Ceramides and Cardiovascular Risk Factors, Inflammatory Parameters and Left Ventricular Function in AMI Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population Characteristics and Data Acquisition
2.2. Plasma Processing
2.3. HPLC-MS/MS Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
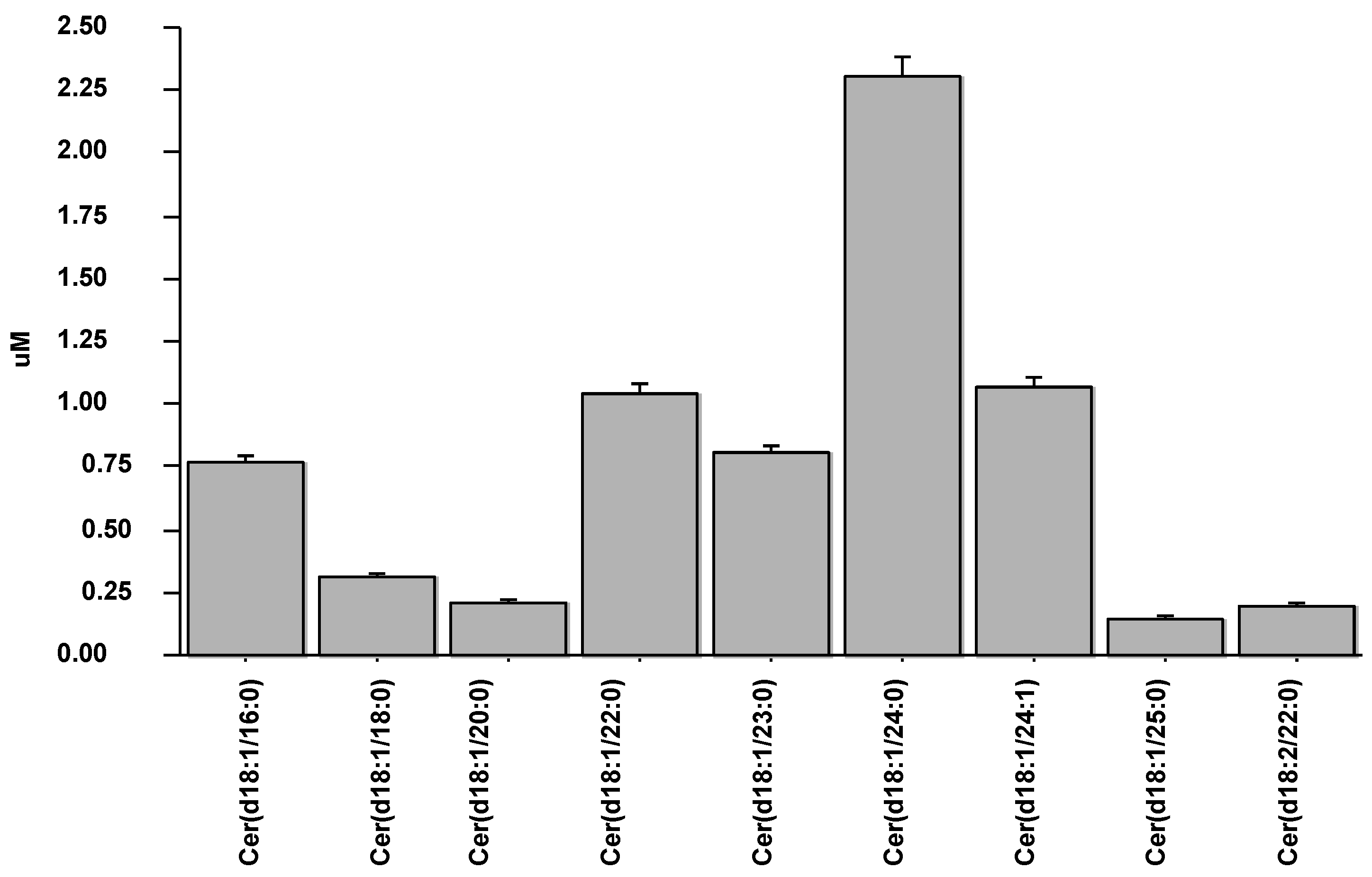
3.2. Ceramide Distribution and Levels in AMI
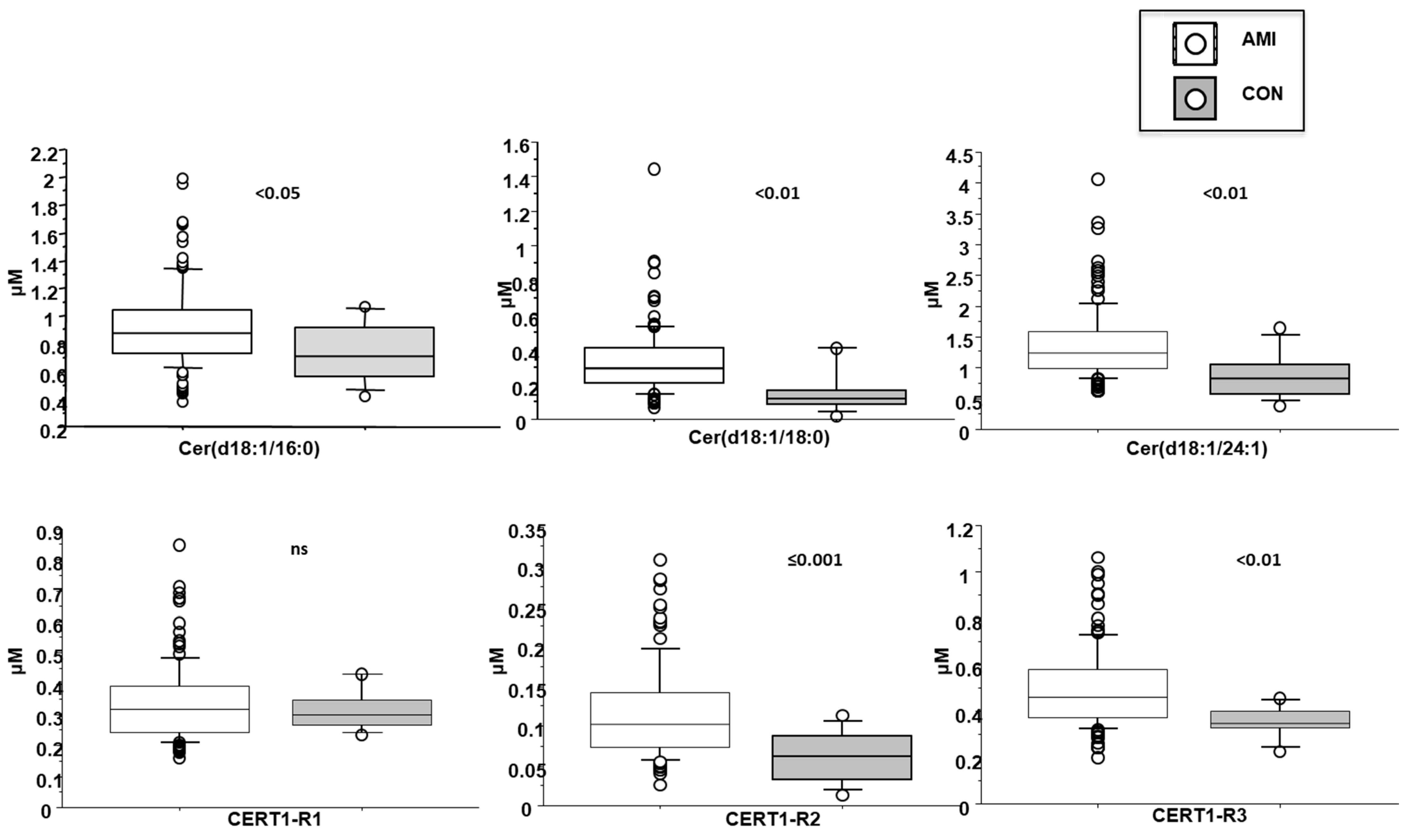
3.3. Cer(d18:1/16:0)
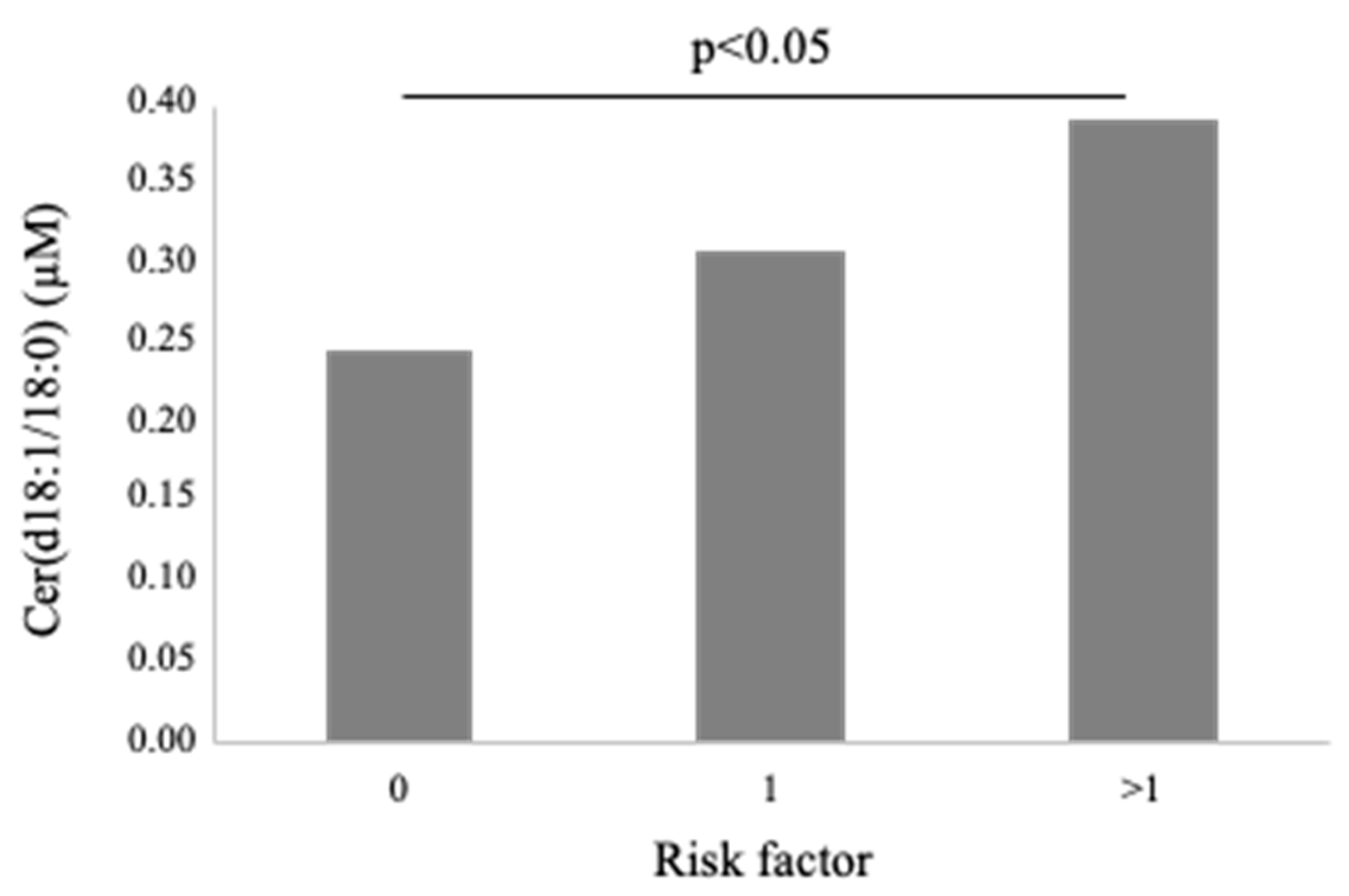
3.4. Cer(d18:1/18:0)
3.5. Cer(d18:1/24:1)
3.6. Cer(d18:1/16:0)/Cer(d18:1/24:0) (CERT-R1)
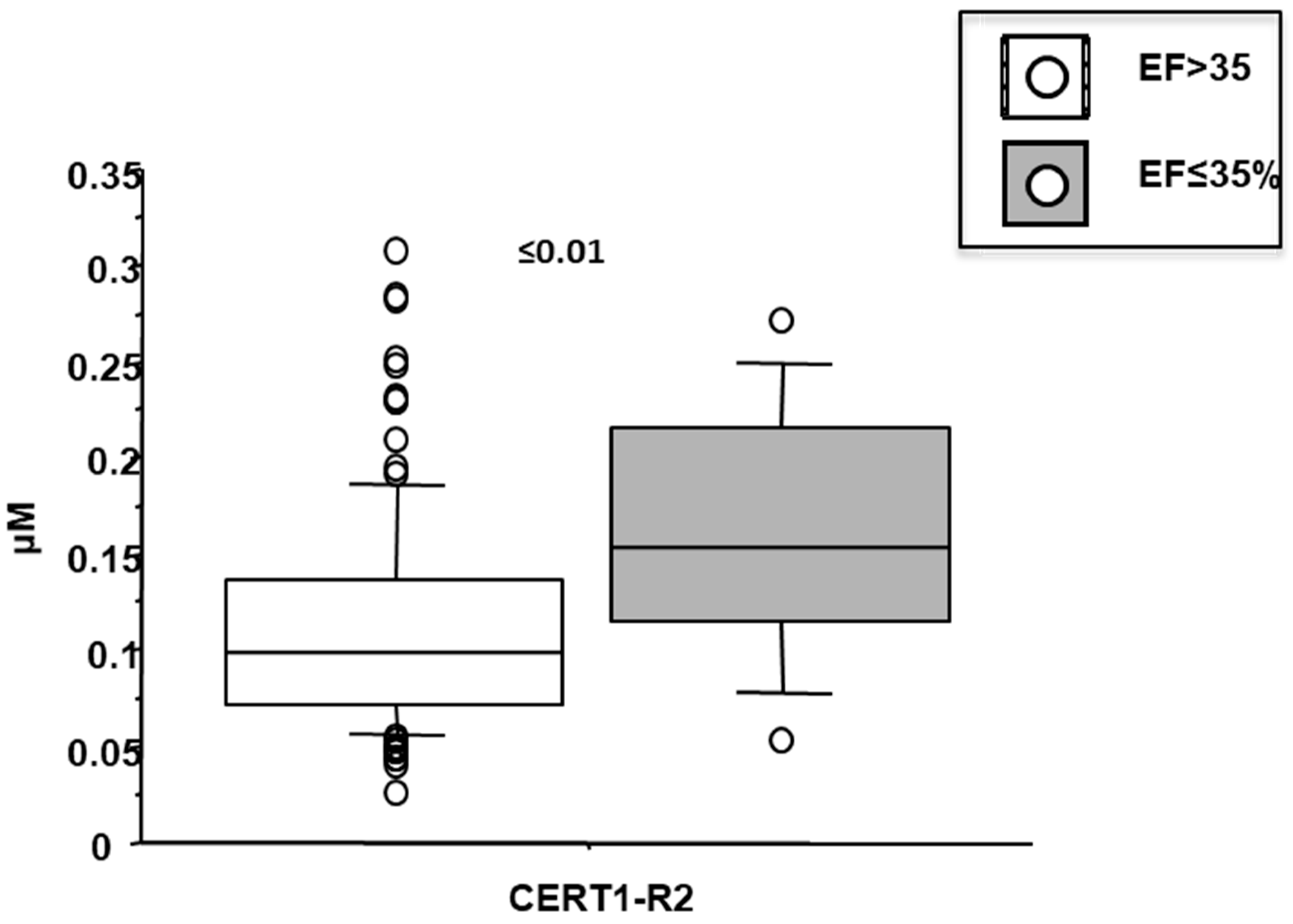
3.7. Cer(d18:1/18:0)/Cer(d18:1/24:0) (CERT-R2)
3.8. Cer(d18:1/18:0)/Cer(d18:1/24:0 (CERT-R3)
3.9. Ceramides as Deteminants of Echocardiographic Left Ventricular Dysfunction in AMI Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaggini, M.; Pingitore, A.; Vassalle, C. Plasma Ceramides Pathophysiology, Measurements, Challenges, and Opportunities. Metabolites 2021, 11, 719. [Google Scholar] [CrossRef] [PubMed]
- Havulinna, A.S.; Sysi-Aho, M.; Hilvo, M.; Kauhanen, D.; Hurme, R.; Ekroos, K.; Salomaa, V.; Laaksonen, R. Circulating Ceramides Predict Cardiovascular Outcomes in the Population-Based FINRISK 2002 Cohort. Arter. Thromb. Vasc. Biol. 2016, 36, 2424–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vozella, V.; Basit, A.; Piras, F.; Realini, N.; Armirotti, A.; Bossù, P.; Assogna, F.; Sensi, S.L.; Spalletta, G.; Piomelli, D. Elevated plasma ceramide levels in post-menopausal women: A cross-sectional study. Aging 2019, 11, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, X.; Pang, J.; Zhang, Y.; Zhang, H.; Xu, Z.; Chen, Q.; Ling, W. Associations between plasma ceramides and mortality in patients with coronary artery disease. Atherosclerosis 2020, 314, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Yu, J.; Shi, R.; Yan, L.; Yang, T.; Li, Y.; Zhang, Z.; Yu, G.; Bai, Y.; Schuchman, E.H.; et al. Elevation of ceramide and activation of secretory acid sphingomyelinase in patients with acute coronary syndromes. Coron. Artery Dis. 2014, 25, 230–235. [Google Scholar] [CrossRef]
- Mantovani, A.; Bonapace, S.; Lunardi, G.; Canali, G.; Dugo, C.; Vinco, G.; Calabria, S.; Barbieri, E.; Laaksonen, R.; Bonnet, F.; et al. Associations between specific plasma ceramides and severity of coronary- artery stenosis assessed by coronary angiography. Diabetes Metab. 2020, 46, 150–157. [Google Scholar] [CrossRef]
- Alshehry, Z.H.; Mundra, P.A.; Barlow, C.K.; Mellett, N.A.; Wong, G.; McConville, M.J.; Simes, J.; Tonkin, A.M.; Sullivan, D.R.; Barnes, E.H.; et al. Plasma Lipidomic Profiles Improve on Traditional Risk Factors for the Prediction of Cardiovascular Events in Type 2 Diabetes Mellitus. Circulation 2016, 134, 1637–1650. [Google Scholar] [CrossRef] [Green Version]
- Stegemann, C.; Pechlaner, R.; Willeit, P.; Langley, S.R.; Mangino, M.; Mayr, U.; Menni, C.; Moayyeri, A.; Santer, P.; Rungger, G.; et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation 2014, 129, 1821–1831. [Google Scholar] [CrossRef] [Green Version]
- Hla, T.; Dannenberg, A.J. Sphingolipid signaling in metabolic disorders. Cell Metab. 2012, 16, 420–434. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Junk, P.; Huwiler, A.; Burkhardt, C.; Wallerath, T.; Pfeilschifter, J.; Förstermann, U. Dual effect of ceramide on human endothelial cells: Induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circulation 2002, 106, 2250–2256. [Google Scholar] [CrossRef] [Green Version]
- Bielawska, A.E.; Shapiro, J.P.; Jiang, L.; Melkonyan, H.S.; Piot, C.; Wolfe, C.L.; Tomei, L.D.; Hannun, Y.A.; Umansky, S.R. Ceramide is involved in triggering of cardiomyocyte apoptosis induced by ischemia and reperfusion. Am. J. Pathol. 1997, 151, 1257–1263. [Google Scholar] [PubMed]
- Delpy, E.; Hatem, S.N.; Andrieu, N.; de Vaumas, C.; Henaff, M.; Rücker-Martin, C.; Jaffrézou, J.P.; Laurent, G.; Levade, T.; Mercadier, J.J. Doxorubicin induces slow ceramide accumulation and late apoptosis in cultured adult rat ventricular myocytes. Cardiovasc. Res. 1999, 43, 398–407. [Google Scholar] [CrossRef]
- Pchejetski, D.; Kunduzova, O.; Dayon, A.; Calise, D.; Seguelas, M.H.; Leducq, N.; Seif, I.; Parini, A.; Cuvillier, O. Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase A-associated cardiac cell apoptosis. Circ. Res. 2007, 100, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, O.M.; Discher, D.J.; Bishopric, N.H.; Webster, K.A. Rapid activation of neutral sphingomyelinase by hypoxia-reoxygenation of cardiac myocytes. Circ. Res. 2000, 86, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Dong, H.; Sun, R.; Zhao, L.; Sun, M.; Li, L.; Yu, X.; Liu, J.; Wu, J.; Yang, F.; et al. Plasma ceramides in relation to coronary plaque characterization determined by optical coherence tomography. J. Cardiovasc. Transl. Res. 2021, 14, 140–149. [Google Scholar] [CrossRef]
- Uchida, Y.; Uchida, Y.; Kobayashi, T.; Shirai, S.; Hiruta, N.; Shimoyama, E.; Tabata, T. Detection of ceramide, a risk factor for coronary artery disease, in human coronary plaques by fluorescent angioscopy. Circ. J. 2017, 81, 1886–1893. [Google Scholar] [CrossRef] [Green Version]
- Park, J.Y.; Lee, S.H.; Shin, M.J.; Hwang, G.S. Alteration in metabolic signature and lipid metabolism in patients with angina pectoris and myocardial infarction. PLoS ONE 2015, 10, e0135228. [Google Scholar] [CrossRef] [Green Version]
- Anroedh, S.; Hilvo, M.; Akkerhuis, K.M.; Kauhanen, D.; Koistinen, K.; Oemrawsingh, R.; Serruys, P.; van Geuns, R.J.; Boersma, E.; Laaksonen, R.; et al. Plasma concentrations of molecular lipid species predict long-term clinical outcome in coronary artery disease patients. J. Lipid Res. 2018, 59, 1729–1737. [Google Scholar] [CrossRef]
- Laaksonen, R.; Ekroos, K.; Sysi-Aho, M.; Hilvo, M.; Vihervaara, T.; Kauhanen, D.; Suoniemi, M.; Hurme, R.; März, W.; Scharnagl, H.; et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J. 2016, 37, 1967–1976. [Google Scholar] [CrossRef]
- Hilvo, M.; Vasile, V.C.; Donato, L.J.; Hurme, R.; Laaksonen, R. Ceramides and Ceramide Scores: Clinical Applications for Cardiometabolic Risk Stratification. Front. Endocrinol. 2020, 11, 570628. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [PubMed]
- Michelucci, E.; Di Giorgi, N.; Finamore, F.; Smit, J.M.; Scholte, A.J.H.A.; Signore, G.; Rocchiccioli, S. Lipid biomarkers in statin users with coronary artery disease annotated by coronary computed tomography angiography. Sci. Rep. 2021, 11, 12899. [Google Scholar] [CrossRef] [PubMed]
- Schissel, S.L.; Tweedie-Hardman, J.; Rapp, J.H.; Graham, G.; Williams, K.J.; Tabas, I. Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J. Clin. Investig. 1996, 98, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Park, L.K.; Garr Barry, V.; Hong, J.; Heebink, J.; Sah, R.; Peterson, L.R. Links between ceramides and cardiac function. Curr. Opin. Lipidol. 2021, 33, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Akashi, H.; Drosatos, K.; Liao, X.; Jiang, H.; Kennel, P.J.; Brunjes, D.L.; Castillero, E.; Zhang, X.; Deng, L.Y.; et al. Increased de novo ceramide synthesis and accumulation in failing myocardium. JCI Insight 2017, 2, e82922. [Google Scholar] [CrossRef] [Green Version]
- Spijkers, L.J.; van den Akker, R.F.; Janssen, B.J.; Debets, J.J.; De Mey, J.G.; Stroes, E.S.; van den Born, B.J.; Wijesinghe, D.S.; Chalfant, C.E.; MacAleese, L.; et al. Hypertension is associated with marked alterations in sphingolipid biology: A potential role for ceramide. PLoS ONE 2011, 6, e21817. [Google Scholar] [CrossRef] [Green Version]
- Haus, J.M.; Kashyap, S.R.; Kasumov, T.; Zhang, R.; Kelly, K.R.; Defronzo, R.A.; Kirwan, J.P. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 2009, 58, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.D.; Toledo, E.; Hruby, A.; Rosner, B.A.; Willett, W.C.; Sun, Q.; Razquin, C.; Zheng, Y.; Ruiz-Canela, M.; Guasch-Ferré, M.; et al. Plasma ceramides, mediterranean diet, and incident cardiovascular disease in the PREDIMED trial (Prevención con Dieta Mediterránea). Circulation 2017, 135, 2028–2040. [Google Scholar] [CrossRef] [Green Version]
- Tanase, D.M.; Gosav, E.M.; Petrov, D.; Jucan, A.E.; Lacatusu, C.M.; Floria, M.; Tarniceriu, C.C.; Costea, C.F.; Ciocoiu, M.; Rezus, C. Involvement of Ceramides in Non-Alcoholic Fatty Liver Disease (NAFLD) Atherosclerosis (ATS) Development: Mechanisms and Therapeutic Targets. Diagnostics 2021, 11, 2053. [Google Scholar] [CrossRef]
- Saleem, M.; Herrmann, N.; Dinoff, A.; Marzolini, S.; Mielke, M.M.; Andreazza, A.; Oh, P.I.; Vattem Venkata, S.L.; Haughey, N.J.; Lanctôt, K.L. Association Between Sphingolipids and Cardiopulmonary Fitness in Coronary Artery Disease Patients Undertaking Cardiac Rehabilitation. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 671–679. [Google Scholar] [CrossRef] [Green Version]
- Bikman, B.T. A role for sphingolipids in the pathophysiology of obesity-induced inflammation. Cell Mol. Life Sci. 2012, 69, 2135–2146. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Canals, D.; Idkowiak-Baldys, J.; Simbari, F.; Roddy, P.; Perry, D.M.; Kitatani, K.; Luberto, C.; Hannun, Y.A. Regulated secretion of acid sphingomyelinase: Implications for selectivity of ceramide formation. J. Biol. Chem. 2010, 285, 35706–35718. [Google Scholar] [CrossRef] [Green Version]
- Lozanski, G.; Berthier, F.; Kushner, I. The sphingomyelin-ceramide pathway participates in cytokine regulation of C-reactive protein and serum amyloid A, but not alpha-fibrinogen. Biochem. J. 1997, 328, 271–275. [Google Scholar] [CrossRef]
- Mantovani, A.; Altomari, A.; Lunardi, G.; Bonapace, S.; Lippi, G.; Bonnet, F.; Targher, G. Association between specific plasma ceramides and high-sensitivity C-reactive protein levels in postmenopausal women with type 2 diabetes. Diabetes Metab. 2020, 46, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, B.; Summers, S.A. Ceramides in Metabolism: Key Lipotoxic Players. Annu. Rev. Physiol. 2021, 83, 303–330. [Google Scholar] [CrossRef]
- Hoffman, M.; Palioura, D.; Kyriazis, I.D.; Cimini, M.; Badolia, R.; Rajan, S.; Gao, E.; Nikolaidis, N.; Schulze, P.C.; Goldberg, I.J.; et al. Cardiomyocyte Krüppel-Like Factor 5 Promotes De Novo Ceramide Biosynthesis and Contributes to Eccentric Remodeling in Ischemic Cardiomyopathy. Circulation 2021, 143, 1139–1156. [Google Scholar] [CrossRef] [PubMed]
- Hadas, Y.; Vincek, A.S.; Youssef, E.; Żak, M.M.; Chepurko, E.; Sultana, N.; Sharkar, M.T.K.; Guo, N.; Komargodski, R.; Kurian, A.A.; et al. Altering Sphingolipid Metabolism Attenuates Cell Death and Inflammatory Response After Myocardial Infarction. Circulation 2020, 141, 916–930. [Google Scholar] [CrossRef]
- Menuz, V.; Howell, K.S.; Gentina, S.; Epstein, S.; Riezman, I.; Fornallaz-mulhauser, M.; Hengartner, M.O.; Gomez, M.; Riezman, H.; Martinou, J. Protection of C. elegans from Anoxia by HYL-2 Ceramide Synthase. Science 2009, 324, 381–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mielke, M.M.; Bandaru, V.V.; Han, D.; An, Y.; Resnick, S.M.; Ferrucci, L.; Haughey, N.J. Demographic and clinical variables affecting mid- to late-life trajectories of plasma ceramide and dihydroceramide species. Aging Cell 2015, 14, 1014–1023. [Google Scholar] [CrossRef]
- Mielke, M.M.; Bandaru, V.V.; Han, D.; An, Y.; Resnick, S.M.; Ferrucci, L.; Haughey, N.J. Factors affecting longitudinal trajectories of plasma sphingomyelins: The Baltimore Longitudinal Study of Aging. Aging Cell 2015, 14, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Neeland, I.J.; Singh, S.; McGuire, D.K.; Vega, G.L.; Roddy, T.; Reilly, D.F.; Castro-Perez, J.; Kozlitina, J.; Scherer, P.E. Relation of plasma ceramides to visceral adiposity, insulin resistance and the development of type 2 diabetes mellitus: The Dallas Heart Study. Diabetologia 2018, 61, 2570–2579. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Males | 87 (71) | |
| Age (years) | 69 ± 12 | |
| Body mass index (kg/m2) | 27 ± 5 | |
| CV risk factors | ||
| Type 2 Diabetes | 22 (18) | |
| Hypertension | 76 (62) | |
| Dyslipidemia | 61 (49) | |
| Current/ex smoking habit | 56 (45) | |
| Ejection fraction (%) | 50 ± 9 | |
| Wall Motion Score Index | 1.2 ± 0.23 | |
| Obesity | 21 (17) | |
| Laboratory parameters | ||
| Creatinine (mg/dL) | 1 ± 0.8 | |
| Brain Natriuretic Peptide (ng/L) | 217 ± 319 | |
| Fibrinogen (mg/dL) | 376 ± 122 | |
| Monocytes (109/L) | 0.9 ± 0.7 | |
| Platelets (109/L) | 231 ± 75 | |
| Lymphocytes (109/L) | 2.1 ± 1.9 | |
| Neutrophils (109/L) | 6.5 ± 2.6 | |
| Erythrocyte Sedimentation Rate (mm/h) | 27 ± 26 | |
| Neutrophil-to-lymphocyte-ratio | 4.0 ± 2.8 | |
| C Reactive Protein (mg/dL) | 3.4 ± 4 | |
| Troponin I (at admission) (μg/L) | 26 ± 53 | |
| Multivessel disease | 59 (48) |
| Cer(d18:1/16:0) | Cer(d18:1/18:0) | Cer(d18:1/24:1) | Cer(d18:1/16:0)/Cer(d18:1/24:0) | Cer(d18:1/18:0)/Cer(d18:1/24:0) | Cer(d18:1/24:1)/ Cer(d18:1/24:0) | |
|---|---|---|---|---|---|---|
| Age (years) | r = 0.22 p < 0.05 | ns | r = 0.17 p ≤ 0.05 | r = 0.50 p < 0.001 | r = 0.29 p < 0.01 | r = 0.49 p < 0.001 |
| Ejection fraction (%) | ns | ns | ns | ns | r = 0.17 p ≤ 0.05 | ns |
| Wall Motion Score Index | ns | ns | ns | r = 0.22 p < 0.05 | r = 0.27 p < 0.01 | r = 0.19 p < 0.05 |
| Brain Natriuretic Peptide (ng/L) | r = 0.25 p < 0.01 | r = 0.28 p < 0.01 | r = 0.29 p < 0.01 | r = 0.45 p < 0.001 | r = 0.45 p < 0.001 | r = 0.55 p < 0.001 |
| C Reactive Protein (mg/dL) | ns | r = 0.37 p < 0.01 | ns | r = 0.31 p < 0.05 | r = 0.50 p < 0.001 | r = 0.30 p < 0.05 |
| Erythrocyte Sedimentation Rate (mm/h) | r = 0.20 p < 0.05 | r = 0.23 p < 0.05 | ns | r = 0.40 p < 0.001 | r = 0.41 p < 0.001 | r = 0.37 p < 0.001 |
| Fibrinogen (mg/dL) | r = 0.28 p < 0.01 | r = 0.41 p < 0.001 | r = 0.25 p < 0.01 | r = 0.25 p < 0.01 | r = 0.47 p < 0.001 | r = 0.31 p < 0.001 |
| Neutrophils (109/L) | ns | ns | r = 0.19 p ≤ 0.05 | ns | r = 0.20 p < 0.05 | r = 0.24 p < 0.05 |
| Platelets (109/L) | ns | ns | ns | ns | r = 0.18 p ≤ 0.05 | ns |
| Neutrophil-to- lymphocyte-ratio | ns | ns | ns | r = 0.20 p < 0.05 | r = 0.18 p ≤ 0.05 | r = 0.24 p < 0.05 |
| Troponin I (at admission) (μg/L) | ns | r = 0.24 p < 0.05 | ns | ns | r = 0.34 p < 0.001 | r = 0.19 p < 0.05 |
| Variable | STD Coefficient | T Value | p | STD Coefficient | T Value | p |
|---|---|---|---|---|---|---|
| CERT-R1 | 0.17 | 1.9 | ≤0.05 | |||
| CERT-R2 | 0.21 | 2.26 | <0.05 | |||
| T2D | 0.27 | 2.9 | <0.05 | 0.29 | 3.1 | <0.01 |
| Platelet count | 0.13 | 1.5 | ns | 0.16 | 1.9 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michelucci, E.; Rocchiccioli, S.; Gaggini, M.; Ndreu, R.; Berti, S.; Vassalle, C. Ceramides and Cardiovascular Risk Factors, Inflammatory Parameters and Left Ventricular Function in AMI Patients. Biomedicines 2022, 10, 429. https://doi.org/10.3390/biomedicines10020429
Michelucci E, Rocchiccioli S, Gaggini M, Ndreu R, Berti S, Vassalle C. Ceramides and Cardiovascular Risk Factors, Inflammatory Parameters and Left Ventricular Function in AMI Patients. Biomedicines. 2022; 10(2):429. https://doi.org/10.3390/biomedicines10020429
Chicago/Turabian StyleMichelucci, Elena, Silvia Rocchiccioli, Melania Gaggini, Rudina Ndreu, Sergio Berti, and Cristina Vassalle. 2022. "Ceramides and Cardiovascular Risk Factors, Inflammatory Parameters and Left Ventricular Function in AMI Patients" Biomedicines 10, no. 2: 429. https://doi.org/10.3390/biomedicines10020429
APA StyleMichelucci, E., Rocchiccioli, S., Gaggini, M., Ndreu, R., Berti, S., & Vassalle, C. (2022). Ceramides and Cardiovascular Risk Factors, Inflammatory Parameters and Left Ventricular Function in AMI Patients. Biomedicines, 10(2), 429. https://doi.org/10.3390/biomedicines10020429









