Urinary KIM-1 Correlates with the Subclinical Sequelae of Tubular Damage Persisting after the Apparent Functional Recovery from Intrinsic Acute Kidney Injury
Abstract
1. Introduction
2. Materials and Methods
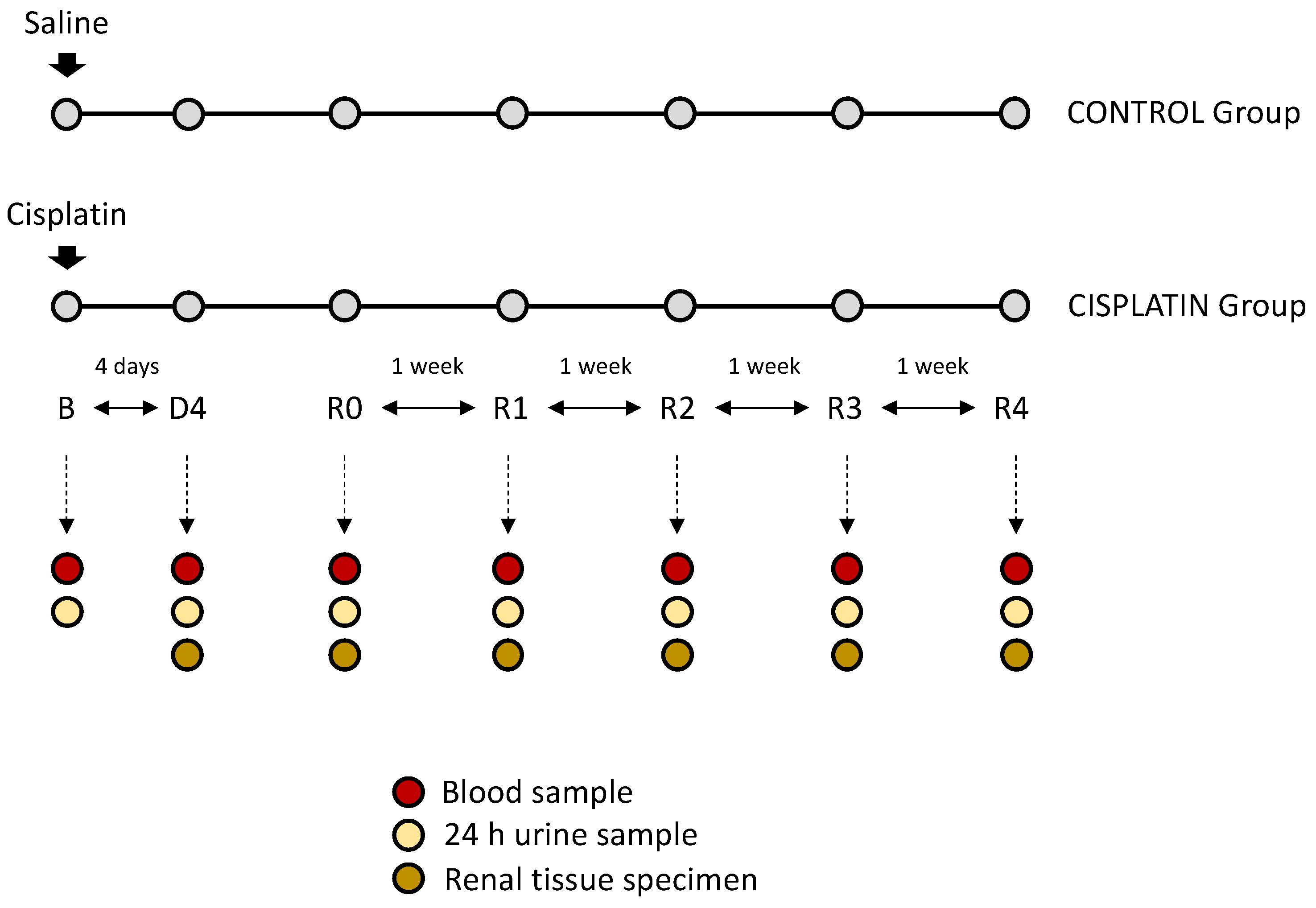
2.1. In Vivo Experimental Model
2.2. Sample Collection
2.3. Renal Function Studies
2.4. Histological Studies
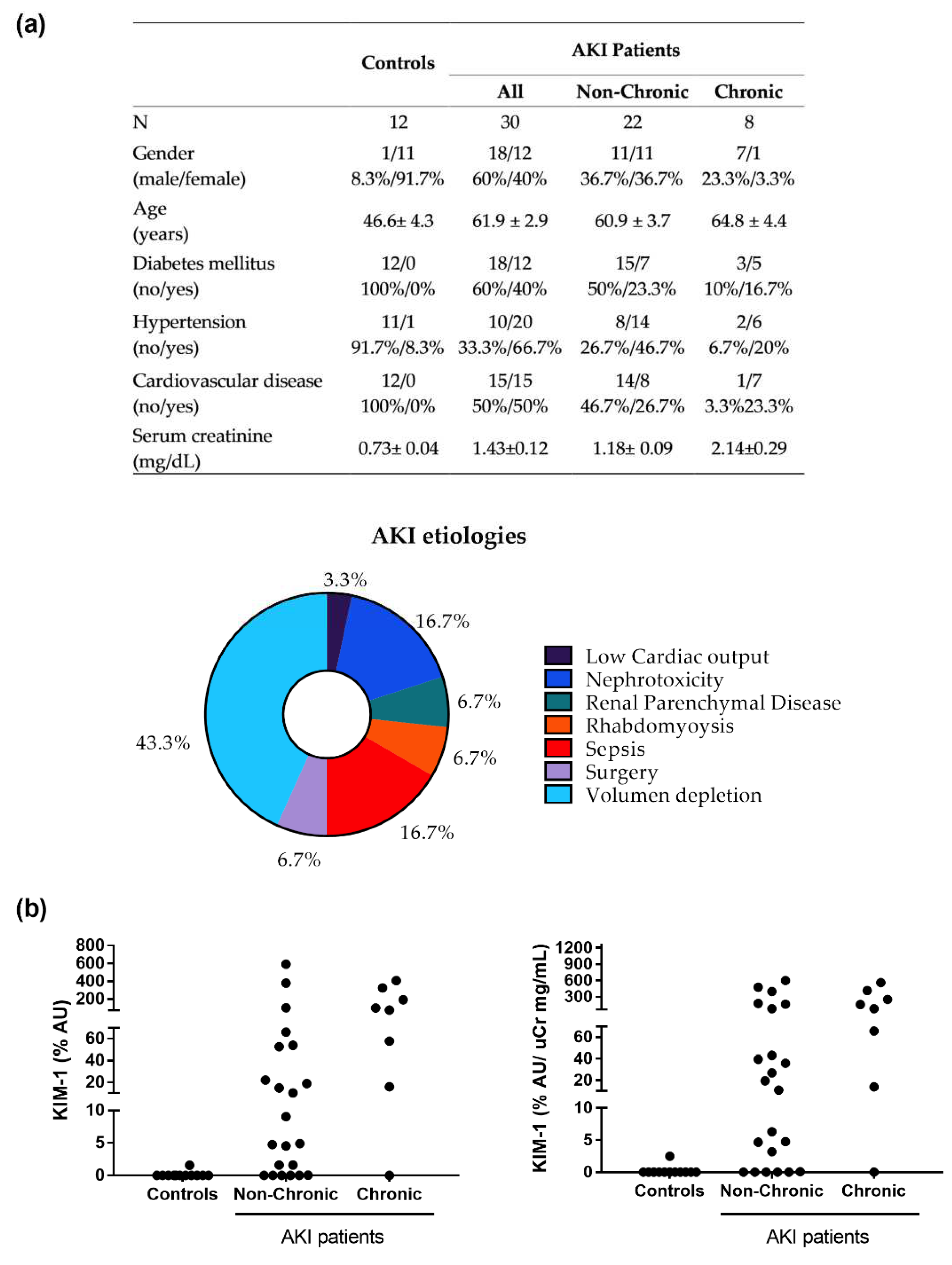
2.5. Patients and Clinical Protocol
2.6. Analysis of Urinary KIM-1 Excretion
2.7. Renal Gene Expression Analysis
2.8. Statistical Analysis
3. Results
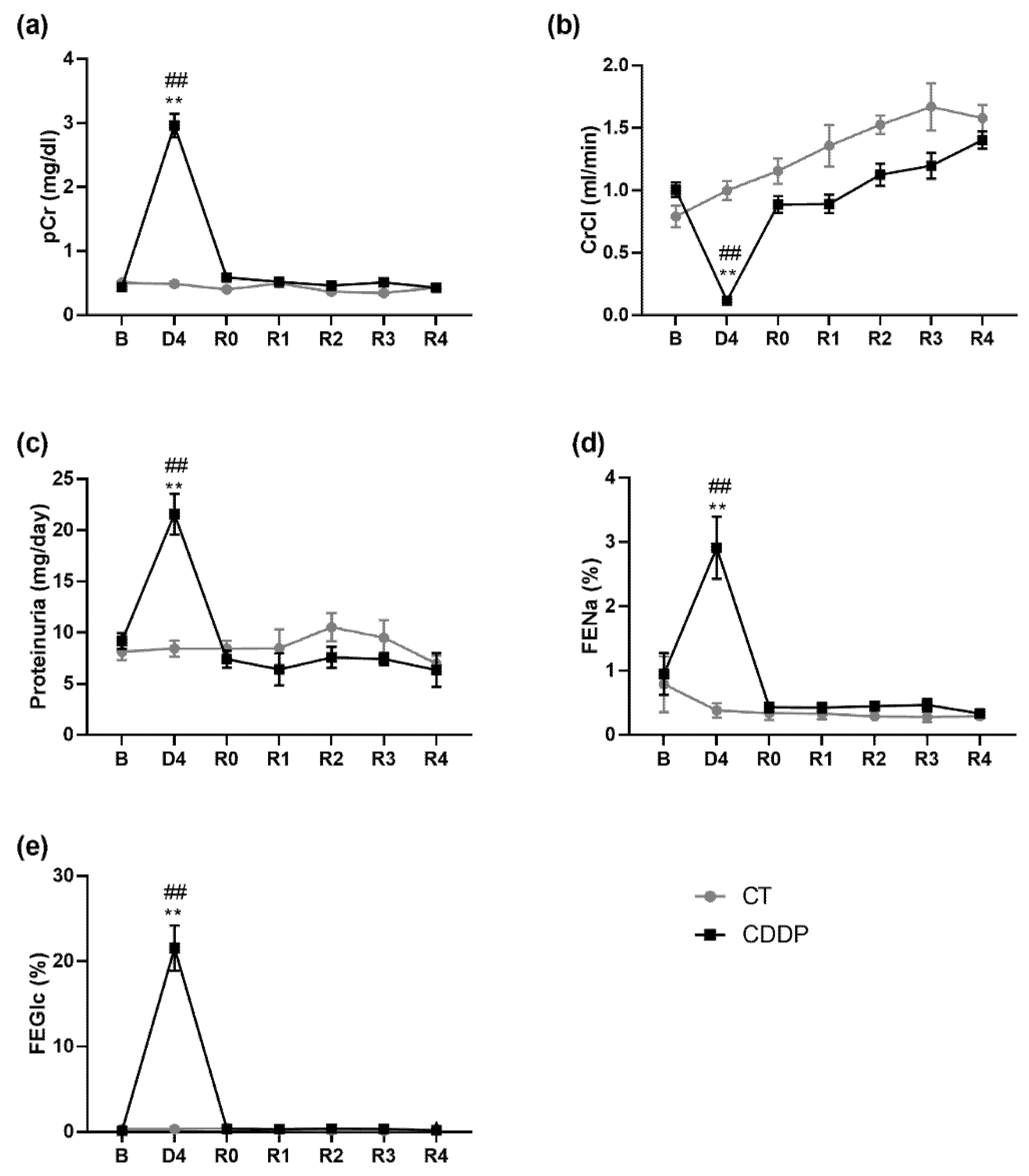
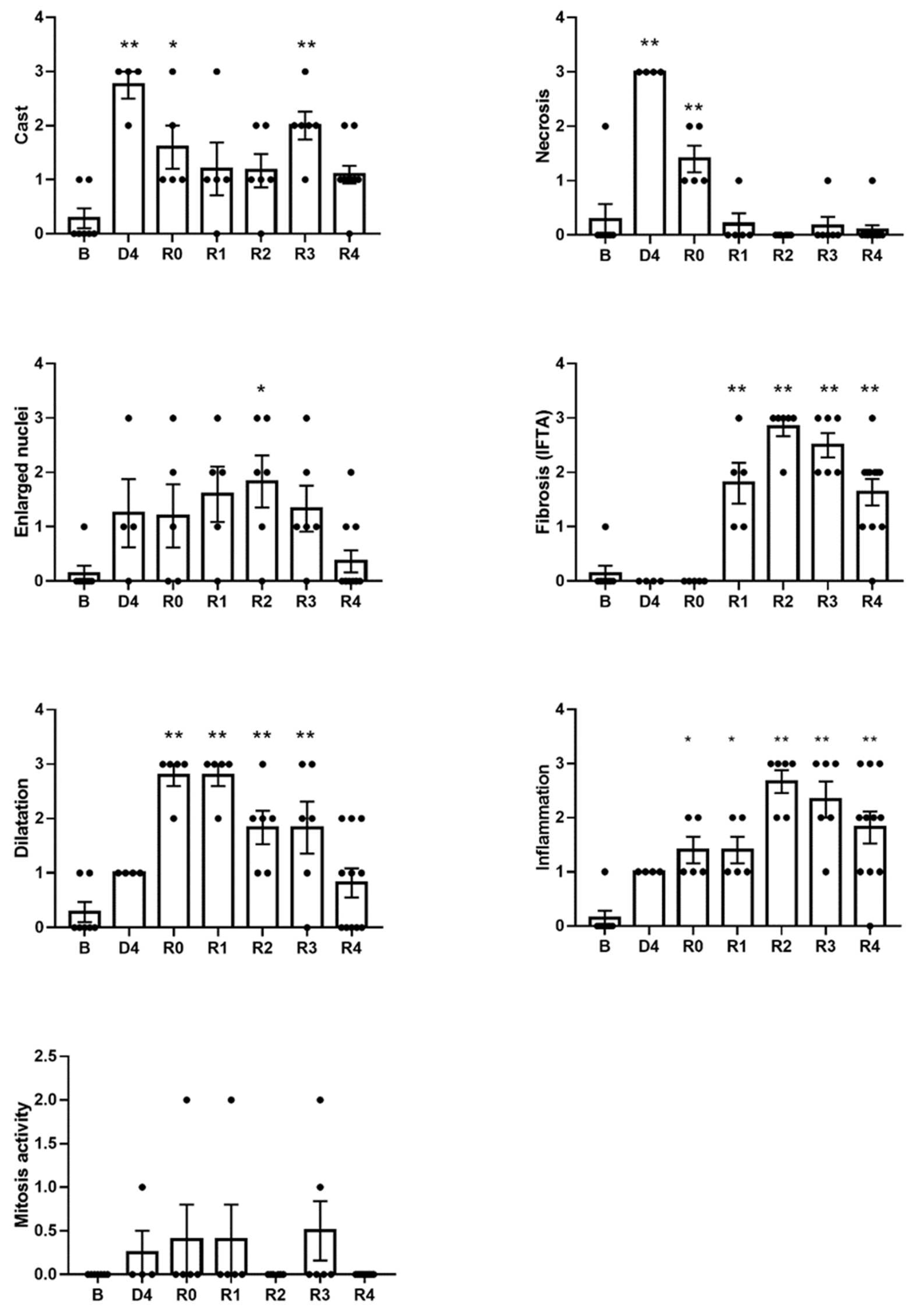
3.1. Kidneys Show Structural Sequelae That Remain after the Normalization of the Glomerular Filtration Rate and Tubular Function
3.2. KIM-1 Urinary Excretion Remains Elevated Three Weeks after the Apparent Normalization of Renal Function
3.3. Urinary KIM-1 Excretion Correlates with the Degree of Histological Damage
3.4. KIM-1 Is Elevated in the Urine of Patients Apparently Recovered from an Episode of AKI
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomas, M.E.; Blaine, C.; Dawnay, A.; Devonald, M.A.J.; Ftouh, S.; Laing, C.; Latchem, S.; Lewington, A.; Milford, D.V.; Ostermann, M. The definition of acute kidney injury and its use in practice. Kidney Int. 2015, 87, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Siew, E.D.; Davenport, A. The growth of acute kidney injury: A rising tide or just closer attention to detail? Kidney Int. 2015, 87, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Macedo, E.; Garcia-Garcia, G.; Mehta, R.L.; Rocco, M.V. International Society of Nephrology 0 by 25 Project: Lessons Learned. Ann. Nutr. Metab. 2019, 74, 45–50. [Google Scholar] [CrossRef]
- Melo, F.D.A.F.; Macedo, E.; Bezerra, A.C.F.; De Melo, W.A.L.; Mehta, R.L.; Burdmann, E.D.A.; Zanetta, D.M.T. A systematic review and meta-analysis of acute kidney injury in the intensive care units of developed and developing countries. PLoS ONE 2020, 15, e0226325. [Google Scholar] [CrossRef]
- Kellum, J.A.; Sileanu, F.E.; Bihorac, A.; Hoste, E.A.J.; Chawla, L.S. Recovery after acute kidney injury. Am. J. Respir. Crit. Care Med. 2017, 195, 784–791. [Google Scholar] [CrossRef]
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef]
- Kashani, K.; Kellum, J.A. Novel biomarkers indicating repair or progression after acute kidney injury. Curr. Opin. Nephrol. Hypertens. 2015, 24, 21–27. [Google Scholar] [CrossRef]
- Bihorac, A.; Yavas, S.; Subbiah, S.; Hobson, C.E.; Schold, J.D.; Gabrielli, A.; Layon, A.J.; Segal, M.S. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann. Surg. 2009, 249, 851–858. [Google Scholar] [CrossRef]
- Nejat, M.; Pickering, J.W.; Devarajan, P.; Bonventre, J.V.; Edelstein, C.L.; Walker, R.J.; Endre, Z.H. Some biomarkers of acute kidney injury are increased in pre-renal acute injury. Kidney Int. 2012, 81, 1254–1262. [Google Scholar] [CrossRef]
- Heung, M.; Steffick, D.E.; Zivin, K.; Gillespie, B.W.; Banerjee, T.; Hsu, C.Y.; Powe, N.R.; Pavkov, M.E.; Williams, D.E.; Saran, R.; et al. Acute Kidney Injury Recovery Pattern and Subsequent Risk of CKD: An Analysis of Veterans Health Administration Data. Am. J. Kidney Dis. 2016, 67, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Haase, M.; Kellum, J.A.; Ronco, C. Subclinical AKI—An emerging syndrome with important consequences. Nat. Rev. Nephrol. 2012, 8, 735–739. [Google Scholar] [CrossRef] [PubMed]
- De Geus, H.R.; Haase, M.; Jacob, L. The cardiac surgery-associated neutrophil gelatinase-associated lipocalin score for postoperative acute kidney injury: Does subclinical acute kidney injury matter? J. Thorac. Cardiovasc. Surg. 2017, 154, 939–940. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef]
- Pfaller, W.; Gstraunthaler, G. Nephrotoxicity testing in vitro—What we know and what we need to know. Environ. Health Perspect. 1998, 106, 559–569. [Google Scholar] [CrossRef]
- Lopez-Novoa, J.M.; Quiros, Y.; Vicente, L.; Morales, A.I.; Lopez-Hernandez, F.J. New insights into the mechanism of aminoglycoside nephrotoxicity: An integrative point of view. Kidney Int. 2011, 79, 33–45. [Google Scholar] [CrossRef]
- Blanco-Gozalo, V.; Casanova, A.G.; Sancho-Martínez, S.M.; Prieto, M.; Quiros, Y.; Morales, A.I.; Martínez-Salgado, C.; Agüeros-Blanco, C.; Benito-Hernández, A.; Ramos-Barron, M.A.; et al. Combined use of GM2AP and TCP1-eta urinary levels predicts recovery from intrinsic acute kidney injury. Sci. Rep. 2020, 10, 11599. [Google Scholar] [CrossRef]
- Bonventre, J.V. Kidney injury molecule-1: A translational journey. Trans. Am. Clin. Climatol. Assoc. 2014, 125, 293–299. [Google Scholar]
- Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A specific and sensitive biomarker of kidney injury. Scand. J. Clin. Lab. Invest. Suppl. 2009, 68, 78–83. [Google Scholar] [CrossRef]
- Ko, G.J.; Grigoryev, D.N.; Linfert, D.; Jang, H.R.; Watkins, T.; Cheadle, C.; Racusen, L.; Rabb, H. Transcriptional analysis of kidneys during repair from AKI reveals possible roles for NGAL and KIM-1 as biomarkers of AKI-to-CKD transition. Am. J. Physiol. Ren. Physiol. 2010, 298, 2009. [Google Scholar] [CrossRef]
- Ferreira, L.; Quiros, Y.; Sancho-Martínez, S.M.; García-Sánchez, O.; Raposo, C.; López-Novoa, J.M.; González-Buitrago, J.M.; López-Hernández, F.J. Urinary levels of regenerating islet-derived protein III Β and gelsolin differentiate gentamicin from cisplatin-induced acute kidney injury in rats. Kidney Int. 2011, 79, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Stokman, G.; Leemans, J.C.; Claessen, N.; Weening, J.J.; Florquin, S. Hematopoietic stem cell mobilization therapy accelerates recovery of renal function independent of stem cell contribution. J. Am. Soc. Nephrol. 2005, 16, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Perretta-Tejedor, N.; Muñoz-Félix, J.M.; Düwel, A.; Quiros-Luis, Y.; Fernández-Martín, J.L.; Morales, A.I.; López-Hernández, F.J.; López-Novoa, J.M.; Martínez-Salgado, C. Cardiotrophin-1 opposes renal fibrosis in mice: Potential prevention of chronic kidney disease. Acta Physiol. 2019, 226. [Google Scholar] [CrossRef] [PubMed]
- Karmakova, T.A.; Sergeeva, N.S.; Kanukoev, K.Y.; Alekseev, B.Y.; Kaprin, A.D. Kidney Injury Molecule 1 (KIM-1): A Multifunctional Glycoprotein and Biological Marker (Review). Sovrem. Tekhnologii Med. 2021, 13, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, T.; Bonventre, J.V.; Bailly, V.; Wei, H.; Hession, C.A.; Cate, R.L.; Sanicola, M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 1998, 273, 4135–4142. [Google Scholar] [CrossRef]
- Ichimura, T.; Hung, C.C.; Yang, S.A.; Stevens, J.L.; Bonventre, J.V. Kidney injury molecule-1: A tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am. J. Physiol. Ren. Physiol. 2004, 286, 2002. [Google Scholar] [CrossRef]
- Zhang, P.L.; Rothblum, L.I.; Han, W.K.; Blasick, T.M.; Potdar, S.; Bonventre, J.V. Kidney injury molecule-1 expression in transplant biopsies is a sensitive measure of cell injury. Kidney Int. 2008, 73, 608–614. [Google Scholar] [CrossRef]
- Bonventre, J.V. Kidney injury molecule-1 (KIM-1): A urinary biomarker and much more. Nephrol. Dial. Transpl. 2009, 24, 3265–3268. [Google Scholar] [CrossRef]
- Ichimura, T.; Asseldonk, E.J.P.V.; Humphreys, B.D.; Gunaratnam, L.; Duffield, J.S.; Bonventre, J.V. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J. Clin. Investig. 2008, 118, 1657–1668. [Google Scholar] [CrossRef]
- Brooks, C.R.; Yeung, M.Y.; Brooks, Y.S.; Chen, H.; Ichimura, T.; Henderson, J.M.; Bonventre, J. V KIM-1-/TIM-1-mediated phagocytosis links ATG5-/ULK1-dependent clearance of apoptotic cells to antigen presentation. EMBO J. 2015, 34, 2441–2464. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, C.X. Kidney injury molecule-1 (KIM-1) mediates renal epithelial cell repair via ERK MAPK signaling pathway. Mol. Cell. Biochem. 2016, 416, 109–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- George, B.; Joy, M.S.; Aleksunes, L.M. Urinary protein biomarkers of kidney injury in patients receiving cisplatin chemotherapy. Exp. Biol. Med. 2018, 243, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Griffin, B.R.; Faubel, S.; Edelstein, C.L. Biomarkers of Drug-Induced Kidney Toxicity. Ther. Drug Monit. 2019, 41, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Van Timmeren, M.M.; van den Heuvel, M.C.; Bailly, V.; Bakker, S.J.L.; van Goor, H.; Stegeman, C.A. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J. Pathol. 2007, 212, 209–217. [Google Scholar] [CrossRef]
- Cai, J.; Jiao, X.; Luo, W.; Chen, J.; Xu, X.; Fang, Y.; Ding, X.; Yu, X. Kidney injury molecule-1 expression predicts structural damage and outcome in histological acute tubular injury. Ren. Fail. 2019, 41, 80–87. [Google Scholar] [CrossRef]



| KIM1 (µg/Day) | KIM1 (ng/mg CrU) | |
|---|---|---|
| Dilatation | 0.4957 (0.001) | 0.6286 (<0.0001) |
| Cast | 0.2209 (0.1651) | 0.4809 (0.0022) |
| Enlarged nuclei | 0.3694 (0.0175) | 0.4750 (0.0026) |
| Mitosis activity | 0.2678 (0.0993) | 0.3094 (0.0587) |
| Inflammation | −0.0904 (0.5741) | −0.0330 (0.8441) |
| Necrosis | 0.5282 (0.0004) | 0.6403 (<0.0001) |
| Fibrosis (IFTA) | −0.3031 (0.054) | −0.2099 (0.206) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuesta, C.; Fuentes-Calvo, I.; Sancho-Martinez, S.M.; Valentijn, F.A.; Düwel, A.; Hidalgo-Thomas, O.A.; Agüeros-Blanco, C.; Benito-Hernández, A.; Ramos-Barron, M.A.; Gómez-Alamillo, C.; et al. Urinary KIM-1 Correlates with the Subclinical Sequelae of Tubular Damage Persisting after the Apparent Functional Recovery from Intrinsic Acute Kidney Injury. Biomedicines 2022, 10, 1106. https://doi.org/10.3390/biomedicines10051106
Cuesta C, Fuentes-Calvo I, Sancho-Martinez SM, Valentijn FA, Düwel A, Hidalgo-Thomas OA, Agüeros-Blanco C, Benito-Hernández A, Ramos-Barron MA, Gómez-Alamillo C, et al. Urinary KIM-1 Correlates with the Subclinical Sequelae of Tubular Damage Persisting after the Apparent Functional Recovery from Intrinsic Acute Kidney Injury. Biomedicines. 2022; 10(5):1106. https://doi.org/10.3390/biomedicines10051106
Chicago/Turabian StyleCuesta, Cristina, Isabel Fuentes-Calvo, Sandra M. Sancho-Martinez, Floris A. Valentijn, Annette Düwel, Omar A. Hidalgo-Thomas, Consuelo Agüeros-Blanco, Adalberto Benito-Hernández, María A. Ramos-Barron, Carlos Gómez-Alamillo, and et al. 2022. "Urinary KIM-1 Correlates with the Subclinical Sequelae of Tubular Damage Persisting after the Apparent Functional Recovery from Intrinsic Acute Kidney Injury" Biomedicines 10, no. 5: 1106. https://doi.org/10.3390/biomedicines10051106
APA StyleCuesta, C., Fuentes-Calvo, I., Sancho-Martinez, S. M., Valentijn, F. A., Düwel, A., Hidalgo-Thomas, O. A., Agüeros-Blanco, C., Benito-Hernández, A., Ramos-Barron, M. A., Gómez-Alamillo, C., Arias, M., Nguyen, T. Q., Goldschmeding, R., Martínez-Salgado, C., & López-Hernández, F. J. (2022). Urinary KIM-1 Correlates with the Subclinical Sequelae of Tubular Damage Persisting after the Apparent Functional Recovery from Intrinsic Acute Kidney Injury. Biomedicines, 10(5), 1106. https://doi.org/10.3390/biomedicines10051106






