A Small Molecule Promoting Neural Differentiation Suppresses Cancer Stem Cells in Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Sphere Forming Assay
2.3. Screen for Drugs That Promote Neuronal Differentiation
2.4. High-Contents Screening
2.5. Immunoblotting Assay
2.6. Immunohistochemistry and Immunocytochemistry
2.7. Fluorescence-Activated Cell Sorting Analysis
2.8. Animal Studies
2.9. Antibodies and Reagents
2.10. Statistical Analysis
3. Results
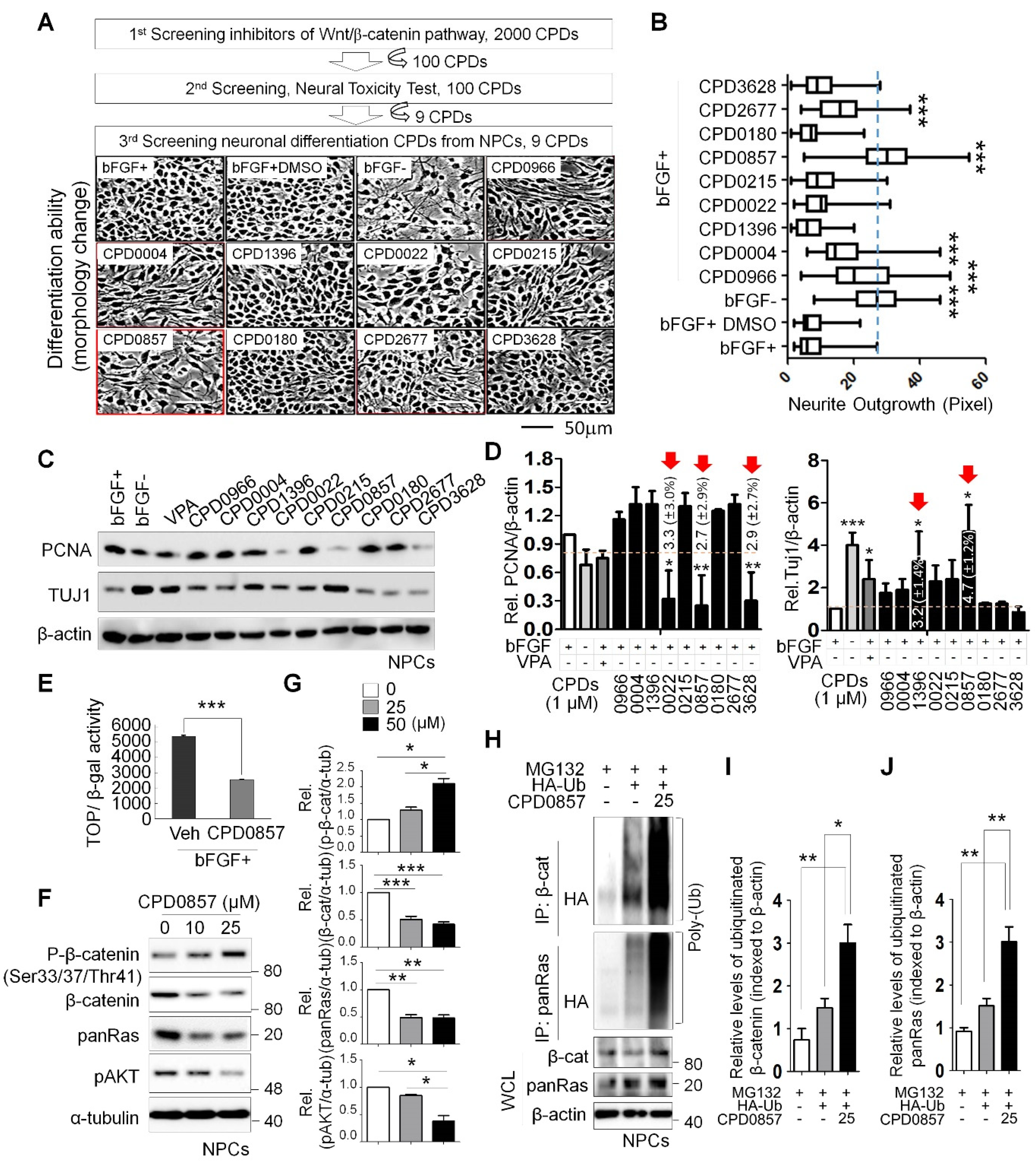
3.1. Neuronal Differentiation Activity of Various Compounds following Inhibition of Wnt/β-Catenin Signaling
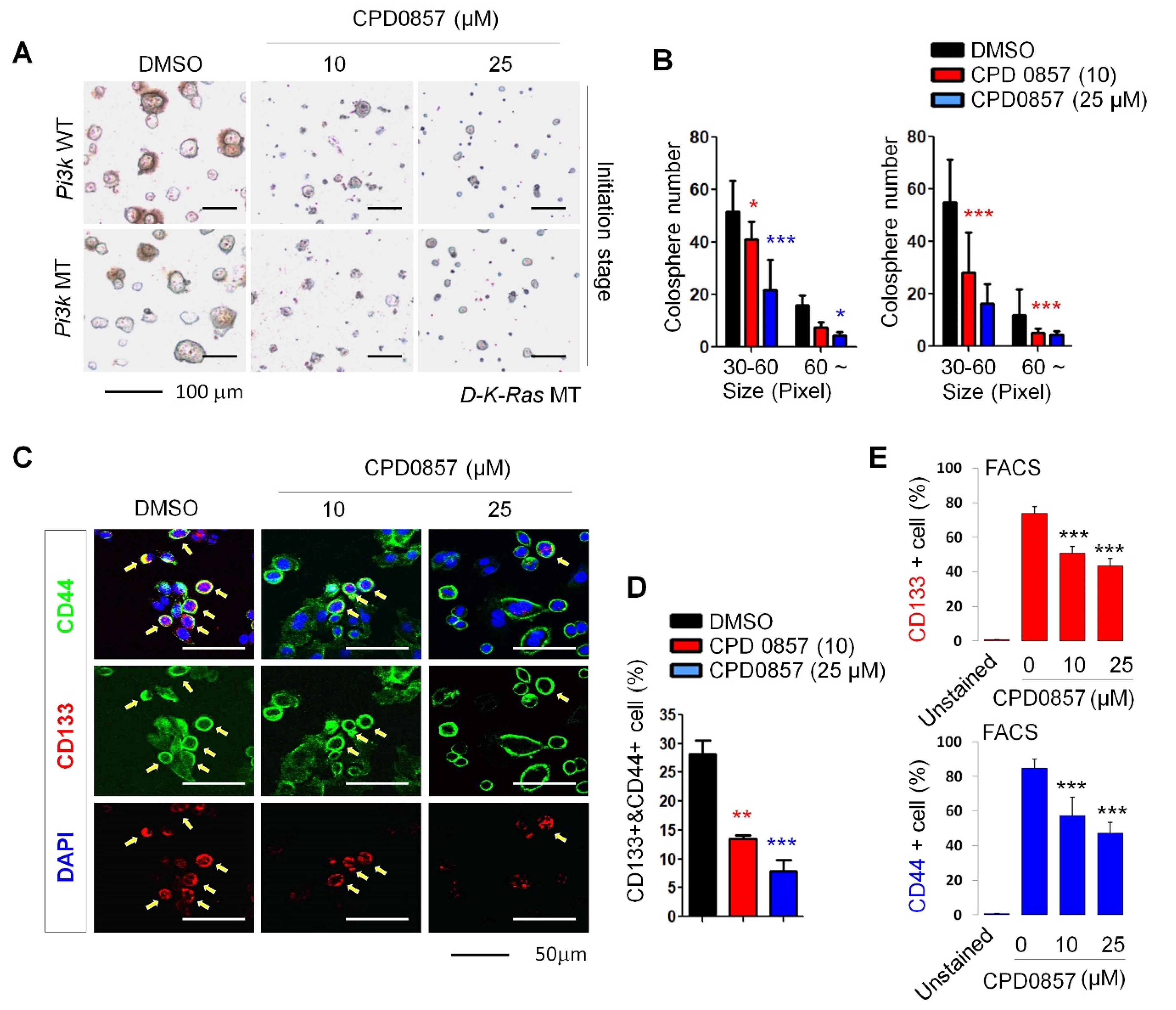
3.2. CPD0857 Treatment Alters NPC Proliferation and Differentiation
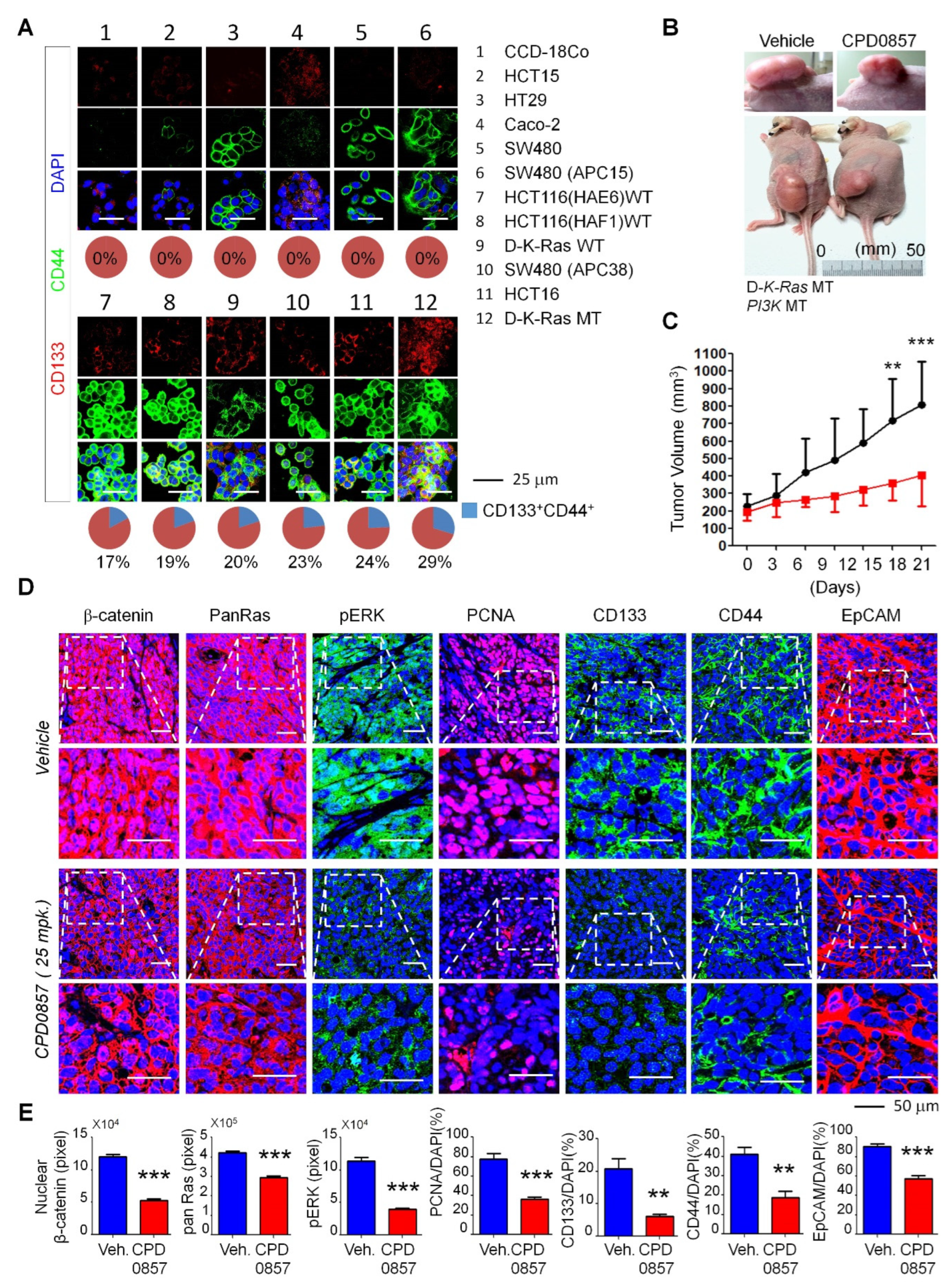
3.3. Effects of CPD0857 on CRC Cells Carrying K-Ras, APC and PI3K Mutations
3.4. CPD0857 Inhibits Tumorigenesis in CSCs Harboring K-Ras, APC and PI3K Mutations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Barker, N.; Ridgway, R.A.; van Es, J.H.; van de Wetering, M.; Begthel, H.; van den Born, M.; Danenber, E.; Clarke, A.E.; Sanson, O.J.; Clevers, H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009, 457, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Merlos-Suarez, A.; Barriga, F.M.; Jung, P.; Iglesias, M.; Cespedes, M.V.; Rossell, D.; Sevillano, M.; Hernando-Momblona, X.; da Silva-Diz, V.; Muñoz, P.; et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell 2011, 8, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Zeuner, A.; De Maria, R. Not so lonely at the top for cancer stem cells. Cell Stem Cell 2011, 9, 289–290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ro, E.J.; Cho, Y.H.; Jeong, W.J.; Park, J.C.; Min, D.S.; Choi, K.-Y. WDR76 degrades RAS and suppresses cancer stem cell activation in colorectal cancer. Cell Commun. Signal. 2019, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.M.L.; Lee, T.K.W. Cancer stem cells: Emerging key players in immune evasion of cancers. Front. Cell. Dev. Biol. 2021, 9, 692940. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.B.; Chaffer, C.L.; Weinberg, R.A. Cancer stem cells: Mirage or reality? Nat. Med. 2009, 15, 1010–1012. [Google Scholar] [CrossRef] [PubMed]
- Pattabiraman, D.R.; Weinberg, R.A. Tackling the cancer stem cells—What challenges do they pose? Nat. Rev. Drug Discov. 2014, 13, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ouyang, G. Periostin: A bridge between cancer stem cells and their metastatic niche. Cell Stem Cell 2012, 10, 111–112. [Google Scholar] [CrossRef]
- Flemming, A. Cancer stem cells: Targeting the root of cancer relapse. Nat. Rev. Drug Discov. 2015, 14, 165. [Google Scholar] [CrossRef] [PubMed]
- Ayob, A.Z.; Ramasamy, T.S. Cancer stem cells as key drivers of tumour progression. J. Biomed. Sci. 2018, 25, 20. [Google Scholar] [CrossRef] [PubMed]
- Plaks, V.; Kong, N.; Werb, Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Vaiopoulos, A.G.; Kostakis, I.D.; Koutsilieris, M.; Papavassiliou, A.G. Colorectal cancer stem cells. Stem Cells 2012, 30, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Gjorevski, N.; Ordonez-Moran, P. Intestinal stem cell niche insights gathered from both in vivo and novel in vitro models. Stem Cells Int. 2017, 2017, 8387297. [Google Scholar] [CrossRef]
- Ong, B.A.; Vega, K.J.; Houchen, C.W. Intestinal stem cells and the colorectal cancer microenvironment. World J. Gastroenterol. 2014, 20, 1898–1909. [Google Scholar] [CrossRef]
- Fevr, T.; Robine, S.; Louvard, D.; Huelsken, J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol. Cell. Biol. 2007, 27, 7551–7559. [Google Scholar] [CrossRef]
- Kinzler, K.W.; Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 1996, 87, 159–170. [Google Scholar] [CrossRef]
- Zhang, L.; Shay, J.W. Multiple roles of APC and its therapeutic implications in colorectal cancer. J. Natl. Cancer Inst. 2017, 2017, 109. [Google Scholar] [CrossRef]
- Solberg, N.T.; Melheim, M.; Strand, M.F.; Olsen, P.A.; Krauss, S. MEK Inhibition Induces Canonical WNT Signaling through YAP in KRAS Mutated HCT-15 Cells, and a Cancer Preventive FOXO3/FOXM1 Ratio in Combination with TNKS Inhibition. Cancers 2019, 11, 164. [Google Scholar] [CrossRef]
- Oliveira, L.F.S.; Predes, D.; Borges, H.L.; Abreu, J.G. Therapeutic potential of naturally occurring small molecules to target the Wnt/beta-Catenin signaling pathway in colorectal cancer. Cancers 2022, 14, 403. [Google Scholar] [CrossRef]
- Hwang, J.H.; Yoon, J.; Cho, Y.H.; Cha, P.H.; Park, J.C.; Choi, K.-Y. A mutant KRAS-induced factor REG4 promotes cancer stem cell properties via Wnt/beta-catenin signaling. Int. J. Cancer 2020, 146, 2877–2890. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Bommer, G.T.; Zhao, J.; Green, M.; Sands, E.; Zhai, Y.; Brown, K.; Burberry, A.; Cho, K.; Fearon, E.R. Mutant KRAS promotes hyperplasia and alters differentiation in the colon epithelium but does not expand the presumptive stem cell pool. Gastroenterology 2011, 141, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Snippert, H.J.; Schepers, A.G.; van Es, J.H.; Simons, B.D.; Clevers, H. Biased competition between Lgr5 intestinal stem cells driven by oncogenic mutation induces clonal expansion. EMBO Rep. 2014, 15, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Moon, B.S.; Cho, Y.H.; Jeong, W.J.; Choi, K.Y. Response. J. Natl. Cancer Inst. 2014, 106, dju197. [Google Scholar] [CrossRef]
- Moon, B.S.; Jeong, W.J.; Park, J.; Kim, T.I.; Min, D.S.; Choi, K.-Y. Role of oncogenic K-Ras in cancer stem cell activation by aberrant Wnt/beta-catenin signaling. J. Natl. Cancer Inst. 2014, 106, djt373. [Google Scholar] [CrossRef] [PubMed]
- Jobling, P.; Pundavela, J.; Oliveira, S.M.; Roselli, S.; Walker, M.M.; Hondermarck, H. Nerve-Cancer Cell Cross-talk: A Novel Promoter of Tumor Progression. Cancer Res. 2015, 75, 1777–1781. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Fan, C.; Shangguan, W.; Liu, Y.; Li, Y.; Shang, Y.; Yin, D.; Zhang, S.; Huang, Q.; Li, X.; et al. Neurons generated from carcinoma stem cells support cancer progression. Signal. Transduct. Target Ther. 2017, 2, 16036. [Google Scholar] [CrossRef]
- Pundavela, J.; Demont, Y.; Jobling, P.; Lincz, L.F.; Roselli, S.; Thorne, R.F.; Bond, D.; Bradshaw, R.A.; Walker, M.M.; Hondermarck, H. ProNGF correlates with Gleason score and is a potential driver of nerve infiltration in prostate cancer. Am. J. Pathol. 2014, 184, 3156–3162. [Google Scholar] [CrossRef]
- Dobrenis, K.; Gauthier, L.R.; Barroca, V.; Magnon, C. Granulocyte colony-stimulating factor off-target effect on nerve outgrowth promotes prostate cancer development. Int. J. Cancer 2015, 136, 982–988. [Google Scholar] [CrossRef]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef]
- Lange, C.; Mix, E.; Rateitschak, K.; Rolfs, A. Wnt signal pathways and neural stem cell differentiation. Neurodegener. Dis. 2006, 3, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Cho, H.; Moon, B.S. Small Molecule Destabilizer of beta-Catenin and Ras Proteins Antagonizes Growth of K-Ras Mutation-Driven Colorectal Cancers Resistant to EGFR Inhibitors. Target. Oncol. 2020, 15, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Cha, P.H.; Cho, Y.H.; Lee, S.K.; Lee, J.; Jeong, W.J.; Moon, B.S.; Yun, J.H.; Yang, J.S.; Choi, S.; Yoon, J.; et al. Small-molecule binding of the axin RGS domain promotes beta-catenin and Ras degradation. Nat. Chem. Biol. 2016, 12, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Namiki, J.; Shibata, S.; Mastuzaki, Y.; Okano, H. The neural stem/progenitor cell marker nestin is expressed in proliferative endothelial cells, but not in mature vasculature. J. Histochem. Cytochem. 2010, 58, 721–730. [Google Scholar] [CrossRef]
- Baccelli, I.; Trumpp, A. The evolving concept of cancer and metastasis stem cells. J. Cell Biol. 2012, 198, 281–293. [Google Scholar] [CrossRef]
- Janssen, K.P.; Alberici, P.; Fsihi, H.; Gaspar, C.; Breukel, C.; Franken, P.; Rosty, C.; Abal, M.; El Marjou, F.; Smits, R.; et al. APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology 2006, 131, 1096–1109. [Google Scholar] [CrossRef]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Lee, S.K.; Cho, Y.H.; Cha, P.H.; Yoon, J.S.; Ro, E.J.; Jeong, W.J.; Park, J.; Kim, H.; Kim, T.I.; Min, D.S.; et al. A small molecule approach to degrade RAS with EGFR repression is a potential therapy for KRAS mutation-driven colorectal cancer resistance to cetuximab. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef]
- Yoon, J.; Koo, K.H.; Choi, K.Y. MEK1/2 inhibitors AS703026 and AZD6244 may be potential therapies for KRAS mutated colorectal cancer that is resistant to EGFR monoclonal antibody therapy. Cancer Res. 2011, 71, 445–453. [Google Scholar] [CrossRef]
- Liebl, F.; Demir, I.E.; Rosenberg, R.; Boldis, A.; Yildiz, E.; Kujundzic, K.; Kehl, T.; Dischl, D.; Schuster, T.; Maak, M.; et al. The severity of neural invasion is associated with shortened survival in colon cancer. Clin. Cancer Res. 2013, 19, 50–61. [Google Scholar] [CrossRef]
- Xiao, Z.; Kong, Y.; Yang, S.; Li, M.; Wen, J.; Li, L. Upregulation of Flk-1 by bFGF via the ERK pathway is essential for VEGF-mediated promotion of neural stem cell proliferation. Cell Res. 2007, 17, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.A.; Yoon, J.Y.; Moon, B.S.; Yang, D.H.; Kim, H.Y.; Lee, S.H.; Bryja, V.; Arenas, E.; Choi, K.Y. Valproic acid induces differentiation and inhibition of proliferation in neural progenitor cells via the beta-catenin-Ras-ERK-p21Cip/WAF1 pathway. BMC Cell Biol. 2008, 9, 66. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.K.; Kwak, I.-S.; Yoon, S.-B.; Cho, H.; Moon, B.-S. A Small Molecule Promoting Neural Differentiation Suppresses Cancer Stem Cells in Colorectal Cancer. Biomedicines 2022, 10, 859. https://doi.org/10.3390/biomedicines10040859
Choi JK, Kwak I-S, Yoon S-B, Cho H, Moon B-S. A Small Molecule Promoting Neural Differentiation Suppresses Cancer Stem Cells in Colorectal Cancer. Biomedicines. 2022; 10(4):859. https://doi.org/10.3390/biomedicines10040859
Chicago/Turabian StyleChoi, Jung Kyu, Ihn-Sil Kwak, Sae-Bom Yoon, Heeyeong Cho, and Byoung-San Moon. 2022. "A Small Molecule Promoting Neural Differentiation Suppresses Cancer Stem Cells in Colorectal Cancer" Biomedicines 10, no. 4: 859. https://doi.org/10.3390/biomedicines10040859
APA StyleChoi, J. K., Kwak, I.-S., Yoon, S.-B., Cho, H., & Moon, B.-S. (2022). A Small Molecule Promoting Neural Differentiation Suppresses Cancer Stem Cells in Colorectal Cancer. Biomedicines, 10(4), 859. https://doi.org/10.3390/biomedicines10040859






