pomerantzeff, P.M.; Laurindo, F.R. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 463–470. [Google Scholar] [CrossRef]
- Farrar, E.J.; Huntley, G.D.; Butcher, J. Endothelial-derived oxidative stress drives myofibroblastic activation and calcification of the aortic valve. PLoS ONE 2015, 10, e0123257. [Google Scholar] [CrossRef]
- Das, D.; Holmes, A.; Murphy, G.A.; Mishra, K.; Rosenkranz, A.C.; Horowitz, J.D.; Kennedy, J.A. TGF-beta1-Induced MAPK activation promotes collagen synthesis, nodule formation, redox stress and cellular senescence in porcine aortic valve interstitial cells. J. Heart Valve Dis. 2013, 22, 621–630. [Google Scholar]
- Liu, H.; Wang, L.; Pan, Y.; Wang, X.; Ding, Y.; Zhou, C.; Shah, A.M.; Zhao, G.; Zhang, M. Celastrol Alleviates Aortic Valve Calcification Via Inhibition of NADPH Oxidase 2 in Valvular Interstitial Cells. JACC Basic Transl. Sci. 2020, 5, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.A.; Grant, G.R.; Manduchi, E. Davies, P.F. Spatial heterogeneity of endothelial phenotypes correlates with side-specific vulnerability to calcification in normal porcine aortic valves. Circ. Res. 2005, 96, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Hjortnaes, J.; Shapero, K.; Goettsch, C.; Hutcheson, J.D.; Keegan, J.; Kluin, J.; Mayer, J.E.; Bischoff, J.; Aikawa, E. Valvular interstitial cells suppress calcification of valvular endothelial cells. Atherosclerosis 2015, 242, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Rutkovskiy, A.; Malashicheva, A.; Sullivan, G.; Bogdanova, M.; Kostareva, A.; Stensløkken, K.O.; Fiane, A.; Vaage, J. Valve Interstitial Cells: The Key to Understanding the Pathophysiology of Heart Valve Calcification. J. Am. Heart Assoc. 2017, 6, e006339. [Google Scholar] [CrossRef]
- Gomez-Stallons, M.V.; Wirrig-Schwendeman, E.E.; Hassel, K.R.; Conway, S.J.; Yutzey, K.E. Bone Morphogenetic Protein Signaling Is Required for Aortic Valve Calcification. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1398–1405. [Google Scholar] [CrossRef]
- Edep, M.E.; Shirani, J.; Wolf, P.; Brown, D.L. Matrix metalloproteinase expression in nonrheumatic aortic stenosis. Cardiovasc. Pathol. 2000, 9, 281–286. [Google Scholar] [CrossRef]
- Satta, J.; Oiva, J.; Salo, T.; Eriksen, H.; Ohtonen, P.; Biancari, F.; Juvonen, T.S.; Soini, Y. Evidence for an altered balance between matrix metalloproteinase-9 and its inhibitors in calcific aortic stenosis. Ann. Thorac. Surg. 2003, 76, 681–688. [Google Scholar] [CrossRef]
- Fondard, O.; Detaintm, D.; Iung, B.; Choqueux, C.; Adle-Biassette, H.; Jarraya, M.; Hvass, U.; Couetil, J.P.; Henin, D.; Michel, J.B.; et al. Extracellular matrix remodelling in human aortic valve disease: The role of matrix metalloproteinases and their tissue inhibitors. Eur. Heart J. 2005, 26, 1333–1341. [Google Scholar] [CrossRef]
- Kaden, J.J.; Dempfle, C.E.; Grobholz, R.; Fischer, C.S.; Vocke, D.C.; Kiliç, R.; Sarikoç, A.; Piñol, R.; Hagl, S.; Lang, S.; et al. Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovasc. Pathol. 2005, 14, 80–87. [Google Scholar] [CrossRef]
- Helske, S.; Syväranta, S.; Lindstedt, K.A.; Lappalainen, J.; Oörni, K.; Mäyränpää, M.I.; Lommi, J.; Turto, H.; Werkkala, K.; Kupari, M.; et al. Increased expression of elastolytic cathepsins S, K, and V and their inhibitor cystatin C in stenotic aortic valves. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1791–1798. [Google Scholar] [CrossRef]
- Latif, N.; Sarathchandra, P.; Taylor, P.M.; Antoniw, J.; Yacoub, M.H. Localization and pattern of expression of extracellular matrix components in human heart valves. J. Heart Valve Dis. 2005, 14, 218–227. [Google Scholar] [PubMed]
- Hutson, H.N.; Marohl, T.; Anderson, M.; Eliceiri, K.; Campagnola, P.; Masters, K.S. Calcific Aortic Valve Disease Is Associated with Layer-Specific Alterations in Collagen Architecture. PLoS ONE 2016, 11, e0163858. [Google Scholar]
- Archer, G.T.; Elhawaz, A.; Barker, N.; Fidock, B.; Rothman, A.; van der Geest, R.J.; Hose, R.; Briffa, N.; Hall, I.R.; Grech, E.; et al. Validation of four-dimensional flow cardiovascular magnetic resonance for aortic stenosis assessment. Sci. Rep. 2020, 10, 10569. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Patel, A.; Ali, Z.; Abu-Omar, Y.; Saeed, A.; Athanasiou, T.; Pepper, J. Enhanced left ventricular mass regression after aortic valve replacement in patients with aortic stenosis is associated with improved long-term survival. J. Thorac. Cardiovasc. Surg. 2011, 142, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Binter, C.; Gotschy, A.; Sündermann, S.H.; Frank, M.; Tanner, F.C.; Lüscher, T.F.; Manka, R.; Kozerke, S. Turbulent Kinetic Energy Assessed by Multipoint 4-Dimensional Flow Magnetic Resonance Imaging Provides Additional Information Relative to Echocardiography for the Determination of Aortic Stenosis Severity. Circ. Cardiovasc. Imaging 2017, 10, e005486. [Google Scholar] [CrossRef]
- Von Knobelsdorff-Brenkenhoff, F.; Karunaharamoorthy, A.; Trauzeddel, R.F.; Barker, A.J.; Blaszczyk, E.; Markl, M.; Schulz-Menger, J. Evaluation of Aortic Blood Flow and Wall Shear Stress in Aortic Stenosis and Its Association with Left Ventricular Remodeling. Circ. Cardiovasc. Imaging 2016, 9, e004038. [Google Scholar] [CrossRef] [PubMed]
- Can Ooij, P.; Markl, M.; Collins, J.D.; Carr, J.C.; Rigsby, C.; Bonow, R.O.; Malaisrie, S.C.; McCarthy, P.M.; Fedak, P.W.M.; Barker, A.J. Aortic Valve Stenosis Alters Expression of Regional Aortic Wall Shear Stress: New Insights from a 4-Dimensional Flow Magnetic Resonance Imaging Study of 571 Subjects. J. Am. Heart Assoc. 2017, 6, e005959. [Google Scholar] [CrossRef]
| Study Name and NCT Identifier | Principal Investigator and Institution | Pharmacological Target and Treatment Arms | Recruitment Status |
|---|---|---|---|
| A study evaluating the effects of Ataciguat (HMR1766) on aortic valve calcification (CAVS) NCT02481258 | Jordan D Miller; Mayo Clinic, Rochester, Minnesota, United States | Nitric oxide-independent soluble guanylate cyclase activator Ataciguat (HMR1766) 200 mg daily for 12 months vs. placebo | Completed, results awaited |
| Effect of PCSK9 inhibitors on calcific aortic valve disease (EPISODE) NCT04968509 | Zhijian Wang; Beijing Anzhen Hospital, China | PCSK9 inhibitors (lipid lowering) 140 mg PCSK9 inhibitors (140 mg Evolocumab or Alirocumab subcutaneously every two weeks and conventional lipid lowering therapy (Atorvastatin 40–80 mg or Rosuvastatin 20–40 mg or Atorvastatin 20 mg+ Ezetimibe 10 mg or Rosuvastatin 10 mg+ Ezetimibe 10 mg vs. Conventional lipid-lowering therapy (Atorvastatin 40–80 mg or Rosuvastatin 20–40 mg or Atorvastatin 20 mg+ Ezetimibe 10 mg or Rosuvastatin 10 mg+ Ezetimibe) | Recruiting |
| A Study to evaluate the efficacy and safety of DA-1229 (Evogliptin) in patients with calcific aortic valve disease with mild to moderate stenosis (EVOID-AS) NCT05143177 | REDNVIA Co., Ltd. | DA-1229 anti-diabetic Three arm study where patients will receive one of the three treatments orally once daily for period of 104 weeks; DA-1229 5 mg once vs. GroupDA-1229 10 mg vs. Placebo | Not yet recruiting |
| Colchicine and Inflammation in Aortic Stenosis (CHIANTI) NCT05162742 | Radboud University Medical Centre | Colchicine vs. placebo | Not yet recruiting |
| Slower progress of calcification with Vitamin K2 (SLOW) NCT04429035 | First Department of Cardiology, University of Athens Athens, Attiki, Greece Hippokration Hospital 1st Department of Cardiology, National and Kapodistrian University of Athens, Medical School Athens, Attiki, Greece | Dietary Supplement: Vitamin K2 vs. Dietary Supplement: Placebo | Recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanna, L.; Armour, C.; Xu, X.Y.; Gibbs, R. The Haemodynamic and Pathophysiological Mechanisms of Calcific Aortic Valve Disease. Biomedicines 2022, 10, 1317. https://doi.org/10.3390/biomedicines10061317
Hanna L, Armour C, Xu XY, Gibbs R. The Haemodynamic and Pathophysiological Mechanisms of Calcific Aortic Valve Disease. Biomedicines. 2022; 10(6):1317. https://doi.org/10.3390/biomedicines10061317
Chicago/Turabian StyleHanna, Lydia, Chlöe Armour, Xiao Yun Xu, and Richard Gibbs. 2022. "The Haemodynamic and Pathophysiological Mechanisms of Calcific Aortic Valve Disease" Biomedicines 10, no. 6: 1317. https://doi.org/10.3390/biomedicines10061317
APA StyleHanna, L., Armour, C., Xu, X. Y., & Gibbs, R. (2022). The Haemodynamic and Pathophysiological Mechanisms of Calcific Aortic Valve Disease. Biomedicines, 10(6), 1317. https://doi.org/10.3390/biomedicines10061317






