Drug Reaction with Eosinophilia and Systemic Symptoms (DReSS)/Drug-Induced Hypersensitivity Syndrome (DiHS)—Readdressing the DReSS
Abstract
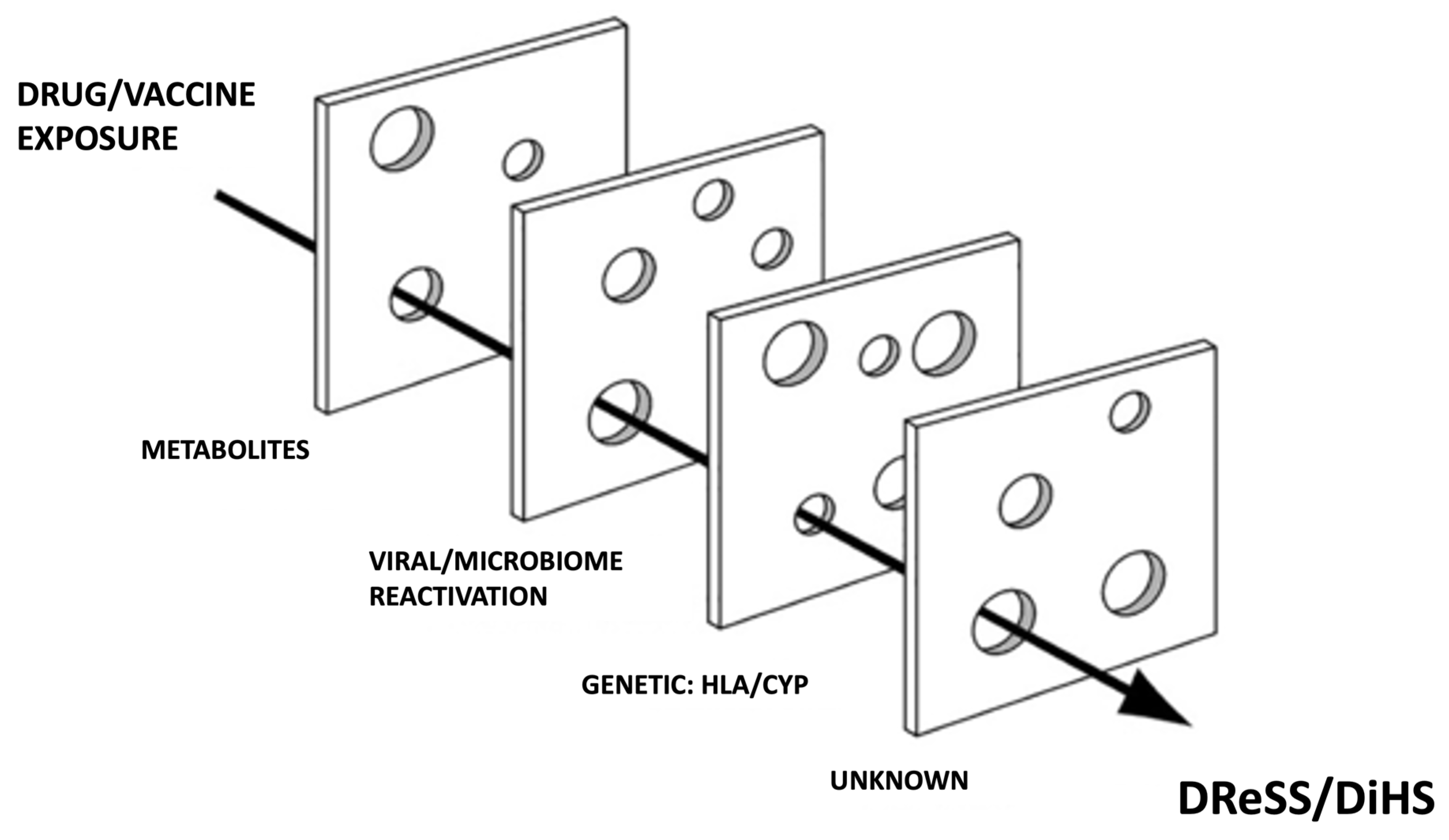
:1. Introduction
2. Literature Search Methods
3. Epidemiology
4. Pathogenesis
4.1. Viral Reactivation
4.2. Drugs
4.3. Immune Changes
4.4. Genetic Predisposition
| Drug | HLA Allele | Ethnicity | References |
|---|---|---|---|
| Allopurinol | B*58:01 | Han Chinese, Korean, Taiwanese, Thai | [66,67,74,75,76,77,78] |
| Carbamazepine | A*31:01 | European, Chinese, Korean, Japanese | [68,69,70,71,79,80] |
| Dapsone | B*13:01 | Chinese, Taiwanese, Thai | [81,82,83,84] |
| Salazosulfapyridine | B*13:01 | Han Chinese | [85] |
| Phenytoin | A*24:02 | European (Spanish) | [80] |
| B*15:13 | Malaysian | [86] | |
| B* 51:01 | Thai | [87] | |
| C*14:02 | Thai | [87] | |
| Lamotrigine | B*51:01 and A*24:02 | European (Spanish) | [80] |
| Piperacillin/tazobactam | B*62 | UK caucasian | [88] |
| Vancomycin | A*32:01 | North American | [89] |
| Abacavir * | B*57:01 | European, African, North American | [64,65,73,90] |
| Nevirapine * | CW*04:01 | Han Chinese, Thai, Malawian | [91,92,93,94] |
| Cw*8/Cw*08-B*14 | Italian, Japanese | [95,96] | |
| B* 35:05 | Asian (Thai) | [92,97] | |
| B*35:01 | Australian | [98] | |
| DRB1∗01:01 | Australian | [97] | |
| Raltegravir | B*53:01 | African, Hispanic | [99] |
5. Clinical Features
6. Diagnosis
6.1. DReSS Minor/DReSS Major
6.2. Causality Assessment and Confirmatory Testing
7. Histopathologic Findings
8. Differential Diagnosis
9. Prognosis and Long-Term Outcomes
10. Treatment
11. Limitations
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shear, N.H.; Dodiuk-Gad, R. Advances in Diagnosis and Management of Cu-Taneous Adverse Drug Reactions: Current and Future Trends; Shear, N., Dodi-uk-Gad, R., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2019; pp. 87–104. ISBN 9789811314889. [Google Scholar]
- Klimas, N.; Quintanilla-Dieck, J.; Vandergriff, T. Drug-Induced Delayed Mul-ti-Organ Hypersensitivity Syndrome. In Cutaneous Drug Eruptions: Diagnosis, Histopathology and Therapy; Hall, B.J., Hall, J.C., Eds.; Springer: London, UK, 2015; pp. 271–279. [Google Scholar]
- Martínez-Cabriales, S.A.; Rodríguez-Bolaños, F.; Shear, N.H. Drug Reaction with Eosinophilia and Systemic Symptoms (DReSS): How Far Have We Come? Am. J. Clin. Dermatol. 2019, 20, 217–236. [Google Scholar] [CrossRef]
- Bocquet, H.; Bagot, M.; Roujeau, J.C. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: DRESS). Semin. Cutan. Med. Surg. 1996, 15, 250–257. [Google Scholar] [CrossRef]
- Kardaun, S.; Sidoroff, A.; Valeyrie-Allanore, L.; Halevy, S.; Davidovici, B.; Mockenhaupt, M.; Roujeau, J. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: Does a DRESS syndrome really exist? Br. J. Dermatol. 2007, 156, 609–611. [Google Scholar] [CrossRef]
- Shiohara, T.; Mizukawa, Y. Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): An update in 2019. Allergol. Int. 2019, 68, 301–308. [Google Scholar] [CrossRef]
- Griffin, D.W.; Martin, G.E.; McLean, C.; Cheng, A.C.; Giles, M.L. A case of drug reaction with eosinophilia and systemic symptoms (DRESS) without a typical precipitant. Med. J. Aust. 2020, 212, 300–301.e1. [Google Scholar] [CrossRef]
- Lospinoso, K.; Nichols, C.S.; Malachowski, S.J.; Mochel, M.C.; Nutan, F. A case of severe cutaneous adverse reaction following administration of the Janssen Ad26.COV2.S COVID-19 vaccine. JAAD Case Rep. 2021, 13, 134–137. [Google Scholar] [CrossRef]
- Di Palma-Grisi, J.C.; Vijayagopal, K.; Muslimani, M.A. Case Reports of DRESS Syndrome and Symptoms Consistent with DRESS Syndrome Following Treatment with Recently Marketed Monoclonal Antibodies. Autoimmune Dis. 2019, 2019, 7595706. [Google Scholar] [CrossRef]
- Vittorio, C.; Muglia, J. Anticonvulsant Hypersensitivity Syndrome. Arch. Intern. Med. 1995, 155, 2285–2290. [Google Scholar] [CrossRef]
- Ramírez, E.; Medrano-Casique, N.; Tong, H.Y.; Bellón, T.; Cabañas, R.; Fiandor, A.; González-Ramos, J.; Herranz, P.; Trigo, E.; Muñoz, M.; et al. Eosinophilic drug reactions detected by a prospective pharmacovigilance programme in a tertiary hospital. Br. J. Clin. Pharmacol. 2016, 83, 400–415. [Google Scholar] [CrossRef] [Green Version]
- Muller, P.; Dubreil, P.; Mahé, A.; Lamaury, I.; Salzer, B.; Deloumeaux, J.; Strobel, M. Drug Hypersensitivity Syndrome in a West-Indian population. Eur. J. Dermatol. 2003, 13, 478–481. [Google Scholar]
- Hiransuthikul, A.; Rattananupong, T.; Klaewsongkram, J.; Rerknimitr, P.; Pongprutthipan, M.; Ruxrungtham, K. Drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms (DIHS/DRESS): 11 years retrospective study in Thailand. Allergol. Int. 2016, 65, 432–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfson, A.R.; Zhou, L.; Li, Y.; Phadke, N.A.; Chow, O.A.; Blumenthal, K.G. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome Identified in the Electronic Health Record Allergy Module. J. Allergy Clin. Immunol. Pract. 2019, 7, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, M.P.; Gameiro, A.; Coutinho, I.; Pereira, N.; Cardoso, J.; Gonçalo, M. Overlap between maculopapular exanthema and drug reaction with eosinophilia and systemic symptoms among cutaneous adverse drug reactions in a dermatology ward. Br. J. Dermatol. 2016, 175, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Kardaun, S.H.; Sekula, P.; Valeyrie-Allanore, L.; Liss, Y.; Chu, C.-Y.; Creamer, D.; Sidoroff, A.; Naldi, L.; Mockenhaupt, M.; Roujeau, J.C.; et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): An original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br. J. Dermatol. 2013, 169, 1071–1080. [Google Scholar] [CrossRef]
- Mizukawa, Y.; Hirahara, K.; Kano, Y.; Shiohara, T. Drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms severity score: A useful tool for assessing disease severity and predicting fatal cytomegalovirus disease. J. Am. Acad. Dermatol. 2019, 80, 670–678.e2. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-B.; Abe, R.; Pan, R.-Y.; Wang, C.-W.; Hung, S.-I.; Tsai, Y.-G.; Chung, W.-H. An Updated Review of the Molecular Mechanisms in Drug Hypersensitivity. J. Immunol. Res. 2018, 2018, 6431694. [Google Scholar] [CrossRef]
- Metterle, L.; Hatch, L.; Seminario-Vidal, L. Pediatric drug reaction with eosinophilia and systemic symptoms: A systematic review of the literature. Pediatr. Dermatol. 2019, 37, 124–129. [Google Scholar] [CrossRef]
- Miyagawa, F.; Asada, H. Current Perspective Regarding the Immunopathogenesis of Drug-Induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms (Dihs/Dress). Int. J. Mol. Sci. 2021, 22, 2147. [Google Scholar] [CrossRef]
- Seishima, M.; Yamanaka, S.; Fujisawa, T.; Tohyama, M.; Hashimoto, K. Reactivation of human herpesvirus (HHV) family members other than HHV-6 in drug-induced hypersensitivity syndrome. Br. J. Dermatol. 2006, 155, 344–349. [Google Scholar] [CrossRef]
- Drago, F.; Cogorno, L.; Broccolo, F.; Ciccarese, G.; Parodi, A. A fatal case of DRESS induced by strontium ranelate associated with HHV-7 reactivation. Osteoporos. Int. 2015, 27, 1261–1264. [Google Scholar] [CrossRef]
- Shiohara, T.; Iijima, M.; Ikezawa, Z.; Hashimoto, K. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br. J. Dermatol. 2007, 156, 1083–1084. [Google Scholar] [CrossRef] [PubMed]
- Descamps, V.; Brunet-Possenti, F. Human Herpesvirus 6 Reactivation in DRESS with Acute Liver Failure. Transplant. 2017, 101, e224–e225. [Google Scholar] [CrossRef] [PubMed]
- Roujeau, J.-C.; Dupin, N. Virus Reactivation in Drug Reaction with Eosinophilia and Systemic Symptoms (Dress) Results from a Strong Drug-Specific Immune Response. J. Allergy Clin. Immunol. Pract. 2017, 5, 811–812. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, M.; Hashimoto, K.; Yasukawa, M.; Kimura, H.; Horikawa, T.; Nakajima, K.; Urano, Y.; Matsumoto, K.; Iijima, M.; Shear, N. Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br. J. Dermatol. 2007, 157, 934–940. [Google Scholar] [CrossRef]
- Cacoub, P.; Musette, P.; Descamps, V.; Meyer, O.; Speirs, C.; Finzi, L.; Roujeau, J.C. The DRESS Syndrome: A Literature Review. Am. J. Med. 2011, 124, 588–597. [Google Scholar] [CrossRef]
- Picard, D.; Janela, B.; Descamps, V.; D’Incan, M.; Courville, P.; Jacquot, S.; Rogez, S.; Mardivirin, L.; Moins-Teisserenc, H.; Toubert, A.; et al. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): A Multiorgan Antiviral T Cell Response. Sci. Transl. Med. 2010, 2, 46ra62. [Google Scholar] [CrossRef]
- Tohyama, M.; Hashimoto, K.; Oda, F.; Namba, C.; Sayama, K. Influence of corticosteroid therapy on viral reactivation in drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms. J. Dermatol. 2020, 47, 476–482. [Google Scholar] [CrossRef]
- Shiohara, T.; Kano, Y.; Hirahara, K.; Aoyama, Y. Prediction and management of drug reaction with eosinophilia and systemic symptoms (DRESS). Expert Opin. Drug Metab. Toxicol. 2017, 13, 701–704. [Google Scholar] [CrossRef] [Green Version]
- Ichai, P.; Laurent-Bellue, A.; Saliba, F.; Moreau, D.; Besch, C.; Francoz, C.; Valeyrie-Allanore, L.; Bretagne, S.R.; Boudon, M.; Antonini, T.M.; et al. Acute Liver Failure/Injury Related to Drug Reaction with Eosinophilia and Systemic Symptoms. Transplant. 2017, 101, 1830–1837. [Google Scholar] [CrossRef]
- Descamps, V.; Brunet-Possenti, F. Monitoring of human herpesvirus 6 infection in the management of drug reaction with eosinophilia and systemic symptoms. Clin. Exp. Dermatol. 2021, 46, 351–352. [Google Scholar] [CrossRef]
- Kano, Y.; Hiraharas, K.; Sakuma, K.; Shiohara, T. Several herpesviruses can reactivate in a severe drug-induced multiorgan reaction in the same sequential order as in graft-versus-host disease. Br. J. Dermatol. 2006, 155, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Kano, Y.; Yamazaki, Y.; Kimishima, M.; Mizukawa, Y.; Shiohara, T. Defective Regulatory T Cells In Patients with Severe Drug Eruptions: Timing of the Dysfunction Is Associated with the Pathological Phenotype and Outcome. J. Immunol. 2009, 182, 8071–8079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ushigome, Y.; Kano, Y.; Ishida, T.; Hirahara, K.; Shiohara, T. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J. Am. Acad. Dermatol. 2013, 68, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Shiohara, T.; Kurata, M.; Mizukawa, Y.; Kano, Y. Recognition of Immune Reconstitution Syndrome Necessary for Better Management of Patients with Severe Drug Eruptions and Those under Immunosuppressive Therapy. Allergol. Int. 2010, 59, 333–343. [Google Scholar] [CrossRef] [Green Version]
- Shiohara, T.; Inaoka, M.; Kano, Y. Drug-Induced Hypersensitivity Syndrome (DIHS): A Reaction Induced by a Complex Interplay among Herpesviruses and Antiviral and Antidrug Immune Responses. Allergol. Int. 2006, 55, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Schunkert, E.M.; Divito, S.J. Updates and Insights in the Diagnosis and Management of DRESS Syndrome. Curr. Dermatol. Rep. 2021, 10, 192–204. [Google Scholar] [CrossRef]
- Almeida, C.-A.; van Miert, P.; O’Driscoll, K.; Zoet, Y.M.; Chopra, A.; Witt, C.; John, M.; Claas, F.H.J.; D’Orsogna, L.J. Virus-Specific T-Cell Clonotypes Might Contribute to Drug Hypersensitivity Reactions through Heterologous Immunity. J. Allergy Clin. Immunol. 2019, 144, 608–611.e4. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.Y.; Kobayashi, T.; Voisin, B.; Jo, J.-H.; Sakamoto, K.; Jin, S.-P.; Kelly, M.; Pasieka, H.B.; Naff, J.L.; Meyerle, J.H.; et al. Targeted therapy guided by single-cell transcriptomic analysis in drug-induced hypersensitivity syndrome: A case report. Nat. Med. 2020, 26, 236–243. [Google Scholar] [CrossRef]
- Descamps, V.; Brunet-Possenti, F. Drug Reaction with Eosinophilia and Sys-temic Symptoms or Virus Reactivation with Eosinophilia and Systemic Symptoms. Pediatr. Dermatol. 2016, 33, 562. [Google Scholar] [CrossRef]
- Sharifzadeh, S.; Mohammadpour, A.H.; Tavanaee, A.; Elyasi, S. Antibacterial antibiotic-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: A literature review. Eur. J. Clin. Pharmacol. 2021, 77, 275–289. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chiu, H.-C.; Chu, C.-Y. Drug Reaction with Eosinophilia and Systemic Symptoms. Arch. Dermatol. 2010, 146, 1373–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bommersbach, T.J.; Lapid, M.I.; Leung, J.G.; Cunningham, J.L.; Rummans, T.A.; Kung, S. Management of Psychotropic Drug–Induced DRESS Syndrome: A Systematic Review. Mayo Clin. Proc. 2016, 91, 787–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Fadhel, N.; Ben Romdhane, H.; Chaabane, A.; Ali, H.B.; Boughattas, N.; Aouam, K.; Ben Fredj, N. DRESS syndrome following furosemide administration: An unusual association. Néphrologie Thérapeutique 2020, 16, 437–438. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mei, X.-L. Drug Reaction with Eosinophilia and Systemic Symptoms. Chin. Med. J. 2017, 130, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Cabañas, R.; Ramírez, E.; Sendagorta, E.; Alamar, R.; Barranco, R.; Blanca-López, N.; Doña, I.; Fernández, J.; Garcia-Nunez, I.; García-Samaniego, J.; et al. Spanish Guidelines for Diagnosis, Management, Treatment, and Prevention of DRESS Syndrome. J. Investig. Allergy Clin. Immunol. 2020, 30, 229–253. [Google Scholar] [CrossRef] [Green Version]
- James, J.; Sammour, Y.M.; Virata, A.R.; Nordin, T.A.; Dumic, I. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome Secondary to Furosemide: Case Report and Review of Literature. Am. J. Case Rep. 2018, 19, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Walsh, S.A.; Creamer, D. Drug reaction with eosinophilia and systemic symptoms (DRESS): A clinical update and review of current thinking. Clin. Exp. Dermatol. 2010, 36, 6–11. [Google Scholar] [CrossRef]
- Beeler, A.; Engler, O.; Gerber, B.O.; Pichler, W.J. Long-lasting reactivity and high frequency of drug-specific T cells after severe systemic drug hypersensitivity reactions. J. Allergy Clin. Immunol. 2006, 117, 455–462. [Google Scholar] [CrossRef]
- Hansel, K.; Bellini, V.; Bianchi, L.; Brozzi, J.; Stingeni, L. Drug reaction with eosinophilia and systemic symptoms from ceftriaxone confirmed by positive patch test: An immunohistochemical study. J. Allergy Clin. Immunol. Pract. 2017, 5, 808–810. [Google Scholar] [CrossRef]
- Kang, S.-Y.; Kim, J.; Ham, J.; Cho, S.-H.; Kang, H.-R.; Kim, H.Y. Altered T cell and monocyte subsets in prolonged immune reconstitution inflammatory syndrome related with DRESS (drug reaction with eosinophilia and systemic symptoms). Asia Pac. Allergy 2020, 10, e2. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, K.; Morito, H.; Hasegawa, A.; Miyagawa, F.; Kobayashi, N.; Watanabe, H.; Sueki, H.; Tohyama, M.; Hashimoto, K.; Kano, Y.; et al. Elevated serum thymus and activation-regulated chemokine (TARC/CCL17) relates to reactivation of human herpesvirus 6 in drug reaction with eosinophilia and systemic symptoms (DRESS)/drug-induced hypersensitivity syndrome (DIHS). Br. J. Dermatol. 2014, 171, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Morito, H.; Hasegawa, A.; Daikoku, N.; Miyagawa, F.; Okazaki, A.; Fukumoto, T.; Kobayashi, N.; Kasai, T.; Watanabe, H.; et al. Identification of thymus and activation-regulated chemokine (TARC/CCL17) as a potential marker for early indication of disease and prediction of disease activity in drug-induced hypersensitivity syndrome (DIHS)/drug rash with eosinophilia and systemic symptoms (DRESS). J. Dermatol. Sci. 2013, 69, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Nishio, D.; Izu, K.; Kabashima, K.; Tokura, Y. T cell populations propagating in the peripheral blood of patients with drug eruptions. J. Dermatol. Sci. 2007, 48, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Weinborn, M.; Barbaud, A.; Truchetet, F.; Beurey, P.; Germain, L.; Cribier, B. Histopathological study of six types of adverse cutaneous drug reactions using granulysin expression. Int. J. Dermatol. 2016, 55, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Fujita, A.; Yagami, A.; Suzuki, K.; Matsunaga, K.; Ihira, M.; Asano, Y. Human herpesvirus 6 reactivation and inflammatory cytokine production in patients with drug-induced hypersensitivity syndrome. J. Clin. Virol. 2006, 37, S92–S96. [Google Scholar] [CrossRef]
- Dosch, H.-M.; Jason, J.; Gelfand, E.W. Transient Antibody Deficiency and Abnormal T Suppressor Cells Induced by Phenytoin. N. Engl. J. Med. 1982, 306, 406–409. [Google Scholar] [CrossRef]
- Kumar, P.; Saini, S.; Khan, S.; Lele, S.S.; Prabhakar, B.S. Restoring self-tolerance in autoimmune diseases by enhancing regulatory T-cells. Cell. Immunol. 2019, 339, 41–49. [Google Scholar] [CrossRef]
- Matta, J.M.R.; Flores, S.M.; Cherit, J.D. Drug reaction with eosinophilia and systemic symptoms (DRESS) and its relation with autoimmunity in a reference center in Mexico. An. Bras. Dermatol. 2017, 92, 30–33. [Google Scholar] [CrossRef] [Green Version]
- Deng, M.; Wu, H.; Yu, M.; Tian, Y.; Li, Y.; Xiao, X. Co-Occurrence of Multiple Endocrine Abnormalities Induced by the DIHS/DRESS. Int. J. Endocrinol. 2019, 2019, 7959615. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, P.; Hertzman, R.J.; Palubinsky, A.M.; Giles, J.B.; Karnes, J.H.; Gibson, A.; Phillips, E.J. Immunopharmacogenomics: Mechanisms of HLA-Associated Drug Reactions. Clin. Pharmacol. Ther. 2021, 110, 607–615. [Google Scholar] [CrossRef]
- Husain, Z.; Reddy, B.Y.; Schwartz, R.A. DRESS syndrome. J. Am. Acad. Dermatol. 2013, 68, 693.e1–693.e14. [Google Scholar] [CrossRef] [PubMed]
- Saag, M.; Balu, R.; Phillips, E.; Brachman, P.; Martorell, C.; Burman, W.; Stancil, B.; Mosteller, M.; Brothers, C.; Wannamaker, P.; et al. High Sensitivity of Human Leukocyte Antigen–B*5701 as a Marker for Immunologically Confirmed Abacavir Hypersensitivity in White and Black Patients. Clin. Infect. Dis. 2008, 46, 1111–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hetherington, S.; Hughes, A.R.; Mosteller, M.; Shortino, D.; Baker-Neblett, K.; Spreen, W.; Lai, E.; Davies, K.; Handley, A.; Dow, D.J.; et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet 2002, 359, 1121–1122. [Google Scholar] [CrossRef]
- Hung, S.-I.; Chung, W.-H.; Liou, L.-B.; Chu, C.-C.; Lin, M.; Huang, H.-P.; Lin, Y.-L.; Lan, J.-L.; Yang, L.-C.; Hong, H.-S.; et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc. Natl. Acad. Sci. USA 2005, 102, 4134–4139. [Google Scholar] [CrossRef] [Green Version]
- Ponzo, M.G.; Miliszewski, M.; Kirchhof, M.G.; Keown, P.A.; Dutz, J.P. HLA-B*58:01 Genotyping to Prevent Cases of DRESS and SJS/TEN in East Asians Treated with Allopurinol—A Canadian Missed Opportunity. J. Cutan. Med. Surg. 2019, 23, 595–601. [Google Scholar] [CrossRef]
- Genin, E.; Chen, D.-P.; Hung, S.-I.; Sekula, P.; Schumacher, M.E.; Chang, P.-Y.; Tsai, S.-H.; Wu, T.-L.; Bellón, T.; Tamouza, R.; et al. HLA-A*31:01 and different types of carbamazepine-induced severe cutaneous adverse reactions: An international study and meta-analysis. Pharm. J. 2014, 14, 281–288. [Google Scholar] [CrossRef]
- McCormack, M.; Alfirevic, A.; Bourgeois, S.; Farrell, J.J.; Kasperavičiūtė, D.; Carrington, M.; Sills, G.J.; Marson, T.; Jia, X.; De Bakker, P.I.; et al. HLA-A*3101 and Carbamazepine-Induced Hypersensitivity Reactions in Europeans. N. Engl. J. Med. 2011, 364, 1134–1143. [Google Scholar] [CrossRef] [Green Version]
- Ozeki, T.; Mushiroda, T.; Yowang, A.; Takahashi, A.; Kubo, M.; Shirakata, Y.; Ikezawa, Z.; Iijima, M.; Shiohara, T.; Hashimoto, K.; et al. Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum. Mol. Genet. 2011, 20, 1034–1041. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-H.; Lee, K.W.; Song, W.-J.; Kim, S.-H.; Jee, Y.-K.; Lee, S.-M.; Kang, H.-R.; Park, H.-W.; Cho, S.-H.; Park, S.-H.; et al. Carbamazepine-induced severe cutaneous adverse reactions and HLA genotypes in Koreans. Epilepsy Res. 2011, 97, 190–197. [Google Scholar] [CrossRef]
- Amstutz, U.; Shear, N.H.; Rieder, M.J.; Hwang, S.; Fung, V.; Nakamura, H.; Connolly, M.; Ito, S.; Carleton, B.C.; The CPNDS Clinical Recommendation Group. Recommendations for HLA-B*15:02 and HLA-A*31:01 genetic testing to reduce the risk of carbamazepine-induced hypersensitivity reactions. Epilepsia 2014, 55, 496–506. [Google Scholar] [CrossRef]
- Mallal, S.; Nolan, D.; Witt, C.; Masel, G.; Martin, A.; Moore, C.; Sayer, D.; Castley, A.; Mamotte, C.; Maxwell, D.; et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet 2002, 359, 727–732. [Google Scholar] [CrossRef]
- Kang, H.-R.; Jee, Y.K.; Kim, Y.-S.; Lee, C.H.; Jung, J.-W.; Kim, S.H.; Park, H.-W.; Chang, Y.-S.; Jang, I.-J.; Cho, S.-H.; et al. Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharm. Genom. 2011, 21, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.L.S.; Hu, M.; Ng, M.H.L.; Yeung, C.; Chan, J.-Y.; Chang, M.; Cheng, S.; Li, L.; Tomlinson, B. Association between HLA-B*58:01 allele and severe cutaneous adverse reactions with allopurinol in Han Chinese in Hong Kong. Br. J. Dermatol. 2012, 167, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Ko, T.-M.; Tsai, C.-Y.; Chen, S.-Y.; Chen, K.-S.; Yu, K.-H.; Chu, C.-S.; Huang, C.-M.; Wang, C.-R.; Weng, C.-T.; Yu, C.-L.; et al. Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: National prospective cohort study. BMJ 2015, 351, h4848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, C.Y.; Yeh, Y.-T.; Wang, C.-W.; Hung, S.-I.; Yang, C.-H.; Chang, Y.-C.; Chang, W.-C.; Lin, Y.-J.; Chang, C.-J.; Su, S.-C.; et al. Impact of the HLA-B58:01 Allele and Renal Impairment on Allopurinol-Induced Cutaneous Adverse Reactions. J. Investig. Dermatol. 2016, 136, 1373–1381. [Google Scholar] [CrossRef] [Green Version]
- Sukasem, C.; Jantararoungtong, T.; Kuntawong, P.; Puangpetch, A.; Koomdee, N.; Satapornpong, P.; Supapsophon, P.; Klaewsongkram, J.; Rerkpattanapipat, T. HLA-B*58:01 for Allopurinol-Induced Cutaneous Adverse Drug Reactions: Implication for Clinical Interpretation in Thailand. Front. Pharmacol. 2016, 7, 186. [Google Scholar] [CrossRef] [Green Version]
- Kashiwagi, M.; Aihara, M.; Takahashi, Y.; Yamazaki, E.; Yamane, Y.; Song, Y.; Muramatsu, M.; Ikezawa, Z. Human Leukocyte Antigen Genotypes in Carbamazepine-Induced Severe Cutaneous Adverse Drug Response in Japanese Patients. J. Dermatol. 2008, 35, 683–685. [Google Scholar] [CrossRef]
- Ramírez, E.; Bellón, T.; Tong, H.Y.; Borobia, A.M.; de Abajo, F.J.; Lerma, V.; Hidalgo, M.A.M.; Castañer, J.L.; Cabañas, R.; Fiandor, A.; et al. Significant HLA class I type associations with aromatic antiepileptic drug (AED)-induced SJS/TEN are different from those found for the same AED-induced DRESS in the Spanish population. Pharmacol. Res. 2017, 115, 168–178. [Google Scholar] [CrossRef]
- Zhang, F.-R.; Liu, H.; Irwanto, A.; Fu, X.-A.; Li, Y.; Yu, G.-Q.; Yu, Y.-X.; Chen, M.-F.; Low, H.-Q.; Li, J.-H.; et al. HLA-B*13:01 and the Dapsone Hypersensitivity Syndrome. N. Engl. J. Med. 2013, 369, 1620–1628. [Google Scholar] [CrossRef] [Green Version]
- Tangamornsuksan, W.; Lohitnavy, M. Association Between HLA-B*1301 and Dapsone-Induced Cutaneous Adverse Drug Reactions. JAMA Dermatol. 2018, 154, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Tempark, T.; Satapornpong, P.; Rerknimitr, P.; Nakkam, N.; Saksit, N.; Wattanakrai, P.; Jantararoungtong, T.; Koomdee, N.; Mahakkanukrauh, A.; Tassaneeyakul, W.; et al. Dapsone-induced severe cutaneous adverse drug reactions are strongly linked with HLA-B*13. Pharm. Genom. 2017, 27, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-T.; Wang, C.-W.; Lu, C.-W.; Chen, C.-B.; Lee, H.-E.; Hung, S.-I.; Choon, S.-E.; Yang, C.-H.; Liu, M.-T.; Chen, T.J.; et al. The Function of HLA-B*13:01 Involved in the Pathomechanism of Dapsone-Induced Severe Cutaneous Adverse Reactions. J. Investig. Dermatol. 2018, 138, 1546–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Gu, B.; Zhang, L.; Xuan, J.; Luo, H.; Zhou, P.; Zhu, Q.; Yan, S.; Chen, S.-A.; Cao, Z.; et al. HLA-B*13:01 is associated with salazosulfapyridine-induced drug rash with eosinophilia and systemic symptoms in Chinese Han population. Pharmacogenomics 2014, 15, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Ng, C.-C.; Too, C.-L.; Choon, S.-E.; Lee, C.-K.; Chung, W.-H.; Hussein, S.H.; Lim, K.-S.; Murad, S. Association of HLA-B*15:13 and HLA-B*15:02 with phenytoin-induced severe cutaneous adverse reactions in a Malay population. Pharm. J. 2016, 17, 170–173. [Google Scholar] [CrossRef]
- Manuyakorn, W.; Likkasittipan, P.; Wattanapokayakit, S.; Suvichapanich, S.; Inunchot, W.; Wichukchinda, N.; Khongkhatithuml, C.; Thampratankul, L.; Kamchaisatian, W.; Benjaponpitak, S.; et al. Association of HLA genotypes with phenytoin induced severe cutaneous adverse drug reactions in Thai children. Epilepsy Res. 2020, 162, 106321. [Google Scholar] [CrossRef]
- Rutkowski, K.; Taylor, C.; Wagner, A. HLA B62 as a possible risk factor for drug reaction with eosinophilia and systemic symptoms to piperacillin/tazobactam. J. Allergy Clin. Immunol. Pract. 2017, 5, 829–830. [Google Scholar] [CrossRef]
- Konvinse, K.C.; Trubiano, J.A.; Pavlos, R.; James, I.; Shaffer, C.M.; Bejan, C.A.; Schutte, R.J.; Ostrov, D.A.; Pilkinton, M.A.; Rosenbach, M.; et al. HLA-A*32:01 is strongly associated with vancomycin-induced drug reaction with eosinophilia and systemic symptoms. J. Allergy Clin. Immunol. 2019, 144, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Mounzer, K.; Hsu, R.; Fusco, J.S.; Brunet, L.; Henegar, C.E.; Vannappagari, V.; Stainsby, C.M.; Shaefer, M.S.; Ragone, L.; Fusco, G.P. HLA-B*57:01 screening and hypersensitivity reaction to abacavir between 1999 and 2016 in the OPERA® observational database: A cohort study. AIDS Res. Ther. 2019, 16, 1. [Google Scholar] [CrossRef]
- Gao, S.; Gui, X.-E.; Liang, K.; Liu, Z.; Hu, J.; Dong, B. HLA-Dependent Hypersensitivity Reaction to Nevirapine in Chinese Han HIV-Infected Patients. AIDS Res. Hum. Retrovir. 2012, 28, 540–543. [Google Scholar] [CrossRef]
- Yuan, J.; Guo, S.; Hall, D.; Cammett, A.M.; Jayadev, S.; Distel, M.; Storfer, S.; Huang, Z.; Mootsikapun, P.; Ruxrungtham, K.; et al. Toxicogenomics of nevirapine-associated cutaneous and hepatic adverse events among populations of African, Asian, and European descent. AIDS 2011, 25, 1271–1280. [Google Scholar] [CrossRef]
- Likanonsakul, S.; Rattanatham, T.; Feangvad, S.; Uttayamakul, S.; Prasithsirikul, W.; Tunthanathip, P.; Nakayama, E.E.; Shioda, T. HLA-Cw*04 allele associated with nevirapine-induced rash in HIV-infected Thai patients. AIDS Res. Ther. 2009, 6, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, D.F.; Chaponda, M.; Jorgensen, A.L.; Castro, E.C.; van Oosterhout, J.J.; Khoo, S.H.; Lalloo, D.G.; Heyderman, R.S.; Alfirevic, A.; Pirmohamed, M. Association of Human Leukocyte Antigen Alleles and Nevirapine Hypersensitivity in a Malawian HIV-Infected Population. Clin. Infect. Dis. 2013, 56, 1330–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Littera, R.; Carcassi, C.; Masala, A.; Piano, P.; Serra, P.; Ortu, F.; Corso, N.; Casula, B.; La Nasa, G.; Contu, L.; et al. HLA-dependent hypersensitivity to nevirapine in Sardinian HIV patients. AIDS 2006, 20, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Gatanaga, H.; Yazaki, H.; Tanuma, J.; Honda, M.; Genka, I.; Teruya, K.; Tachikawa, N.; Kikuchi, Y.; Oka, S. HLA-Cw8 primarily associated with hypersensitivity to nevirapine. AIDS 2007, 21, 264–265. [Google Scholar] [CrossRef] [PubMed]
- Chantarangsu, S.; Mushiroda, T.; Mahasirimongkol, S.; Kiertiburanakul, S.; Sungkanuparph, S.; Manosuthi, W.; Tantisiriwat, W.; Charoenyingwattana, A.; Sura, T.; Chantratita, W.; et al. HLA-B*3505 allele is a strong predictor for nevirapine-induced skin adverse drug reactions in HIV-infected Thai patients. Pharm. Genom. 2009, 19, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Keane, N.M.; Pavlos, R.K.; McKinnon, E.; Lucas, A.; Rive, C.; Blyth, C.C.; Dunn, D.; Lucas, M.; Mallal, S.; Phillips, E. HLA Class I restricted CD8+ and Class II restricted CD4+ T cells are implicated in the pathogenesis of nevirapine hypersensitivity. AIDS 2014, 28, 1891–1901. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.; Hopkins, C.; Duffy, E.; Lee, D.; Loulergue, P.; Ripamonti, D.; Ostrov, D.A.; Phillips, E. Association of the HLA-B*53:01 Allele with Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome During Treatment of HIV Infection with Raltegravir. Clin. Infect. Dis. 2017, 64, 1198–1203. [Google Scholar] [CrossRef] [Green Version]
- Spielberg, S.P.; Gordon, G.B.; Blake, D.A.; Mellits, E.D.; Bross, D.S. Anticonvulsant Toxicity in Vitro: Possible Role of Arene Oxides. J. Pharmacol. Exp. Ther. 1981, 217, 386–389. [Google Scholar]
- Shear, N.H.; Spielberg, S.P.; Grant, D.M.; Tang, B.K.; Kalow, W. Differences in Metabolism of Sulfonamides Predisposing to Idiosyncratic Toxicity. Ann. Intern. Med. 1986, 105, 179–184. [Google Scholar] [CrossRef]
- Shear, N.H.; Spielberg, S.P. Anticonvulsant hypersensitivity syndrome. In vitro assessment of risk. J. Clin. Investig. 1988, 82, 1826–1832. [Google Scholar] [CrossRef]
- Chung, W.-H.; Chang, W.-C.; Lee, Y.-S.; Wu, Y.-Y.; Yang, C.-H.; Ho, H.-C.; Chen, M.-J.; Lin, J.-Y.; Hui, R.C.-Y.; Ho, J.-C.; et al. Genetic Variants Associated with Phenytoin-Related Severe Cutaneous Adverse Reactions. JAMA J. Am. Med. Assoc. 2014, 312, 525–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, W.-H.; Chang, W.-C.; Stocker, S.L.; Juo, C.-G.; Graham, G.G.; Lee, M.-H.H.; Williams, K.M.; Tian, Y.-C.; Juan, K.-C.; Wu, Y.-J.J.; et al. Insights into the poor prognosis of allopurinol-induced severe cutaneous adverse reactions: The impact of renal insufficiency, high plasma levels of oxypurinol and granulysin. Ann. Rheum. Dis. 2014, 74, 2157–2164. [Google Scholar] [CrossRef]
- Momen, S.E.; Diaz-Cano, S.; Walsh, S.; Creamer, D. Discriminating minor and major forms of drug reaction with eosinophilia and systemic symptoms: Facial edema aligns to the severe phenotype. J. Am. Acad. Dermatol. 2021, 85, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Soria, A.; Bernier, C.; Veyrac, G.; Barbaud, A.; Puymirat, E.; Milpied, B. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: A retrospective study. J. Am. Acad. Dermatol. 2020, 82, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Um, S.J.; Lee, S.K.; Kim, Y.H.; Kim, K.H.; Son, C.H.; Roh, M.S.; Lee, M.K. Clinical features of drug-induced hypersensitivity syndrome in 38 patients. J. Investig. Allergy Clin. Immunol. 2010, 20, 556–562. [Google Scholar]
- Soria, A.; Amsler, E.; Bernier, C.; Milpied, B.; Tétart, F.; Morice, C.; Dezoteux, F.; Bouedec, M.-C.F.-L.; Barbaud, A.; Staumont-Sallé, D.; et al. DRESS and AGEP Reactions to Iodinated Contrast Media: A French Case Series. J. Allergy Clin. Immunol. Pract. 2021, 9, 3041–3050. [Google Scholar] [CrossRef]
- Eshki, M.; Allanore, L.; Musette, P.; Milpied, B.; Grange, A.; Guillaume, J.-C.; Chosidow, O.; Guillot, I.; Paradis, V.; Joly, P.; et al. Twelve-Year Analysis of Severe Cases of Drug Reaction with Eosinophilia and Systemic Symptoms. Arch. Dermatol. 2009, 145, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Walsh, S.; Diaz-Cano, S.; Higgins, E.; Morris-Jones, R.; Bashir, S.; Bernal, W.; Creamer, D. Drug reaction with eosinophilia and systemic symptoms: Is cutaneous phenotype a prognostic marker for outcome? A review of clinicopathological features of 27 cases. Br. J. Dermatol. 2013, 168, 391–401. [Google Scholar] [CrossRef]
- Bedouelle, E.; Ben Said, B.; Tetart, F.; Milpied, B.; Welfringer-Morin, A.; Maruani, A.; Catteau, B.; Dezoteux, F.; Staumont-Sallé, D.; Mazereeuw-Hautier, J.; et al. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): Series of 49 French Pediatric Cases. J. Allergy Clin. Immunol. Pract. 2021, 10, 267–274.e5. [Google Scholar] [CrossRef]
- Lin, I.-C.; Yang, H.-C.; Strong, C.; Yang, C.-W.; Cho, Y.-T.; Chen, K.-L.; Chu, C.-Y. Liver injury in patients with DRESS: A clinical study of 72 cases. J. Am. Acad. Dermatol. 2015, 72, 984–991. [Google Scholar] [CrossRef]
- Kano, Y.; Ishida, T.; Hirahara, K.; Shiohara, T. Visceral Involvements and Long-term Sequelae in Drug-induced Hypersensitivity Syndrome. Med. Clin. N. Am. 2010, 94, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Taweesedt, P.T.; Nordstrom, C.W.; Stoeckel, J.; Dumic, I. Pulmonary Manifestations of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome: A Systematic Review. BioMed Res. Int. 2019, 2019, 7863815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radovanovic, M.; Jevtic, D.; Calvin, A.D.; Petrovic, M.; Paulson, M.; Prada, L.R.; Sprecher, L.; Savic, I.; Dumic, I. “Heart in DRESS&rdquo: Cardiac Manifestations, Treatment and Outcome of Patients with Drug Reaction with Eosinophilia and Systemic Symptoms Syndrome: A Systematic Review. J. Clin. Med. 2022, 11, 704. [Google Scholar] [CrossRef]
- Thongsri, T.; Chularojanamontri, L.; Pichler, W.J. Cardiac involvement in DRESS syndrome. Asian Pac. J. Allergy Immunol. 2016, 35, 3–10. [Google Scholar] [CrossRef]
- Choudhary, R.; Vinay, K.; Srivastava, N.; Bishnoi, A.; Kamat, D.; Parsad, D.; Bhatia, A.; Kumaran, M.S. Clinical, biochemical, and serologic predictors of drug reaction with eosinophilia and systemic symptoms syndrome: A prospective case–control study. J. Am. Acad. Dermatol. 2021, 85, 901–909. [Google Scholar] [CrossRef]
- Skowron, F.; Bensaid, B.; Balme, B.; Depaepe, L.; Kanitakis, J.; Nosbaum, A.; Maucort-Boulch, D.; Berard, F.; D’Incan, M.; Kardaun, S.H.; et al. Comparative histological analysis of drug-induced maculopapular exanthema and DRESS. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2085–2090. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, C.; Celik, G.; Rouzaire, P.; Whitaker, P.; Bonadonna, P.; Rodrigues-Cernadas, J.; Vultaggio, A.; Brockow, K.; Caubet, J.C.; Makowska, J.; et al. In vitro tests for drug hypersensitivity reactions: An ENDA/EAACI Drug Allergy Interest Group position paper. Allergy 2016, 71, 1103–1134. [Google Scholar] [CrossRef]
- Pichler, W.J.; Tilch, J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy 2004, 59, 809–820. [Google Scholar] [CrossRef]
- Cabañas, R.; Calderón, O.; Ramírez, E.; Fiandor, A.; Caballero, T.; Heredia, R.; Herranz, P.; Madero, R.; Quirce, S.; Bellón, T. Sensitivity and specificity of the lymphocyte transformation test in drug reaction with eosinophilia and systemic symptoms causality assessment. Clin. Exp. Allergy 2018, 48, 325–333. [Google Scholar] [CrossRef]
- Karami, Z.; Mesdaghi, M.; Karimzadeh, P.; Mansouri, M.; Taghdiri, M.M.; Kayhanidoost, Z.; Jebelli, B.; Foumani, R.S.; Babaie, D.; Chavoshzadeh, Z. Evaluation of Lymphocyte Transformation Test Results in Patients with Delayed Hypersensitivity Reactions following the Use of Anticonvulsant Drugs. Int. Arch. Allergy Immunol. 2016, 170, 158–162. [Google Scholar] [CrossRef]
- Barbaud, A.; Collet, E.; Milpied, B.; Assier, H.; Staumont, D.; Avenel-Audran, M.; Grange, A.; Amarger, S.; Girardin, P.; Guinnepain, M.-T.; et al. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br. J. Dermatol. 2013, 168, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Ben Said, B.; Berard, F.; Hacard, F.; Pralong, P.; Balme, B.; Nicolas, J.F. Skin tests may induce DRESS relapse. Clin. Transl. Allergy 2014, 4, P136. [Google Scholar] [CrossRef] [Green Version]
- Allouchery, M.; Logerot, S.; Cottin, J.; Pralong, P.; Villier, C.; Ben Saïd, B. Antituberculosis Drug-Associated DRESS: A Case Series. J. Allergy Clin. Immunol. Pract. 2018, 6, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Ortonne, N.; Valeyrie-Allanore, L.; Bastuji-Garin, S.; Wechsler, J.; De Feraudy, S.; Duong, T.-A.; Delfau-Larue, M.-H.; Chosidow, O.; Wolkenstein, P.; Roujeau, J.-C. Histopathology of drug rash with eosinophilia and systemic symptoms syndrome: A morphological and phenotypical study. Br. J. Dermatol. 2015, 173, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.-H.; Hui, R.-Y.; Yang, C.-H.; Lin, J.-Y.; Lin, Y.-T.; Ho, H.-C.; Hung, S.-I.; Kuo, T.-T. Histopathological analysis and clinical correlation of drug reaction with eosinophilia and systemic symptoms (DRESS). Br. J. Dermatol. 2014, 170, 866–873. [Google Scholar] [CrossRef]
- Gonçalo, M.M.; Cardoso, J.C.; Gouveia, M.P.; Coutinho, I.; Gameiro, A.R.; Brites, M.M.; Tellechea Óscar, E. Histopathology of the Exanthema in DRESS Is Not Specific but May Indicate Severity of Systemic Involvement. Am. J. Dermatopathol. 2016, 38, 423–433. [Google Scholar] [CrossRef]
- Saltzstein, S.L.; Ackerman, L.V. Lymphadenopathy induced by anticonvulsant drugs and mimicking clinically and pathologically malignant lymphomas. Cancer 1959, 12, 164–182. [Google Scholar] [CrossRef]
- Augusto, J.-F.; Sayegh, J.; Simon, A.; Croue, A.; Chennebault, J.-M.; Cousin, M.; Subra, J.-F. A case of sulphasalazine-induced DRESS syndrome with delayed acute interstitial nephritis. Nephrol. Dial. Transplant. 2009, 24, 2940–2942. [Google Scholar] [CrossRef] [Green Version]
- Esposito, A.J.; Murphy, R.C.; Toukatly, M.N.; Amro, O.W.; Kestenbaum, B.R.; Najafian, B. Acute kidney injury in allopurinol-induced DRESS syndrome: A case report of concurrent tubulointerstitial nephritis and kidney-limited necrotizing vasculitis. Clin. Nephrol. 2017, 87, 316–319. [Google Scholar] [CrossRef]
- Diazmancebo, R.; Costerofernandez, O.; Vegacabrera, C.; Oleatejero, T.; Yebenes, L.; Picazo, M.L.; Selgasgutierrez, R. Síndrome de DRESS y nefritis tubulointersticial aguda tras tratamiento con vancomicina y betalactámicos. Descripción de un caso y revisión de la literatura. Case Rep. Lit. Review. Nefrol. 2012, 32, 685–687. [Google Scholar]
- Ramirez, G.; Della-Torre, E.; Tresoldi, M.; Scarpellini, P.; Ciceri, F.; Dagna, L.M.-R. Exanthema and Eosinophilia in COVID-19 Patients: Has Viral Infection a Role in Drug Induced Exanthemas? J. Eur. Acad. Dermatol. Venereol. 2020, 34, 559–561. [Google Scholar]
- de Matos, J.N.; Sendino Áurea, R.; Teruel, A.P.; Sendino, J.I.R. Síndrome de DRESS simulando enfermedad por coronavirus 2019-NcoV. Semer.-Med. Fam. 2021, 47, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, A.; Abe, R. Recent advances in managing and understanding Stevens-Johnson syndrome and toxic epidermal necrolysis. F1000Research 2020, 9, 612. [Google Scholar] [CrossRef] [PubMed]
- Teraki, Y.; Shibuya, M.; Izaki, S. Stevens-Johnson syndrome and toxic epidermal necrolysis due to anticonvulsants share certain clinical and laboratory features with drug-induced hypersensitivity syndrome, despite differences in cutaneous presentations. Clin. Exp. Dermatol. 2010, 35, 723–728. [Google Scholar] [CrossRef]
- Szatkowski, J.; Schwartz, R.A. Acute generalized exanthematous pustulosis (AGEP): A review and update. J. Am. Acad. Dermatol. 2015, 73, 843–848. [Google Scholar] [CrossRef]
- Bouvresse, S.; Valeyrie-Allanore, L.; Ortonne, N.; Konstantinou, M.P.; Kardaun, S.H.; Bagot, M.; Wolkenstein, P.; Roujeau, J.-C. Toxic epidermal necrolysis, DRESS, AGEP: Do overlap cases exist? Orphanet J. Rare Dis. 2012, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Curtis, C.; Ogbogu, P. Hypereosinophilic Syndrome. Clin. Rev. Allergy Immunol. 2016, 50, 240–251. [Google Scholar] [CrossRef]
- Choudhary, S.; McLeod, M.; Torchia, D.; Romanelli, P. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome. J. Clin. Aesthetic Dermatol. 2013, 6, 31–37. [Google Scholar]
- Intarasupht, J.; Kanchanomai, A.; Leelasattakul, W.; Chantrarat, T.; Nakakes, A.; Tiyanon, W. Prevalence, risk factors, and mortality outcome in the drug reaction with eosinophilia and systemic symptoms patients with cardiac involvement. Int. J. Dermatol. 2018, 57, 1187–1191. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chang, C.-Y.; Cho, Y.-T.; Chiu, H.-C.; Chu, C.-Y. Long-term sequelae of drug reaction with eosinophilia and systemic symptoms: A retrospective cohort study from Taiwan. J. Am. Acad. Dermatol. 2013, 68, 459–465. [Google Scholar] [CrossRef]
- Tempark, T.; Deekajorndech, T.; Chatproedprai, S.; Supornsilchai, V.; Wananukul, S. Late sequelae of drug reaction with eosinophilia and systemic symptoms (DRESS) cause thyroid dysfunction and thyroiditis: Review of literature. J. Pediatr. Endocrinol. Metab. 2022. [Google Scholar] [CrossRef]
- Jörg, L.; Helbling, A.; Yerly, D.; Pichler, W.J. Drug-related relapses in drug reaction with eosinophilia and systemic symptoms (DRESS). Clin. Transl. Allergy 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Descamps, V.; ben Saïd, B.; Sassolas, B.; Truchetet, F.; Avenel-Audran, M.; Girardin, P.; Guinnepain, M.T.; Mathelier-Fusade, P.; Assier, H.; Milpied, B.; et al. Management of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS). Ann. Dermatol. Venereol. 2010, 137, 703–708. [Google Scholar] [CrossRef]
- Heelan, K.; Shear, N.H. Cutaneous Drug Reactions in Children: An Update. Pediatr. Drugs 2013, 15, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Sasidharanpillai, S.; Chathoth, A.; Khader, A.; Mariyath, O.R.; Riyaz, N.; Binitha, M.; Muhammed, K.; George, B.; Santhosh, P.; Roslind, S.; et al. Predictors of disease severity in drug reaction with eosinophilia and systemic symptoms. Indian J. Dermatol. Venereol. Leprol. 2019, 85, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Funck-Brentano, E.; Duong, T.-A.; Bouvresse, S.; Bagot, M.; Wolkenstein, P.; Roujeau, J.-C.; Chosidow, O.; Valeyrie-Allanore, L. Therapeutic management of DRESS: A retrospective study of 38 cases. J. Am. Acad. Dermatol. 2015, 72, 246–252. [Google Scholar] [CrossRef]
- Brin, C.; Bernigaud, C.; Hua, C.; Duong, T.-A.; Gaudin, O.; Colin, A.; de Prost, N.; Wolkenstein, P.; Chosidow, O.; Ingen-Housz-Oro, S. Impact of systemic to topical steroids switch on the outcome of drug reaction with eosinophilia and systemic symptoms (DRESS): A monocenter retrospective study of 20 cases. Ann. Dermatol. Vénéréologie 2021, 148, 168–171. [Google Scholar] [CrossRef]
- Uhara, H.; Saiki, M.; Kawachi, S.; Ashida, A.; Oguchi, S.; Okuyama, R. Clinical course of drug-induced hypersensitivity syndrome treated without systemic corticosteroids. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 722–726. [Google Scholar] [CrossRef]
- A Martinez-Cabriales, S.; Shear, N.H.; Gonzalez-Moreno, E.I. Liver involvement in the drug reaction, eosinophilia, and systemic symptoms syndrome. World J. Clin. Cases 2019, 7, 705–716. [Google Scholar] [CrossRef]
- Yu, W.; Zhu, J.; Han, Y.; Chang, S.-J.; Shang, Y.; Ma, G.; Lin, X. Assessment of Outcomes with Pulsed Dye Laser Treatment of Port-Wine Stains Located Proximally vs. Distally on Extremities. JAMA Dermatol. 2020, 156, 702. [Google Scholar] [CrossRef]
- Kirchhof, M.G.; Wong, A.; Dutz, J.P. Cyclosporine Treatment of Drug-Induced Hypersensitivity Syndrome. JAMA Dermatol. 2016, 152, 1254–1257. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, E.; Zwahlen, H.; Gilliet, F.; Marone, C. Vancomycin-Induced Hypersensitivity Reaction with Acute Renal Failure: Resolution Following Cyclosporine Treatment. Clin. Nephrol. 2005, 64, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Joly, P.; Janela, B.; Tetart, F.; Rogez, S.; Picard, D.; D’Incan, M.; Descamps, V.; Collet, E.; Roujeau, J.C.; Musette, P. Poor Benefit/Risk Balance of Intravenous Immunoglobulins in DRESS. Arch. Dermatol. 2011, 148, 542–544. [Google Scholar] [CrossRef]
| Drug Category | Drug Name |
|---|---|
| Anticonvulsant | Carbamazepine *, lamotrigine *, phenobarbital *, levetiracetam *, valproate, phenytoin *, oxcarbazepine, ethosuximide, zonisamide, gabapentin |
| Anti-infective | Vancomycin *, minocycline *, ampicillin/amoxicillin, ampicillin/sulbactam, amoxicillin-clavulanic acid, cefadroxil, cefepime, cefixime, cefotaxime, ceftazidime, imipenem, meropenem, piperacillin/tazobactam * metronidazole, linezolid, azithromycin, levofloxacin, benznidazole *, clindamycin, hydroxychloroquine, teicoplanin, voriconazole |
| Anti-tuberculosis | Rifampin *, isoniazid, pyrazinamide, streptomycin, ethambutol |
| Anti-viral | Abacavir *, nevirapine *, boceprevir, telaprevir, and zalcitabine |
| Antidepressant and antipsychotic | Bupropion, fluoxetine, olanzapine, amitriptyline, clomipramine |
| Sulfonamide | Trimethoprim-sulfamethoxazole *, dapsone *, sulfasalazine *, salazosulfapyridine *, furosemide |
| Antineoplastic and immunomodulators | Sorafenib, vismodegib, vemurafenib, efalizumab and imatinib, azathioprine, chlorambucil, leflunomide, lenalidomide |
| Antihypertensive | Amlodipine, captopril, diltiazem, spironolactone |
| Analgesics | Diclofenac, celecoxib, ibuprofen, aspirin, metamizole, phenylbutazone, dexketoprofen, codeine phosphate |
| Miscellaneous | Allopurinol *, atorvastatin, Traditional Chinese Medicine (see Wang and Mei for specific products [46]), quinine, mexiletine, omeprazole, esomeprazole, strontium ranelate, epoetin alfa, ranitidine, thiamine, cyanamide, vitamin B12, sitagliptin, tribenoside, iodinated contrast media, rivaroxaban |
| Diagnostic Criteria |
|---|
| 1. Maculopapular rash developing > 3 weeks after starting a limited number of drugs |
| 2. Prolonged clinical symptoms after discontinuation of the causative drug |
| 3. Fever (>38 °C) |
| 4. Liver abnormalities (ALT > 100 U/L) or other organ involvement |
5. Leukocyte abnormalities (at least one present)
|
| 6. Lymphadenopathy |
| 7. HHV-6 reactivation |
| The diagnosis is confirmed by presence of all seven criteria above (typical DiHS) or five of seven criteria (atypical DiHS) |
| RegiSCAR Criteria | Score | |||||
|---|---|---|---|---|---|---|
| −1 | 0 | 1 | 2 | Min | Max | |
| Fever ≥ 38.5 °C | N/U | Y | −1 | 0 | ||
| Enlarged lymph nodes) (>1 cm size, at least 2 sites) | N/U | Y | 0 | 1 | ||
| Eosinophilia | N/U | 700–1499/μL (10–19.9% if leukopenia) | ≥1500/μL (≥20% if leukopenia) | 0 | 2 | |
| Atypical lymphocytes | N/U | Y | 0 | 1 | ||
| Skin involvement | −2 | 2 | ||||
| N/U | Y | ||||
| N | U | Y | |||
| N | Y/U | ||||
| Organ involvement -liver, kidney, lung, muscle/heart, pancreas, and other organ(s) | N/U | Y/Y/Y/Y/Y/Y | 0 | 2 | ||
| Resolution ≥ 15 days | N/U | Y | −1 | 0 | ||
| Evaluation other potential causes (ANA, blood culture, serology for HAV/HBV/HCV, Chlamydia, Mycoplasma pneumoniae) If none positive and ≥3 negative | Y | 0 | 1 | |||
| Total score | <2, Excluded; 2–3, Possible; 4–5, Probable; >5, Definite | −4 | 9 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stirton, H.; Shear, N.H.; Dodiuk-Gad, R.P. Drug Reaction with Eosinophilia and Systemic Symptoms (DReSS)/Drug-Induced Hypersensitivity Syndrome (DiHS)—Readdressing the DReSS. Biomedicines 2022, 10, 999. https://doi.org/10.3390/biomedicines10050999
Stirton H, Shear NH, Dodiuk-Gad RP. Drug Reaction with Eosinophilia and Systemic Symptoms (DReSS)/Drug-Induced Hypersensitivity Syndrome (DiHS)—Readdressing the DReSS. Biomedicines. 2022; 10(5):999. https://doi.org/10.3390/biomedicines10050999
Chicago/Turabian StyleStirton, Hannah, Neil H. Shear, and Roni P. Dodiuk-Gad. 2022. "Drug Reaction with Eosinophilia and Systemic Symptoms (DReSS)/Drug-Induced Hypersensitivity Syndrome (DiHS)—Readdressing the DReSS" Biomedicines 10, no. 5: 999. https://doi.org/10.3390/biomedicines10050999
APA StyleStirton, H., Shear, N. H., & Dodiuk-Gad, R. P. (2022). Drug Reaction with Eosinophilia and Systemic Symptoms (DReSS)/Drug-Induced Hypersensitivity Syndrome (DiHS)—Readdressing the DReSS. Biomedicines, 10(5), 999. https://doi.org/10.3390/biomedicines10050999






