The Antiplatelet Action of S-Nitroso Human Serum Albumin in Whole Blood
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Subjects
2.3. Blood Collection and Preparation
2.4. Sampling
2.5. Analysis of Nitrite and Nitrate
2.6. Impedance Aggregation Assay
2.7. Platelet Function Analyzer 200
2.8. Whole Blood Platelet Adhesion/Aggregation Assay
2.9. Whole Blood Tissue Factor-Triggered TEM Assay
2.10. Statistics
3. Results
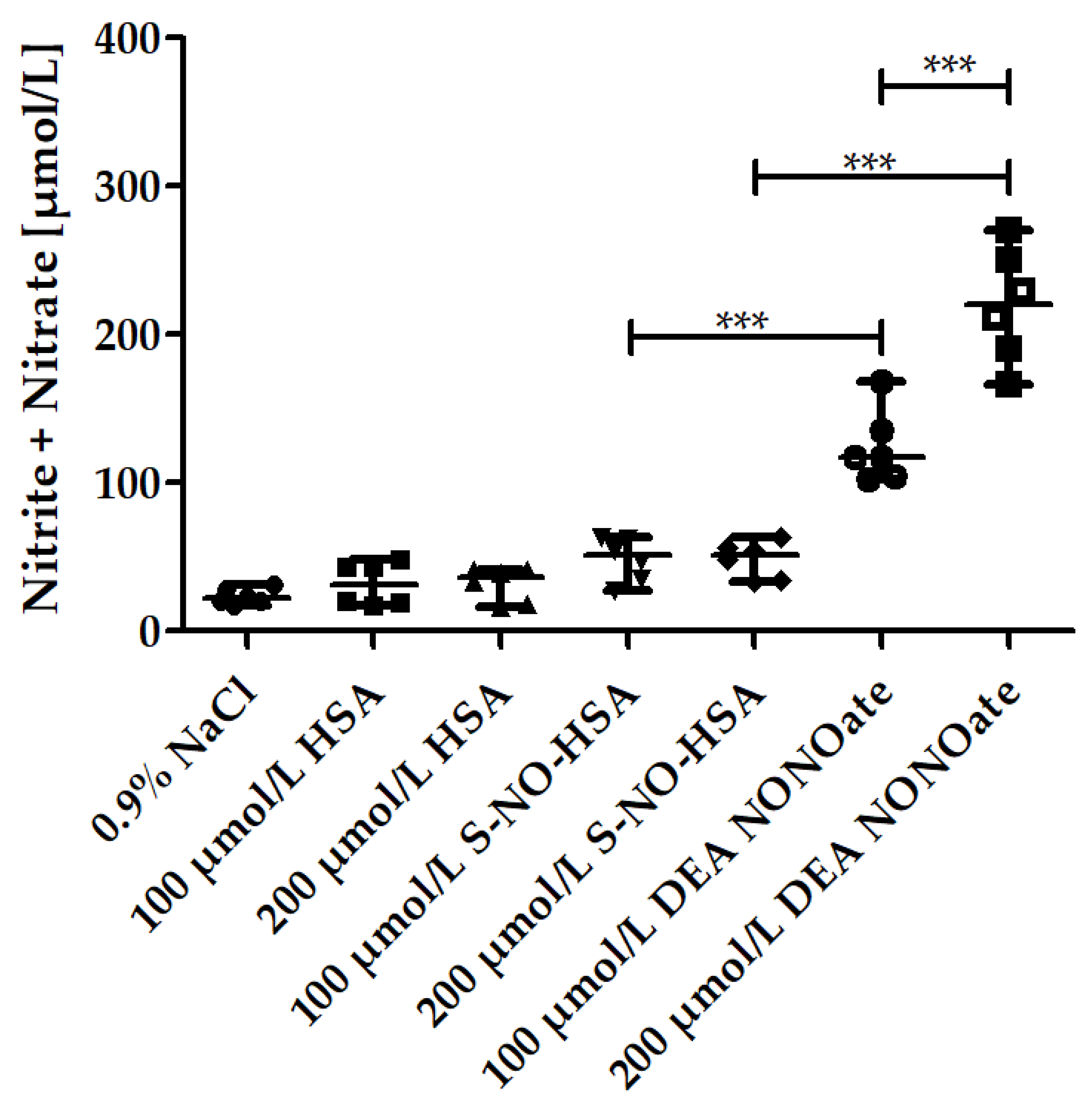
3.1. Nitrite/Nitrate Amounts in the Presence of Increasing Concentrations of NO-Donors in WB
3.2. Effects of Increasing Concentrations of NO-Donors on Impedance Aggregometry Values in WB
3.3. Effects of Increasing Concentrations of NO-Donors on PFA 200 Values in WB
3.4. Effects of Increasing Concentrations of NO-Donors on CPA Values in WB
3.5. Effects of Increasing Concentrations of NO-Donors on TEM Values in WB
3.6. Nitrite/Nitrate Levels in the Presence of Increasing Concentrations of NO-donors in PRP
3.7. Effects of Increasing Concentrations of NO-Donors on Impedance Aggregometry Values in PRP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pautz, A.; Li, H.; Kleinert, H. Regulation of NOS expression in vascular diseases. Front. Biosci. 2021, 26, 85–101. [Google Scholar] [CrossRef]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [PubMed]
- Macrae, D.J.; Field, D.; Mercier, J.C.; Moller, J.; Stiris, T.; Biban, P.; Cornick, P.; Goldman, A.; Gothberg, S.; Gustafsson, L.E.; et al. Inhaled nitric oxide therapy in neonates and children: Reaching a European consensus. Intensiv. Care Med. 2004, 30, 372–380. [Google Scholar] [CrossRef]
- Akinosoglou, K.; Alexopoulos, D. Use of antiplatelet agents in sepsis: A glimpse into the future. Thromb. Res. 2014, 133, 131–138. [Google Scholar] [CrossRef]
- Roberts, B.W.; Mitchell, J.; Kilgannon, J.H.; Chansky, M.E.; Trzeciak, S. Nitric oxide donor agents for the treatment of ischemia/reperfusion injury in human subjects: A systematic review. Shock 2013, 39, 229–239. [Google Scholar] [CrossRef]
- Oliveira, C.; Benfeito, S.; Fernandes, C.; Cagide, F.; Silva, T.; Borges, F. NO and HNO donors, nitrones, and nitroxides: Past, present, and future. Med. Res. Rev. 2018, 38, 1159–1187. [Google Scholar] [CrossRef]
- Simon, D.I.; Stamler, J.S.; Jaraki, O.; Keaney, J.F.; Osborne, J.A.; Francis, S.A.; Singel, D.J.; Loscalzo, J. Antiplatelet properties of protein S-nitrosothiols derived from nitric oxide and endothelium-derived relaxing factor. Arterioscler. Thromb. 1993, 13, 791–799. [Google Scholar] [CrossRef]
- Dutra, L.A.; Guanaes, J.F.O.; Johmann, N.; Lopes Pires, M.E.; Chin, C.M.; Marcondes, S.; Dos Santos, J.L. Synthesis, antiplatelet and antithrombotic activities of resveratrol derivatives with NO-donor properties. Bioorg. Med. Chem. Lett. 2017, 27, 2450–2453. [Google Scholar] [CrossRef]
- Mellion, B.T.; Ignarro, L.J.; Ohlstein, E.H.; Pontecorvo, E.G.; Hyman, A.L.; Kadowitz, P.J. Evidence for the inhibitory role of guanosine 3′, 5′-monophosphate in ADP-induced human platelet aggregation in the presence of nitric oxide and related vasodilators. Blood 1981, 57, 946–955. [Google Scholar] [CrossRef]
- Mendelsohn, M.E.; O’Neill, S.; George, D.; Loscalzo, J. Inhibition of fibrinogen binding to human platelets by S-nitroso-N-acetylcysteine. J. Biol. Chem. 1990, 265, 19028–19034. [Google Scholar] [CrossRef]
- Zang, Y.; Popat, K.C.; Reynolds, M.M. Nitric oxide-mediated fibrinogen deposition prevents platelet adhesion and activation. Biointerphases 2018, 13, 06E403. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, S.; Tohmatsu, T.; Hattori, H.; Okano, Y.; Nozawa, Y. Inhibitory action of cyclic GMP on secretion, polyphosphoinositide hydrolysis and calcium mobilization in thrombin-stimulated human platelets. Biochem. Biophys. Res. Commun. 1986, 135, 1099–1104. [Google Scholar] [CrossRef]
- Sen, L.; Zuo, S.J.; Cao, L.; Liu, D.Z.; Zhang, S.Q.; Cao, Y.X. Vasodilation and hypotension of a novel 3-benzylquinazolin- 4(3H)-one derivative via the inhibition of calcium flux. Eur. J. Pharmacol. 2016, 791, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, E.H.; O’Neill, S.; Mendelsohn, M.E. S-nitrosocysteine inhibition of human platelet secretion is correlated with increases in platelet cGMP levels. Circ. Res. 1991, 68, 1722–1728. [Google Scholar] [CrossRef] [PubMed]
- Gries, A.; Bode, C.; Peter, K.; Herr, A.; Bohrer, H.; Motsch, J.; Martin, E. Inhaled nitric oxide inhibits human platelet aggregation, P-selectin expression, and fibrinogen binding in vitro and in vivo. Circulation 1998, 97, 1481–1487. [Google Scholar] [CrossRef]
- Ferrer, R.; Masclans, J.R.; Angles, R.; Pico, M.; Millan, B.; Planas, M.; de Latorre, F.J. Anticoagulative effect of nitric oxide inhalation in ARDS. Intensiv. Care Med. 1998, 24, 837–838. [Google Scholar] [CrossRef] [PubMed]
- Akmal, A.H.; Hasan, M. Role of nitric oxide in management of acute respiratory distress syndrome. Ann. Thorac. Med. 2008, 3, 100–103. [Google Scholar] [CrossRef]
- Van Meurs, K.P.; Rhine, W.D.; Asselin, J.M.; Durand, D.J. Response of premature infants with severe respiratory failure to inhaled nitric oxide. Preemie NO Collaborative Group. Pediatr. Pulmonol. 1997, 24, 319–323. [Google Scholar] [CrossRef]
- Hallstrom, S.; Franz, M.; Gasser, H.; Vodrazka, M.; Semsroth, S.; Losert, U.M.; Haisjackl, M.; Podesser, B.K.; Malinski, T. S-nitroso human serum albumin reduces ischaemia/reperfusion injury in the pig heart after unprotected warm ischaemia. Cardiovasc. Res. 2008, 77, 506–514. [Google Scholar] [CrossRef]
- Tsikas, D. Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic. Res. 2005, 39, 797–815. [Google Scholar] [CrossRef] [PubMed]
- Hallstrom, S.; Gasser, H.; Neumayer, C.; Fugl, A.; Nanobashvili, J.; Jakubowski, A.; Huk, I.; Schlag, G.; Malinski, T. S-nitroso human serum albumin treatment reduces ischemia/reperfusion injury in skeletal muscle via nitric oxide release. Circulation 2002, 105, 3032–3038. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Romitelli, F.; Santini, S.A.; Chierici, E.; Pitocco, D.; Tavazzi, B.; Amorini, A.M.; Lazzarino, G.; Di Stasio, E. Comparison of nitrite/nitrate concentration in human plasma and serum samples measured by the enzymatic batch Griess assay, ion-pairing HPLC and ion-trap GC-MS: The importance of a correct removal of proteins in the Griess assay. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 851, 257–267. [Google Scholar] [CrossRef]
- Li, H.; Meininger, C.J.; Wu, G. Rapid determination of nitrite by reversed-phase high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 2000, 746, 199–207. [Google Scholar] [CrossRef]
- Radulovic, S.; Gottschalk, B.; Horl, G.; Zardoya-Laguardia, P.; Schilcher, I.; Hallstrom, S.; Vujic, N.; Schmidt, K.; Trieb, M.; Graier, W.F.; et al. Endothelial lipase increases eNOS activating capacity of high-density lipoprotein. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158612. [Google Scholar] [CrossRef] [PubMed]
- Morel-Kopp, M.C.; Tan, C.W.; Brighton, T.A.; McRae, S.; Baker, R.; Tran, H.; Mollee, P.; Kershaw, G.; Joseph, J.; Ward, C.; et al. Validation of whole blood impedance aggregometry as a new diagnostic tool for HIT: Results of a large Australian study. Thromb. Haemost. 2012, 107, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Cvirn, G.; Gallistl, S.; Rehak, T.; Jurgens, G.; Muntean, W. Elevated thrombin-forming capacity of tissue factor-activated cord compared with adult plasma. J. Thromb. Haemost. 2003, 1, 1785–1790. [Google Scholar] [CrossRef]
- Cvirn, G.; Gallistl, S.; Kutschera, J.; Wagner, T.; Ferstl, U.; Jurgens, G.; Koestenberger, M. Collagen/endogenous thrombin-induced platelet aggregation in whole blood samples. Blood Coagul. Fibrinolysis 2007, 18, 585–588. [Google Scholar] [CrossRef]
- Chang, Y.W.; Liao, C.H.; Day, Y.J. Platelet function analyzer (PFA-100) offers higher sensitivity and specificity than thromboelastography (TEG) in detection of platelet dysfunction. Acta Anaesthesiol. Taiwanica 2009, 47, 110–117. [Google Scholar] [CrossRef]
- Varon, D.; Dardik, R.; Shenkman, B.; Kotev-Emeth, S.; Farzame, N.; Tamarin, I.; Savion, N. A new method for quantitative analysis of whole blood platelet interaction with extracellular matrix under flow conditions. Thromb. Res. 1997, 85, 283–294. [Google Scholar] [CrossRef]
- Sorensen, B.; Johansen, P.; Christiansen, K.; Woelke, M.; Ingerslev, J. Whole blood coagulation thrombelastographic profiles employing minimal tissue factor activation. J. Thromb. Haemost. 2003, 1, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.S.; Jaraki, O.; Osborne, J.; Simon, D.I.; Keaney, J.; Vita, J.; Singel, D.; Valeri, C.R.; Loscalzo, J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc. Natl. Acad. Sci. USA 1992, 89, 7674–7677. [Google Scholar] [CrossRef] [PubMed]
- Maragos, C.M.; Morley, D.; Wink, D.A.; Dunams, T.M.; Saavedra, J.E.; Hoffman, A.; Bove, A.A.; Isaac, L.; Hrabie, J.A.; Keefer, L.K. Complexes of ·NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. J. Med. Chem. 1991, 34, 3242–3247. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P.; Dejam, A.; Lauer, T.; Rassaf, T.; Schindler, A.; Picker, O.; Scheeren, T.; Godecke, A.; Schrader, J.; Schulz, R.; et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic. Biol. Med. 2003, 35, 790–796. [Google Scholar] [CrossRef]
- Scorza, G.; Pietraforte, D.; Minetti, M. Role of ascorbate and protein thiols in the release of nitric oxide from S-nitroso-albumin and S-nitroso-glutathione in human plasma. Free Radic. Biol. Med. 1997, 22, 633–642. [Google Scholar] [CrossRef]
- Miranda, K.M.; Katori, T.; Torres de Holding, C.L.; Thomas, L.; Ridnour, L.A.; McLendon, W.J.; Cologna, S.M.; Dutton, A.S.; Champion, H.C.; Mancardi, D.; et al. Comparison of the NO and HNO donating properties of diazeniumdiolates: Primary amine adducts release HNO in vivo. J. Med. Chem. 2005, 48, 8220–8228. [Google Scholar] [CrossRef]
- Mondoro, T.H.; Ryan, B.B.; Hrinczenko, B.W.; Schechter, A.N.; Vostal, J.G.; Alayash, A.I. Biological action of nitric oxide donor compounds on platelets from patients with sickle cell disease. Br. J. Haematol. 2001, 112, 1048–1054. [Google Scholar] [CrossRef]
- Shah, C.M.; Locke, I.C.; Chowdrey, H.S.; Gordge, M.P. Rapid S-nitrosothiol metabolism by platelets and megakaryocytes. Biochem. Soc. Trans. 2003, 31, 1450–1452. [Google Scholar] [CrossRef]
- Rungatscher, A.; Milani, E.; Covajes, C.; Hallstrom, S.; Gottin, L.; Guidi, G.C.; Luciani, G.B.; Faggian, G. Blood transfusions may impair endothelium-dependent vasodilatation during coronary artery bypass surgery. Microvasc. Res. 2017, 112, 109–114. [Google Scholar] [CrossRef]
- Mathews, W.R.; Kerr, S.W. Biological activity of S-nitrosothiols: The role of nitric oxide. J. Pharmacol. Exp. Ther. 1993, 267, 1529–1537. [Google Scholar]
- Rukoyatkina, N.; Walter, U.; Friebe, A.; Gambaryan, S. Differentiation of cGMP-dependent and -independent nitric oxide effects on platelet apoptosis and reactive oxygen species production using platelets lacking soluble guanylyl cyclase. Thromb. Haemost. 2011, 106, 922–933. [Google Scholar] [CrossRef]
- Korthuis, R.J.; Granger, D.N.; Townsley, M.I.; Taylor, A.E. The role of oxygen-derived free radicals in ischemia-induced increases in canine skeletal muscle vascular permeability. Circ. Res. 1985, 57, 599–609. [Google Scholar] [CrossRef]
- Elzein, C.; Urbas, C.; Hughes, B.; Li, Y.; Lefaiver, C.; Ilbawi, M.; Vricella, L. Efficacy of Nitric Oxide Administration in Attenuating Ischemia/Reperfusion Injury During Neonatal Cardiopulmonary Bypass. World J. Pediatr. Congenit. Heart Surg. 2020, 11, 417–423. [Google Scholar] [CrossRef]
- Baue, A.E. The horror autotoxicus and multiple-organ failure. Arch. Surg. 1992, 127, 1451–1462. [Google Scholar] [CrossRef]
- Grace, P.A. Ischaemia-reperfusion injury. Br. J. Surg. 1994, 81, 637–647. [Google Scholar] [CrossRef]
- Aslam, M.; Gunduz, D.; Troidl, C.; Heger, J.; Hamm, C.W.; Schulz, R. Purinergic Regulation of Endothelial Barrier Function. Int. J. Mol. Sci. 2021, 22, 1207. [Google Scholar] [CrossRef]
- Huk, I.; Nanobashvili, J.; Neumayer, C.; Punz, A.; Mueller, M.; Afkhampour, K.; Mittlboeck, M.; Losert, U.; Polterauer, P.; Roth, E.; et al. L-arginine treatment alters the kinetics of nitric oxide and superoxide release and reduces ischemia/reperfusion injury in skeletal muscle. Circulation 1997, 96, 667–675. [Google Scholar] [CrossRef]
- Pou, S.; Pou, W.S.; Bredt, D.S.; Snyder, S.H.; Rosen, G.M. Generation of superoxide by purified brain nitric oxide synthase. J. Biol. Chem. 1992, 267, 24173–24176. [Google Scholar] [CrossRef]
- Samama, C.M.; Diaby, M.; Fellahi, J.L.; Mdhafar, A.; Eyraud, D.; Arock, M.; Guillosson, J.J.; Coriat, P.; Rouby, J.J. Inhibition of platelet aggregation by inhaled nitric oxide in patients with acute respiratory distress syndrome. Anesthesiology 1995, 83, 56–65. [Google Scholar] [CrossRef]
- Frostell, C.; Fratacci, M.D.; Wain, J.C.; Jones, R.; Zapol, W.M. Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation 1991, 83, 2038–2047. [Google Scholar] [CrossRef]
- Mittermayr, R.; Valentini, D.; Fitzal, F.; Hallstrom, S.; Gasser, H.; Redl, H. Protective effect of a novel NO-donor on ischemia/reperfusion injury in a rat epigastric flap model. Wound Repair Regen. 2003, 11, 3–10. [Google Scholar] [CrossRef]
- Rungatscher, A.; Hallstrom, S.; Linardi, D.; Milani, E.; Gasser, H.; Podesser, B.K.; Scarabelli, T.M.; Luciani, G.B.; Faggian, G. S-nitroso human serum albumin attenuates pulmonary hypertension, improves right ventricular-arterial coupling, and reduces oxidative stress in a chronic right ventricle volume overload model. J. Heart Lung Transplant. 2015, 34, 479–488. [Google Scholar] [CrossRef]
- Jakubowski, A.; Maksimovich, N.; Olszanecki, R.; Gebska, A.; Gasser, H.; Podesser, B.K.; Hallstrom, S.; Chlopicki, S. S-nitroso human serum albumin given after LPS challenge reduces acute lung injury and prolongs survival in a rat model of endotoxemia. Naunyn. Schmiedebergs Arch. Pharmacol. 2009, 379, 281–290. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsiountsioura, M.; Cvirn, G.; Schlagenhauf, A.; Haidl, H.; Zischmeier, K.; Janschitz, N.; Koestenberger, M.; Wonisch, W.; Paar, M.; Wagner, T.; et al. The Antiplatelet Action of S-Nitroso Human Serum Albumin in Whole Blood. Biomedicines 2022, 10, 649. https://doi.org/10.3390/biomedicines10030649
Tsiountsioura M, Cvirn G, Schlagenhauf A, Haidl H, Zischmeier K, Janschitz N, Koestenberger M, Wonisch W, Paar M, Wagner T, et al. The Antiplatelet Action of S-Nitroso Human Serum Albumin in Whole Blood. Biomedicines. 2022; 10(3):649. https://doi.org/10.3390/biomedicines10030649
Chicago/Turabian StyleTsiountsioura, Melina, Gerhard Cvirn, Axel Schlagenhauf, Harald Haidl, Kathrin Zischmeier, Nicole Janschitz, Martin Koestenberger, Willibald Wonisch, Margret Paar, Thomas Wagner, and et al. 2022. "The Antiplatelet Action of S-Nitroso Human Serum Albumin in Whole Blood" Biomedicines 10, no. 3: 649. https://doi.org/10.3390/biomedicines10030649
APA StyleTsiountsioura, M., Cvirn, G., Schlagenhauf, A., Haidl, H., Zischmeier, K., Janschitz, N., Koestenberger, M., Wonisch, W., Paar, M., Wagner, T., Weiss, E.-C., & Hallström, S. (2022). The Antiplatelet Action of S-Nitroso Human Serum Albumin in Whole Blood. Biomedicines, 10(3), 649. https://doi.org/10.3390/biomedicines10030649







