Abstract
MicroRNAs are short, non-coding RNA molecules regulating gene expression by inhibiting the translation of messenger RNA (mRNA) or leading to degradation. The miRNAs are encoded in the nuclear genome and exported to the cytosol. However, miRNAs have been found in mitochondria and are probably derived from mitochondrial DNA. These miRNAs are able to directly regulate mitochondrial genes and mitochondrial activity. Mitochondrial dysfunction is the cause of many diseases, including cancer. In this review, we consider the role of mitochondrial miRNAs in the pathogenesis of lung cancer with particular reference to radon exposure.
1. Radon-Induced Lung Cancer Epidemiology
Lung cancer is a malignant neoplasm that forms in the epithelial cells of the bronchus. Lung tumors have a high risk of invasion and metastasis.
In the structure of oncological diseases in general, lung cancer had a leading position in the number of deaths among men and women in 2020. In terms of the number of cases, lung cancer is in first place among men (15.4%) and in third place among women (8.8%), after breast and colorectal cancer (https://gco.iarc.fr/) (accessed on 26 December 2021).
Lung cancer includes two basic histological types: small-cell lung cancer (SCLC) and non-small-cell lung carcinoma (NSCLC). SCLC stands at 15% of all lung cancers. It is the most differentiated type and it metastasizes faster, mainly to the lymph nodes [1]. Squamous cell lung cancers (SQCLC), adenocarcinoma, and large-cell anaplastic carcinoma (LCAC) are subtypes of NSCLC [2]. Adenocarcinoma is the least aggressive type of tumor that develops mainly in the peripheral bronchus and is mostly found among nonsmokers [3]. SQCLC develops in the main bronchus. Lung cancer usually has no overt signs and symptoms in its early stages. Therefore, the formation of a tumor in the lung is often diagnosed when it is already in the late stages of the disease, which affects the treatment, prognosis, and the survival of the patients [4]. Lung cancer cases are projected to increase by 70% by 2040 (https://gco.iarc.fr/) (accessed on 26 December 2021).
Lung cancer is a multifactorial disease, and various genetic, epigenetic, and environmental variations play a key role in its manner of development. However, smoking is the first environmental risk factor for the development of lung cancer in smokers. The risk of developing a lung tumor depends on the duration of smoking and the number of cigarettes smoked. Cancer can develop among passive and former smokers. In general, it should be noted that a lung tumor can be formed among people who have never smoked or been exposed to tobacco smoke [5].
However, in the case of lung cancer in non-smokers, exposure to radon is found to be a second environmental risk factor [6]. The International Agency for Research on Cancer (IARC) has also recognized radon as a carcinogen and a leading factor in the development of lung tumors in nonsmoking patients [7]. The increased risk of lung cancer is associated with high levels of radon exposure among underground miners [8]. Previously, it was assumed that exposure to radon is only an occupational risk. However, recent studies have shown a significant association between residential exposure to radon and lung cancer [9]. The WHO estimates that radon is responsible for up to 15% of lung cancer cases worldwide (www.who.int) (accessed on 26 December 2021). Residential radon exposure is a major factor in lung cancer mortality in Spain [10]. A study by Lorenzo-Gonzalez et al. confirmed that residential radon exposure increases the risk of lung cancer at concentrations above 50 Bq/m3 [11]. In Italy, the overall proportion of lung cancer deaths attributable to radon is about 10%. It is estimated that the majority of radon-associated lung cancer cases occur among smokers (up to 72%) [12]. There is a higher concentration of radon in new buildings compared to older homes in Canada [13]. There are 350 cases of lung cancer and 255 deaths each year in Ireland due to radon exposure [14].
2. Cellular Effects of Radon Exposure
There are three uranium isotopes in nature: uranium-238, uranium-235, and uranium-234. Uranium isotopes are radioactive and unstable, which means that they decay and turn into other elements, which are accompanied by the emission of particles.
The most common isotope found in uranium ore is uranium-238. This isotope has a half-life of about 4.47 billion years. Uranium-238 decays into unstable isotopes of thorium-234 with the formation of alpha radiation. Further decay leads to the formation of isotopes of uranium-234, which continue to decay until the formation of a stable isotope of lead-206 [15]. One of the key elements in the decay of uranium is radon-222, a radioactive unstable isotope, the decay of which occurs quite quickly for about 4 days and is accompanied by alpha radiation. The elements that are formed during the decay of radon isotopes are called “daughter products”. Starting with radon, further decay occurs rather quickly (in up to 30 min) with the emission of beta particles [16].
Most of the natural background radiation in the air comes from radon annually. Radon is a gas that enters the biosphere through cracks in the lithosphere and constitutes the Earth’s natural background radiation. When cells are exposed to densely ionizing radiation, such as radon alpha particles, a cascade of molecular and cellular events can occur, eventually leading to the formation of lung and other malignancies. Starting with the deposition of clusters of ionizations and concluding with the development of cancer, that flow may now be outlined. Cellular damage, DNA breakage, accurate or inaccurate repair, apoptosis, gene mutations, chromosomal alteration, and genetic instability are all caused by ionization. However, this can lead to the production of hazardous byproducts. Radon isotopes reach the lungs by inhalation and cause the formation of toxic byproducts. Endogenous radon exposure harms biomolecules.
According to the International Commission on Radiological Protection (ICRP), the relative biological effectiveness (RBE) of radon is 20, which means that, in living tissue, radon is estimated to create 20 times more damage than equal doses of beta or gamma radiation [17]. Thus, endogenous exposure to radon can cause various cytotoxic effects, such as chromosomal aberrations, the formation of reactive oxygen species, cell cycle disturbances, double-strand DNA breaks, and so on [18]. Meenakshi et al., using chromosomal aberrations as a marker of the risk of exposure to radon, showed that the RBE value for radon can be more than 38 [19].
Radon particles can damage cellular components by two mechanisms: linear energy transfer (LET) and the oxidation of cell components by reactive oxygen species (ROS). As they pass through the cell, the movement speed of alpha particles decreases, resulting in more energy releasing per unit of track length, which leads to the damage of cellular components. The path of the particle through the nucleus of the cell crosses many strands of DNA, and the energy released during this breaks the phosphodiester bond, resulting in the formation of double-stranded DNA breaks (DSBs) [20]. This leads to the most cytotoxic lesions caused by radon, and, in the case of defects in the work of the reparative systems, the formation of such breaks can lead to chromosomal instability [21]. Chromosomal instability is not only one of the causes of carcinogenesis but also contributes to tumor adaptation to cytotoxic anticancer drugs [22].
Endogenous factors of a different nature, including ionizing radiation, can trigger DSBs in mtDNA. However, DSBs, which are formed in mtDNA by radiation or ROS, do not lead to the loss of mitochondrial functional activity or cell death. These cells show the ability to survive with the severe loss of mtDNA, as well as a compensatory mechanism, reflected in the increase in the number of mitochondria [23].
The irradiation of cells stimulates mitochondria to increase the production of ROS [24]; therefore, it is mtDNA that is the closest target for their action. Previously, it was argued that mitochondria lack mtDNA repair mechanisms but recent studies report their presence [25]. RAD23A is a protein involved in NER in the nucleus of cells. It has been shown that RAD23A is involved in mitochondria following the induction of oxidative damage to mtDNA [26]. Protein XRCC4, in combination with DNA ligase IV and DNA-dependent protein kinase, is involved in the repair of double-stranded DNA breaks (NHEJ) and has also been found in mitochondria. However, despite the discovery of repair proteins for mtDNA oxidative damage, the mtDNA repair mechanisms themselves remain unknown [27].
Since water is the main component of cells, because of ionizing radiation absorption by the cell, radiolysis of water occurs, which leads to the formation of ROS [28]. Exceeding the level of cellular levels of ROS can cause damage to mitochondrial membranes [29] and, therefore, causes a violation of the membrane potential of mitochondria, which is the main symptom of mitochondrial dysfunction. Mitochondrial dysfunction leads to the release of pro-apoptotic cytochrome C, the activation of caspases-3 and -9, and further cell apoptosis [30].
3. Mitochondrial MicroRNAs
Mitochondria are important cellular organelles that support the life of cells by providing them with energy and play a key role in the process of cell death [31]. Mitochondrial dysfunction can cause serious diseases such as diabetes [32], Alzheimer disease, [33], and cancer [34,35]. MicroRNAs play an important role in the pathogenesis of various diseases. For example, by regulating oncogenes and tumor suppressor genes, microRNAs can control the process of carcinogenesis [36].
The proteome of human mitochondria includes more than 1000 proteins [37], of which only 13 are encoded in the mitochondrial genome and are the main components of the electron transport chain (ETC). The rest of the proteins are encoded in the nucleus and imported into the mitochondria [38].
The presence of pre-miRNAs, as well as mature miRNAs, in mitochondria has been reported, raising the possibility of mitochondrial miRNA synthesis. Some pre-miRNA variants seem to be metabolized in the mitochondria and may be used to produce mature miRNAs, which might be active in mitochondrial transcripts or transferred to the cytosol to interact with genomic mRNA. As a result, mitochondrial-processed miRNAs are anticipated to have a role in post-transcriptional gene regulation linked to mitochondrial functions [39].
They play their role in the normal functioning of mitochondria by regulating the mitochondrial genes themselves or by controlling the expression of nuclear transcripts involved in mitochondrial processes. This cluster of regulatory molecules is called mitochondrial miRNAs (mitomiR) [40]. There are three known miRNAs that are mapped in the mitochondrial genome: miR-1974, miR-1977, and miR-1978 [41].
The miRNAs regulate gene expression through association with the RNA-induced silencing complex (RISC). Dicer forms a RISC complex with RNA-binding proteins, TRBP (HIV-1 transactivating response (TAR) RNA-binding protein), PACT, and Ago-2. Ago-2 is the catalytic center of the RISC complex. Mature miRNAs loaded into the RISC complex bind to the 3′-untranslated region (3’-UTR) of mRNA. Furthermore, the translation inhibition or complete degradation of mRNA occurs [42]. Protein GW182 stabilizes the RISC complex by binding to Ago-2 [43]. The interaction of the GW18 protein with Ago-2 is important for the regulation of translation in the cytoplasm. However, for the translocation of microRNA into mitochondria, it is necessary to separate GW182 from the Ago-2 protein. Zhang et al. found that all three members of the GW182 family were excluded from mitochondria, in contrast to Ago-2 [44].
Usually, the transportation of mitochondrial proteins from the cytoplasm is carried out through special, highly specific transport complexes that are located on the outer (TOM40) and inner (TIM23) mitochondrial membranes. However, some proteins avoid granzymes (they lack the N-terminal mitochondrial target sequence) and use channels SAM50 and TIM22 to cross the OMM and IMM, instead of the canonical TOM40–TIM23 pathway. It should be noted that SAM50 has no channel translocase activity [45].
Cellular stress leads to the thermodynamic instability of GW182 and the release of microRNA/Ago-2 from the RISC complex. The proposed mechanisms for the translocation of the free microRNA/Ago-2 complex into mitochondria include both the TOM20–TIM and SAM50–TIM pathways [46]. However, it remains unknown what mechanism provides the transport of miRNAs into mitochondria.
4. The Role of MicroRNA in the Functional Activity of Mitochondria
Studies have shown that many different miRNAs play a key role in the regulation of mitochondrial functional activity (Table 1, Figure 1). There are identified microRNAs that are involved in the expression of mitochondrial proteins in the cell cytoplasm and mitochondrial matrix [47].

Table 1.
The role of various miRNAs in the regulation of mitochondrial genes.
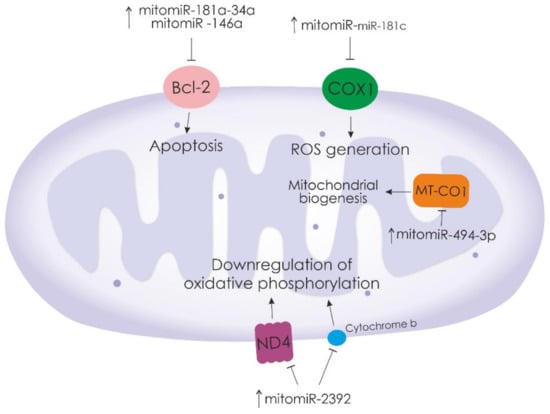
Figure 1.
Mitochondrial miRNAs that regulate the expression of major mitochondrial proteins. “ ”—activation of downstream signaling pathways.
”—activation of downstream signaling pathways.  —inhibition of downstream signaling pathways.
—inhibition of downstream signaling pathways.
 ”—activation of downstream signaling pathways.
”—activation of downstream signaling pathways.  —inhibition of downstream signaling pathways.
—inhibition of downstream signaling pathways.
The regulatory role of mitomiR in the mitochondrial metabolism is known. Thus, miR-194 plays an important role in glucose metabolism and is associated with the progression from insulin resistance to type 2 diabetes. Latouche et al. showed that miR-194 is suppressed in the skeletal muscle of insulin-resistant rats, mice with high fat levels, and people with prediabetes and type 2 diabetes [60]. The important role of miR-33 is known in the regulation of lipoprotein metabolism and associated disorders, including metabolic syndrome, obesity, and atherosclerosis [61]. The miR-128-1, miR-148a, miR-130b, and miR-301b control the expression of key proteins involved in cholesterol and lipoprotein transfer, such as the low-density lipoprotein (LDL) receptor (LDLR) and cholesterol transporter ATP-binding cassette A1 (ABCA1) [62]. Changes in mitomiR expression can have a protective effect in the event of cell damage by oxidative stress. Thus, miR-21 mediates a pronounced myocardial protective effect of kaempferol against damage caused by hypoxia and reoxygenation by reducing oxidative stress and activating the Notch1/PTEN/Akt signaling pathway [63]. The overexpression of miR-140 reduces ischemia–reperfusion damage to the myocardium and can also inhibit the expression of Drp1 and Fis1 proteins, which provoke mitochondrial division [64]. The increased expression of miR-200a-3p in proximal renal tubular epithelial cells (TECs) stimulates mitochondrial antioxidant protection and ATP production through the activation of the Keap1–Nrf2 signaling pathway that protects TECs from oxidative effects by normalizing the mitochondrial membrane potential and increasing the mitochondrial DNA copy number [65]. The exposure of HT22 cells to hydrogen peroxide leads to changes in the mitochondrial morphology and dynamic. The administration of exogenous miR-125b decreases cell viability and mitochondrial damage. Against the background of a decrease in miR-125b, an increase in the level of the p53 protein was noted, which led to mitochondrial damage and cell apoptosis. Thus, it was shown that the protective effect of miR-125b is due to the direct targeting of p53 [66].
The role of mitomiR in the regulation of apoptosis is known. For example, the protective properties of miRNA-214 result from a decreased apoptosis of cardiomyocytes in myocardial infarction in the elderly. Four target genes for miRNA-214, PUMA, PTEN, Bax, and caspase-7 were identified, the suppression of the expression of which leads to the inhibition of apoptosis [67]. In addition, an increase in the level of miRNA-214 was observed with a prolonged exposure to 2% sevoflurane, which led to the degeneration of brain neurons and the disruption of mitochondrial morphology. Furthermore, miRNA-214 suppresses the expression of the Mfn2 protein. The decrease in miRNA-214, in turn, led to the activation of the interaction between Mfn2 and Pkm2, which promotes the mitochondrial fusion and decreases brain damage [68].
5. The Role of Mitochondria in Oncogenesis and Tumor Development
Cancer is recognized as a multifactorial disease; this definition is consistent with the theory of somatic mutations in tumor initiation. However, there is evidence that cancer is a metabolic disease resulting from disturbances in the metabolic activity of mitochondria. As Warburg suggested, it is the change in mitochondrial respiration that leads to the malignant transformation of cells [69]. However, there is a possibility that the so-called Warburg effect is just a phenotype encoded by the nuclear genome. To determine the possible effect of cytoplasm on oncogenesis, several different experiments have been carried out. Defects in mitochondrial function have long been suspected of playing a role in cancer genesis and progression. All the experiments carried out with the development of cybrid systems clearly showed that tumor development depends on the cytoplasm and cellular components and not the cell nucleus [70,71,72].
Modern research has shown more than once that it is the disturbances in the functional activity of mitochondria that can lead to the malignant transformation of cells. In general, five possible pathways for the participation of mitochondria in carcinogenesis and the further development of the tumor can be identified. First, as we stated earlier, a change in cellular metabolism is one of the causes of malignant cell transformation. Many studies have confirmed this, identifying disorders of mitochondrial metabolism as the cause of cancer development [73,74].
The second cause of carcinogenesis is oxidative cellular stress. A result of metabolism is that mitochondria produce free radicals, which are subsequently capable of attacking the cell. In the process of energy synthesis, the last electron leaving the electron transport chain is oxygen O2, which is further reduced to water. However, about 2% of molecular oxygen is reduced to superoxide, which, in turn, is the precursor to many forms of ROS [75]. Moreover, although ROS are normal products of cellular respiration and are always present in the cell, an increase in their amount, for example, because of the disturbances in mitochondria, leads to damage to cellular organelles and DNA [76]. In addition, the active production of ROS is closely related to the effect of external factors on cells, such as radon and asbestos [32]. It is now evident that ROS induction is associated with the development of cancer at different stages [77], metastasis, progression, and the survival of tumor cells [78,79].
Third, the appearance of a tumor is associated with the suppression of apoptosis. In a way, mitochondria and ROS play the role of a two-faced Janus in carcinogenesis. As a result of overload in the mitochondrial electric transport chain, there is an increased formation of free radicals and ROS, which, in some cases, can lead to carcinogenesis; in others, ROS are effectors for activating the mitochondrial apoptotic pathway. ROS damage the lipid bilayer of the outer mitochondrial membrane, resulting in the release of an apoptogenic protein (CytC) into the cytoplasm. In the cytosol, CytC participates in the assembly of the apoptosome, which activates the initiator caspase-9 and effector caspase-3, leading to cell death [80]. Apoptosis suppresses tumorigenesis by removing damaged cells from the healthy pool. However, cancer cells can inhibit apoptosis, leading to tumor growth. A whole family of apoptosis inhibitor proteins (IAPs) is known, among which IAP is a potent inhibitor of caspases [81]. Many studies show increased IAP expression in various types of cancer, such as cancers of the lung [82], prostate [83], chest [84], bile [85], ovaries [86], and bladder [87], as well as melanoma [88], lymphoma [89], glioblastoma [90], and hepatocellular carcinoma [91]. It is noted that the overexpression of XIAP promotes the survival of cancer cells and the development of cancer [92]; however, mitochondria, in this case, may have a protective function. In response to apoptogenic stimuli, the ARTS protein is released from mitochondria and binds to XIAP, thereby promoting apoptosis [93].
Mitochondria are organelles that are unique in nature. As discussed above, the role of mitochondria in the cell is quite diverse, from providing energy to cell death. Since mitochondria are autonomous organelles with their own ribosomes and DNA, the D-loop region of mitochondrial DNA (mtDNA) contains important regulatory elements, and mutations in this region can lead to certain consequences for the cell. Moreover, the localization of mtDNA in the immediate vicinity of the source of the formation of free radicals and ROS makes it the most vulnerable to damage. Thus, it was studied that somatic mutations of the Mnl I site can be the cause of the pathogenesis of breast cancer [94]. The somatic deletion (50 bp) of the mtDNA D-loop was found in gastric adenocarcinoma [95]. A total of 48 mutant D-loop sites were found from the analysis of brain cancer samples [96]. Three D-loop mutation hotspots at sites 146, 152, and 186 are associated with the risk of oral cancer [97]. Thus, we can conclude that the mutation in mtDNA is the fourth variant of oncogenesis. There is also evidence that not only are mutations in mtDNA involved in the process of cell transformation, but also in changing the number of free circulating mtDNA in the blood plasma of healthy people and cancer patients. Moreover, CS-mtDNA is a molecular marker of oncology [98,99].
Given the participation of mitochondria in apoptosis, it is valid to assume that they are a therapeutic target in the treatment of cancer [100,101]. Nevertheless, mitochondria in this case also exhibit opposite functions and provide cancer cells with drug resistance, which can be attributed to the fifth variant of the participation of mitochondria in the development of cancer. For example, it is known that the overexpression of the mitochondrial protein OPA 1 leads to resistance to cisplatin treatment. At the same time, the suppression of OPA 1 correlates with a high release of cytochrome c into the cytoplasm and promotes apoptosis. Accordingly, the suppression of OPA 1 expression can reduce cisplatin resistance [102]. Another mechanism for the survival of cancer cells after chemotherapy is the transfer of mitochondria; Moschoi R et al. showed this well in their research. Acute myeloid leukemia cells can capture mitochondria from a healthy environment after being treated with cytarabine, which leads to their survival [103].
6. The Role of Mitochondrial miRNAs in Oncogenesis and the Development of Various Forms of Cancer
Due to their diverse functions, mitochondria are involved in various mechanisms of cell stress, malignant transformation, and the adaptation of cancer cells, ensuring their survival and tumor development. However, many mitochondrial processes can be controlled and regulated by microRNA molecules. As mentioned above, mitomiR is a separate group of microRNA molecules that can regulate the expression of mitochondrial proteins encoded by nuclear genomes or penetrate mitochondria and control the main regulatory elements of the mitochondrial genome [39].
The miR-1 is an oncosuppressive miRNA; miR-1 expression is known to be suppressed in various types of cancer. A decrease in miR-1 has been noted in prostate cancer. The suppressive function of miR-1, in this case, was achieved by the inhibition of E2F5 and PFTK1, which promote cell proliferation and cell cycle progression [104]. The overexpression of miR-1-3p significantly suppressed proliferation and induced the apoptosis of hepatocellular carcinoma cells by inhibiting SOX9, which reduced tumor volume in vivo [105]. The miR-1 may suppress tumor growth and metastasis in breast and stomach cancers by acting on six target genes (CDK4, TWF1, CNN3, CORO1C, WASF2, and TMSB4X) [106]. Liu C. et al. identified miR-1 as mitomiR because the overexpression of miR-1 caused mitochondrial damage in melanoma and breast cancer stem cells. The miR-1 binds to 3HTO MINOS1, which is a component of the MICOS complex of the inner mitochondrial membrane, maintains the structure of the inner membrane, and participates in the formation of contact sites with the outer membrane, GPD2 (the protein encoded by this gene is localized on the inner mitochondrial membrane and catalyzes the conversion of glycerol-3-phosphate to dihydroxyacetone phosphate), and LRPPRC (in the mitochondria, it binds to mRNA poly (A) and participates in the regulation of transcription). Interestingly, in normal cancer cells, miR-1 activity did not result in mitochondrial destruction [107].
There was a decrease in miR-29b-3p expression in gastric cancer cells compared to normal epithelial cells of the human gastric mucosa. Myc-associated zinc finger protein (MAZ) has been identified as a direct target for miR-29b-3p. The suppression of MAZ led to the inhibition of the proliferation and migration of cancer cells [108]. The miR-29b-3p also inhibits angiogenesis by binding to transcripts of VEGFA and PDGFB genes in endothelial cells of retinal microvessels [109]. Mao A. et al. observed that miR-29b-3p enhances the therapeutic effect of X-ray and radioactive carbon ion irradiation in the treatment of prostate cancer. The miR-29b-3p negatively regulates the expression of WISP1 and, as a result, the anti-apoptotic protein Bcl-XL is suppressed and the mitochondrial apoptosis pathway is triggered. It should be noted that a decrease in miR-29b-3p levels leads to cancer radioresistance [110].
Several studies reported an association between miR-155 and lung cancer [111], esophageal cancer [112], and breast cancer [113]. Changes in the metabolism of cancer cells contribute to the transformation, survival, and invasion of tumor cells. A study by Kim S. et al. showed how oncomiR-155 promotes breast tumor growth by regulating glucose metabolism. The miR-155, through the regulation of the p85α–AKT–FOXO3a pathway, controls cMYC, which is a major regulator of glycolysis. The c-MYC activates glucose transporter proteins and its metabolic enzymes HK2, PKM2, and LDHA. As is known, cancer cells change their metabolism by receiving energy through glycolysis, and miR-155 increases glucose uptake and enhances glycolysis, thereby ensuring the viability of cancer cells [114].
The miR-181a-5p also contributes to the change in glucose metabolism in liver cancer. The overexpression of mitomiR-181a-5p decreases the level of protein components of the electron transport chain mt-CYB and mt-CO2, which results in the decrease in the membrane potential of mitochondria. However, the expression of HK2 (glycolysis regulator) and GLUT1 (glucose transporter) increases. As a result, the increased consumption of glucose and the activation of glycolysis contribute to the proliferation and metastasis of cancer cells [115].
It is known that miR-145 can suppress cell proliferation and migration in melanoma [116] and endometrial cancer [117] as well as stomach cancer [118]. According to a study by Zhao Sh. et al., it can be concluded that miR-145 can be attributed to mitomiR because this mitomiR suppresses the function of mitochondria in ovarian cancer cells. First, miR-145 overexpression correlates inversely with ATP and mtDNA levels in cancer cells due to ARL5B targeting. Second, an increase in the level of miR-145 leads to a decrease in the membrane potential of mitochondria and promotes the release of cytochrome C into the cytoplasm. Thirdly, miR-145 inhibits SDHA (one of the main components of the mitochondrial respiratory chain complex) and HSP60 (a chaperone involved in the correct folding and import of mitochondrial proteins) [119].
The miR-125b is known as a tumor suppressor in breast cancer [120], ovarian cancer [121], hepatocellular carcinoma [122], and esophageal cancer [123]. Xie X. et al. reported that the decreased expression of miR-125b resulted in cell resistance to doxorubicin. A normal miR-125b level increases cell cytotoxicity to doxorubicin and activates cell apoptosis by inhibiting anti-apoptotic protein Mcl-1. The treatment of cells with miR-125b and doxorubicin causes a loss of the mitochondrial membrane potential, which ultimately leads to an increase in the permeability and activation of caspases [124].
Chen W. et al. determined that a decreased expression of mito-miR-5787 leads to chemoresistance to cisplatin by altering glucose metabolism and the negative regulation of MT-CO3. MT-CO3 is a component of cytochrome-c-oxidase of the OXPHOS system, and its suppression weakens OXPHOS. Cytochrome-c-oxidase is a component of the respiratory chain that catalyzes the reduction of oxygen to water, which explains why miR-5787 knockdown increases ROS generation. Moreover, miR-5787 knockdown increases the expression of HK2 and PKM2, which contributes to the increase in glucose uptake and, therefore, to an increased production of lactate. This change in the pH of the cell balance leads to the resistance of the cells to cisplatin [125].
Thus, based on a review of the available information on this subject, it can be concluded that mitomiR can not only be biological markers of oncology but also participate in the survival and development of cancer cells, controlling mitochondrial functional activity.
7. The Role of Mitochondria and mtDNA in the Development of Lung Cancer
According to GLOBOCAN data in 2020, out of 36 types of cancer in 185 countries, lung cancer is the most commonly diagnosed form and the leading cause of cancer death among men in 96 countries. Among women, lung cancer is the second most common and the leading cause of cancer death (https://gco.iarc.fr/) (accessed on 26 December 2021).
Although most of the data report that lung cancer is a multifactorial disease, the pathogenesis of which is caused by a genetic predisposition (polymorphisms in the genes for DNA repair and the detoxification of xenobiotics, the spontaneous inactivation of tumor suppressor genes) [126] and exposure to external factors, such as smoking, asbestos [127], and radon [128] in the case of lung cancer.
There are data that show the different roles of mitochondria in the emergence and development of lung tumors [129]. An increase in glucose uptake by cells leads to the synthesis of insulin, which increases the IGF-1 level and suppresses the production of IGF-binding proteins. Typically, tumor cells, including lung cancer cells, have a high level of IGF-1R expression. The IGF-1/IGF-1R complex increases the expression of VEGF, which promotes angiogenesis and tumor development. The knockdown of the SNCG gene leads to the inhibition of IGF-1 and IGF-1R, and, consequently, cell proliferation and tumor growth [130].
It is worth noting that mitochondrial damage leads to tumor progression. Lung cancer metastases showed a lower membrane potential with reduced mitochondrial functionality compared to non-metastatic primary tumors. Electron microscopy of the metastases showed irregular shapes of mitochondria in which transverse bridges were formed between the membranes, which led to a change in the mitochondrial structure [131].
With the growth of lung tumors, mtDNA depletion is also observed, which was associated with a low metastatic potential and an unfavorable prognosis of survival for patients with lung cancer [132]. However, as for freely circulating mtDNA, it is worth noting that in the case of exposure to aggressive factors, such as tobacco smoke, radon, or asbestos, an increase in the level of cf-MtDNA and proinflammatory cytokines in the plasma of patients with NSCLC occurs, which correlates with the poor prognosis of the survival rate [133]. It was noted that cf-mtDNA levels increased in patients with radon-induced lung cancer and in healthy donors living in areas with high radon levels [134]. This can be explained by the fact that the main provocateurs of lung tissue carcinogenesis (smoking and radon) increase the production of ROS, which, in turn, damages various structural parts of cells including the lipid bilayer of mitochondrial membranes. Such damage to mitochondria causes disturbances in their functional activity, affecting the work of the electron transport chain and the oxidation of mtDNA. In response to oxidative stress, mitochondria seek to increase the number of copies of their DNA [135]. Long-term exposure to negative factors contributes to the constant production of ROS, which leads to greater consequences: the apoptosis [136] or necrosis [137] of cells.
In the case of apoptosis, the BAX (Bcl-2-associated X protein) and BAK (BCL2 Antagonist/Killer 1) proteins invading the mitochondrial membrane increase its penetrating ability. As a result, intracellular mtDNA released into the cytosol can be associated with cGAS–STING, TLR9 (Toll Like Receptor 9), and the inflammasomes NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3), NLRC4 (NLR Family Pyrin Domain Containing 4), and (absent in melanoma 2), which promotes an enhanced immune response [138]. Similar to bacterial DNA, mitochondrial DNA is hypomethylated at CpG islands, which explains its recognition by TLR9 receptors [139]. After binding to mtDNA, TLR9 recruits its intracellular adapter MyD88, which activates a number of signaling proteins such as MAPK and NF-κB [140]. NF-κB is a central participant in many inflammatory processes; after activation, it is translocated into the nucleus and, acting as a transcription factor, triggers the synthesis of pro-inflammatory cytokines and chemokines [141]. However, genes activated by NF-κB can be involved in various processes of cell carcinogenesis. For example, anti-apoprotein, Fas, BCL-2, and c-FLIP promote cell survival and avoid apoptosis; BCL-2L1, PAL2 b, and cyclins regulate cell proliferation; cytokines IL-1, IL-2, IL-6, IL-8, IL -12, and TNF-a support inflammation; chemokines MCP-1, IL-18, CXCL1m and CXCL10 cause angiogenesis; and ICAM-1, VCAM-1, and ECAM-1 promote invasion and metastasis [141]. Dimitrakopoulos et al. reported that the dysregulation of NF-κB in NSCLC plays a negative role in predicting patient survival and increases the chance of disease recurrence [142]. It is known that mtDNA can move to neighboring cells to spread the inflammation focus. Horizontal transfer of mtDNA is carried out using several possible mechanisms: directly through gap junctions between cells, nanotubules formed between neighboring cells in the tumor microarray, or packing into extracellular vesicles [143]. Maintaining inflammation is very important for the onset and development of lung cancer. COPD is known to be associated with chronic inflammation and is a precancerous disease. The mtDNA profile was significantly increased in both COPD and lung cancer [144]. However, inflammation can express a protective effect against tumors. After activation, neutrophils create antitumor immunity, which is aimed in suppressing cancer [145]. A low index of inflammation is associated with poor overall survival of cancer patients with NSCLC and squamous cell carcinoma of the head and neck as well as with diffuse large-cell lymphoma [146]. Prolonged systemic inflammation results in mtDNA being released into the bloodstream, stimulates the neutrophil activity through TLR9 binding and p38 MAPK activation, and causes inflammation [147]. Lai et al. determined that cc-mtDNA can be identified as a diagnostic biomarker for NSCLC. In addition, different levels of cytokine expression induced by TLR9 activation can be an important criterion for determining the stages of cancer and making a prognosis for lung cancer patients [148].
Since mitochondria are generators of ROS, it is mtDNA that is the closest target for their action. As a result, various mutations accumulate in mtDNA. The mtDNA mutations are common in lung cancer. Raghav et al. found that the mean germline mtDNA mutation rate in stage 1 lung adenocarcinoma was 2.29 mutations per kbp. Most of the mutations occurred in the D-loop and the CYTB gene (which are part of the mitochondrial respiratory chain) [149].
8. Mitochondrial miRNAs Are Involved in the Development of Lung Cancer through the Regulation of Mitochondrial Genes
It is impossible to answer the question of how exactly a tumor arises and develops. There are many mechanisms underlying carcinogenesis. This is a rather complex process that includes several successive stages necessary for the full growth of the tumor. Intracellular changes or cell damage initiates the process of the malignant transformation of cells. Furthermore, there is a change in the phenotype of cancer cells, cells stop responding to cellular signals and lose control over proliferation, and the tumor begins to actively grow and form its own microenvironment. After the formation of a non-invasive tumor, which is also called “cancer in place”, angiogenesis begins, new blood vessels are formed, which directly feed the growing tumor, and the growing tumor progresses [150].
Each stage from the initiation of tumorigenesis to metastasis is accompanied by various biochemical changes and is initiated by cellular signals. For example, the oxygen starvation of a tumor activates HIF-1α, which, in turn, induces the expression of VEGF and its receptor, which eventually stimulates the formation of new blood vessels [151].
MicroRNA molecules can regulate any pathway of cell signaling and, accordingly, they can control the stages of carcinogenesis [152]. The role of microRNA in the development of radon-induced lung cancer as well as asbestos-induced lung cancer is already known [153]. Therefore, it is of interest to consider the role of mitomiR in the pathogenesis and development of lung cancer.
Transcription factors also play a vital role in cancer proliferation; the overexpression of the transcription factor NFAT5 promotes the proliferation and migration of lung adenocarcinoma cells [154]. Phosphoglycerate kinase 1 (PGK-1), one of two ATP-generating enzymes during glycolysis, is the target gene for NFAT5 [155]. The miR-194 suppresses the migration and proliferation of NICRL cells, inhibiting the expression of NFAT5, whereas the level of miR-194 decreases in NSCLC cells with a high content of glucose [156].
Oxidative stress induces the expression of miR-200c, which leads to cell death. The transcriptional repressor ZEB1 is a direct target for miR-200c [157]. Decreased ZEB1 expression in NSCLC correlates with overall patient survival. The lower the expression of ZEB1, the better the survival rate with NSCLC [158]. MaD’Almeida et al. studied the effect of miR-200c encapsulated in cholesterol carriers in the cells. Given the nature of the carrier, the Nano miR-200c was designed to penetrate the mitochondria and the cell nucleus. As a result, an increase in the expression of mitochondrial genes was observed, which can later be used to restore the membrane potential of mitochondria and normalize the work of the electron transport chain (ETC) [159].
The miR-214 is an oncosuppressive agent in the advanced types of cancer; however, studies show it to be an inducer of cell proliferation and metastasis in lung cancer. The miR-214 regulates hypoxia-inducible factor 1 (HIF-1α), matrix metalloproteinase-2 (MMP2), and vascular endothelial growth factor (VEGF) by inhibiting the inhibitor of growth protein 4 (ING4) [160]. The suppression of miR-214 expression reduces the rate of glycolysis and lactate production by decreasing the level of hexokinase-2 (HK2), which plays a key role in maintaining the integrity of the outer mitochondrial membrane and pyruvate kinase (PKM2) in NSCLC cells. The miR-214 is capable of increasing cell proliferation by targeting PTEN and, consequently, regulates the PI3K/AKT/ mTOR signaling pathway [161].
9. Effect of Radon on Both MicroRNA Profile and Lung Cancer Risk
MicroRNAs regulate a variety of cellular processes, including those induced by the effects of radiation exposure. Several studies recognize microRNAs as biomarkers for assessing the degree of radiation contamination with radon. They are important regulators of various genes associated with the risk of lung cancer.
Sun et al. identified miR-19a, miR-30e, miR-335, and miR-451a as potential biomarkers of radiation damage by radon. These microRNAs were reduced in the peripheral blood of miners who had been exposed to radon for a long time, whereas the level of cyclin A2, cyclin D1, and cyclin E1 was significantly increased [162].
Several studies have been performed using the BEAS-2B healthy cell line model, and long-term exposure to radon led to an increase in miR-34a expression in it. As a result, the levels of the anti-apoptotic proteins Bcl-2 and PARP-1 were decreased, and the expression of the pro-apoptotic protein Bax was increased. As a result, an increase in the apoptosis of BEAS-2B cells was observed [163]. Cui et al. conducted a study of the change in the levels of microRNA in cells after irradiating with a high dose of radon (20,000 Bq/ m3) five times. Screening for differential miRNA expression was performed after the first passage (Rn5-1 cells) and after the 20th passage (Rn5-20 cells). As the outcome, 163 increased and 155 decreased Rn5-1163 cells were found. Rn5-20 cells had 30 upregulated and 28 downregulated miRNAs [164]. Dang et al. studied the effect of radon alpha radiation on the microRNA profile, using cell models with different radiation doses of 0.1, 0.5, and 2 Gy; a total of 24 and 41 miRNAs showed a change in the microRNA profile in the 10th and 40th generations of cells, respectively [165] (Table 2, Table 3).

Table 2.
The miRNAs that were significantly increased after exposure to different concentrations of radon in cells.

Table 3.
The miRNAs that were significantly reduced after exposure to different concentrations of radon in cells.
Another study showed a decrease in the microRNA profile (hsa-miR-16-2-3p, hsa-miR-182-3phsa-miR-221-5p, hsa-miR-30c-2-3p, hsa-miR-3660, hsa-miR-4306, hsa-miR-4440, hsa-miR-4443, hsa-miR-452-5p, hsa-miR-454-3p, hsa-miR-455-3p, hsa-miR-4793-3p, hsa-miR-598-3p, hsa-miR-6500-5p, hsa-miR-6826-5p, hsa-miR-6872-3p 0, hsa-miR-7159-5p, hsa-miR-98-5p) of the patients with diagnosed lung cancer who live in areas with high levels of radon in the air [166].
Possible microRNA target genes whose expression is affected by radon exposure were predicted using TargetScan software (Release 7.2). A total of seven of them are presumed to play a role in mitochondrial protein expression (Figure 2). These data can be confirmed by further experiments.
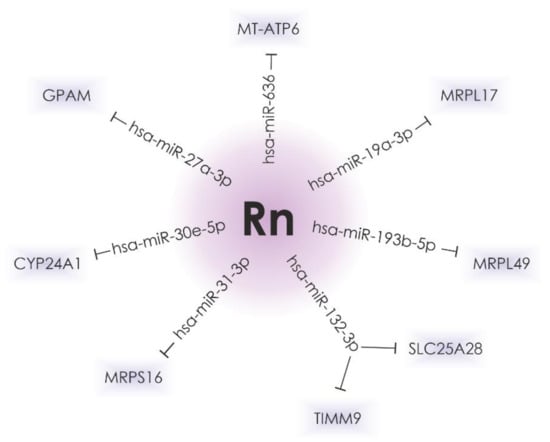
Figure 2.
The hsa-miR-636, hsa-miR-27a-3p, hsa-miR-30e-5p, hsa-miR-31-3p, hsa-miR-132-3p, hsa-miR-193b-5p, and hsa-miR-19a-3p, the expression of which is significantly altered in lung cancer and after radon irradiation, can possibly regulate the expression of genes that encode mitochondrial proteins.
Alpha radiation is able to activate autophagy and promote ROS accumulation by upregulating miR-22. Regulated in development and DNA damage response 1 (REDD1) is known as a gene that is activated in response to DNA damage [167]. REDD1 has been identified as a direct target gene for miR-22. REDD1 accumulates in mitochondria and serves as a regulator of mitochondrial metabolism. During irradiation, miR-22 blocks the expression of REDD1, thereby promoting the accumulation of DNA damage and active ROS generation and enhancing apoptosis [168].
In mammals, one important epigenetic mechanism for regulating chromatin structure and gene expression is DNA methylation [169]. Many CpG sites regulate miRNA expression in cells. Changes in DNA methylation can lead to the differentiated expression of miRNA in cancer cells [170]. Hypermethylation in p16INK4 and O6-MGMT is proportional to the effect of radon among uranium miners. However, ionizing radiation above 1 Gy is often characterized by a loss of global DNA methylation [171]. If ionizing radiation and radon, among other things, can influence methylation patterns, then it is possible this causes the alteration of microRNA expression after exposure to radiation. It is possible that hypomethylation leads to overexpression, and hypermethylation, on the other hand, reduces the microRNA profile.
10. Conclusions
Radon-induced lung cancer is a significant problem worldwide. Radon is the main component of the radioactive background of our planet. The population is exposed to ionizing radiation at home and in the workplace. Despite the fact that the WHO has determined the permissible radon standards to be 100 Bq/m3, the risk of lung cancer increases from 50 Bq/m3. Prolonged exposure to radon causes damage to cellular components, mitochondria, and oxidative stress, which leads to damage to lung tissue [172]. The extensive role of microRNA molecules in the regulation of carcinogenesis processes induced by radon has long been known. MitomiR is a class of microRNA molecules that can regulate the expression of mitochondrial proteins and control the functional activity of mitochondria. Therefore, it was of interest to investigate the participation of mitomiR in the development of lung cancer. In our review, it was shown that various mitomiRs are involved in the pathogenesis and progression of lung cancer acting as regulators of mitochondrial processes. As a result, it is possible to state the study of mitomiRs as new biomarkers of radon-induced lung cancer is a relevant and promising area.
Funding
This study was partially supported by the Ministry of Education and Science of the Republic of Kazakhstan (Grant No. AP08856116).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, S.; Zhang, Z.; Wang, Q. Emerging therapies for small cell lung cancer. J. Hematol. Oncol. 2019, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer, current treatment, and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G. The distinctive nature of adenocarcinoma of the lung. OncoTargets Ther. 2015, 8, 2399–2406. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gandara, D.R.; Hammerman, P.S.; Sos, M.L.; Lara, P.N.; Hirsch, F.R. Squamous cell lung cancer, from tumor genomics to cancer therapeutics. Clin. Cancer Res. 2015, 21, 2236–2243. [Google Scholar] [CrossRef] [PubMed]
- Latimer, K.M. Lung cancer, clinical presentation and diagnosis. FP Essent. 2018, 464, 23–26. [Google Scholar] [PubMed]
- Abdel-Rahman, O. Incidence and mortality of lung cancer among never smokers in relationship to secondhand smoking, findings from the PLCO trial. Clin. Lung Cancer 2020, 21, 415–420. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Man-Made Mineral Fibers, and Radon; IARC Press: Lyon, France, 1988; Volume 43, pp. 1–300. [Google Scholar]
- National Research Council; Committee on Health Risks of Exposure to Radon; Board on Radiation Effects Research; Commission on Life Sciences. Health Effects of Exposure to Radon; BEIR-VI; National Academy Press: Washington, DC, USA, 1999. [Google Scholar]
- Cheng, E.S.; Egger, S.; Hughes, S.; Weber, M.; Steinberg, J.; Rahman, B.; Worth, H.; Ruano-Ravina, A.; Rawstorne, P.; Yu, X.Q. Systematic review and meta-analysis of residential radon and lung cancer in never-smokers. Eur. Respir. Rev. 2021, 30, 200230. [Google Scholar] [CrossRef] [PubMed]
- Ruano-Ravina, A.; Varela Lema, L.; García Talavera, M.; García Gómez, M.; González Muñoz, S.; Santiago-Pérez, M.I.; Rey-Brandariz, J.; Barros-Dios, J.; Pérez-Ríos, M. Lung cancer mortality attributable to residential radon exposure in Spain and its regions. Environ. Res. 2021, 199, 111372. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Gonzalez, M.; Ruano-Ravina, A.; Torres-Duran, M.; Kelsey, K.T.; Provencio, M.; Parente-Lamelas, I.; Piñeiro-Lamas, M.; Varela-Lema, L.; Perez-Rios, M.; Fernandez-Villar, A.; et al. Lung cancer risk and residential radon exposure: A pooling of case-control studies in northwestern Spain. Environ. Res. 2020, 189, 109968. [Google Scholar] [CrossRef]
- Bochicchio, F.; Antignani, S.; Venoso, G.; Forastiere, F. Quantitative evaluation of the lung cancer deaths attributable to residential radon: A simple method and results for all the 21 Italian Regions. Radiat. Meas. 2012, 50, 121–126. [Google Scholar] [CrossRef]
- Simms, J.A.; Pearson, D.D.; Cholowsky, N.L.; Irvine, J.L.; Nielsen, M.E.; Jacques, W.R.; Taron, J.M.; Peters, C.E.; Carlson, L.E.; Goodarzi, A.A. Younger North Americans are exposed to more radon gas due to occupancy biases within the residential built environment. Sci. Rep. 2021, 11, 6724. [Google Scholar] [CrossRef]
- Murphy, P.; Dowdall, A.; Long, S.; Curtin, B.; Fenton, D. Estimating population lung cancer risk from radon using a resource efficient stratified population weighted sample survey protocol-Lessons and results from Ireland. J. Environ. Radioact. 2021, 233, 106582. [Google Scholar] [CrossRef] [PubMed]
- Vogiannis, E.G.; Nikolopoulos, D. Radon sources and associated risk in terms of exposure and dose. Environ. Health 2015, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bersimbaev, R.; Pulliero, A.; Bulgakova, O.; Kussainova, A.A.; Aripova, A.; Izzotti, A. Radon biomonitoring and microRNA in lung cancer. Int. J. Mol. Sci. 2020, 21, 2154. [Google Scholar] [CrossRef] [PubMed]
- Valentin, J. Relative biological effectiveness (RBE), quality factor (Q), and radiation weighting factor (wR): ICRP Publication 92. Ann. ICRP 2003, 33, 1–121. [Google Scholar]
- Robertson, A.; Allen, J.; Laney, R.; Curnow, A. The cellular and molecular carcinogenic effects of radon exposure, a review. Int. J. Mol. Sci. 2013, 14, 14024–14063. [Google Scholar] [CrossRef]
- Meenakshi, C.; Mohankumar, M.N. Relative biological effectiveness of radon. An in vitro study using chromosome aberrations as a biomarker. Int. J. Radiat. Biol. 2015, 91, 681–685. [Google Scholar] [CrossRef]
- National Research Council (U.S.). Committee on Risk Assessment of exposure to radon in drinking water. In Risk Assessment of Radon in Drinking Water; National Academies Press: Washington, DC, USA, 1999; p. 6. [Google Scholar]
- Huang, L.; Snyder, A.; Morgan, W. Radiation-induced genomic instability, and its implications for radiation carcinogenesis. Oncogene 2003, 22, 5848–5854. [Google Scholar] [CrossRef]
- Vargas-Rondón, N.; Villegas, V.E.; Rondón-Lagos, M. The role of chromosomal instability in cancer and therapeutic responses. Cancers 2017, 10, 4. [Google Scholar] [CrossRef]
- Moretton, A.; Morel, F.; Macao, B. Selective mitochondrial DNA degradation following double-strand breaks. PLoS ONE 2017, 12, e0176795. [Google Scholar] [CrossRef]
- Kostyuk, S.V.; Proskurnina, E.V.; Konkova, M.S.; Abramova, M.S.; Kalianov, A.A.; Ershova, E.S.; Izhevskaya, V.L.; Kutsev, S.I.; Veiko, N.N. Effect of low-dose ionizing radiation on the expression of mitochondria-related genes in human mesenchymal stem cells. Int. J. Mol. Sci. 2022, 23, 261. [Google Scholar] [CrossRef]
- Rong, Z.; Tu, P.; Xu, P.; Sun, Y.; Yu, F.; Tu, N.; Guo, L.; Yang, Y. The mitochondrial response to DNA damage. Front. Cell Dev. Biol. 2021, 9, 669379. [Google Scholar] [CrossRef]
- Fontana, G.A.; Gahlon, H.L. Mechanisms of replication and repair in mitochondrial DNA deletion formation. Nucleic Acids Res. 2020, 48, 11244–11258. [Google Scholar] [CrossRef]
- Wisnovsky, S.; Jean, S.; Liyanage, S. Mitochondrial DNA repair and replication proteins revealed by targeted chemical probes. Nat. Chem. Biol. 2016, 12, 567–573. [Google Scholar] [CrossRef]
- Azzam, E.I.; Jay-Gerin, J.P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef]
- Maureen, R.D. Activation of apoptosis signaling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1863, 12, 2977–2992. [Google Scholar]
- Li, X.; Fang, F.; Gao, Y.; Tang, G.; Xu, W.; Wang, Y.; Kong, R. ROS induced by killerred targeting mitochondrion (mtKR) enhances apoptosis caused by radiation via Cyt 3 pathc. Oxid. Med. Cell. Longev. 2019, 11, 4528616. [Google Scholar]
- Kushnareva, Y.; Newmeyer, D.D. Bioenergetics and cell death. Ann. N. Y. Acad. Sci. 2010, 1201, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Sivitz, W.I.; Yorek, M.A. Mitochondrial dysfunction in diabetes, from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid. Redox. Signal. 2010, 12, 537–577. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, F.; Ma, X. Mitochondrion dysfunction in the pathogenesis of Alzheimer’s disease, recent advances. Mol. Neurodegener. 2020, 15, 30. [Google Scholar] [CrossRef]
- Księżakowska-Łakoma, K.; Żyła, M.; Wilczyński, J.R. Mitochondrial dysfunction in cancer. Prz. Menopauzalny 2014, 13, 136–144. [Google Scholar] [CrossRef]
- Boland, M.L.; Chourasia, A.H.; Macleod, K.F. Mitochondrial dysfunction in cancer. Front. Oncol. 2013, 3, 292. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C. The role of MicroRNAs in human cancer. Sig. Transduct. Target Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Pfanner, N.; Warscheid, B.; Wiedemann, N. Mitochondrial proteins, from biogenesis to functional networks. Nat. Rev. Mol. Cell Biol. 2019, 20, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Calvo, S.E.; Clauser, K.R.; Mootha, V.K. MitoCarta2.0, an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 2016, 44, D1251–D1257. [Google Scholar] [CrossRef] [PubMed]
- Bienertova-Vasku, J.; Sana, J.; Slaby, O. The role of microRNAs in mitochondrion in cancer. Cancer Lett. 2013, 9, 1–7. [Google Scholar] [CrossRef]
- Srinivasan, H.; Das, S. Mitochondrial miRNA (MitomiR), a new player in cardiovascular health. Can. J. Physiol. Pharmacol. 2015, 93, 855–861. [Google Scholar] [CrossRef]
- Bandiera, S.; Rüberg, S.; Girard, M.; Cagnard, N.; Hanein, S.; Chrétien, D.; Munnich, A.; Lyonnet, S.; Henrion-Caude, A. Nuclear outsourcing of RNA interference components to human mitochondrion. PLoS ONE 2011, 6, e20746. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Rossi, J. RNAi mechanisms and applications. Biotechniques 2008, 44, 613–616. [Google Scholar] [CrossRef]
- Eulalio, A.; Tritschler, F.; Izaurralde, E. The GW182 protein family in animal cells, new insights into domains required for miRNA-mediated gene silencing. RNA J. 2009, 15, 1433–1442. [Google Scholar] [CrossRef]
- Zhang, X.; Zuo, X.; Yang, B. MicroRNA directly enhances mitochondrial translation during muscle differentiation. Cell 2014, 158, 607–619. [Google Scholar] [CrossRef]
- Lionello, S.; Marzaro, G.; Martinvalet, D. SAM50, a side door to the mitochondrion, The case of cytotoxic proteases. Pharmacol. Res. 2020, 160, 105196. [Google Scholar] [CrossRef] [PubMed]
- Macgregor-Das, A.M.; Das, S. A microRNA’s journey to the center of the mitochondrion. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H206–H215. [Google Scholar] [CrossRef]
- Duarte, F.V.; Palmeira, C.M.; Rolo, A.P. The role of microRNAs in mitochondrion, small players acting wide. Genes 2014, 5, 865–886. [Google Scholar] [CrossRef]
- Giuliani, A.; Cirilli, I.; Prattichizzo, F. The mitomiR/Bcl-2 axis affects mitochondrial function and autophagic vacuole formation in senescent endothelial cells. Aging 2018, 10, 2855–2873. [Google Scholar] [CrossRef]
- Das, S.; Bedja, D.; Campbell, N. miR-181c regulates the mitochondrial genome, bioenergetics, and propensity for heart failure in vivo. PLoS ONE 2014, 9, e96820. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Tian, T.; Chen, W.; Lv, X.; Lei, X.; Zhang, H.; Sun, S.; Cai, L.; Pan, G.; He, L.; et al. Mitochondrial miRNA determines chemoresistance by reprogramming metabolism and regulating mitochondrial transcription. Cancer Res. 2019, 15, 1069–1084. [Google Scholar] [CrossRef]
- Machado, I.F.; Teodoro, J.S.; Palmeira, C.M. Rolo AP. miR-378a, a new emerging microRNA in metabolism. Cell Mol. Life Sci. 2020, 77, 1947–1958. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, H.; Pattnaik, B.; Datta, M. Inhibition of mitochondrialβ-oxidation by miR-107 promotes hepatic lipid accumulation and impairs glucose tolerance in vivo. Int. J. Obes. 2016, 40, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Lemecha, M.; Morino, K.; Imamura, T. MiR-494-3p regulates mitochondrial biogenesis and thermogenesis through PGC1-αsignalling in beige adipocytes. Sci. Rep. 2018, 8, 15096. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Tang, Q.; Zhang, X.; Shang, F. MicroRNA-410-3p binds to TLR2 and alleviates myocardial mitochondrial dysfunction and chemokine production in LPS-induced sepsis. Mol. Ther. Nucleic Acids 2020, 22, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhao, L.; Song, X.; Zhang, J.; Xing, Y.; Liu, N.; Yan, Y.; Li, Z.; Lu, Y.; Wu, J.; et al. MicroRNA-210 modulates the cellular energy metabolism shift during H2O2-induced oxidative stress by repressing ISCU in H9c2 cardiomyocytes. Cell Physiol. Biochem. 2017, 43, 383–394. [Google Scholar] [CrossRef]
- Zheng, Y.; Dong, L.; Liu, N.; Luo, X.; He, Z. Mir-141-3p regulates apoptosis and mitochondrial membrane potential via targeting Sirtuin1 in a 1-methyl-4-phenylpyridinium in vitro model of Parkinson’s disease. Biomed. Res. Int. 2020, 6, 7239895. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Venkata Subbaiah, K.C.; Jiang, F. MicroRNA-574 regulates FAM210A expression and influences pathological cardiac remodeling. EMBO Mol. Med. 2021, 13, e12710. [Google Scholar] [CrossRef] [PubMed]
- Houzelle, A.; Dahlmans, D.; Nascimento, E.B.M. MicroRNA-204-5p modulates mitochondrial biogenesis in C2C12 myotubes and associates with oxidative capacity in humans. J. Cell Physiol. 2020, 235, 9851–9863. [Google Scholar] [CrossRef]
- Yan, K.; An, T.; Zhai, M. Mitochondrial miR-762 regulates apoptosis and myocardial infarction by impairing ND2. Cell Death. Dis. 2019, 10, 500. [Google Scholar] [CrossRef] [PubMed]
- Latouche, C.; Natoli, A.; Reddy-Luthmoodoo, M.; Heywood, S.E.; Armitage, J.A.; Kingwell, B.A. MicroRNA-194 modulates glucose metabolism and its skeletal muscle expression is reduced in diabetes. PLoS ONE 2016, 11, e0155108. [Google Scholar] [CrossRef]
- Aryal, B.; Singh, A.K.; Rotllan, N.; Price, N.; Fernández-Hernando, C. MicroRNAs and lipid metabolism. Curr. Opin. Lipidol. 2017, 28, 273–280. [Google Scholar] [CrossRef]
- Wagschal, A.; Najafi-Shoushtari, S.H.; Wang, L.; Goedeke, L.; Sinha, S.; de Lemos, A.S.; Black, J.C.; Ramírez, C.M. Genome-wide identification of microRNAs regulating cholesterol and triglyceride homeostasis. Nat. Med. 2015, 21, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Qi, Z. MiR-21 mediates the protection of kaempferol against hypoxia/reoxygenation-induced cardiomyocyte injury via promoting Notch1/PTEN / AKT signaling pathway. PLoS ONE 2020, 15, e0241007. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, H.; Chen, L. MicroRNA-140 attenuates myocardial ischemia-reperfusion injury through suppressing mitochondrion-mediated apoptosis by targeting YES1. J. Cell Biochem. 2019, 120, 3813–3821. [Google Scholar] [CrossRef]
- Cao, H.; Cheng, Y.; Gao, H.; Zhuang, J.; Zhang, W.; Bian, Q.; Wang, F.; Du, Y.; Li, Z.; Kong, D.; et al. In vivo tracking of mesenchymal stem cell-derived extracellular vesicles improving mitochondrial function in renal ischemia-reperfusion injury. ACS Nano 2020, 14, 4014–4026. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Deng, S.; Ai, Y.; Mo, Y.; Li, W.; Peng, Q.; Huang, L.; Zhang, L. MicroRNA-125b alleviates hydrogen-peroxide-induced abnormal mitochondrial dynamics in HT22 cells by inhibiting p53. Metab. Brain. Dis. 2021, 36, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Lv, L.; Wang, W. Expression of miRNA-214 in the sera of elderly patients with acute myocardial infarction and its effect on cardiomyocyte apoptosis. Exp. Ther. Med. 2019, 17, 4657–4662. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, B.; Li, G. Disruption of microRNA-214 during general anesthesia prevents brain injury and maintains mitochondrial fusion by promoting Mfn2 interaction with Pkm2. J. Cell Mol. Med. 2020, 24, 13589–13599. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Koura, M.; Isaka, H.; Yoshida, M.C.; Tosu, M.; Sekiguchi, T. Suppression of tumorigenicity in interspecific reconstituted cells and cybrids. Gan 1982, 73, 574–580. [Google Scholar]
- Israel, B.A.; Schaeffer, W.I. Cytoplasmic suppression of malignancy. In Vitro Cell Dev. Biol. 1987, 23, 627–632. [Google Scholar] [CrossRef]
- Shay, J.W.; Liu, Y.N.; Werbin, H. Cytoplasmic suppression of tumor progression in reconstituted cells. Somat Cell Mol. Genet. 1988, 14, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, T.N. Cancer as a mitochondrial metabolic disease. Front. Cell Dev. Biol. 2015, 3, 43. [Google Scholar] [CrossRef]
- Sun, L.; Suo, C.; Li, S.T.; Zhang, H.; Gao, P. Metabolic reprogramming for cancer cells and their microenvironment. Beyond the Warburg Effect. Biochim. Biophys. Acta. Rev. Cancer 2018, 1870, 51–66. [Google Scholar] [CrossRef]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef]
- Klaunig, J.E. Oxidative stress and cancer. Curr. Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef]
- Assi, M. The differential role of reactive oxygen species in early and late stages of cancer. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R646–R653. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 10, 167–197. [Google Scholar] [CrossRef]
- Zeng, J.; Li, M.; Xu, J.Y.; Xiao, H.; Yang, X.; Fan, J.X.; Wu, K.; Chen, S. Aberrant ROS mediate cell cycle and motility in colorectal cancer cells through an oncogenic CXCL14 signaling pathway. Front. Pharmacol. 2021, 12, 764015. [Google Scholar] [CrossRef] [PubMed]
- Burke, P.J. Mitochondrion, bioenergetics and apoptosis in cancer. Trends Cancer 2017, 3, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Dubrez, L.; Berthelet, J.; Glorian, V. IAP proteins as targets for drug development in oncology. OncoTargets Ther 2013, 9, 1285–1304. [Google Scholar] [CrossRef]
- Krepela, E.; Dankova, P.; Moravcikov, E.; Krepelova, A.; Prochazka, J.; Cermak, J.; Schützner, J.; Zatloukal, P.; Benkova, K. Increased expression of inhibitor of apoptosis proteins, survivin and XIAP, in non-small cell lung carcinoma. Int. J. Oncol. 2009, 35, 1449–1462. [Google Scholar] [CrossRef]
- Zhuang, W.; Zhang, C.; Hao, F.; Sun, X. Baculoviral IAP repeat containing 6 (BIRC6) is a predictor of prognosis in prostate cancer. Med. Sci. Monit. 2018, 10, 839–845. [Google Scholar] [CrossRef]
- Delbue, D.; Mendonça, B.S.; Robaina, M.C.; Lemos, L.G.T.; Lucena, P.I.; Viola, J.P.B.; Magalhães, L.M.; Crocamo, S.; Oliveira, C.A.B.; Teixeira, F.R.; et al. Expression of nuclear XIAP associates with cell growth and drug resistance and confers poor prognosis in breast cancer. Biochim. Biophys. Acta. Mol. Cell Res. 2020, 1867, 118761. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, W.; Ma, Y.; Zhu, G.; Chen, F.; Qu, H. Dual targeting of survivin and X-linked inhibitor of apoptosis protein suppresses the growth and promotes the apoptosis of gastric cancer HGC-27 cells. Oncol. Lett. 2018, 16, 3489–3498. [Google Scholar] [CrossRef] [PubMed]
- Rada, M.; Nallanthighal, S.; Cha, J.; Ryan, K.; Sage, J.; Eldred, C.; Ullo, M.; Orsulic, S.; Cheon, D.J. Inhibitor of apoptosis proteins (IAPs) mediate collagen type XI alpha 1-driven cisplatin resistance in ovarian cancer. Oncogene 2018, 37, 4809–4820. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Singh, P.K.; Singh, D.; Dalela, D.; Rath, S.K.; Goel, M.M.; Bhatt, M.L. Evaluation of urinary XIAP as a diagnostic biomarker of carcinoma of urinary bladder. Tumour Biol. 2014, 35, 8243–8248. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yuen, N.K.; Zhan, Q. Immunity to the melanoma inhibitor of apoptosis protein (ML-IAP; livin) in patients with malignant melanoma. Cancer Immunol Immunother 2012, 61, 655–665. [Google Scholar] [CrossRef][Green Version]
- Akyurek, N.; Ren, Y.; Rassidakis, G.Z.; Schlette, E.J.; Medeiros, L.J. Expression of inhibitor of apoptosis proteins in B-cell non-Hodgkin and Hodgkin lymphomas. Cancer 2006, 15, 1844–1851. [Google Scholar] [CrossRef] [PubMed]
- Tchoghandjian, A.; Soubéran, A.; Tabouret, E. Inhibitor of apoptosis protein expression in glioblastomas and their in vitro and in vivo targeting by SMAC mimetic GDC-0152. Cell Death Dis. 2016, 7, e2325. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Ding, W.X.; Zhou, J. Expression of X-linked inhibitor-of-apoptosis protein in hepatocellular carcinoma promotes metastasis and tumor recurrence. Hepatology 2008, 48, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Costa, M. XIAP’s profile in human cancer. Biomolecules 2020, 10, 1493. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, Y.; Rotem, A.; Lotan, R.; Steller, H.; Larisch, S. The mitochondrial ARTS protein promotes apoptosis through targeting XIAP. EMBO J. 2004, 23, 1627–1635. [Google Scholar] [CrossRef]
- Ye, C.; Shu, X.O.; Pierce, L. Mutations in the mitochondrial DNA D-loop region and breast cancer risk. Breast Cancer Res. Treat. 2010, 119, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Burgart, L.J.; Zheng, J.; Shu, Q.; Strickler, J.G.; Shibata, D. Somatic mitochondrial mutation in gastric cancer. Am. J. Pathol. 1995, 147, 1105–1111. [Google Scholar] [PubMed]
- Mohamed Yusoff, A.A.; Mohd Nasir, K.N.; Haris, K.; MohdKhair, S.Z.; Abdul Ghani, A.R.; Idris, Z.; Abdullah, J.M. Detection of somatic mutations in the mitochondrial DNA control region D—Loop in brain tumors, The first report in Malaysian patients. Oncol Lett. 2017, 14, 5179–5188. [Google Scholar] [CrossRef]
- Prior, S.L.; Griffiths, A.P.; Baxter, J.M.; Baxter, P.W.; Hodder, S.C.; Silvester, K.C.; Lewis, P.D. Mitochondrial DNA mutations in oral squamous cell carcinoma. Carcinogenesis 2006, 27, 945–950. [Google Scholar] [CrossRef]
- Afrifa, J.; Zhao, T.; Yu, J. Circulating mitochondrion DNA, a non-invasive cancer diagnostic biomarker candidate. Mitochondrion 2019, 47, 238–243. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, K.; Guo, S.; Wang, Y.; Ji, X.; Yuan, Q.; Su, L.; Guo, X.; Gu, X.; Xing, J. NGS-based accurate and efficient detection of circulating cell-free mitochondrial DNA in cancer patients. Mol. Ther. Nucleic Acids 2021, 23, 657–666. [Google Scholar] [CrossRef]
- Klein, K.; He, K.; Younes, A.I. Role of mitochondrion in cancer immune evasion and potential therapeutic approaches. Front. Immunol. 2020, 11, 573326. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Zhou, Y.; Lin, X.; Kou, J.; Lin, N. Cyclometalated iridium (III) complexes as mitochondrion-targeting photosensitizers against Cisplatin-resistant cells. Photochem. Photobiol. 2021, 98, 85–91. [Google Scholar] [CrossRef]
- Cocetta, V.; Ragazzi, E.; Montopoli, M. Mitochondrial involvement in cisplatin resistance. Int. J. Mol. Sci. 2019, 20, 3384. [Google Scholar] [CrossRef]
- Moschoi, R.; Imbert, V.; Nebout, M.; Chiche, J.; Mary, D.; Prebet, T.; Saland, E.; Griessinger, E. Protective mitochondrial transfer from bone marrow stromal cells to acute myeloid leukemic cells during chemotherapy. Blood 2016, 128, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Li, S.M.; Wu, H.L.; Yu, X. The putative tumor suppressor miR-1-3p modulates prostate cancer cell aggressiveness by repressing E2F5 and PFTK1. J. Exp. Clin. Cancer Res. 2018, 37, 219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Z.; Gao, L.; Qiao, Z.; Yu, M.; Yu, B.; Yang, T. miR-1-3p suppresses proliferation of hepatocellular carcinoma through targeting SOX9. OncoTargets Ther. 2019, 22, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, S.; Wang, Q.; Zhang, X. Tumor suppressor miR-1 inhibits tumor growth and metastasis by simultaneously targeting multiple genes. Oncotarget 2017, 8, 42043–42060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, C.; Zhang, X. Mitochondrial damage mediated by miR-1 overexpression in cancer stem cells. Mol. Ther. Nucleic Acids 2019, 18, 938–953. [Google Scholar] [CrossRef]
- Zhao, X.; Ye, N.; Feng, X.; Ju, H.; Liu, R.; Lu, W. MicroRNA-29b-3p inhibits the migration, and invasion of gastric cancer cells by regulating the autophagy-associated protein MAZ. OncoTargets Ther. 2021, 14, 3239–3249. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Guo, J.; Gu, R. MicroRNA-29b-3p inhibits cell proliferation and angiogenesis by targeting VEGFA and PDGFB in retinal microvascular endothelial cells. Mol. Vis. 2020, 26, 64–75. [Google Scholar] [PubMed]
- Mao, A.; Tang, J.; Tang, D. MicroRNA-29b-3p enhances radiosensitivity through modulating WISP1-mediated mitochondrial apoptosis in prostate cancer cells. J. Cancer 2020, 11, 6356–6364. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Yang, F.; Qin, Z. The value of miR-155 as a biomarker for the diagnosis and prognosis of lung cancer, a systematic review with meta-analysis. BMC Cancer 2019, 19, 1103. [Google Scholar] [CrossRef]
- Nariman-Saleh-Fam, Z.; Saadatian, Z.; Daraei, A.; Mansoori, Y.; Bastami, M.; Tavakkoli-Bazzaz, J. The intricate role of miR-155 in carcinogenesis, potential implications for esophageal cancer research. Biomark. Med. 2019, 13, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Mattiske, S.; Suetani, R.J.; Neilsen, P.M.; Callen, D.F. The oncogenic role of miR-155 in breast cancer. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, E.; Jung, J.; Lee, J.W.; Kim, H.J.; Kim, J.; Yoo, H.J.; Lee, H.; Chae, S.Y.; Jeon, S.M.; et al. microRNA-155 positively regulates glucose metabolism via PIK3R1-FOXO3a-cMYC axis in breast cancer. Oncogene 2018, 37, 2982–2991. [Google Scholar] [CrossRef]
- Zhuang, X.; Chen, Y.; Wu, Z.; Xu, Q.; Chen, M.; Shao, M.; Cao, X.; Zhou, Y.; Xie, M.; Shi, Y.; et al. Mitochondrial miR-181a-5p promotes glucose metabolism reprogramming in liver cancer by regulating the electron transport chain. Carcinogenesis 2020, 41, 972–983. [Google Scholar] [CrossRef]
- Liu, S.; Gao, G.; Yan, D.; Chen, X.; Yao, X.; Guo, S.; Li, G.; Zhao, Y. Effects of miR-145-5p through NRAS on the cell proliferation, apoptosis, migration, and invasion in melanoma by inhibiting MAPK and PI3K / AKT pathways. Cancer Med. 2017, 6, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Yuan, Z.; Shi, H.; Bian, Y.; Guo, R. miR-145 targets the SOX11 3’UTR to suppress endometrial cancer growth. Am. J. Cancer Res. 2017, 7, 2305–2317. [Google Scholar]
- Zhou, K.; Song, B.; Wei, M. MiR-145-5p suppresses the proliferation, migration, and invasion of gastric cancer epithelial cells via the ANGPT2/NOD_LIKE_RECEPTOR axis. Cancer Cell Int. 2020, 20, 416. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Y.; Pei, M. MiR-145 inhibits mitochondrial function of ovarian cancer by targeting ARL5B. J Ovarian Res. 2021, 14, 8. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Q.; Wang, Y. MiR-125b-5p suppresses the bladder cancer progression via targeting HK2 and suppressing PI3K/AKT pathway. Hum. Cell 2020, 33, 185–194. [Google Scholar] [CrossRef]
- Luo, S.; Wang, J.; Ma, Y.; Yao, Z.; Pan, H. PPARγ inhibits ovarian cancer cells proliferation through upregulation of miR-125b. Biochem. Biophys. Res. Commun. 2015, 462, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Yang, Y.; Liu, M.; Liu, B.; Yang, X.; Yu, M.; Qi, H.; Ren, M.; Wang, Z.; Zou, J.; et al. MiR-125b attenuates human hepatocellular carcinoma malignancy through targeting SIRT6. Am. J. Cancer Res. 2018, 8, 993–1007. [Google Scholar]
- Mei, L.L.; Wang, W.J.; Qiu, Y.T.; Xie, X.F.; Bai, J.; Shi, Z.Z. MiR-125b-5p functions as a tumor suppressor gene partially by regulating HMGA2 in esophageal squamous cell carcinoma. PLoS ONE 2017, 12, e0185636. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Hu, Y.; Xu, L.; Fu, Y.; Tu, J.; Zhao, H.; Zhang, S.; Hong, R.; Gu, X. The role of miR-125b-mitochondrion-caspase-3 pathway in doxorubicin resistance and therapy in human breast cancer. Tumour Biol. 2015, 36, 7185–7194. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, P.; Lu, Y. Decreased expression of mitochondrial miR-5787 contributes to chemoresistance by reprogramming glucose metabolism and inhibiting MT-CO3 translation. Theranostics 2019, 9, 5739–5754. [Google Scholar] [CrossRef] [PubMed]
- Cocco, P. Multifactorial etiology of lung cancer among silica-exposed workers. Ann. Acad. Med. Singap. 2001, 30, 468–474. [Google Scholar] [PubMed]
- Klebe, S.; Leigh, J.; Henderson, D.W.; Nurminen, M. Asbestos, smoking and lung cancer. An update. Int. J. Environ. Res. Public Health 2019, 17, 258. [Google Scholar] [CrossRef] [PubMed]
- Bersimbaev, R.; Bulgakova, O. Residential radon exposure and lung cancer risk in Kazakhstan. In Radon; Adrovic, F., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Cloonan, S.M.; Kim, K.; Esteves, P.; Trian, T.; Barnes, P.J. Mitochondrial dysfunction in lung aging and disease. Eur. Respir. Rev. 2020, 29, 200165. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.; Xiao, C.; Yang, M.; Zhou, X.; Gong, P. Inhibition of SNCG suppresses the proliferation of lung cancer cells induced by high glucose. Mol. Med. Rep. 2021, 23, 138. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.H.; Dorsch, M.; Dujardin, P.; Silas, S.; Ueffing, K.; Hölken, J.M.; Yang, D.; Winslow, M.M.; Grüner, B.M. Altered mitochondrion functionality defines a metastatic cell state in lung cancer and creates an exploitable vulnerability. Cancer Res. 2021, 81, 567–579. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Yu, X.; Zhou, H.; Luo, Y.; Wang, W.; Wang, L. Clinical application of plasma mitochondrial DNA content in patients with lung cancer. Oncol. Lett. 2018, 16, 7074–7081. [Google Scholar] [CrossRef]
- Bulgakova, O.; Kausbekova, A.; Kussainova, A.; Kalibekov, N.; Serikbaiuly, D.; Bersimbaev, R. Involvement of circulating cell-free mitochondrial DNA and Proinflammatory cytokines in pathogenesis of chronic obstructive pulmonary disease and lung cancer. Asian Pac. J. Cancer Prev. 2021, 22, 1927–1933. [Google Scholar] [CrossRef]
- Bulgakova, O.; Kussainova, A.; Kakabayev, A.; Aripova, A.; Baikenova, G.; Izzotti, A.; Bersimbaev, R. The level of free-circulating mtDNA in patients with radon-induced lung cancer. Environ. Res. 2021, 207, 112215. [Google Scholar] [CrossRef]
- Berger, R.; Fiegl, H.; Goebel, G.; Obexer, P.; Ausserlechner, M.; Doppler, W.; Hauser-Kronberger, C.; Reitsamer, R.; Egle, D.; Reimer, D. Toll-like receptor 9 expression in breast and ovarian cancer is associated with poorly differentiated tumors. Cancer Sci. 2010, 101, 1059–1066. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signaling pathways by reactive oxygen species. Biochim. Biophys. Acta. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Kim, J.; Kim, G.W.; Choi, C. Oxidative stress-induced necrotic cell death via mitochondira-dependent burst of reactive oxygen species. Curr. Neurovasc. Res. 2009, 6, 213–222. [Google Scholar] [CrossRef] [PubMed]
- West, A.P.; Shadel, G.S. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat. Rev. Immunol. 2017, 17, 363–375. [Google Scholar] [CrossRef] [PubMed]
- De Dios, R.; Nguyen, L.; Ghosh, S.; McKenna, S.; Wright, C.J. CpG-ODN-mediated TLR9 innate immune signalling and calcium dyshomeostasis converge on the NFκB inhibitory protein IκBβ to drive IL1α and IL1β expression. Immunology 2020, 160, 64–77. [Google Scholar] [CrossRef]
- Sugiyama, K.; Muroi, M.; Kinoshita, M.; Hamada, O.; Minai, Y.; Sugita-Konishi, Y.; Kamata, Y.; Tanamoto, K. NF-κB activation via MyD88-dependent Toll-like receptor signaling is inhibited by trichothecene mycotoxin deoxynivalenol. J. Toxicol. Sci. 2016, 41, 273–279. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Dimitrakopoulos, F.I.D.; Antonacopoulou, A.G.; Kottorou, A.E. Expression of intracellular components of the NF-κB alternative pathway (NF-κB2, RelB, NIK and Bcl3) is associated with clinical outcome of NSCLC patients. Sci. Rep. 2019, 9, 14299. [Google Scholar] [CrossRef]
- Riley, J.S.; Tait, S.W. Mitochondrial DNA in inflammation and immunity. EMBO Rep. 2020, 21, e49799. [Google Scholar] [CrossRef]
- Nam, H.S.; Izumchenko, E.; Dasgupta, S.; Hoque, M.O. Mitochondrion in chronic obstructive pulmonary disease and lung cancer, where are we now? Biomark. Med. 2017, 11, 475–489. [Google Scholar] [CrossRef]
- Alifano, M. Systemic immune-inflammation index and prognosis of advanced non-small cell lung cancer. Ann. Transl. Med. 2020, 8, 667. [Google Scholar] [CrossRef]
- Hua, X.; Chen, J.; Wu, Y. Prognostic role of the advanced lung cancer inflammation index in cancer patients, a meta-analysis. World J. Surg. Oncol. 2019, 17, 177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Itagaki, K.; Hauser, C.J. Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock 2010, 34, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Liu, H.; Huang, C.; Chau, Y.; Wu, S. Mitochondrial-DNA-associated TLR9 signaling is a potential serological biomarker for non-small cell lung cancer. Oncol. Rep. 2019, 41, 999–1006. [Google Scholar] [PubMed]
- Raghav, L.; Chang, Y.H.; Hsu, Y.C. Landscape of mitochondrion genome and clinical outcomes in stage 1 lung adenocarcinoma. Cancers 2020, 12, 755. [Google Scholar] [CrossRef]
- Cooper, G.M. The Cell, a Molecular Approach. The Development and Causes of Cancer, 2nd ed.; Sunderland, M.A., Ed.; Sinauer Associates: Washington, DC, USA, 2000. [Google Scholar]
- Cheng, J.; Yang, H.; Gu, C.; Liu, Y.; Shao, J.; Zhu, R.; Li, M. Melatonin restricts the viability and angiogenesis of vascular endothelial cells by suppressing HIF-1α / ROS / VEGF. Int. J. Mol. Med. 2019, 3, 945–955. [Google Scholar] [CrossRef]
- Zhu, X.; Kudo, M.; Huang, X.; Sui, H.; Tian, H.; Croce, C.M.; Cui, R. Frontiers of MicroRNA signature in non-small cell lung cancer. Front. Cell Dev. Biol 2021, 9, 643942. [Google Scholar] [CrossRef]
- Bersimbaev, R.; Bulgakova, O.; Aripova, A.; Kussainova, A.; Ilderbayev, O. Role of microRNAs in lung carcinogenesis induced by asbestos. J. Pers. Med. 2021, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Jin, F. NFAT5 promotes proliferation and migration of lung adenocarcinoma cells in part through regulating AQP5 expression. Biochem. Biophys. Res. Commun. 2015, 465, 644–649. [Google Scholar] [CrossRef]
- Jiang, Y.; He, R.; Jiang, Y. Transcription factor NFAT5 contributes to the glycolytic phenotype rewiring and pancreatic cancer progression via transcription of PGK1. Cell Death Dis. 2019, 10, 948. [Google Scholar] [CrossRef]
- Meng, X.; Li, Z.; Zhou, S.; Xiao, S.; Yu, P. miR-194 suppresses high glucose-induced non-small cell lung cancer cell progression by targeting NFAT5. Thorac Cancer 2019, 10, 1051–1059. [Google Scholar] [CrossRef]
- Magenta, A.; Cencioni, C.; Fasanaro, P. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ. 2011, 18, 1628–1639. [Google Scholar] [CrossRef]
- Ma, D.J.; Liu, H.S.; Li, S.Q.; Qin, Y.Z.; He, J.; Li, L.; Cui, Y.S. Correlations of the ZEB1 expression with the incidence and prognosis of non-small cell lung cancer. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1528–1535. [Google Scholar]
- D’Almeida, O.; Mothar, O.; Bondzie, E.A. Encapsulated miR-200c and Nkx2.1 in a nuclear mitochondrion transcriptional regulatory network of non-metastatic and metastatic lung cancer cells. BMC Cancer 2019, 19, 136. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Qi, Y.; Yang, X. MicroRNA-214 upregulates HIF-1α and VEGF by targeting ING4 in lung cancer cells. Mol. Med. Rep. 2019, 19, 4935–4945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, M.; Jiang, H.; Liu, F.; Liu, H.; Li, Y. Down-regulation of miR-214 inhibits proliferation and glycolysis in non-small-cell lung cancer cells via down-regulating the expression of hexokinase 2 and pyruvate kinase isozyme M2. Biomed. Pharmacother. 2018, 105, 545–552. [Google Scholar] [CrossRef]
- Sun, L.; Pan, Y.; Wang, X. Screening for potential biomarkers in peripheral blood from miners exposed to radon radiation. Dose Resp. 2020, 18, 1559325820904600. [Google Scholar] [CrossRef]
- Wu, J.; Sun, B.; Zhang, S.; Zhang, J.; Tong, J.; Nie, J.; Li, J. Effects of radon on miR-34a-induced apoptosis in human bronchial epithelial BEAS-2B cells. J. Toxicol. Environ. Health A 2019, 82, 913–919. [Google Scholar] [CrossRef]
- Cui, F.M.; Li, J.X.; Chen, Q.; Du, H.B.; Zhang, S.Y.; Nie, J.H.; Cao, J.P.; Zhou, P.K.; Hei, T.K.; Tong, J. Radon-induced alterations in micro-RNA expression profiles in transformed BEAS2B cells. J. Toxicol. Environ. Health A 2013, 76, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.; Lin, H.; Li, Y.; Guo, X.; Yuan, Y.; Zhang, R.; Li, X.; Chai, D.; Zuo, Y. MicroRNA profiling in BEAS-2B cells exposed to alpha radiation reveals potential biomarkers for malignant cellular transformation. Toxicol. Res. 2020, 9, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Izzotti, A.; Coronel Vargas, G.; Pulliero, A.; Coco, S.; Vanni, I.; Colarossi, C.; Blanco, G.; Agodi, A.; Barchitta, M.; Maugeri, A.; et al. Relationship between the miRNA profiles and oncogene mutations in non-smoker lung cancer. Relevance for lung cancer personalized screenings and treatments. J. Pers. Med. 2021, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Ellisen, L.W.; Ramsayer, K.D.; Johannessen, C.M.; Yang, A.; Beppu, H.; Minda, K.; Oliner, J.D.; McKeon, F.; Haber, D.A. REDD1, a developmentally regulated transcriptional target of p63 and p53, links p63 to regulation of reactive oxygen species. Mol. Cell 2002, 10, 995–1005. [Google Scholar] [CrossRef]
- Liu, Z.; Li, T.; Zhu, F. Regulatory roles of miR-22/Redd1-mediated mitochondrial ROS and cellular autophagy in ionizing radiation-induced BMSC injury. Cell Death Dis. 2019, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.; Le, T.; Fan, G. DNA methylation, and its basic function. Neuropsychopharmacol. 2013, 38, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.I.H.N.; Othman, I.; Naidu, R. The role of MicroRNAs in lung cancer metabolism. Cancers 2021, 13, 1716. [Google Scholar] [CrossRef] [PubMed]
- Miousse, I.R.; Kutanzi, K.R.; Koturbash, I. Effects of ionizing radiation on DNA methylation, from experimental biology to clinical applications. Int. J. Radiat. Biol 2017, 93, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Sun, J.; Zhai, X.; Chen, X.; Wan, J.; Tian, H. Repeated radon exposure induced lung damage via oxidative stress-mediated mitophagy in human bronchial epithelial cells and mice. Environ. Toxicol. Pharmacol. 2022, 90, 103812. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).