Abstract
In December 2019, the first case of COVID-19 was reported and since then several groups have already published that the virus can be present in the testis. To study the influence of SARS-CoV-2 which cause a dysregulation of the androgen receptor (AR) level, thereby leading to fertility problems and inducing germ cell testicular changes in patients after the infection. Formalin-Fixed-Paraffin-Embedded (FFPE) testicular samples from patients who died with or as a result of COVID-19 (n = 32) with controls (n = 6), inflammatory changes (n = 9), seminoma with/without metastasis (n = 11) compared with healthy biopsy samples (n = 3) were analyzed and compared via qRT-PCR for the expression of miR-371a-3p. An immunohistochemical analysis (IHC) and ELISA were performed in order to highlight the miR-371a-3p targeting the AR. Serum samples of patients with mild or severe COVID-19 symptoms (n = 34) were analyzed for miR-371a-3p expression. In 70% of the analyzed postmortem testicular tissue samples, a significant upregulation of the miR-371a-3p was detected, and 75% of the samples showed a reduced spermatogenesis. In serum samples, the upregulation of the miR-371a-3p was also detectable. The upregulation of the miR-371a-3p is responsible for the downregulation of the AR in SARS-CoV-2-positive patients, resulting in decreased spermatogenesis. Since the dysregulation of the AR is associated with infertility, further studies have to confirm if the identified dysregulation is regressive after a declining infection.
1. Introduction
In December 2019, the first case of the new coronavirus, called SARS-CoV-2 was described in Wuhan, China [1,2] and subsequently spread all over the world; the disease caused by SARS-CoV-2 is called COVID-19. The most common symptoms of SARS-CoV-2 infection include fever, pneumonia, shortness of breath and cough. From its initial presentation as a respiratory tract infection, it can affect multiple other internal organs and the brain or eyes [3]. The involvement of multiple organs frequently results in hospitalization and intensive care unit (ICU) admittance, which is often associated with poor outcome in patients [3]. Furthermore, SARS-CoV-2 can infect healthy people as well as those with pre-existing medical conditions, e.g., diabetes and obesity [4]. The risk of infection and mortality also increases with higher age and male sex [4,5]. Since the first documentation of the virus, several mutations have developed with different levels of infectiousness [6].
Regarding the urogenital organs, it is discussed that the testes can act as a reservoir for viruses, and some of these viral infections are associated with the development of testicular cancer [7]. It is known that the entry point for SARS-CoV-2 is the angiotensin converting enzyme 2 (ACE2). In the testis, a high expression level of ACE2 is detected in Leydig and Sertoli cells [7,8].
Seminomas are germ cell testicular cancers (GCTC). The average age for developing GCTC is between 20 to 40 years [9]. The epigenetic predisposition for the development of testicular cancer can be traced back to the first trimester of intrauterine life [10]. Nevertheless, the development of testicular cancer depends on several different environmental factors [11,12]. Recently, Dieckmann et al. published that in the serum of GCTC patients, a significant increase in the microRNA (miR) 371a-3p can be detected [13]. This result led to the incorporation of this specific miR as a diagnostic marker and care for GCTC in German guidelines. However, an upregulation of this miR is also described in other cancer entities [14] and therefore it can be assumed that the miR acts as an additional risk factor for the development of cancer. Since the connection of testicular tissue with the miR-371a-3p is established and since miRNAs can be influenced by infections, we investigated whether SARS-CoV-2 can also influence the expression pattern of the miR-371a-3p [15].
MicroRNAs (miRs) are small non-coding RNAs which either bind to the 3′ UTR of target messenger RNAs (mRNA), leading to a decrease in translation, destabilization and faster degradation or transporting the target mRNAs into P-bodies for storing [16]. It is known that the miR-371a-3p binds to the 3′ UTR of the androgen receptor (AR), leading to decreased protein levels [17]. A reduced AR level is frequently associated with infertility and testicular cancer development [18].
We hypothesized that COVID-19 could be a possible trigger for the development of infertility and therefore investigated if an associated increase in miR-371a-3p levels exists, resulting in the downregulation of the AR. We analyzed testicular formalin fixed paraffinized embedded (FFPE) tissue sections from patients dying with or as a result of SARS-CoV-2 infection and compared them with seminoma tissues, tissue from patients dying in the ICU with or without extracorporeal membrane oxygenation (ECMO) and biopsied inflamed testicular tissue (Table 1 for detailed information). Testicular tissue samples from autopsy patients (for detailed information see Table 2) and testicular tissue samples from patients without inflammatory history or cancer (Table 2) were used as further controls. We tried to distinguish whether the miR-371a-3p up- and AR downregulation is specific to COVID-19 infection or if there is a general reaction pattern in inflammation and/or intensive care therapy. All of the correlating control patients were of the same age so that age-related dysregulations of spermatogenesis could be excluded.

Table 1.
Concluding the used samples and correlating controls.

Table 2.
Patient characteristic. Abbreviations: n.d. = not determined, neg = negative, IC = internal control, GCNIS: germ cell neoplasia in-situ, nl= normal, (+) = slightly positive, + = positive.
Furthermore, we evaluated the levels of miR-371a-3p serum samples of COVID-19 patients as a possible future biomarker for late onset complications.
2. Materials and Methods
2.1. Testicular Histological Changes
We analyzed testicular tissue fixed in 4% buffered formalin (Sigma-Aldrich, Taufkirchen, Germany) obtained either by surgery or at autopsy and grouped them into the following five categories: patients who died with a positive SARS-CoV-2 qPCR test (n = 32); patients without SARS-CoV-2 infection but in need of critical care at the time of death with or without ECMO (n = 4); patients with non-SARS-CoV-2 dependent inflammation, such as orchitis, who underwent orchiectomy (n = 9); patients who died from different causes without need of critical care (n = 6), and from surgically resected testicular tissue for causes other than inflammation or cancer (n = 3). Patients with seminoma either with metastasis (n = 5) or without (n = 6) served as controls for miR-371a-3p levels.
2.2. FFPE Tissue Samples
Testicular formalin fixed paraffin embedded (FFPE) tissue sections from patients who died as a result of COVID-19 (n = 32) were compared with testicular autopsy tissue from patients who received intensive care with/without ECMO (n = 4) and surgically resected inflamed testicular tissue (n = 9). We chose testicular tissue samples from autopsy patients (average 68.5 years, n = 6) as the control group for COVID-19 infected patients. Surgically resected testicular tissue samples from patients without a history of inflammation or cancer (n = 3) were included in the seminoma control group, with an average age 37.5 years consisted of seminoma tissues, either with (n = 5) or without metastasis (n = 6). (For further explanation please see Table 1). In our COVID-19-collective, no orchitis was noted. The COVID-19 samples were fixed for up to 10 days in 4% buffered formalin prior to the embedding procedure according to a safety protocol used for these specific autopsies. Other tumor entities were excluded in this study. Patients’ agreement was obtained (BioMASOTA, University Hospital of Cologne, file reference 12–163, informed consent or coroner approved). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
2.3. Serum Samples
Serum samples were collected from patients with mild or severe COVID-19 symptoms (female and male). All patients signed the BioMASOTA protocol. The average age of females with mild symptoms was 67.12 ± 17.34 (n = 10), and with severe symptoms 59.16 ± 5.83 (n = 6), males with mild symptoms 62.1 ± 6.5 years (n = 11) and with severe symptoms 71.4 ± 8.72 years (n = 7). From 250 µL serum, miRNA and mRNA was isolated according to Qiagen miRNA or mRNA protocol (Qiagen, Hilden, Germany).
2.4. Light Microscopy and Immunohistochemistry of FFPE Tissues
The morphology of the FFPE tissues was examined using 1 µm sections stained with routine H&E. Spermiogenesis was examined and classified according to Sigg et al. [19,20]. Further tissue sections (3 µm thick) were immunohistochemically stained for the diagnostically used androgen receptor (AR, monoclonal mouse anti-human androgen receptor, clone AR441, Code M3562, Agilent Dako, Santa Clara, CA, USA) using a BOND immunostainer at a dilution of 1: 400 and citrate epitope retrieval. Semi quantitative grading of AR immunoreactivity was minimal (+), medium + and strong ++.
Normal testis samples as well as intratesticular structures such as rete testis or epididymis served as (internal) positive controls (int con). Negative controls for the AR receptor IHC are not a part of a diagnostically stained specimen since the antibody was extensively validated before use in the diagnostics panel (golden standard of a pathological institute).
2.5. Double Labelling of AR Immunohistochemistry of FFPE Tissues and by miR-371a-3p In Situ PCR
AR staining was performed as previously described. After the final IHC step, slides were stored in tap water and immediately in situ PCR was performed as described in Nuovo et al. [21], with slight differences as follows: For the digestion of the tissue was performed with Proteinase K was used. The RT-PCR reaction was done with a one-step kit (QPO15, Biocat, Heidelberg, Germany). The PCR conditions were as recommended by the company. After the PCR step, slides were washed, counterstained and mounted with DAPI medium.
2.6. Immunofluorescence of ACE2 Protein
The 3 μm thick FFPE sections were deparaffinized in 1 × 10 min at room temperature (RT) in xylene (Sigma-Aldrich, Taufkirchen, Germany), followed by 1 × 5 min in 100% ethanol (Sigma-Aldrich, Taufkirchen, Germany) and 1 min in 70% ethanol and rinsed with distilled water at RT. The specimens were digested with Proteinase K (Qiagen, Hilden, Germany) for 30 min at RT. Blocking was performed for 30 min in 3% milk in PBS (Gibco, Darmstadt, Germany) and slides were incubated for 1 h at RT with specific primary antibodies (ACE2, ab15348, Abcam, Cambridge, UK) 1:500 in 3% milk in PBS. Following washing with PBS, the sections were incubated for 1 h with a secondary antibody (Goat-anti-rabbit IgG-FITC, Santa Cruz, sc2359, Heidelberg, Germany). Then, 1:1000 in PBS at RT (rinsed with PBS). Counterstaining with the DAPI mounting medium (nuclear staining, Thermo Scientific, Schwerte, Germany) was performed and slides were cover slipped. Negative controls were performed by omitting the primary antibody.
2.7. Protein Extraction from FFPE
As described in Ikeda et al., the protein extraction from FFPE tissue was performed [22]. Then, 10 μm sections were incubated for 15 sec in Xylol, mixed, and centrifuged for 2 min at full speed at RT. Furthermore, 100% ethanol was added for 2 min to the pellet and mixed, after which it was centrifuged for 2 min at full speed. The supernatant was discarded and the pellet was air dried. They were incubated for 20 min at 100 °C in 40 μL of RIPA buffer (Santa Cruz, Heidelberg, Germany), followed by an incubation period of 2 h at 60 °C. The samples were centrifuged at full speed at 4 °C for 20 min and stored at −80 °C until further use. As previously described, protein quantification was performed [23].
2.8. ELISA
To check for the AR of the autopsy tissue by a different technique, an ELISA was performed in non-coated 96 well plates (Brand GmbH & Co KG, Wertheim, Germany). A total of 1 µg of each patient sample was incubated in 50 µL PBS for 2 h at RT. The washing step was performed thrice with 1× PBS followed by an incubation step with an androgen-specific antibody (1:500) (Agilent Dako, Clone AR441, M3562, Madrid, Spain) in the test plate and ß-actin (1:500) (Santa Cruz, SC 47778, Heidelberg, Germany) in the control plate for 1 hr at RT. Afterwards, a 3× washing step was followed by incubation with the secondary antibody (Advansta, R-05071-500 goat anti mouse HRP conjugated, San Jose, CA, USA) (1:5000) for 1 hr at RT. After washing thrice, 50 µL substrate solution (Invitrogen, Schwerte, Germany) was added for 15 min and the reaction was stopped with a 50 µL stop solution (Bethyl Laboratories. Inc., Montgomery, TX, USA). The outread of the ELISA was performed at 450 nm.
2.9. miR Extraction from FFPE Tissue and RT-PCR
Furthermore, miRNA extraction from FFPE tissue was performed according to the miRNeasy FFPE kit (Qiagen, Hilden, Germany). For RNA quantification, NanoDrop technology was used.
The cDNA was obtained from 100 ng of RNA using MystiCq microRNA Primers (Sigma-Aldrich, St. Louis, MO, USA) and the miRScript miRNA reverse transcription kit (Qiagen, Hilden, Germany), according to the manufacturer’s protocol.
2.10. Quantitative Real-Time PCR (qRT-PCR)
The qRT-PCR was performed as previously described [24,25]. All samples were normalized to 5 s rRNA (miR-371a-3p) and to β-actin (AR wild type and AR TC as the reference gene). In order to calculate the relative fluorescence the ΔΔ-CT method was used, as outlined in User Bulletin 2 (PE Applied Biosystems, Darmstadt, Germany). Primer information is as follows: All PCRs were performed at 50 °C and 40 cycles. 5s rRNA: Forw 5′-GGCCAUACCACCCUGAACGC-3′, miR-371a-3p: Forw 5′-CACCGCGGTAA CACTCAAAAGAT-3′, ß-actin: Forw 5′-TTGGCAATGA GCGGTTCCGCTG-3′, Rev 5′-GACAGCACTGTGTTGCGTA-3′, AR: Forw 5′-CGGAAGCTGAAGAAACTTGG -3′, Rev 5′-ATGGCTTCCAGGACATTCAG -3′.
2.11. Statistical Analysis
For the statistical analysis, GraphPad Prism 5 (v5.0, San Diego, CA, USA) program was used and the analysis of variance (ANOVA) was performed. Significant differences were indicated by stars (* p < 0.05, ** p < 0.01, and *** p < 0.001).
3. Results
3.1. Immunofluorescence ACE2 in Testicular Tissue from Healthy Donors and COVID-19 Patients
To visualize the level of the ACE2 Receptor, the entry point of SARS-CoV-2-, FFPE tissue sections were subjected to immunofluorescence staining. In Figure 1 ACE2 expression is depicted in tissue samples of COVID-19 patients, autopsy samples and testicular biopsy samples (TESE). In all three samples, the expression of the ACE2 receptor was detected.
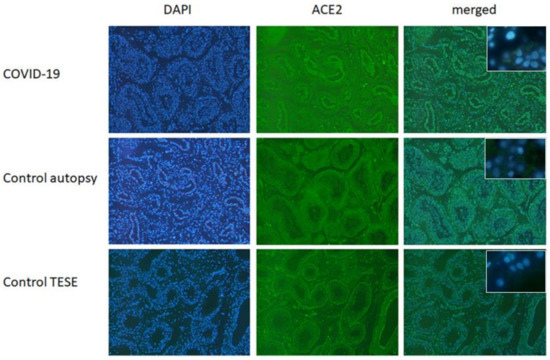
Figure 1.
ACE2 in testicular FFPE samples from control (autopsy and testicular biopsy (TESE)) and COVID-19 patients. In all three depicted samples, ACE2 expression could be found. Shown here is a representative example out of five (40× magnification). Insert shows respective negative controls (63×).
3.2. Histology and Expression of the Androgen Receptor in Testicular Tissue
In 75% of all specimens from COVID-19-positive patients, reduced spermatogenesis was detectable (Figure 2C) compared to COVID-19-negative patients (Figure 2A), inflammatory changes (Figure 2E) or previous intensive care controls (Figure 2G). For a detailed description see Table 2. In the two vaccinated patients who died with SARS-CoV-2, a normal spermatogenesis (yellow arrowheads) was detectable as well as the expression of the AR (Figure 2I,J). For evaluation of spermatogenesis, we used the diagnostically used classification by Sigg et al. [19].
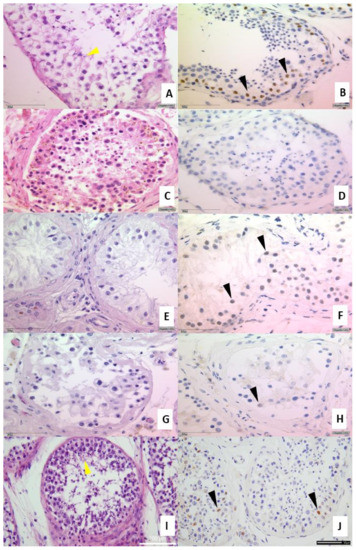
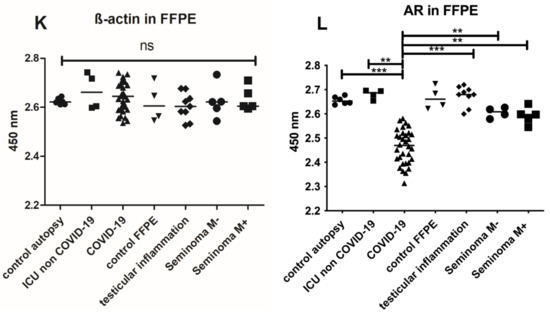
Figure 2.
Upper part: IHC from testicular tissue samples. (A,B) present a slightly reduced spermatogenesis and normal AR expression in healthy control tissue sample (brownish marked nuclei, clone AR441) (C,D) Example of COVID-19 positive patient with no expression of the AR as well as a reduced spermatogenesis. (E,F) are examples of inflammatory changes control and (G,H) from autopsy of a case of previous intensive care treatment. (I,J) autopsy of vaccinated man dying of COVID-19. Figures exemplified a collective of each analyzed group used. Left side H&E (spermiogenesis yellow arrowheads), right side IHC for androgen receptor AR441 (black arrowheads), each at 400× magnification. Lower part: Androgen receptor ELISA from FFPE tissue. Total protein was isolated from FFPE tissue and 1 µg of total protein was used for the analysis by ELISA. Significant decreased AR values were found in testicular tissue samples compared with control group. *** p < 0.0001, ** p < 0.001, unmarked = ns. Control ELISA for ß-actin to control isolation quantity (K). Detection of AR protein in FFPE samples (L). Significant reduction in the AR levels in COVID-19 samples (±30%) compared to control autopsy and ICU non COVID-19 were detectable (*** p < 0.001).
Recently, a correlation between ACE2 and the AR was described [26]. Therefore, IHC-stainings of testicular tissues were performed (Figure 2B,D,F,H,J). The testicular tissues from COVID-19 negative patients (Figure 2B), inflammatory changes (Figure 2F) or previous intensive care controls (Figure 2H) were positive for the AR protein (indicated by the brown nuclei (black arrowheads, Figure 2B) albeit diminished in F and H. Samples from a patients with COVID-19 were negative for AR (Figure 2D). To address a possible degradation of the AR protein due to autolysis or prolonged fixation, we also examined the presence of AR by ELISA and demonstrated the protein in the autopsy cases also with a reduction in COVID-19 cases (Figure 2L). Since the same amount of protein was used for all samples (2 µg), a significant reduction (p < 0.001) could be detected in COVID-19 compared to the corresponding control (autopsy). A reduction of ±30% was notable in COVID-19 samples.
3.3. AR IHC Followed by miR-371a-3p In Situ PCR
To show the localization of the AR as well the as the miR-371a-3p and the co-regulation of both, IHC followed by in situ PCR was performed. Since the AR is a target of the miR-371a-3p, as published by Fletcher et al. [17], the miR levels should be low in samples where the AR is present and vice versa. As depicted in Figure 3, in samples (controls) showing high AR levels, the miR-371a-p expression is reduced. In COVID-19 samples where the AR levels were reduced, the miR-371a-3p was significantly upregulated. Figure 4 presents the results of the three independent experiments.
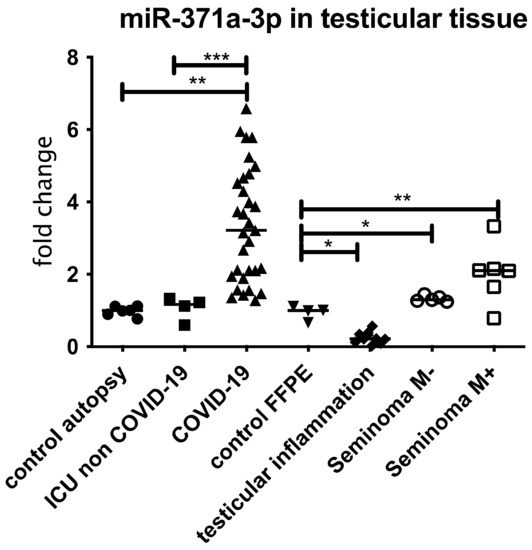
Figure 3.
miR 371a-3p in FFPE testicular tissue samples from COVID-19 patients compared to patients with ICU/ECMO, inflamed testis and Seminoma patients without metastasis (M−) or with metastasis (M+). All samples were normalized to 5s rRNA and corresponding controls (for COVID-19 and ICU controls from autopsy patients and for inflamed testis and Seminoma controls from testicular surgery without inflammatory disease or tumor). An increased expression, comparable with the miR expression of Seminoma patients with metastasis was detectable. In case of ICU non COVID-19, Seminoma without metastasis and inflamed, a significant reduction compared to COVID-19 was detectable. *** p < 0.0001, ** p < 0.001, * p < 0.01, unmarked = ns.
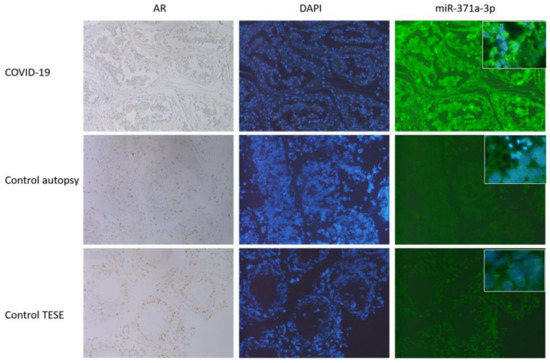
Figure 4.
IHC followed by miR-371a-3p in situ PCR was performed. As it can be seen, those samples (controls, autopsy and TESE) showing high AR levels (here seen in brownish coloration of the nuclei, 40×) the miR-371a-3p expression is reduced (FITC staining right row, 40×, inserts 63×). DAPI staining was included to visualize the nuclei. In COVID-19 samples were the AR levels were reduced (nuclei appears blue, first row) the miR-371a-3p was significantly upregulated (last row). Here, results out of three independent experiments are presented.
3.4. Expression of miR 371a-3p in Testicular Tissue Samples and Serum from COVID-19 Patients
The previously described finding of decreased AR levels in COVID-19 patients’ cases compared to patients within the same age group indicates that a possible Sars-CoV-2-induced mechanism is involved. We therefore examined the miR-371a-3p expression and found a significant overexpression in the COVID-19-positive tissue samples (Figure 3) but not in the control samples collected from patients who were treated in the ICU/with ECMO treatment or in tissue sample showing inflammation (Figure 5). None of the COVID-19-positive patients had been diagnosed with tumors (of any kind, as listed in Table 2).
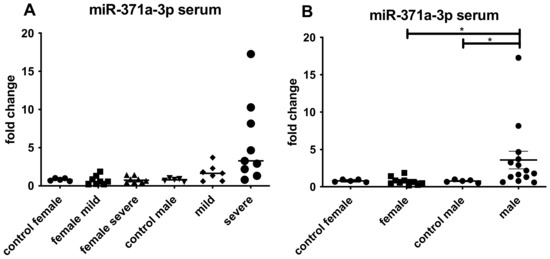
Figure 5.
qRT-PCR results for the detection of miR-371a-3p in serum from patients with mild or severe COVID-19. (A) depicted the miR-371a-3p expression female and male. Upregulation was found in case of male patients either with mild or severe symptoms. (B) summarized all female and male cases showing the expression of the miR-371a-3p. Increase in male patients was detectable (* p < 0.01).
4. Discussion
4.1. COVID-19 and Infiltration of Testes
Since the first documentation of COVID-19 cases in December 2019, different organs have been associated with SARS-CoV-2 infection apart from the respiratory system [1]. The association between COVID-19 and decreasing sperm quality was observed [27,28].
So far, we know (i) that testis, an immunological privileged organ, can serve as a reservoir for viruses [7]. For example, the Zika virus is detectable in ejaculates even after the infection is over [29]. (ii) In the testis, a high level of the ACE2 protein—the target protein of SARS-CoV-2—could be detected but with a decrease in older men [27,30] and it correlates with the AR in these testes. (iii) The spermatogenesis is reduced in older men [31]. As the analyzed COVID-19 patients were 73 years old on average, it is possible that the reduced spermatogenesis is due to the increased age. Nevertheless, in the control group from patients without COVID-19, a slightly higher spermatogenesis was seen by using the Sigg-classification.
Bhowmick et al. published a correlation between the AR and ACE2 [32]. The AR is a promotor for the transmembrane protease serine 2 (TMPRSS2), which is a co-receptor necessary for the internalization of SARS-CoV-2 [33]. Interestingly, at least in the lungs, a downregulation of ACE2 was observed following infection with SARS-CoV-2, which resulted in an increase in disease severity [34]. In the COVID-19 samples, the ACE2 was found to be present. However, as these patients suffered from an acute infection by SARS-CoV-2, it is possible that the levels decrease in testes as well after the infection.
It is postulated that the testosterone levels influence the degree of the SARS-CoV-2 infection and in some cases of severe infection, hypogonadism was detectable [27]. In general, females have lower levels of AR than older males (>70 years), this possibly explains the correlation that more males become infected than females [35]. Additionally, it is known that patients with AR deprivation therapy have a decreased risk of COVID-19 [36].
4.2. COVID-19 and Androgen Receptor (AR)
As mentioned, the testis can serve as reservoir for viruses [7] e.g., the Zika virus which is detectable in ejaculates after the symptomatic phase [29] and Epstein–Barr-Virus, which may play a role in tumor development [37]. Different groups published that SARS-CoV-2 infection is correlated to a decreased sperm quality or even a decreased fertility [38,39]. In older men, spermatogenesis is generally reduced [40,41]. This is most likely caused by a decreased protein level of AR. Since COVID-19-positive patients have a mean age of 73 years, the higher age could explain the reduced spermatogenesis. However, AR was detectable by IHC in age-matched controls so we speculate that a reduction in AR in COVID-19-positive patients could—at least in part—be due to infection with SARS-CoV-2. A reduction in spermatogenesis can be associated with a decreasing presence of the AR [41]. In most of our COVID-19 patients, a reduced spermatogenesis was detectable (Table 2) although the patients were older [37]. This would correlate well with our IHC, showing that in most COVID-19 testis the AR level is absent/reduced (Figure 2). However, it would be possible that due to the longer formalin fixation, the antibody binding was either compromised [42] or that the autolysis led to a degradation of the AR protein. This could be excluded, due to the ELISA against ß-actin and AR serving as an isolation/proof of principle control (Figure 2I,J). Figure 2J visualized the presence and a significant reduction in the AR level in COVID-19 patients, whereas ECMO-/autopsy specimens and inflamed testicular tissue indicated that IHC staining can be interpreted as specific and reliable. As mentioned, the AR mediates the expression of ACE2 and TMPRSS2, which explains that in most patients with AR deprivation therapy, a reduced risk of getting SARS-CoV-2 infections is notable [43]. However, the following question arises: why could we identify decreased AR levels in the analyzed COVID-19 patients. Is there another mechanism involved?
4.3. COVID-19 and miR-371a-3p Overexpression
It is shown that some virus infections are associated with a dysregulation of miRNAs [15]. The GCTC-specific miRNA, called miR-371a-3p, is responsible for the regulation of AR expression [44] and is also used as diagnostic marker for testicular cancer [13] according to the S3 guidelines of the testicular cancer diagnostics. Furthermore, it has also been published that the upregulation of miR-371a-3p in ejaculates can be used as a non-invasive marker for male infertility [45]. We therefore analyzed miR-371a-3p and were able to demonstrate a significant dysregulated expression of the miR-371a-3p (upregulation) in COVID-19 patients but not in the control groups (Figure 3). The dysregulation was even higher than in the seminoma control group (* p < 0.05). Since the miR-371a-3p is also upregulated in other cancer entities [14], we excluded tumor patients (apart from the seminoma group) in all patient groups (Table 2).
Until now, we have had no definitive timeline of SARS-CoV-2 presence or detectability in the body. Our findings of the increased miR level in postmortem COVID-19 testis and in the serum of living COVID-19 patients could be related either to the infection and might normalize over time or it can be the onset process that changes cell morphogenesis, resulting in reduced sperm production. Possibly, as a late outcome of COVID-19 infection and its upregulation of miR-371a-3p it could even be a risk factor for the development of a testicular germ cell cancer. Germ cell tumors arise from a primordial germ cell/gonocyte, which is arrested in development. These cells display a PGC-like expression pattern and remain within the testes environment. In young men during puberty, these precursors eventually start to proliferate to give rise to seminoma or embryonal carcinomas [46]. The exact cause of the developmental arrest as well as the nature of the events leading to the initiation of proliferation during puberty are only poorly understood [47]. It has been demonstrated that Germ Cell Tumors display elevated levels of miR-371a-3p. In fact, miR-371a-3p levels serve as a biomarker for detecting GCT [13]. In our study here, we have demonstrated, that an infection with SARS-CoV-2 leads to an increase in miR-371a-3p levels in serum. To exclude the described complications, a (long-time) follow up of patients after SARS-CoV2-infection would be a good strategy. Due to the low number of vaccinated patients in the study, we can only assume that the vaccine can prevent a reduced spermatogenesis and the correlating reduced expression of the AR receptor (n = 2). It has been shown in the literature that vaccination has no influence on sperm quality which correlates with our results [48].
4.4. AR Expression in COVID-19 Patients
The reduction in AR levels are possibly linked to the increased miR-371a-3p binding to the 3′ UTR of the AR, resulting in the downregulation of that protein [49]. Why should COVID-19 reduce AR which is necessary for the production of the docking and internalization receptors (TMPRSS2 and ACE2)? We speculate that the virus acts similarly to HI-Virus, which also downregulates these important receptors to prevent a super infection event [50]. Figure 4 depicts the results from three independent experiments, showing that the miR-371a-3p was significantly increased in COVID-19 samples, where the AR levels were reduced or absent and vice versa in the corresponding controls. Here, the TESE was used as high control, since in those samples no degradation of AR is expected as it could sometimes be in autopsy controls.
4.5. Detection of miR-371a-3p in Blood Samples
Since an upregulation of the miR-371a-3p was detectable in postmortem testicular tissue of the older male patients, we investigated the miR-371a-3p also in serum of younger COVID-19 male patients where it was also significantly increased (Figure 5). We postulate that in the setting of a SARS-CoV2-infection, this miR-371a-3p could be used to monitor a possible long COVID-like disease development, however, to be confident in this conclusion, further long-term investigations are needed. Since it also is a serum marker for the detection of testicular germ cell tumors [13], a urological follow-up examination should be included to prevent overseeing a seminoma by attributing the higher levels of miR-371a-3p only to COVID-19 and vice versa.
Nevertheless, a slight upregulation of the miR-371a-3p was also detectable in females since females also produce AR. The increase in testosterone results in higher levels of AR in females [51]. Higher levels of COVID-19 infections in older men compared to females could be explained by hormonal status.
5. Conclusions
We present here for the first time an association of SARS-CoV-2 infection involving the testes resulting in a downregulation of the AR by miR-371a-3p. This could influence the spermatogenesis and fertility after SARS-CoV-2 infection. Whether these increased levels of the miR-371a-3p are a long-term effect is still under investigation.
Author Contributions
Conception and design: A.H., M.v.B. and H.G.; Administrative support: D.N., T.N.; P.K., H.S., P.B., Provision of study materials or patients: D.N., T.N., T.K., J.F., H.G., O.A.C., E.K. and C.P., T.O.; A.Q., Collection and assembly of data: B.K., M.H. (Manuel Huerta), M.H. (Matthias Hamdorf), M.M.-R., M.v.B. and C.P.; H.S., Data analysis and interpretation: All authors; Manuscript writing: All authors; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of University of Cologne (protocol code BioMASOTA, file reference 12–163, 8 July 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Beate Rygol and Larissa-Marie Hamacher for expert technical assistance.
Conflicts of Interest
O.A.C. reports grants and personal fees from Actelion, Amplyx, Astellas, Basilea, Cidara, Da Volterra, F2G, Gilead, MedPace, Merck/MSD, Pfizer, Scynexis; grants from DFG (German Research Foundation), German Federal Ministry of Research and Education, Immunic, Janssen, Medicines Company, Melinta Therapeutics; personal fees from Allecra Therapeutics, Al-Jazeera Pharmaceuticals, Biocon, Biosys, CoRe Consulting, Entasis, Grupo Biotoscana, IQVIA, Matinas, Menarini, Molecular Partners, MSG-ERC, Mylan, Nabriva, Noxxon, Octapharma, Paratek, PSI, Roche Diagnostics, Seres, Shionogi; others from Wiley (Blackwell); outside the submitted work. P.K. is supported by the German Federal Ministry of Research and Education and the State of North Rhine-Westphalia, Germany and has received non-financial scientific grants from Miltenyi Biotec GmbH, Bergisch Gladbach, Germany, and the Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases, University of Cologne, Cologne, Germany, and received lecture honoraria from and/or is advisor to Akademie für Infektionsmedizin e.V., Ambu GmbH, Astellas Pharma, European Confederation of Medical Mycology, Gilead Sciences, GPR Academy Ruesselsheim, MSD Sharp & Dohme GmbH, Noxxon N.V., Pfizer Pharma GmbH and University Hospital, LMU Munich outside the submitted work.
References
- Zhang, Y.; Geng, X.; Tan, Y.; Li, Q.; Xu, C.; Xu, J.; Hao, L.; Zeng, Z.; Luo, X.; Liu, F.; et al. New understanding of the damage of SARS-CoV-2 infection outside the respiratory system. Biomed. Pharmacother. 2020, 127, 110195. [Google Scholar] [CrossRef] [PubMed]
- Kirtipal, N.; Kumar, S.; Dubey, S.K.; Dwivedi, V.D.; Gireesh Babu, K.; Maly, P.; Bharadwaj, S. Understanding on the possible routes for SARS CoV-2 invasion via ACE2 in the host linked with multiple organs damage. Infect. Genet. Evol. 2022, 99, 105254. [Google Scholar] [CrossRef] [PubMed]
- Salamanna, F.; Maglio, M.; Landini, M.P.; Fini, M. Body Localization of ACE-2: On the Trail of the Keyhole of SARS-CoV-2. Front. Med. 2020, 7, 594495. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.A.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020, 13, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Migliore, L.; Nicoli, V.; Stoccoro, A. Gender Specific Differences in Disease Susceptibility: The Role of Epigenetics. Biomedicines 2021, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Cosar, B.; Karagulleoglu, Z.Y.; Unal, S.; Ince, A.T.; Uncuoglu, D.B.; Tuncer, G.; Kilinc, B.R.; Ozkan, Y.E.; Ozkoc, H.C.; Demir, I.N.; et al. SARS-CoV-2 Mutations and their Viral Variants. Cytokine Growth Factor Rev. 2021, 63, 10–22. [Google Scholar] [CrossRef]
- Barbagallo, F.; Calogero, A.E.; Cannarella, R.; Condorelli, R.A.; Mongioi, L.M.; Aversa, A.; La Vignera, S. The testis in patients with COVID-19: Virus reservoir or immunization resource? Transl. Androl. Urol. 2020, 9, 1897–1900. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz-Gospodarek, A.; Wdowiak, P.; Czeczelewski, M.; Forma, A.; Flieger, J.; Januszewski, J.; Radzikowska-Buchner, E.; Baj, J. The Impact of SARS-CoV-2 Infection on Fertility and Female and Male Reproductive Systems. J. Clin. Med. 2021, 10, 4520. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, A.; Bokemeyer, C.; Souchon, R. Stage-specific treatment for testicular germ cell tumours. Urol. A 2009, 48, 377–385. [Google Scholar] [CrossRef]
- Clark, A.T. The stem cell identity of testicular cancer. Stem Cell Rev. 2007, 3, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Giannandrea, F.; Fargnoli, S. Environmental Factors Affecting Growth and Occurrence of Testicular Cancer in Childhood: An Overview of the Current Epidemiological Evidence. Children 2017, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Richiardi, L.; Pettersson, A.; Akre, O. Genetic and environmental risk factors for testicular cancer. Int. J. Androl. 2007, 30, 230–240. [Google Scholar] [CrossRef]
- Dieckmann, K.P.; Radtke, A.; Geczi, L.; Matthies, C.; Anheuser, P.; Eckardt, U.; Sommer, J.; Zengerling, F.; Trenti, E.; Pichler, R.; et al. Serum Levels of MicroRNA-371a-3p (M371 Test) as a New Biomarker of Testicular Germ Cell Tumors: Results of a Prospective Multicentric Study. J. Clin. Oncol. 2019, 37, 1412–1423. [Google Scholar] [CrossRef]
- Pan, B.; He, B.; Xu, X.; Liu, X.; Xu, T.; Xu, M.; Chen, X.; Zeng, K.; Lin, K.; Hu, X.; et al. MicroRNA-371-3 cluster as biomarkers for the diagnosis and prognosis of cancers. Cancer Manag. Res. 2019, 11, 5437–5457. [Google Scholar] [CrossRef] [PubMed]
- Skalsky, R.L.; Cullen, B.R. Viruses, microRNAs, and host interactions. Annu. Rev. Microbiol. 2010, 64, 123–141. [Google Scholar] [CrossRef]
- Von Brandenstein, M.; Richter, C.; Fries, J.W. MicroRNAs: Small but amazing, and their association with endothelin. Life Sci. 2012, 91, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.E.; Sulpice, E.; Combe, S.; Shibakawa, A.; Leach, D.A.; Hamilton, M.P.; Chrysostomou, S.L.; Sharp, A.; Welti, J.; Yuan, W.; et al. Androgen receptor-modulatory microRNAs provide insight into therapy resistance and therapeutic targets in advanced prostate cancer. Oncogene 2019, 38, 5700–5724. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Ueda, T.; Ito, S.; Shiraishi, T.; Taniguchi, H.; Kayukawa, N.; Nakanishi, H.; Ushijima, S.; Kanazawa, M.; Nakamura, T.; et al. Androgen suppresses testicular cancer cell growth in vitro and in vivo. Oncotarget 2016, 7, 35224–35232. [Google Scholar] [CrossRef] [PubMed]
- Sigg, C. Classification of tubular testicular atrophies in the diagnosis of sterility. Significance of the so-called “bunte Atrophie”. Schweiz. Med. Wochenschr. 1979, 109, 1284–1293. [Google Scholar] [PubMed]
- Sigg, C.; Hedinger, C. Testicular germ cell tumors and atypical germ cells. Schweiz. Med. Wochenschr. 1980, 110, 801–806. [Google Scholar] [PubMed]
- Nuovo, G.J.; Elton, T.S.; Nana-Sinkam, P.; Volinia, S.; Croce, C.M.; Schmittgen, T.D. A methodology for the combined in situ analyses of the precursor and mature forms of microRNAs and correlation with their putative targets. Nat. Protoc. 2009, 4, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Monden, T.; Kanoh, T.; Tsujie, M.; Izawa, H.; Haba, A.; Ohnishi, T.; Sekimoto, M.; Tomita, N.; Shiozaki, H.; et al. Extraction and analysis of diagnostically useful proteins from formalin-fixed, paraffin-embedded tissue sections. J. Histochem. Cytochem. 1998, 46, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Gerstung, M.; Roth, T.; Dienes, H.P.; Licht, C.; Fries, J.W. Endothelin-1 induces NF-kappaB via two independent pathways in human renal tubular epithelial cells. Am. J. Nephrol. 2007, 27, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Von Brandenstein, M.; Puetz, K.; Schlosser, M.; Loser, H.; Kallinowski, J.P.; Godde, D.; Buettner, R.; Storkel, S.; Fries, J.W. Vimentin 3, the new hope, differentiating RCC versus oncocytoma. Dis. Markers 2015, 2015, 368534. [Google Scholar] [CrossRef]
- Von Brandenstein, M.; Depping, R.; Schafer, E.; Dienes, H.P.; Fries, J.W. Protein kinase C alpha regulates nuclear pri-microRNA 15a release as part of endothelin signaling. Biochim. Biophys. Acta 2011, 1813, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Samuel, R.M.; Majd, H.; Richter, M.N.; Ghazizadeh, Z.; Zekavat, S.M.; Navickas, A.; Ramirez, J.T.; Asgharian, H.; Simoneau, C.R.; Bonser, L.R.; et al. Androgen Signaling Regulates SARS-CoV-2 Receptor Levels and Is Associated with Severe COVID-19 Symptoms in Men. Cell Stem Cell 2020, 27, 876–889.e12. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. SARS-CoV-2 and Male Infertility: Possible Multifaceted Pathology. Reprod. Sci. 2021, 28, 23–26. [Google Scholar] [CrossRef]
- Wang, N.; Qin, L.; Ma, L.; Yan, H. Effect of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) on reproductive system. Stem Cell Res. 2021, 52, 102189. [Google Scholar] [CrossRef]
- Medina, F.A.; Torres, G.; Acevedo, J.; Fonseca, S.; Casiano, L.; De Leon-Rodriguez, C.M.; Santiago, G.A.; Doyle, K.; Sharp, T.M.; Alvarado, L.I.; et al. Duration of the Presence of Infectious Zika Virus in Semen and Serum. J. Infect. Dis. 2019, 219, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Illiano, E.; Trama, F.; Costantini, E. Could COVID-19 have an impact on male fertility? Andrologia 2020, 52, e13654. [Google Scholar] [CrossRef]
- Almeida, S.; Rato, L.; Sousa, M.; Alves, M.G.; Oliveira, P.F. Fertility and Sperm Quality in the Aging Male. Curr. Pharm. Des. 2017, 23, 4429–4437. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, N.A.; Oft, J.; Dorff, T.; Pal, S.; Agarwal, N.; Figlin, R.A.; Posadas, E.M.; Freedland, S.J.; Gong, J. COVID-19 and androgen-targeted therapy for prostate cancer patients. Endocr. Relat. Cancer 2020, 27, R281–R292. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.S.; Moulin, T.C.; Schioth, H.B. Sex differences in COVID-19: The role of androgens in disease severity and progression. Endocrine 2021, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Stilhano, R.S.; Costa, A.J.; Nishino, M.S.; Shams, S.; Bartolomeo, C.S.; Breithaupt-Faloppa, A.C.; Silva, E.A.; Ramirez, A.L.; Prado, C.M.; Ureshino, R.P. SARS-CoV-2 and the possible connection to ERs, ACE2, and RAGE: Focus on susceptibility factors. FASEB J. 2020, 34, 14103–14119. [Google Scholar] [CrossRef] [PubMed]
- Montopoli, M.; Zumerle, S.; Vettor, R.; Rugge, M.; Zorzi, M.; Catapano, C.V.; Carbone, G.M.; Cavalli, A.; Pagano, F.; Ragazzi, E.; et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: A population-based study (N = 4532). Ann. Oncol. 2020, 31, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Garolla, A.; Vitagliano, A.; Muscianisi, F.; Valente, U.; Ghezzi, M.; Andrisani, A.; Ambrosini, G.; Foresta, C. Role of Viral Infections in Testicular Cancer Etiology: Evidence from a Systematic Review and Meta-Analysis. Front. Endocrinol. 2019, 10, 355. [Google Scholar] [CrossRef]
- Li, H.; Xiao, X.; Zhang, J.; Zafar, M.I.; Wu, C.; Long, Y.; Lu, W.; Pan, F.; Meng, T.; Zhao, K.; et al. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine 2020, 28, 100604. [Google Scholar] [CrossRef]
- Mannur, S.; Jabeen, T.; Khader, M.A.; Rao, L.S.S. Post-Covid-19 Associated Decline in Long-Term Male Fertility and Embryo Quality during Assisted Reproductive Technology. QJM 2021, 114, 328–330. [Google Scholar] [CrossRef]
- Kaufman, J.M.; Vermeulen, A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr. Rev. 2005, 26, 833–876. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.S.; Yeh, S.; Tzeng, C.R.; Chang, C. Androgen receptor roles in spermatogenesis and fertility: Lessons from testicular cell-specific androgen receptor knockout mice. Endocr. Rev. 2009, 30, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Sigg, C.; Hedinger, C. Atypical germ cells in testicular biopsy in male sterility. Int. J. Androl. 1981, 4 (Suppl. S4), 163–170. [Google Scholar] [CrossRef] [PubMed]
- McCoy, J.; Wambier, C.G.; Vano-Galvan, S.; Shapiro, J.; Sinclair, R.; Ramos, P.M.; Washenik, K.; Andrade, M.; Herrera, S.; Goren, A. Racial variations in COVID-19 deaths may be due to androgen receptor genetic variants associated with prostate cancer and androgenetic alopecia. Are anti-androgens a potential treatment for COVID-19? J. Cosmet. Dermatol. 2020, 19, 1542–1543. [Google Scholar] [CrossRef] [PubMed]
- Leite, K.R.; Morais, D.R.; Florez, M.G.; Reis, S.T.; Iscaife, A.; Viana, N.; Moura, C.M.; Silva, I.A.; Katz, B.S.; Pontes, J., Jr.; et al. The role of microRNAs 371 and 34a in androgen receptor control influencing prostate cancer behavior. Urol. Oncol. 2015, 33, 267.e15–267.e22. [Google Scholar] [CrossRef]
- Radtke, A.; Dieckmann, K.P.; Grobelny, F.; Salzbrunn, A.; Oing, C.; Schulze, W.; Belge, G. Expression of miRNA-371a-3p in seminal plasma and ejaculate is associated with sperm concentration. Andrology 2019, 7, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Elzinga-Tinke, J.E.; Dohle, G.R.; Looijenga, L.H. Etiology and early pathogenesis of malignant testicular germ cell tumors: Towards possibilities for preinvasive diagnosis. Asian J. Androl. 2015, 17, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwan, Y.G.; Rijlaarsdam, M.A.; Rossello, F.J.; Notini, A.J.; de Boer, S.; Watkins, D.N.; Gillis, A.J.; Dorssers, L.C.; White, S.J.; Looijenga, L.H. Seminoma and embryonal carcinoma footprints identified by analysis of integrated genome-wide epigenetic and expression profiles of germ cell cancer cell lines. PLoS ONE 2014, 9, e98330. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhu, S.; Dai, Z.; Hao, L.; Luan, C.; Guo, Q.; Meng, C.; Zhang, Y. Effects of COVID-19 and mRNA vaccines on human fertility. Hum. Reprod. 2021, 37, 5–13. [Google Scholar] [CrossRef]
- Eringyte, I.; Zamarbide Losada, J.N.; Powell, S.M.; Bevan, C.L.; Fletcher, C.E. Coordinated AR and microRNA regulation in prostate cancer. Asian J. Urol. 2020, 7, 233–250. [Google Scholar] [CrossRef]
- Tanaka, M.; Ueno, T.; Nakahara, T.; Sasaki, K.; Ishimoto, A.; Sakai, H. Downregulation of CD4 is required for maintenance of viral infectivity of HIV-1. Virology 2003, 311, 316–325. [Google Scholar] [CrossRef]
- Davison, S.L.; Bell, R.; Donath, S.; Montalto, J.G.; Davis, S.R. Androgen levels in adult females: Changes with age, menopause, and oophorectomy. J. Clin. Endocrinol. Metab. 2005, 90, 3847–3853. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).