Hypersensitivity Reactions to Iodinated Contrast Media
Abstract
:1. Introduction
2. Classification of ICM
3. Classification of ADRs of ICM
4. Epidemiology
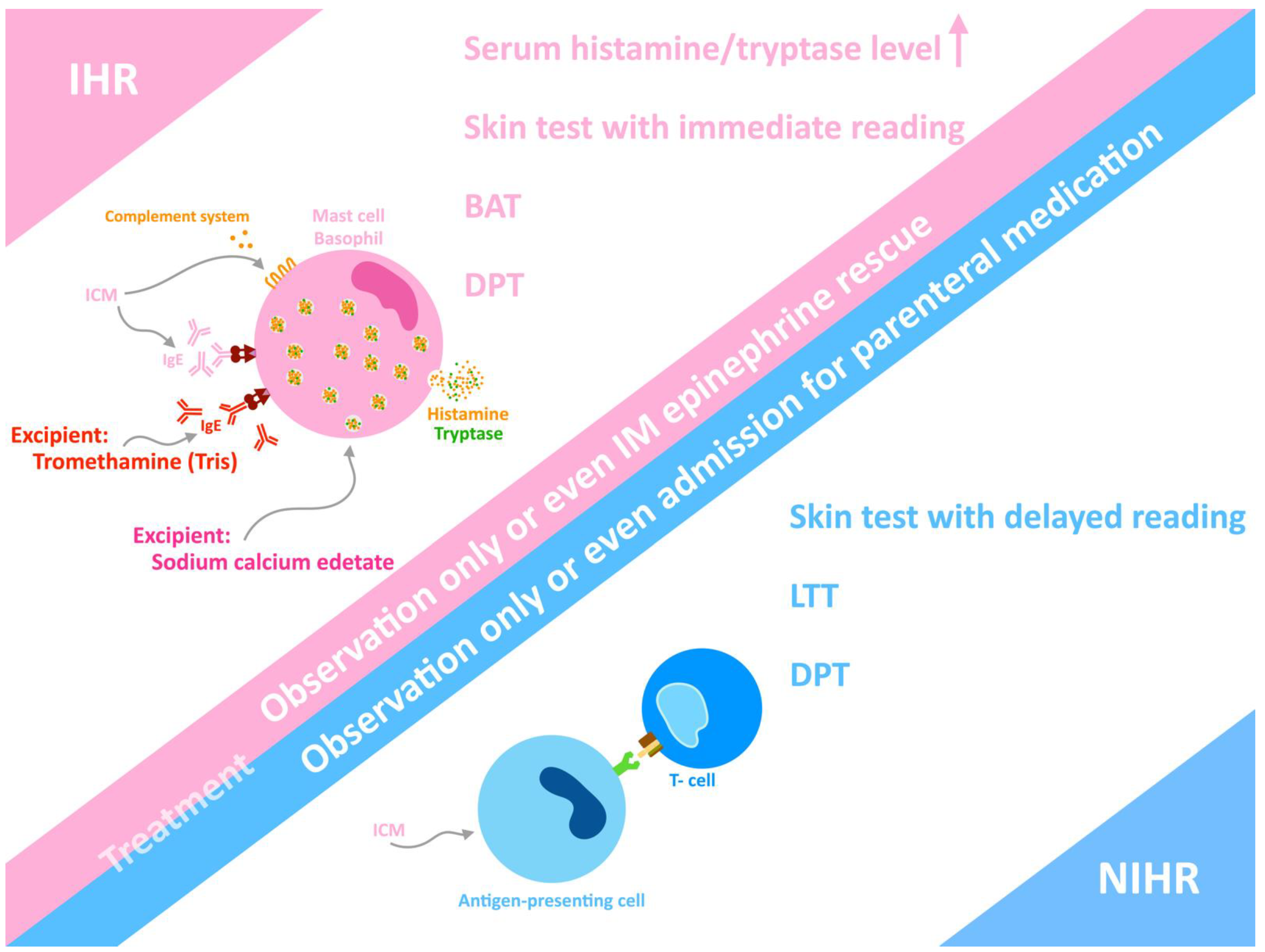
5. Phenotype of Hypersensitivity
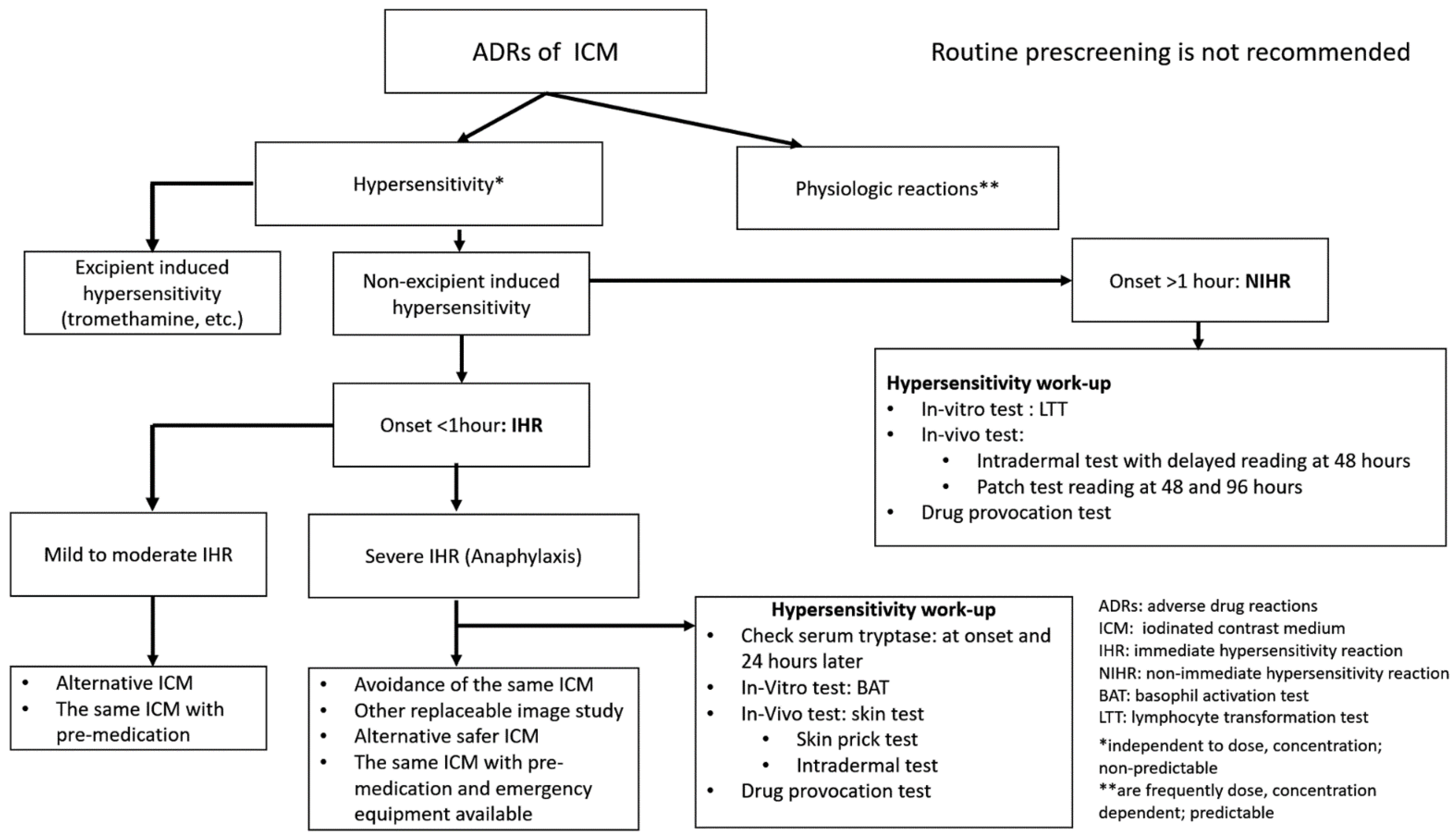
6. Diagnosis
7. Treatment
8. Prescreening, Prevention and Rapid Drug Desensitization
9. Recommendations
10. Standardization of the Documentation of ICM-Induced Hypersensitivity
- The precise name of the injected culprit ICM and its dose (volume, concentration);
- The manifestations of the reaction (e.g., itching, generalized urticaria, dizziness, drop of blood pressure, tachycardia, etc.) to establish the severity of the reaction;
- The chronology of the adverse reaction;
- Specific treatment for the ICM-induced hypersensitivity.
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADR | Adverse drug reaction |
| BAT | Basophil activation test |
| DPT | Drug provocation test |
| ICM | Iodinated contrast media |
| IDT | Intradermal test |
| IHR | Immediate hypersensitivity reaction |
| LTT | Lymphocyte transformation test |
| MPE | Maculopapular exanthema |
| NIHR | Non-immediate hypersensitivity reaction |
| SPT | Skin prick test |
References
- Caschera, L.; Lazzara, A.; Piergallini, L.; Ricci, D.; Tuscano, B.; Vanzulli, A. Contrast agents in diagnostic imaging: Present and future. Pharmacol. Res. 2016, 110, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Radiology, A.C.O. ACR Manual on Contrast Media; American College of Radiology: Reston, VA, USA, 2021; ISBN 978-1-55903-012-0. [Google Scholar]
- O’Connor, S.D.; Summers, R.M. Summers, Revisiting oral barium sulfate contrast agents. Acad. Radiol. 2007, 14, 72–80. [Google Scholar] [CrossRef] [PubMed]
- European Society of Urogenital Radiology. ESUR Guidelines on Contrast Agents 10.0. 2019. Available online: https://www.esur.org/fileadmin/content/2019/ESUR_Guidelines_10.0_Final_Version.pdf (accessed on 15 December 2021).
- Sharafuddin, M.J.; Marjan, A.E. Current status of carbon dioxide angiography. J. Vasc. Surg. 2017, 66, 618–637. [Google Scholar] [CrossRef] [PubMed]
- Lasser, E.C. The multipotential pseudoantigenicity of X-ray contrast media. Pseudoantigen excess may downregulate the release of hypotensive mediators.pdf. Int. Arch. Allergy Immunol. 2000, 123, 282–290. [Google Scholar] [PubMed]
- Arun Kuar Gupta, A.G.; Sandhu, M.S. Iodinated contrast media: An update. In Diagnostic Radiology: Advances in Imaging Technique; Jaypee Brothers Medical Publishers: New Delhi, India, 2019; Chapter 18; p. 374. [Google Scholar]
- Gueant-Rodriguez, R.M.; Romano, A.; Barbaud, A.; Brockow, K.; Gueant, J.-L. Hypersensitivity reactions to iodinated contrast media. Curr. Pharm. Des. 2006, 12, 3359–3372. [Google Scholar] [CrossRef]
- Lukawska, J.; Mandaliya, D.; Chan, A.E.; Foggitt, A.; Bidder, T.; Harvey, J.; Stycharczuk, L.; Bisdas, S. Anaphylaxis to trometamol excipient in gadolinium-based contrast agents for clinical imaging. J. Allergy Clin. Immunol. Pract. 2019, 7, 1086–1087. [Google Scholar] [CrossRef]
- R usso, P.A.; Banovic, T.; Wiese, M.D.; Whyte, A.F.; Smith, W.B. Systemic allergy to EDTA in local anesthetic and radiocontrast media. J. Allergy Clin. Immunol. Pract. 2014, 2, 225–229. [Google Scholar] [CrossRef]
- Ali, S.B.; Perkins, G.; Ryoo, D.; Lee, M.; Tunbridge, M.; Yuson, C.; Smith, W.; Hissaria, P.; Le, T.T. AstraZeneca ChAdOx1-S COVID-19 vaccine can be safely administered in patients with EDTA allergy. Allergy Asthma Clin. Immunol. 2022, 18, 22. [Google Scholar] [CrossRef]
- Rosado Ingelmo, A.; Dona Diaz, I.; Cabanas Moreno, R.; Moya Quesada, M.C.; Garcia-Aviles, C.; Garcia Nunez, I.; Martinez Tadeo, J.I.; Mielgo Ballesteros, R.; Ortega-Rodriguez, N.; Padial Vilchez, M.A.; et al. Clinical Practice Guidelines for Diagnosis and Management of Hypersensitivity Reactions to Contrast Media. J. Investig. Allergol. Clin. Immunol. 2016, 26, 144–155. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, J.; Zhang, L.; Liu, H.; Wang, S.; Chen, X.; Fang, J.; Wang, S.; Zhang, W. Clinical observation of the adverse drug reactions caused by non-ionic iodinated contrast media: Results from 109,255 cases who underwent enhanced CT examination in Chongqing, China. Br. J. Radiol. 2015, 88, 20140491. [Google Scholar] [CrossRef] [Green Version]
- Katayama, H.; Yamaguchi, K.; Kozuka, T.; Takashima, T.; Seez, P.; Matsuura, K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology 1990, 175, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.L.; Arenson, R.L.; Cross, A.P. A prospective trial of ionic vs nonionic contrast agents in routine clinical practice: Comparison of adverse effects. Am. J. Roentgenol. 1989, 152, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Cochran, S.T.; Bomyea, K. Trends in adverse events from iodinated contrast media. Acad. Radiol. 2002, 9, S65–S68. [Google Scholar] [CrossRef]
- Cha, M.J.; Kang, D.Y.; Lee, W.; Yoon, S.H.; Choi, Y.H.; Byun, J.S.; Lee, J.; Kim, Y.H.; Choo, K.S.; Cho, B.S.; et al. Hypersensitivity Reactions to Iodinated Contrast Media: A Multicenter Study of 196,081 Patients. Radiology 2019, 293, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Voltolini, S.; Cofini, V.; Murzilli, F.; Bignardi, D.; Borro, M.; Calamari, M.; Caruso, C.; Cittadini, G.; Contatore, M.; Cortellini, G.; et al. Hypersensitivity reactions to iodinate contrast media in Italy: A retrospective study. Characteristics of patients and risk factors. Eur. Ann. Allergy Clin. Immunol. 2021, 54, 60. [Google Scholar] [CrossRef] [PubMed]
- Klostranec, J.M.; Rohringer, T.; Gerber, R.; Murphy, K.J. The Role of Biologic Sex in Anaphylactoid Contrast Reactions: An Important Consideration for Women of Reproductive Age and Undergoing Hormone Replacement Therapy. Radiology 2021, 299, 272–275. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, J.; Dong, Y.; Guo, B.; Lian, Z.; Yu, H.; Luo, X.; Mo, X.; Zhang, L.; Huang, W.; et al. Extrinsic warming of low-osmolality iodinated contrast media to 37 degrees C reduced the rate of allergic-like reaction. Allergy Asthma Proc. 2018, 39, e55–e63. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Zhao, L.; Liu, J.; Cai, L.; Liu, L.; Zhang, W. Clinical observation of adverse drug reactions to non-ionic iodinated contrast media in population with underlying diseases and risk factors. Br. J. Radiol. 2017, 90, 20160729. [Google Scholar] [CrossRef] [Green Version]
- Brockow, K. Reduced iodinated contrast media dose and injection speed for CT: How much does this decrease the risk of a hypersensitivity reactions? Quant. Imaging Med. Surg. 2020, 10, 537–540. [Google Scholar] [CrossRef]
- Park, H.J.; Son, J.H.; Kim, T.B.; Kang, M.K.; Han, K.; Kim, E.H.; Kim, A.Y.; Park, S.H. Relationship between Lower Dose and Injection Speed of Iodinated Contrast Material for CT and Acute Hypersensitivity Reactions: An Observational Study. Radiology 2019, 293, 565–572. [Google Scholar] [CrossRef]
- Böhm, I.; Heverhagen, J.T.; Klose, K.J. Classification of acute and delayed contrast media-induced reactions: Proposal of a three-step system. Contrast Media Mol. Imaging 2012, 7, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Brockow, K.; Christiansen, C.; Kanny, G.; Clément, O.; Barbaud, A.; Bircher, A.; Dewachter, P.; Guéant, J.L.; Rodriguez Guéant, R.M.; Mouton-Faivre, C.; et al. Management of hypersensitivity reactions to iodinated contrast media. Allergy 2005, 60, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Tanno, L.K.; Torres, M.J.; Castells, M.; Demoly, P. What can we learn in drug allergy management from World Health Organization’s international classifications? Allergy 2018, 73, 987–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brockow, K. Medical algorithm: Diagnosis and treatment of radiocontrast media hypersensitivity. Allergy 2020, 75, 1278–1280. [Google Scholar] [CrossRef]
- Christiansen, C.; Pichler, W.J.; Skotland, T. Delayed allergy-like reactions to X-ray contrast media: Mechanistic considerations. Eur. Radiol. 2000, 10, 1965–1975. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lim, K.W.; Chang, Y.S. Radiocontrast media hypersensitivity in the Asia Pacific region. Asia Pac. Allergy 2014, 4, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Laroche, D. Immediate reactions to contrast media: Mediator release and value of diagnostic testing. Toxicology 2005, 209, 193–194. [Google Scholar] [CrossRef]
- Morcos, S.K. Review article: Acute serious and fatal reactions to contrast media: Our current understanding. Br. J. Radiol. 2005, 78, 686–693. [Google Scholar] [CrossRef]
- Morales-Cabeza, C.; Roa-Medellin, D.; Torrado, I.; De Barrio, M.; Fernandez-Alvarez, C.; Montes-Acenero, J.F.; De La Riva, I.; Prieto-Garcia, A. Immediate reactions to iodinated contrast media. Ann. Allergy Asthma Immunol. 2017, 119, 553–557. [Google Scholar] [CrossRef]
- Zou, W.; Yang, S.; Chen, L.; Hu, S.; Hao, G.; Hu, C. Iodixanol activation of mast cells: Implications in the pathogenesis of iodixanol-induced delayed cutaneous adverse reactions. Toxicology 2022, 465, 153034. [Google Scholar] [CrossRef]
- Böhm, I.; Schild, H.H. Immediate and non-immediate reaction after non-ionic X-ray contrast medium injection: Case report and review of the literature. Eur. J. Radiol. Extra 2007, 61, 129–133. [Google Scholar] [CrossRef]
- Schild, H.H.; Kuhl, C.K.; Hübner-Steiner, U.; Böhm, I.; Speck, U. Adverse events after unenhanced and monomeric and dimeric contrast-enhanced CT: A prospective randomized controlled trial. Radiology 2006, 240, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Brockow, K.; Ardern-Jones, M.R.; Mockenhaupt, M.; Aberer, W.; Barbaud, A.; Caubet, J.C.; Spiewak, R.; Torres, M.J.; Mortz, C.G. EAACI position paper on how to classify cutaneous manifestations of drug hypersensitivity. Allergy 2019, 74, 14–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böhm, I.; Schild, H.H. A practical guide to diagnose lesser-known immediate and delayed contrast media-induced adverse cutaneous reactions. Eur. Radiol. 2006, 16, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Rosado, A.; Canto, G.; Veleiro, B.; Rodríguez, J. Toxic epidermal necrolysis after repeated injections of iohexol. Am. J. Roentgenol. 2001, 176, 262–263. [Google Scholar] [CrossRef] [PubMed]
- Soria, A.; Bernier, C.; Veyrac, G.; Barbaud, A.; Puymirat, E.; Milpied, B. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: A retrospective study. J. Am. Acad. Dermatol. 2020, 82, 606–611. [Google Scholar] [CrossRef]
- Soria, A.; Amsler, E.; Bernier, C.; Milpied, B.; Tetart, F.; Morice, C.; Dezoteux, F.; Ferrier-Le Bouedec, M.C.; Barbaud, A.; Staumont-Salle, D.; et al. DRESS and AGEP Reactions to Iodinated Contrast Media: A French Case Series. J. Allergy Clin. Immunol. Pract. 2021, 9, 3041–3050. [Google Scholar] [CrossRef]
- Tasker, F.; Fleming, H.; McNeill, G.; Creamer, D.; Walsh, S. Contrast media and cutaneous reactions. Part 2: Delayed hypersensitivity reactions to iodinated contrast media. Clin. Exp. Dermatol. 2019, 44, 844–860. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.J.; Gomez, F.; Dona, I.; Rosado, A.; Mayorga, C.; Garcia, I.; Blanca-Lopez, N.; Canto, G.; Blanca, M. Diagnostic evaluation of patients with nonimmediate cutaneous hypersensitivity reactions to iodinated contrast media. Allergy 2012, 67, 929–935. [Google Scholar] [CrossRef]
- Costantino, M.T.; Romanini, L.; Gaeta, F.; Stacul, F.; Valluzzi, R.L.; Passamonti, M.; Bonadonna, P.; Cerri, G.; Pucci, S.; Ricci, P.; et al. SIRM-SIAAIC consensus, an Italian document on management of patients at risk of hypersensitivity reactions to contrast media. Clin. Mol. Allergy 2020, 18, 13. [Google Scholar] [CrossRef]
- Vitte, J.; Amadei, L.; Gouitaa, M.; Mezouar, S.; Zieleskiewicz, L.; Albanese, J.; Bruder, N.; Lagier, D.; Mertès, P.M.; Mège, J.L.; et al. Paired acute-baseline serum tryptase levels in perioperative anaphylaxis: An observational study. Allergy 2019, 74, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Worm, M.; Francuzik, W.; Renaudin, J.M.; Bilo, M.B.; Cardona, V.; Scherer Hofmeier, K.; Köhli, A.; Bauer, A.; Christoff, G.; Cichocka-Jarosz, E.; et al. Factors increasing the risk for a severe reaction in anaphylaxis: An analysis of data from The European Anaphylaxis Registry. Allergy 2018, 73, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Kvedariene, V.; Martins, P.; Rouanet, L.; Demoly, P. Diagnosis of iodinated contrast media hypersensitivity: Results of a 6-year period. Clin. Exp. Allergy. 2006, 36, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Trcka, J.; Schmidt, C.; Seitz, C.S.; Bröcker, E.B.; Gross, G.E.; Trautmann, A. Anaphylaxis to iodinated contrast material: Nonallergic hypersensitivity or IgE-mediated allergy? Am. J. Roentgenol. 2008, 190, 666–670. [Google Scholar] [CrossRef]
- Salas, M.; Gomez, F.; Fernandez, T.D.; Doña, I.; Aranda, A.; Ariza, A.; Blanca-López, N.; Mayorga, C.; Blanca, M.; Torres, M.J. Diagnosis of immediate hypersensitivity reactions to radiocontrast media. Allergy 2013, 68, 1203–1206. [Google Scholar] [CrossRef]
- Mayorga, C.; Fernandez, T.D.; Montañez, M.I.; Moreno, E.; Torres, M.J. Recent developments and highlights in drug hypersensitivity. Allergy 2019, 74, 2368–2381. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.H.; Lee, S.Y.; Kang, H.R.; Kim, J.Y.; Hahn, S.; Park, C.M.; Chang, Y.S.; Goo, J.M.; Cho, S.H. Skin tests in patients with hypersensitivity reaction to iodinated contrast media: A meta-analysis. Allergy 2015, 70, 625–637. [Google Scholar] [CrossRef]
- Goksel, O.; Aydın, O.; Atasoy, C.; Akyar, S.; Demirel, Y.S.; Misirligil, Z.; Bavbek, S. Hypersensitivity reactions to contrast media: Prevalence, risk factors and the role of skin tests in diagnosis—A cross-sectional survey. Int. Arch. Allergy Immunol. 2011, 155, 297–305. [Google Scholar] [CrossRef]
- Brockow, K.; Romano, A.; Aberer, W.; Bircher, A.J.; Barbaud, A.; Bonadonna, P.; Faria, E.; Kanny, G.; Lerch, M.; Pichler, W.J.; et al. Skin testing in patients with hypersensitivity reactions to iodinated contrast media—A European multicenter study. Allergy 2009, 64, 234–241. [Google Scholar] [CrossRef]
- Dewachter, P.; Laroche, D.; Mouton-Faivre, C.; Bloch-Morot, E.; Cercueil, J.P.; Metge, L.; Carette, M.F.; Vergnaud, M.C.; Clément, O. Immediate reactions following iodinated contrast media injection: A study of 38 cases. Eur. J. Radiol. 2011, 77, 495–501. [Google Scholar] [CrossRef]
- Kvedariene, V.; Orvydaite, M.; Petraityte, P.; Rudyte, J.; Edvardas Tamosiunas, A. Inherent clinical properties of non-immediate hypersensitivity to iodinated contrast media. Int. J. Clin. Pract. 2021, 75, e14766. [Google Scholar] [CrossRef] [PubMed]
- Nucera, E.; Parrinello, G.; Gangemi, S.; Buonomo, A.; Aruanno, A.; Lohmeyer, F.M.; Inchingolo, R.; Rizzi, A. Contrast Medium Hypersensitivity: A Large Italian Study with Long-Term Follow-Up. Biomedicines 2022, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Caimmi, S.; Benyahia, B.; Suau, D.; Bousquet-Rouanet, L.; Caimmi, D.; Bousquet, P.J.; Demoly, P. Clinical value of negative skin tests to iodinated contrast media. Clin. Exp. Allergy 2010, 40, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Schrijvers, R.; Breynaert, C.; Ahmedali, Y.; Bourrain, J.L.; Demoly, P.; Chiriac, A.M. Skin Testing for Suspected Iodinated Contrast Media Hypersensitivity. J. Allergy Clin. Immunol. Pract. 2018, 6, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.H.; Koh, Y.I.; Kim, J.H.; Ban, G.Y.; Lee, Y.K.; Hong, G.N.; Jin, U.R.; Choi, B.J.; Shin, Y.S.; Park, H.S.; et al. The potential utility of iodinated contrast media (ICM) skin testing in patients with ICM hypersensitivity. J. Korean Med. Sci. 2015, 30, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Vernassiere, C.; Trechot, P.; Commun, N.; Schmutz, J.L.; Barbaud, A. Low negative predictive value of skin tests in investigating delayed reactions to radio-contrast media. Contact Dermat. 2004, 50, 359–366. [Google Scholar] [CrossRef]
- Bhujoo, Z.; Ingen-Housz-Oro, S.; Gener, G.; Gaudin, O.; Fleck, M.; Verlinde-Carvalho, M.; Paul, M.; Chosidow, O.; Wolkenstein, P.; Assier, H. Patch tests in nonimmediate cutaneous adverse drug reactions: The importance of late readings on day 4. Contact Dermat. 2022, 86, 29–33. [Google Scholar] [CrossRef]
- Gaide, O.; Emerson, R.O.; Jiang, X.; Gulati, N.; Nizza, S.; Desmarais, C.; Robins, H.; Krueger, J.G.; Clark, R.A.; Kupper, T.S. Common clonal origin of central and resident memory T cells following skin immunization. Nat. Med. 2015, 21, 647–653. [Google Scholar] [CrossRef]
- Boehm, I. Limited duration of hypersensitivity reactions to contrast and exact documentation of such adverse events. Reg. Anesth. Pain Med. 2020, 45, 246. [Google Scholar] [CrossRef]
- Steiner, M.; Harrer, A.; Himly, M. Basophil Reactivity as Biomarker in Immediate Drug Hypersensitivity Reactions-Potential and Limitations. Front. Pharmacol. 2016, 7, 171. [Google Scholar] [CrossRef] [Green Version]
- Pinnobphun, P.; Buranapraditkun, S.; Kampitak, T.; Hirankarn, N.; Klaewsongkram, J. The diagnostic value of basophil activation test in patients with an immediate hypersensitivity reaction to radiocontrast media. Ann. Allergy Asthma Immunol. 2011, 106, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.; Ariza, A.; Blanca-Lopez, N.; Torres, M.J. Nonimmediate hypersensitivity reactions to iodinated contrast media. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 345–353. [Google Scholar] [CrossRef]
- Kanny, G.; Pichler, W.; Morisset, M.; Franck, P.; Marie, B.; Kohler, C.; Renaudin, J.M.; Beaudouin, E.; Laudy, J.S.; Moneret-Vautrin, D.A. T cell-mediated reactions to iodinated contrast media: Evaluation by skin and lymphocyte activation tests. J. Allergy Clin. Immunol. 2005, 115, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.J.; Mayorga, C.; Cornejo-Garcia, J.A.; Lopez, S.; Chaves, P.; Rondon, C.; Fernandez, T.; Blanca, M. Monitoring non-immediate allergic reactions to iodine contrast media. Clin. Exp. Immunol. 2008, 152, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Pichler, W.J.; Tilch, J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy 2004, 59, 809–820. [Google Scholar] [CrossRef]
- Hari, Y.; Frutig-Schnyder, K.; Hurni, M.; Yawalkar, N.; Zanni, M.P.; Schnyder, B.; Kappeler, A.; von Greyerz, S.; Braathen, L.R.; Pichler, W.J. T cell involvement in cutaneous drug eruptions. Clin. Exp. Allergy 2001, 31, 1398–1408. [Google Scholar] [CrossRef]
- Sese, L.; Gaouar, H.; Autegarden, J.E.; Alari, A.; Amsler, E.; Vial-Dupuy, A.; Pecquet, C.; Frances, C.; Soria, A. Immediate hypersensitivity to iodinated contrast media: Diagnostic accuracy of skin tests and intravenous provocation test with low dose. Clin. Exp. Allergy 2016, 46, 472–478. [Google Scholar] [CrossRef]
- Soria, A.; Masson, N.; Vial-Dupuy, A.; Gaouar, H.; Amsler, E.; Chollet-Martin, S.; Nicaise-Roland, P.; Autegarden, J.E.; Barbaud, A. Allergological workup with half-dose challenge in iodinated contrast media hypersensitivity. Allergy 2019, 74, 414–417. [Google Scholar] [CrossRef]
- Trautmann, A.; Brockow, K.; Behle, V.; Stoevesandt, J. Radiocontrast Media Hypersensitivity: Skin Testing Differentiates Allergy From Nonallergic Reactions and Identifies a Safe Alternative as Proven by Intravenous Provocation. J. Allergy Clin. Immunol. Pract. 2019, 7, 2218–2224. [Google Scholar] [CrossRef]
- Bansie, R.D.; Karim, A.F.; van Maaren, M.S.; Hermans, M.A.; van Daele, P.; Gerth van Wijk, R.; Rombach, S.M. Assessment of immediate and non-immediate hypersensitivity contrast reactions by skin tests and provocation tests: A review. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211015061. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; de Gregorio, C.; Assimakopoulos, S.F.; Velissaris, D.; Hung, M.Y.; Mplani, V.; Saba, L.; Brinia, A.; Kouni, S.N.; et al. COVID-19 Disease, Women’s Predominant Non-Heparin Vaccine-Induced Thrombotic Thrombocytopenia and Kounis Syndrome: A Passepartout Cytokine Storm Interplay. Biomedicines 2021, 9, 950. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, C.B.; Abraham, R.J.; Mammen, T.; Abdolell, M.; Kapur, S.; Abraham, R.J. Survey of radiologists’ knowledge regarding the management of severe contrast material-induced allergic reactions. Radiology 2009, 251, 691–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieberman, P.; Kemp, S.F.; Oppenheimer, J.; Lang, D.M.; Bernstein, I.L.; Nicklas, R.A.; Anderson, J.A.; Bernstein, D.I.; Bernstein, J.A.; Fink, J.N.; et al. The diagnosis and management of anaphylaxis: An updated practice parameter. J. Allergy Clin. Immunol. 2005, 115, S483–S523. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kwon, O.Y.; Park, S.Y.; Seo, B.; Won, H.K.; Kang, Y.; An, J.; Kwon, H.S.; Song, W.J.; Cho, Y.S.; et al. Validation of the Prescreening Intradermal Skin Test for Predicting Hypersensitivity to Iodinated Contrast Media: A Prospective Study with ICM Challenge. J. Allergy Clin. Immunol. Pract. 2020, 8, 267–272. [Google Scholar] [CrossRef]
- Thong, B.Y.; Vultaggio, A.; Rerkpattanapipat, T.; Schrijvers, R. Prevention of Drug Hypersensitivity Reactions: Prescreening and Premedication. J. Allergy Clin. Immunol. Pract. 2021, 9, 2958–2966. [Google Scholar] [CrossRef]
- Madrigal-Burgaleta, R.; Bernal-Rubio, L.; Berges-Gimeno, M.P.; Carpio-Escalona, L.V.; Gehlhaar, P.; Alvarez-Cuesta, E. A Large Single-Hospital Experience Using Drug Provocation Testing and Rapid Drug Desensitization in Hypersensitivity to Antineoplastic and Biological Agents. J. Allergy Clin. Immunol. Pract. 2019, 7, 618–632. [Google Scholar] [CrossRef]
- Morcos, S.K.; Thomsen, H.S.; Webb, J.A. Prevention of generalized reactions to contrast media: A consensus report and guidelines. Eur. Radiol. 2001, 11, 1720–1728. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yang, M.S.; Choi, Y.H.; Park, C.M.; Park, H.W.; Cho, S.H.; Kang, H.R. Stratified premedication strategy for the prevention of contrast media hypersensitivity in high-risk patients. Ann. Allergy Asthma Immunol. 2017, 118, 339–344.e1. [Google Scholar] [CrossRef]
- Park, S.J.; Kang, D.Y.; Sohn, K.H.; Yoon, S.H.; Lee, W.; Choi, Y.H.; Cho, S.H.; Kang, H.R. Immediate Mild Reactions to CT with Iodinated Contrast Media: Strategy of Contrast Media Readministration without Corticosteroids. Radiology 2018, 288, 710–716. [Google Scholar] [CrossRef] [Green Version]
- Abe, S.; Fukuda, H.; Tobe, K.; Ibukuro, K. Protective effect against repeat adverse reactions to iodinated contrast medium: Premedication vs. changing the contrast medium. Eur. Radiol. 2016, 26, 2148–2154. [Google Scholar] [CrossRef]
- Mervak, B.M.; Davenport, M.S.; Ellis, J.H.; Cohan, R.H. Rates of Breakthrough Reactions in Inpatients at High Risk Receiving Premedication Before Contrast-Enhanced CT. Am. J. Roentgenol. 2015, 205, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Lasser, E.C.; Berry, C.C.; Talner, L.B.; Santini, L.C.; Lang, E.K.; Gerber, F.H.; Stolberg, H.O. Pretreatment with corticosteroids to alleviate reactions to intravenous contrast material. N. Engl. J. Med. 1987, 317, 845–849. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.S.; Larson, N.B.; Kolbe, A.B.; Hunt, C.H.; Schmitz, J.J.; Maddox, D.E.; Hartman, R.P.; Kallmes, D.F.; McDonald, R.J. Prevention of Allergic-like Reactions at Repeat CT: Steroid Pretreatment versus Contrast Material Substitution. Radiology 2021, 301, 133–140. [Google Scholar] [CrossRef]
- Romano, A.; Artesani, M.C.; Andriolo, M.; Viola, M.; Pettinato, R.; Vecchioli-Scaldazza, A. Effective prophylactic protocol in delayed hypersensitivity to contrast media: Report of a case involving lymphocyte transformation studies with different compounds. Radiology 2002, 225, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.J.; Trautmann, A.; Bohm, I.; Scherer, K.; Barbaud, A.; Bavbek, S.; Bonadonna, P.; Cernadas, J.R.; Chiriac, A.M.; Gaeta, F.; et al. Practice parameters for diagnosing and managing iodinated contrast media hypersensitivity. Allergy 2021, 76, 1325–1339. [Google Scholar] [CrossRef]
- Mervak, B.M.; Cohan, R.H.; Ellis, J.H.; Khalatbari, S.; Davenport, M.S. Intravenous Corticosteroid Premedication Administered 5 Hours before CT Compared with a Traditional 13-Hour Oral Regimen. Radiology 2017, 285, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Davenport, M.S.; Mervak, B.M.; Ellis, J.H.; Dillman, J.R.; Dunnick, N.R.; Cohan, R.H. Indirect Cost and Harm Attributable to Oral 13-Hour Inpatient Corticosteroid Prophylaxis before Contrast-enhanced CT. Radiology 2016, 279, 492–501. [Google Scholar] [CrossRef]
- Boehm, I. Three Important Points on the Documentation of Contrast Hypersensitivity Reactions to Improve Contrast Medium Safety. J. Am. Coll. Radiol. 2020, 17, 207. [Google Scholar] [CrossRef]
| Generic Name | Trade Name | Ionic/Non-Ionic | Monomer/Dimer | Iodine Content (mg/mL) | Osmolality (mOsm/kg H2O) | Osmolar | Excipients with Reported Hypersensitivity |
|---|---|---|---|---|---|---|---|
| Iothalamate | Conray | Ionic | Monomer | 325 | 1843 | High | Sodium calcium edetate |
| Amidotrizoate | Urografin 76% | Ionic | Monomer | 370 | 2100 | High | Sodium calcium edetate |
| Ioxithalamate | Telebrix 30 | Ionic | Monomer | 300 | 1710 | High | Sodium calcium edetate |
| Diatrizoate | Hypaque 50 | Ionic | Monomer | 300 | 1550 | High | Sodium calcium edetate |
| Ioxaglate | Hexabrix | Ionic | Dimer | 320 | 580 | Low | Sodium calcium edetate |
| Iopromide | Ultravist 370 | Non-ionic | Monomer | 370 | 774 | Low | Sodium calcium edetate, Tromethamine |
| Iohexol | Omnipaque 350 | Non-ionic | Monomer | 350 | 884 | Low | Sodium calcium edetate, Tromethamine |
| Ioversol | Optiray 300 | Non-ionic | Monomer | 300 | 651 | Low | Sodium calcium edetate, Tromethamine |
| Iopamidol | Isovue-370 | Non-ionic | Monomer | 370 | 796 | Low | Sodium calcium edetate, Tromethamine |
| Iobitridol | Xenetix 350 | Non-ionic | Monomer | 350 | 915 | Low | Sodium calcium edetate, Tromethamine |
| Ioxilan | Oxilan 350 | Non-ionic | Monomer | 350 | 695 | Low | Sodium calcium edetate, Tromethamine |
| Iomeprol | Iomeron 350 | Non-ionic | Monomer | 350 | 618 | Low | Tromethamine |
| Iopentol | Imagopaque 300 | Non-ionic | Monomer | 300 | 640 | Low | Sodium calcium edetate, Tromethamine |
| Iodixanol | Visipaque 320 | Non-ionic | Dimer | 320 | 290 | Iso | Sodium calcium edetate, Tromethamine |
| Iotrolan | Isovist 300 | Non-ionic | Dimer | 300 | 291 | Iso | Sodium calcium edetate |
| Allergic-Like/Hypersensitivity | Chemo-Toxic | |
|---|---|---|
| Mild | ||
| Limited urticaria/pruritis | Limited nausea/vomiting limited | |
| Cutaneous edema | Transient flushing/warmth/chills | |
| Limited “itchy”/”scratchy” throat | Headache/dizziness/anxiety/altered taste | |
| Nasal congestion | Mild hypertension | |
| Sneezing/conjunctivitis/rhinorrhea | Vasovagal reaction that resolves spontaneously | |
| Moderate | ||
| Diffuse urticaria/pruritis | Protracted nausea/vomiting | |
| Diffuse erythema, stable vital signs | Hypertensive urgency | |
| Facial edema without dyspnea | Isolated chest pain | |
| Throat tightness or hoarseness without dyspnea | Vasovagal reaction that requires and is responsive to treatment | |
| Wheezing/bronchospasm, mild or no hypoxia | ||
| Severe | ||
| Diffuse edema, or facial edema with dyspnea | Vasovagal reaction resistant to treatment | |
| Diffuse erythema with hypotension | Arrhythmia | |
| Laryngeal edema with stridor and/or hypoxia | Convulsions, seizures | |
| Wheezing/bronchospasm, significant hypoxia | Hypertensive emergency | |
| Anaphylactic shock (hypotension + tachycardia) |
| Category | Test | Percentage [References] |
|---|---|---|
| IHR | Sensitivity of skin test | 4.2 to 73% (correlate with the severity of the phenotype) [42,43,44,45,46,47,48,49] |
| Specificity of SPT | 94.6% [47,48] | |
| Specificity of IDT | 91.4–96.3% [47,48] | |
| Pooled per-patient positivity rates of skin tests | 17% (95% CI, 10–26%) [46] | |
| Severe IHR-pooled per-patient positivity rates of skin tests | 52% (95% CI, 31–72%) [46] | |
| Negative predictive value of skin test | 93% (95% CI, 86–96%) [46] | |
| Cross-reactivity in skin test | 68% (95% CI, 48–83%) [47,48] | |
| Sensitivity of BAT | 46–63% [40,41,42] | |
| Specificity of BAT | 89–100% [40,41,42] | |
| NIHR | Sensitivity of skin test | 72% [44,50,51] |
| Specificity of skin test | 96% [44,50,51] | |
| Pooled per-patient positivity rates of skin tests | 26% (95% CI, 15–41%) [46] | |
| Pooled per-patient positivity rates of SPT | 7% (95% CI, 1–30%) [46] | |
| Pooled per-patient positivity rates of IDT | 22% (95% CT, 13–34%) [46] | |
| Pooled per-patient positivity rates of patch test | 16% (95% CI, 15–41%) [46] | |
| Cross-reactivity in skin test | 39% (95% CI, 29–50%) [47,48] | |
| Sensitivity of LTT | 13 to 75% [61] | |
| Skin test for alternative ICM | Negative predictive value for IHR | 94.2% (95% CI, 89.6% to 97.2%) [53] |
| Negative predictive value for NIHR | 86.1% (95% CI, 72.1–94.7%) [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, T.-M.; Chu, S.-Y. Hypersensitivity Reactions to Iodinated Contrast Media. Biomedicines 2022, 10, 1036. https://doi.org/10.3390/biomedicines10051036
Chiu T-M, Chu S-Y. Hypersensitivity Reactions to Iodinated Contrast Media. Biomedicines. 2022; 10(5):1036. https://doi.org/10.3390/biomedicines10051036
Chicago/Turabian StyleChiu, Tsu-Man, and Sung-Yu Chu. 2022. "Hypersensitivity Reactions to Iodinated Contrast Media" Biomedicines 10, no. 5: 1036. https://doi.org/10.3390/biomedicines10051036
APA StyleChiu, T.-M., & Chu, S.-Y. (2022). Hypersensitivity Reactions to Iodinated Contrast Media. Biomedicines, 10(5), 1036. https://doi.org/10.3390/biomedicines10051036







