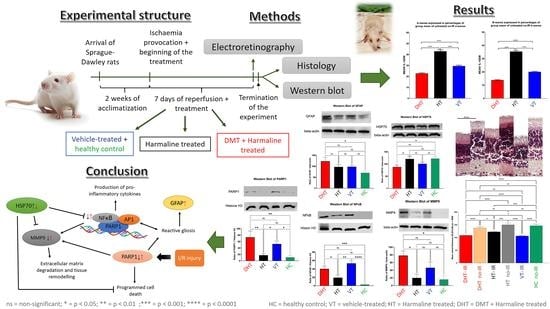
Therapeutic Properties of Ayahuasca Components in Ischemia/Reperfusion Injury of the Eye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Groups
2.2. Ischemia Model
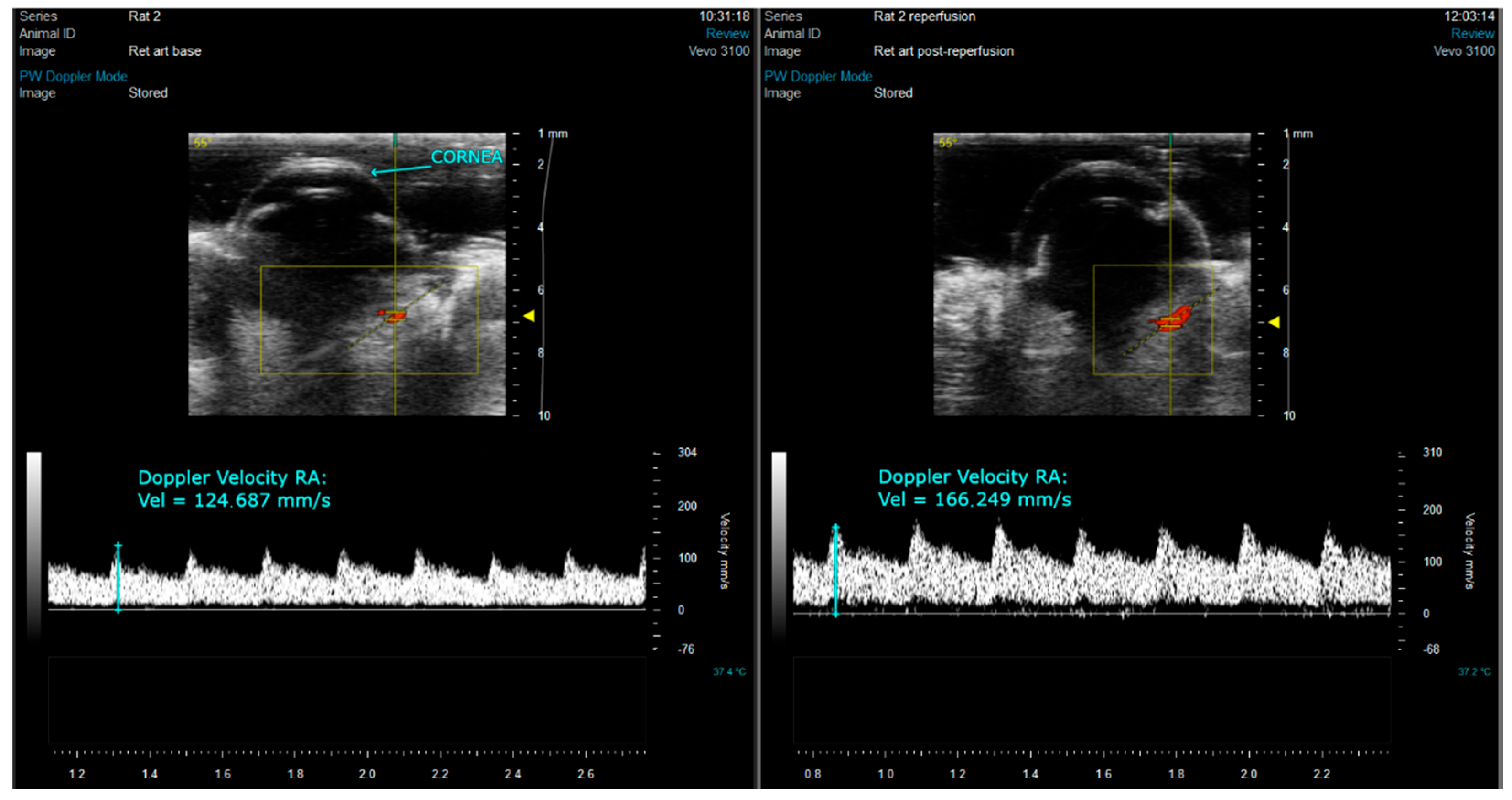
2.3. Ocular Echography
2.4. Osmotic Mini-Pump Implantation
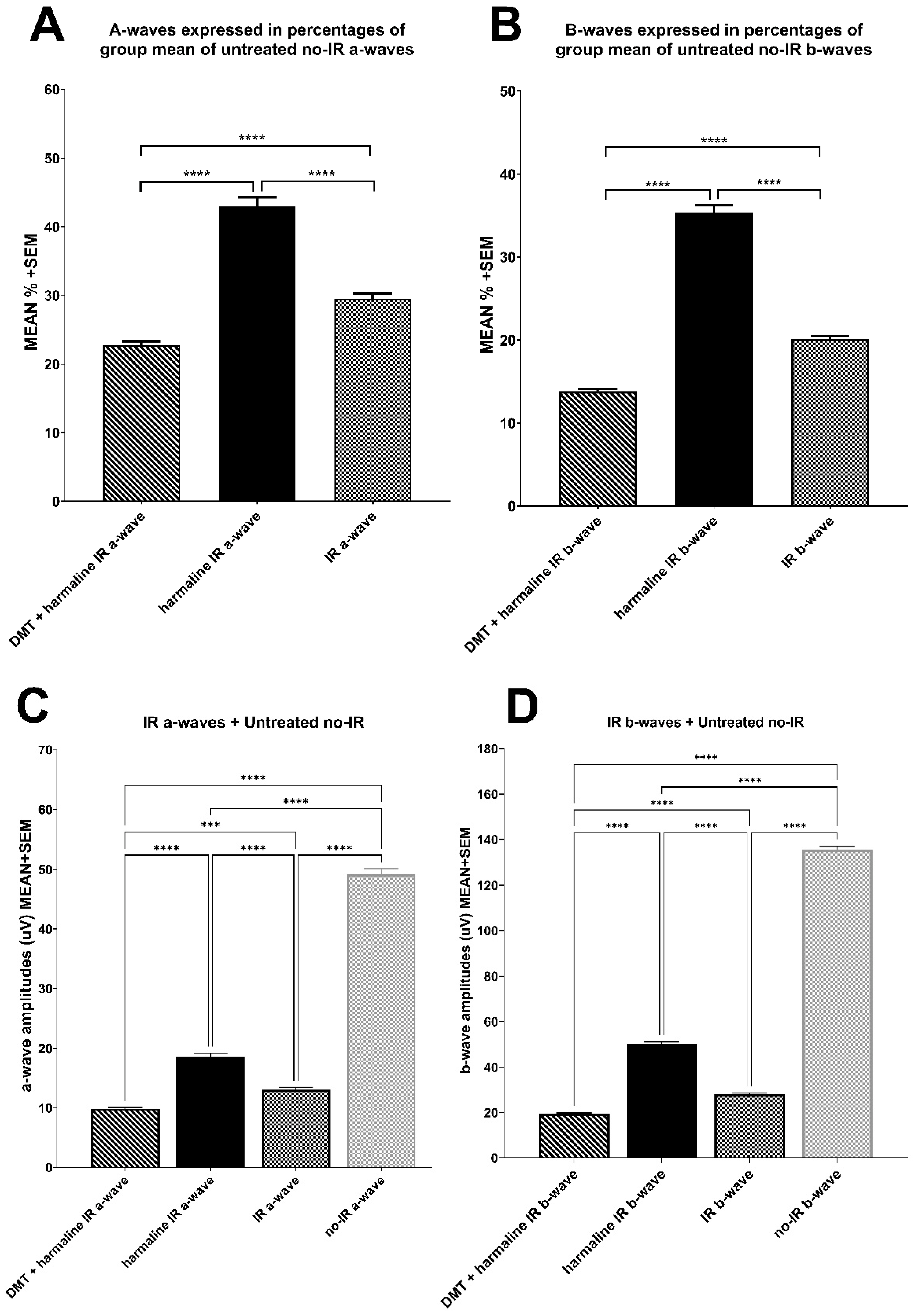
2.5. Electroretinography
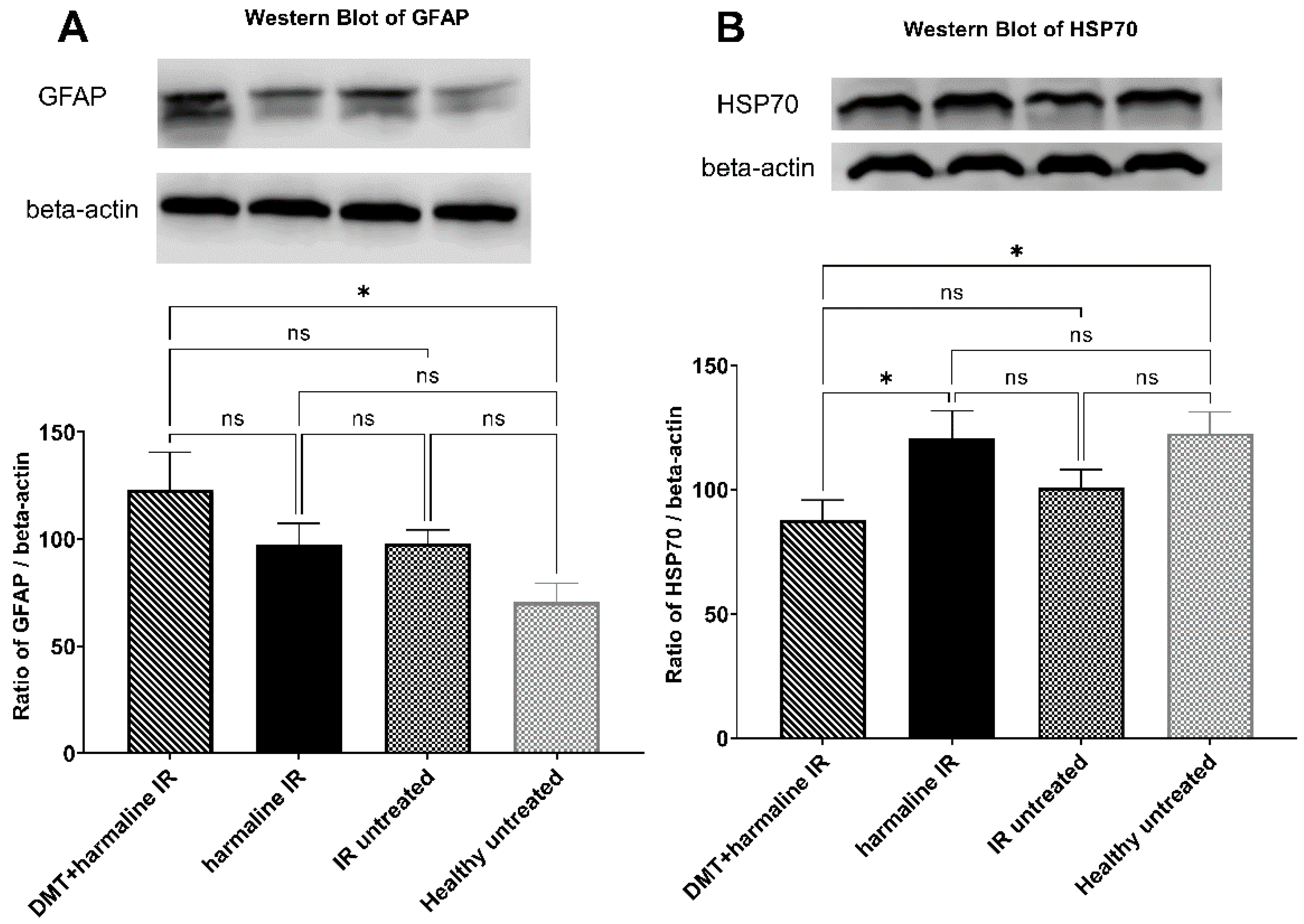
2.6. Western Blot
2.7. Histology: Hematoxylin–Eosin Staining
2.8. Statistical Analyses
3. Results
3.1. Ocular Ultrasound
3.2. Electroretinography
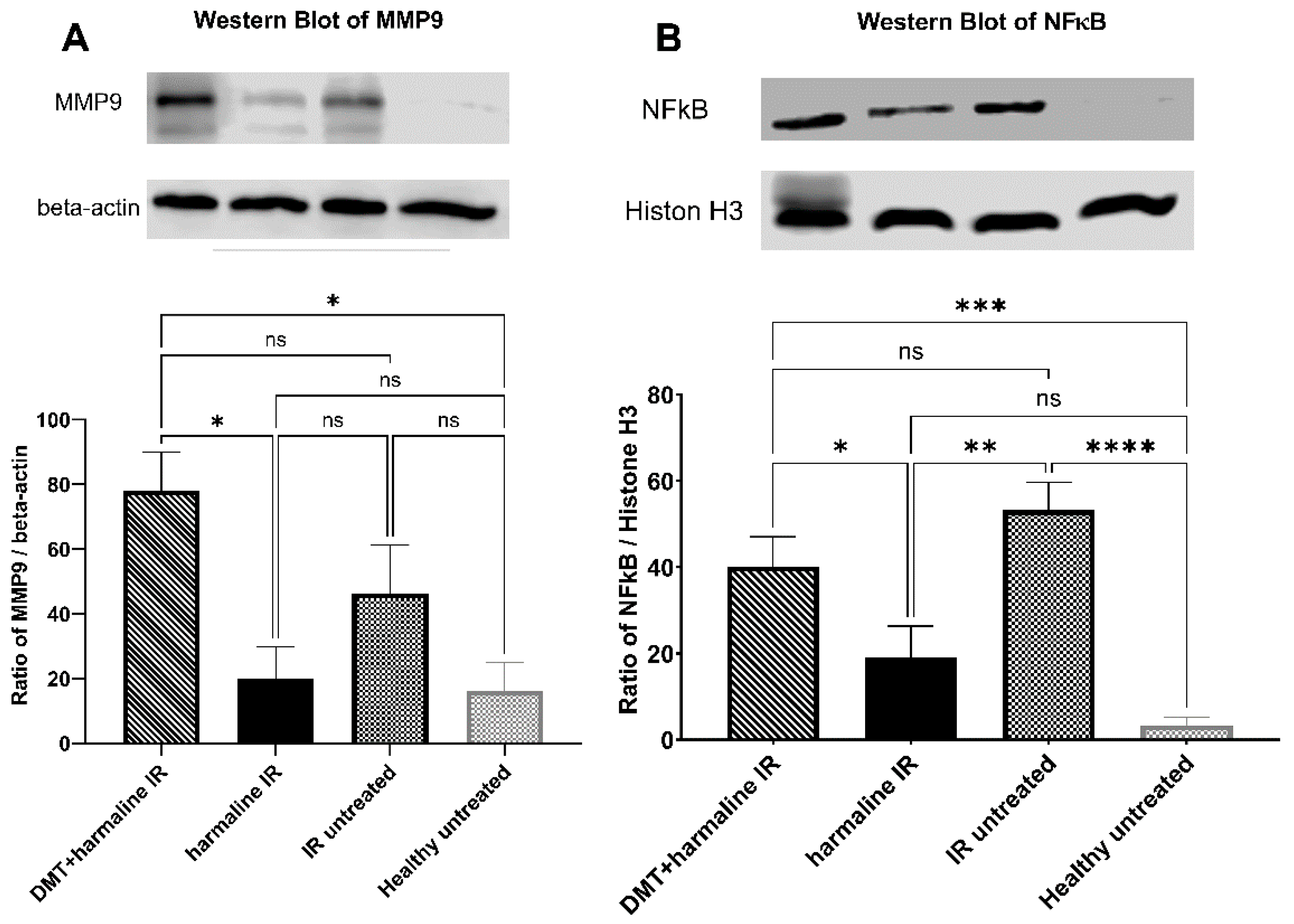
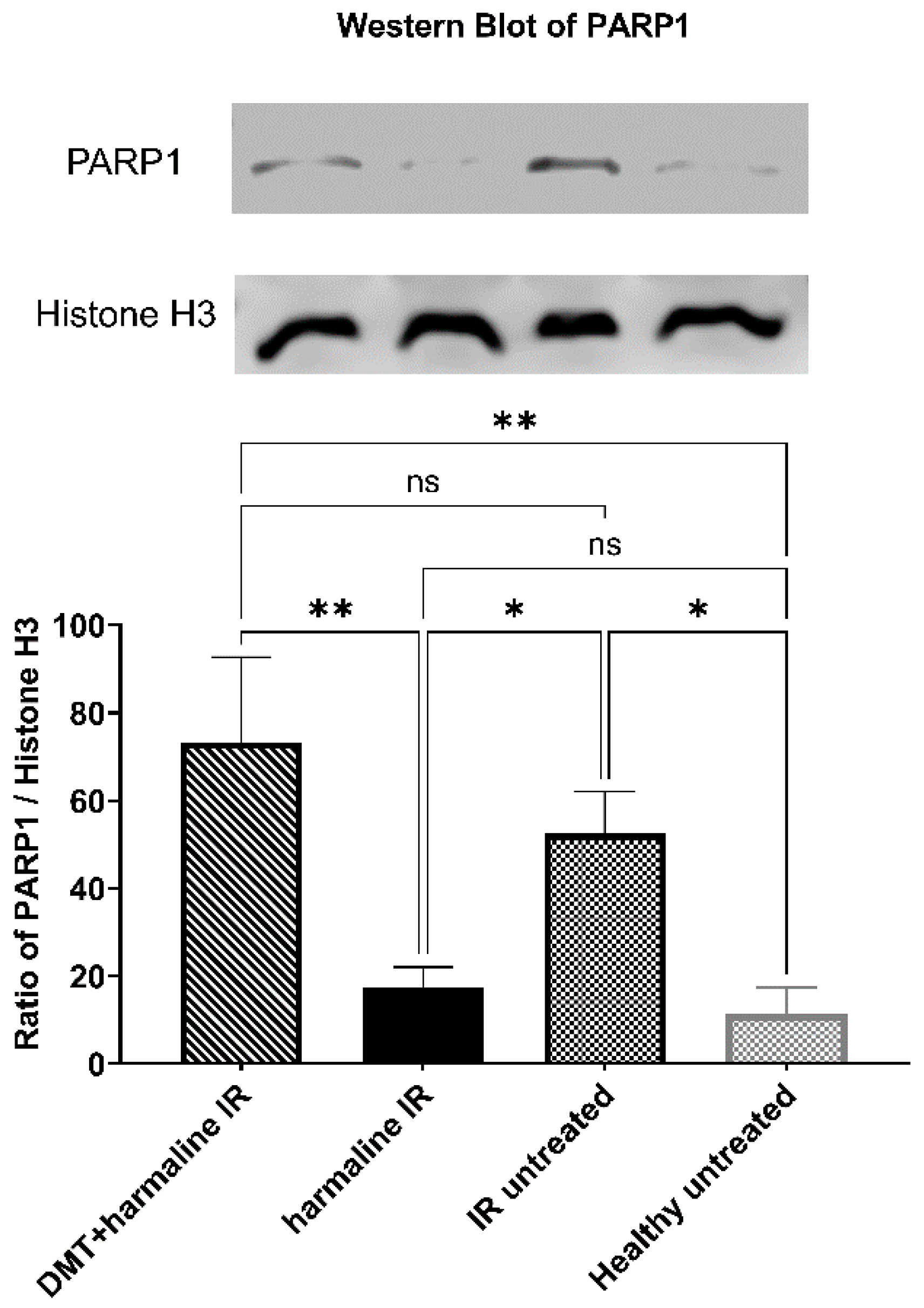
3.3. Western Blot
3.4. Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, N.; Li, Z.; Li, Z.; Zhang, Y.; Yu, Z.; Wan, P.; Zhu, Y.; Li, Y.; Su, W.; Zhuo, Y. Laquinimod exerts anti-inflammatory and antiapoptotic effects in retinal ischemia/reperfusion injury. Int. Immunopharmacol. 2020, 88, 106989. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Li, N.; Zhang, M.; Lin, S.; Zhu, J.; Xiao, D.; Cui, W.; Zhang, T.; Lin, Y.; Cai, X. Tetrahedral framework nucleic acids prevent retina ischemia-reperfusion injury from oxidative stress via activating the Akt/Nrf2 pathway. Nanoscale 2019, 11, 20667–20675. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.M.; Carvalho, F.; Bastos, M.D.L.; de Pinho, P.G.; Carvalho, M. The hallucinogenic world of tryptamines: An updated review. Arch. Toxicol. 2015, 89, 1151–1173. [Google Scholar] [CrossRef]
- Simão, A.Y.; Gonçalves, J.; Gradillas, A.; García, A.; Restolho, J.; Fernández, N.; Rodilla, J.M.; Barroso, M.; Duarte, A.P.; Cristóvão, A.C.; et al. Evaluation of the Cytotoxicity of Ayahuasca Beverages. Molecules 2020, 25, 5594. [Google Scholar] [CrossRef]
- Hamill, J.; Hallak, J.; Dursun, S.M.; Baker, G. Ayahuasca: Psychological and Physiologic Effects, Pharmacology and Potential Uses in Addiction and Mental Illness. Curr. Neuropharmacol. 2019, 17, 108–128. [Google Scholar] [CrossRef]
- Frecska, E.; Bokor, P.; Winkelman, M. The Therapeutic Potentials of Ayahuasca: Possible Effects against Various Diseases of Civilization. Front. Pharmacol. 2016, 7, 35. [Google Scholar] [CrossRef]
- Pal, A.; Fontanilla, D.; Gopalakrishnan, A.; Chae, Y.-K.; Markley, J.L.; Ruoho, A.E. The sigma-1 receptor protects against cellular oxidative stress and activates antioxidant response elements. Eur. J. Pharmacol. 2012, 682, 12–20. [Google Scholar] [CrossRef]
- Jarrott, B.; Williams, S.J. Chronic Brain Inflammation: The Neurochemical Basis for Drugs to Reduce Inflammation. Neurochem. Res. 2016, 41, 523–533. [Google Scholar] [CrossRef]
- Peto, K.; Nemeth, N.; Mester, A.; Magyar, Z.; Ghanem, S.; Somogyi, V.; Tanczos, B.; Deak, A.; Bidiga, L.; Frecska, E.; et al. Hemorheological and metabolic consequences of renal ischemia-reperfusion and their modulation by N,N-dimethyl-tryptamine on a rat model. Clin. Hemorheol. Microcirc. 2018, 70, 107–117. [Google Scholar] [CrossRef]
- Nardai, S.; László, M.; Szabó, A.; Alpár, A.; Hanics, J.; Zahola, P.; Merkely, B.; Frecska, E.; Nagy, Z. N,N-dimethyltryptamine reduces infarct size and improves functional recovery following transient focal brain ischemia in rats. Exp. Neurol. 2020, 327, 113245. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Maalik, A.; Iqbal, Z.; Malik, I. Recent pharmacological developments in β-carboline alkaloid “harmaline”. Eur. J. Pharmacol. 2013, 721, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Kaludercic, N.; Carpi, A.; Menabò, R.; Di Lisa, F.; Paolocci, N. Monoamine oxidases (MAO) in the pathogenesis of heart failure and ischemia/reperfusion injury. Biochim. Biophys. Acta 2011, 1813, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Manni, M.E.; Rigacci, S.; Borchi, E.; Bargelli, V.; Miceli, C.; Giordano, C.; Raimondi, L.; Nediani, C. Monoamine Oxidase Is Overactivated in Left and Right Ventricles from Ischemic Hearts: An Intriguing Therapeutic Target. Oxidative Med. Cell. Longev. 2016, 2016, 4375418. [Google Scholar] [CrossRef] [PubMed]
- Cameron, L.P.; Benson, C.J.; DeFelice, B.C.; Fiehn, O.; Olson, D.E. Chronic, Intermittent Microdoses of the Psychedelic N,N-Dimethyltryptamine (DMT) Produce Positive Effects on Mood and Anxiety in Rodents. ACS Chem. Neurosci. 2019, 10, 3261–3270. [Google Scholar] [CrossRef]
- Cameron, L.P.; Benson, C.J.; Dunlap, L.E.; Olson, D.E. Effects of N,N-Dimethyltryptamine on Rat Behaviors Relevant to Anxiety and Depression. ACS Chem. Neurosci. 2018, 9, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-P.; Wang, Y.-W.; Qi, S.-L.; Zhang, Y.-P.; Deng, G.; Ding, W.-Z.; Ma, C.; Lin, Q.-Y.; Guan, H.-D.; Liu, W.; et al. Analogous β-Carboline Alkaloids Harmaline and Harmine Ameliorate Scopolamine-Induced Cognition Dysfunction by Attenuating Acetylcholinesterase Activity, Oxidative Stress, and Inflammation in Mice. Front. Pharmacol. 2018, 9, 346. [Google Scholar] [CrossRef]
- Szabo, M.E.; Gallyas, E.; Bak, I.; Rakotovao, A.; Boucher, F.; De Leiris, J.; Nagy, N.; Varga, E.; Tosaki, A. Heme Oxygenase-1–Related Carbon Monoxide and Flavonoids in Ischemic/Reperfused Rat Retina. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3727–3732. [Google Scholar] [CrossRef]
- Varga, B.; Gesztelyi, R.; Bombicz, M.; Haines, D.; Szabo, A.M.; Kemeny-Beke, A.; Antal, M.; Vecsernyes, M.; Juhasz, B.; Tosaki, A. Protective Effect of Alpha-Melanocyte-Stimulating Hormone (α-MSH) on the Recovery of Ischemia/Reperfusion (I/R)-Induced Retinal Damage in A Rat Model. J. Mol. Neurosci. 2013, 50, 558–570. [Google Scholar] [CrossRef]
- McCulloch, D.L.; Marmor, M.F.; Brigell, M.G.; Hamilton, R.; Holder, G.E.; Tzekov, R.; Bach, M. ISCEV Standard for full-field clinical electroretinography (2015 update). Doc. Ophthalmol. 2015, 130, 1–12. [Google Scholar] [CrossRef]
- Siu, A.W.; Maldonado, M.; Hidalgo, M.S.; Tan, D.-X.; Reiter, R.J. Protective effects of melatonin in experimental free radical-related ocular diseases. J. Pineal Res. 2006, 40, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Minhas, G.; Sharma, J.; Khan, N. Cellular Stress Response and Immune Signaling in Retinal Ischemia–Reperfusion Injury. Front. Immunol. 2016, 7, 444. [Google Scholar] [CrossRef] [PubMed]
- Palmhof, M.; Frank, V.; Rappard, P.; Kortenhorn, E.; DeMuth, J.; Biert, N.; Stute, G.; Dick, H.B.; Joachim, S.C. From Ganglion Cell to Photoreceptor Layer: Timeline of Deterioration in a Rat Ischemia/Reperfusion Model. Front. Cell. Neurosci. 2019, 13, 174. [Google Scholar] [CrossRef]
- Szabó, I.; Varga, V.E.; Dvorácskó, S.; Farkas, A.E.; Körmöczi, T.; Berkecz, R.; Kecskés, S.; Menyhárt, A.; Frank, R.; Hantosi, D.; et al. N,N-Dimethyltryptamine attenuates spreading depolarization and restrains neurodegeneration by sigma-1 receptor activation in the ischemic rat brain. Neuropharmacology 2021, 192, 108612. [Google Scholar] [CrossRef]
- Da Cruz, R.V.L.; Moulin, T.C.; Petiz, L.L.; Leão, R.N. A Single Dose of 5-MeO-DMT Stimulates Cell Proliferation, Neuronal Survivability, Morphological and Functional Changes in Adult Mice Ventral Dentate Gyrus. Front. Mol. Neurosci. 2018, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Szabo, A.; Kovacs, A.; Riba, J.; Djurovic, S.; Rajnavolgyi, E.; Frecska, E. The Endogenous Hallucinogen and Trace Amine N,N-Dimethyltryptamine (DMT) Displays Potent Protective Effects against Hypoxia via Sigma-1 Receptor Activation in Human Primary iPSC-Derived Cortical Neurons and Microglia-Like Immune Cells. Front. Neurosci. 2016, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Gonsalvez, G.; Bartoli, M.; Mysona, B.A.; Smith, S.B.; Bollinger, K.E. Sigma 1 Receptor Modulates Optic Nerve Head Astrocyte Reactivity. Investig. Ophthalmol. Vis. Sci. 2021, 62, 5. [Google Scholar] [CrossRef]
- Zetler, G.; Back, G.; Iven, H. Pharmacokinetics in the rat of the hallucinogenic alkaloids harmine and harmaline. Naunyn. Schmiedebergs Arch. Pharmacol. 1974, 285, 273–292. [Google Scholar] [CrossRef]
- De Zutter, G.S.; Davis, R.J. Pro-apoptotic gene expression mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Proc. Natl. Acad. Sci. USA 2001, 98, 6168–6173. [Google Scholar] [CrossRef]
- Fitzgerald, J.C.; Klusek, A.U.; Allen, G.; De Girolamo, L.A.; Hargreaves, I.; Ufer, C.; Abramov, A.; Billett, E.E. Monoamine oxidase-A knockdown in human neuroblastoma cells reveals protection against mitochondrial toxins. FASEB J. 2014, 28, 218–229. [Google Scholar] [CrossRef]
- Feng, J.; Xu, J. Identification of pathogenic genes and transcription factors in glaucoma. Mol. Med. Rep. 2019, 20, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Bagès, S.; Guberovic, I.; Buschbeck, M. The MacroH2A1.1—PARP1 Axis at the Intersection Between Stress Response and Metabolism. Front. Genet. 2018, 9, 417. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, S.H.; Pierce, E.A. Parthanatos as a Cell Death Pathway Underlying Retinal Disease. Adv. Exp. Med. Biol. 2019, 1185, 323–327. [Google Scholar] [CrossRef]
- Liu, S.; Luo, W.; Wang, Y. Emerging role of PARP-1 and PARthanatos in ischemic stroke. J. Neurochem. 2022, 160, 74–87. [Google Scholar] [CrossRef] [PubMed]
- De Hoz, R.; Rojas, B.; Ramírez, A.I.; Salazar, J.J.; Gallego, B.I.; Triviño, A.; Ramírez, J.M. Retinal Macroglial Responses in Health and Disease. BioMed Res. Int. 2016, 2016, 2954721. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, T.M.; Gan, L.; Swanson, R.A. Poly(ADP-ribose) polymerase-1-induced NAD+ depletion promotes nuclear factor-κB transcriptional activity by preventing p65 de-acetylation. Biochim. Biophys. Acta 2013, 1833, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Gautel, M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat. Rev. Mol. Cell Biol. 2011, 12, 349–361. [Google Scholar] [CrossRef]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-ΚB P65 and Strategies for Therapeutic Manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef]
- Shih, R.-H.; Wang, C.-Y.; Yang, C.-M. NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef]
- Alomar, S.Y.; Barakat, B.M.; Eldosoky, M.; Atef, H.; Mohamed, A.S.; Elhawary, R.; El-Shafey, M.; Youssef, A.M.; Elkazaz, A.Y.; Gabr, A.M.; et al. Protective effect of metformin on rat diabetic retinopathy involves suppression of toll-like receptor 4/nuclear factor-k B expression and glutamate excitotoxicity. Int. Immunopharmacol. 2021, 90, 107193. [Google Scholar] [CrossRef]
- Arfuzir, N.N.N.; Agarwal, R.; Iezhitsa, I.; Agarwal, P.; Ismail, N.M. Magnesium acetyltaurate protects against endothelin-1 induced RGC loss by reducing neuroinflammation in Sprague dawley rats. Exp. Eye Res. 2020, 194, 107996. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Mishra, M. Regulation of Matrix Metalloproteinase in the Pathogenesis of Diabetic Retinopathy. Prog. Mol. Biol. Transl. Sci. 2017, 148, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.-L.; Cheng, Y.-W.; Yu, M.; Ho, J.-D.; Kuo, Y.-C.; Chiou, G.C.Y.; Chang, H.-M.; Lee, T.-H.; Hsiao, G. The fungus-derived retinoprotectant theissenolactone C improves glaucoma-like injury mediated by MMP-9 inhibition. Phytomedicine 2019, 56, 207–214. [Google Scholar] [CrossRef] [PubMed]
- De Groef, L.; Van Hove, I.; Dekeyster, E.; Stalmans, I.; Moons, L. MMPs in the Neuroretina and Optic Nerve: Modulators of Glaucoma Pathogenesis and Repair? Investig. Ophthalmol. Vis. Sci. 2014, 55, 1953–1964. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kadluczka, J.; Kuter, K.Z. Beyond the GFAP-Astrocyte Protein Markers in the Brain. Biomolecules 2021, 11, 1361. [Google Scholar] [CrossRef] [PubMed]
- Luna, G.; Keeley, P.W.; Reese, B.E.; Linberg, K.A.; Lewis, G.P.; Fisher, S.K. Astrocyte structural reactivity and plasticity in models of retinal detachment. Exp. Eye Res. 2016, 150, 4–21. [Google Scholar] [CrossRef]
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 2019, 20, 665–680. [Google Scholar] [CrossRef]
- Salminen, A.; Lehtonen, M.; Paimela, T.; Kaarniranta, K. Celastrol: Molecular targets of Thunder God Vine. Biochem. Biophys. Res. Commun. 2010, 394, 439–442. [Google Scholar] [CrossRef]
- Kim, J.Y.; Han, Y.; Lee, J.E.; Yenari, M.A. The 70-kDa heat shock protein (Hsp70) as a therapeutic target for stroke. Expert Opin. Ther. Targets 2018, 22, 191–199. [Google Scholar] [CrossRef]
- Collier, R.J.; Patel, Y.; Martin, E.A.; Dembinska, O.; Hellberg, M.; Krueger, D.S.; Kapin, M.A.; Romano, C. Agonists at the Serotonin Receptor (5-HT1A) Protect the Retina from Severe Photo-Oxidative Stress. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2118–2126. [Google Scholar] [CrossRef]
- Doggrell, S.A. The role of 5-HT on the cardiovascular and renal systems and the clinical potential of 5-HT modulation. Expert Opin. Investig. Drugs 2003, 12, 805–823. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Xu, F.; Zhang, Y.; Guan, J.; Liang, X.; Zhang, Y.; Yuan, A.; Liu, R.; Fu, J. Renal ischemia/reperfusion injury in rats is probably due to the activation of the 5-HT degradation system in proximal renal tubular epithelial cells. Life Sci. 2021, 285, 120002. [Google Scholar] [CrossRef] [PubMed]
- Tullis, B.E.; Ryals, R.C.; Coyner, A.S.; Gale, M.J.; Nicholson, A.; Ku, C.; Regis, D.; Sinha, W.; Datta, S.; Wen, Y.; et al. Sarpogrelate, a 5-HT2AReceptor Antagonist, Protects the Retina From Light-Induced Retinopathy. Investig. Opthalmol. Vis. Sci. 2015, 56, 4560–4569. [Google Scholar] [CrossRef]
- Masson, J. Serotonin in retina. Biochimie 2019, 161, 51–55. [Google Scholar] [CrossRef]
- Halberstadt, A.L.; Geyer, M.A. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology 2011, 61, 364–381. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E. N,N-dimethyltryptamine and the pineal gland: Separating fact from myth. J. Psychopharmacol. 2018, 32, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.W.; Sieghart, W. International Union of Pharmacology. LXX. Subtypes of γ-Aminobutyric AcidAReceptors: Classification on the Basis of Subunit Composition, Pharmacology, and Function. Update. Pharmacol. Rev. 2008, 60, 243–260. [Google Scholar] [CrossRef]
- Ghit, A.; Assal, D.; Al-Shami, A.S.; Hussein, D.E.E. GABAA receptors: Structure, function, pharmacology, and related disorders. J. Genet. Eng. Biotechnol. 2021, 19, 123. [Google Scholar] [CrossRef]
- Bergeron, R.; Debonnel, G. Effects of low and high doses of selective sigma ligands: Further evidence suggesting the existence of different subtypes of sigma receptors. Psychopharmacology 1997, 129, 215–224. [Google Scholar] [CrossRef]
- Bergeron, R.; De Montigny, C.; Debonnel, G. Biphasic effects of sigma ligands on the neuronal response to N-methyl-D-aspartate. Naunyn. Schmiedebergs Arch. Pharmacol. 1995, 351, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, C.G.; Greene, S.F. Sigma receptors [sigmaRs]: Biology in normal and diseased states. J. Recept. Signal Transduct. Res. 2016, 36, 327–388. [Google Scholar] [CrossRef] [PubMed]
- Fontanilla, D.; Johannessen, M.; Hajipour, A.R.; Cozzi, N.V.; Jackson, M.B.; Ruoho, A.E. The Hallucinogen N,N -Dimethyltryptamine (DMT) Is an Endogenous Sigma-1 Receptor Regulator. Science 2009, 323, 934–937. [Google Scholar] [CrossRef]
- Jonhede, S.; Petersen, A.; Zetterberg, M.; Karlsson, J.O. Acute effects of the sigma-2 receptor agonist siramesine on lysosomal and extra-lysosomal proteolytic systems in lens epithelial cells. Mol. Vis. 2010, 16, 819–827. [Google Scholar] [PubMed]
- Kargbo, R.B. Sigma-1 and Sigma-2 Receptor Modulators as Potential Therapeutics for Alzheimer’s Disease. ACS Med. Chem. Lett. 2021, 12, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Nemes, B.; Pető, K.; Németh, N.; Mester, A.; Magyar, Z.; Ghanem, S.; Sógor, V.; Tánczos, B.; Deák, A.; Kállay, M.; et al. N,N-dimethyltryptamine Prevents Renal Ischemia-Reperfusion Injury in a Rat Model. Transplant. Proc. 2019, 51, 1268–1275. [Google Scholar] [CrossRef]

| Groups | Number of Animals | Treatment | Examinations |
|---|---|---|---|
| 1. | 10 male SD rats | DMT (sc.) + harmaline (po.) (7 days) = DHT | Electroretinography + Histology + Western blot |
| 2. | 10 male SD rats | harmaline (po.) (7 days) = HT | |
| 3. | 10 male SD rats | vehicle-treated group = VT (left ligated eyes = untreated control; right non-ischemic eyes = healthy control) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szilágyi, A.; Takács, B.; Szekeres, R.; Tarjányi, V.; Bombicz, M.; Priksz, D.; Kovács, A.; Juhász, B.; Frecska, E.; Szilvássy, Z.; et al. Therapeutic Properties of Ayahuasca Components in Ischemia/Reperfusion Injury of the Eye. Biomedicines 2022, 10, 997. https://doi.org/10.3390/biomedicines10050997
Szilágyi A, Takács B, Szekeres R, Tarjányi V, Bombicz M, Priksz D, Kovács A, Juhász B, Frecska E, Szilvássy Z, et al. Therapeutic Properties of Ayahuasca Components in Ischemia/Reperfusion Injury of the Eye. Biomedicines. 2022; 10(5):997. https://doi.org/10.3390/biomedicines10050997
Chicago/Turabian StyleSzilágyi, Anna, Barbara Takács, Réka Szekeres, Vera Tarjányi, Mariann Bombicz, Dániel Priksz, Attila Kovács, Béla Juhász, Ede Frecska, Zoltán Szilvássy, and et al. 2022. "Therapeutic Properties of Ayahuasca Components in Ischemia/Reperfusion Injury of the Eye" Biomedicines 10, no. 5: 997. https://doi.org/10.3390/biomedicines10050997
APA StyleSzilágyi, A., Takács, B., Szekeres, R., Tarjányi, V., Bombicz, M., Priksz, D., Kovács, A., Juhász, B., Frecska, E., Szilvássy, Z., & Varga, B. (2022). Therapeutic Properties of Ayahuasca Components in Ischemia/Reperfusion Injury of the Eye. Biomedicines, 10(5), 997. https://doi.org/10.3390/biomedicines10050997