Ceramic Stereolithography of Bioactive Glasses: Influence of Resin Composition on Curing Behavior and Green Body Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Photocurable Resins
2.3. Fabrication of BAG Scaffolds
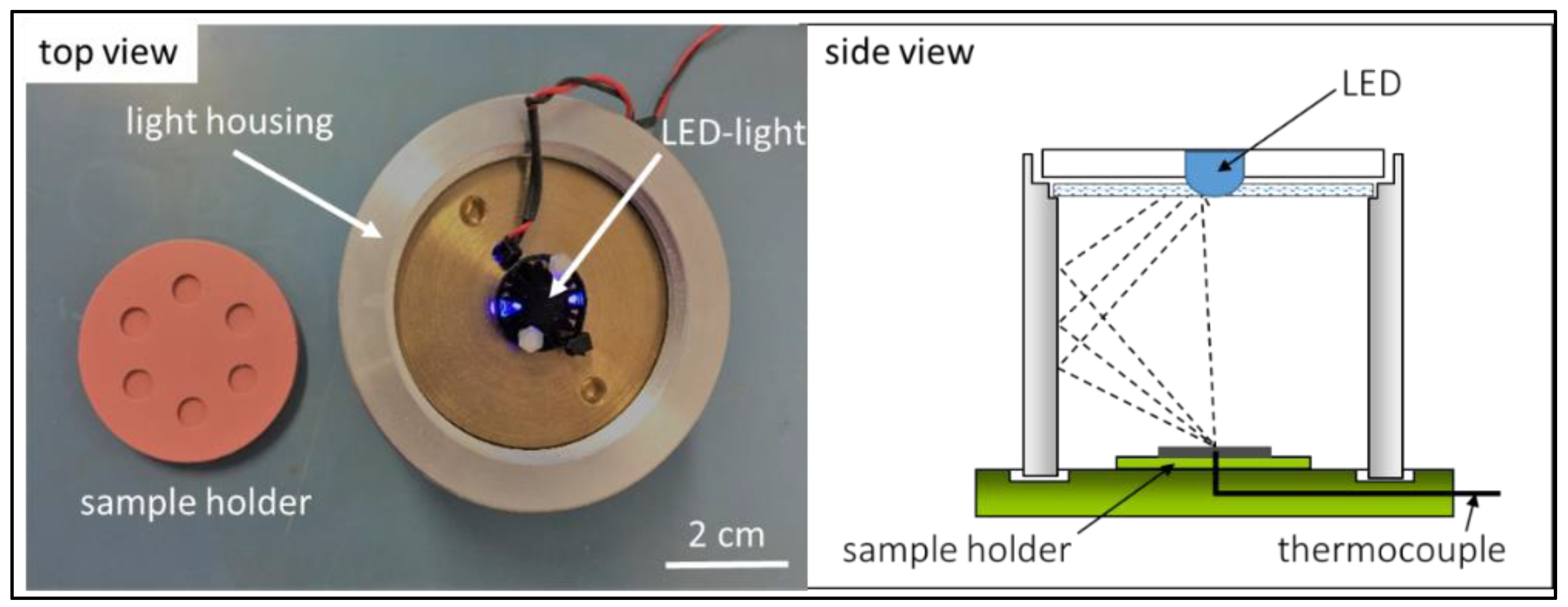
2.4. Characterization Techniques
3. Results and Discussion
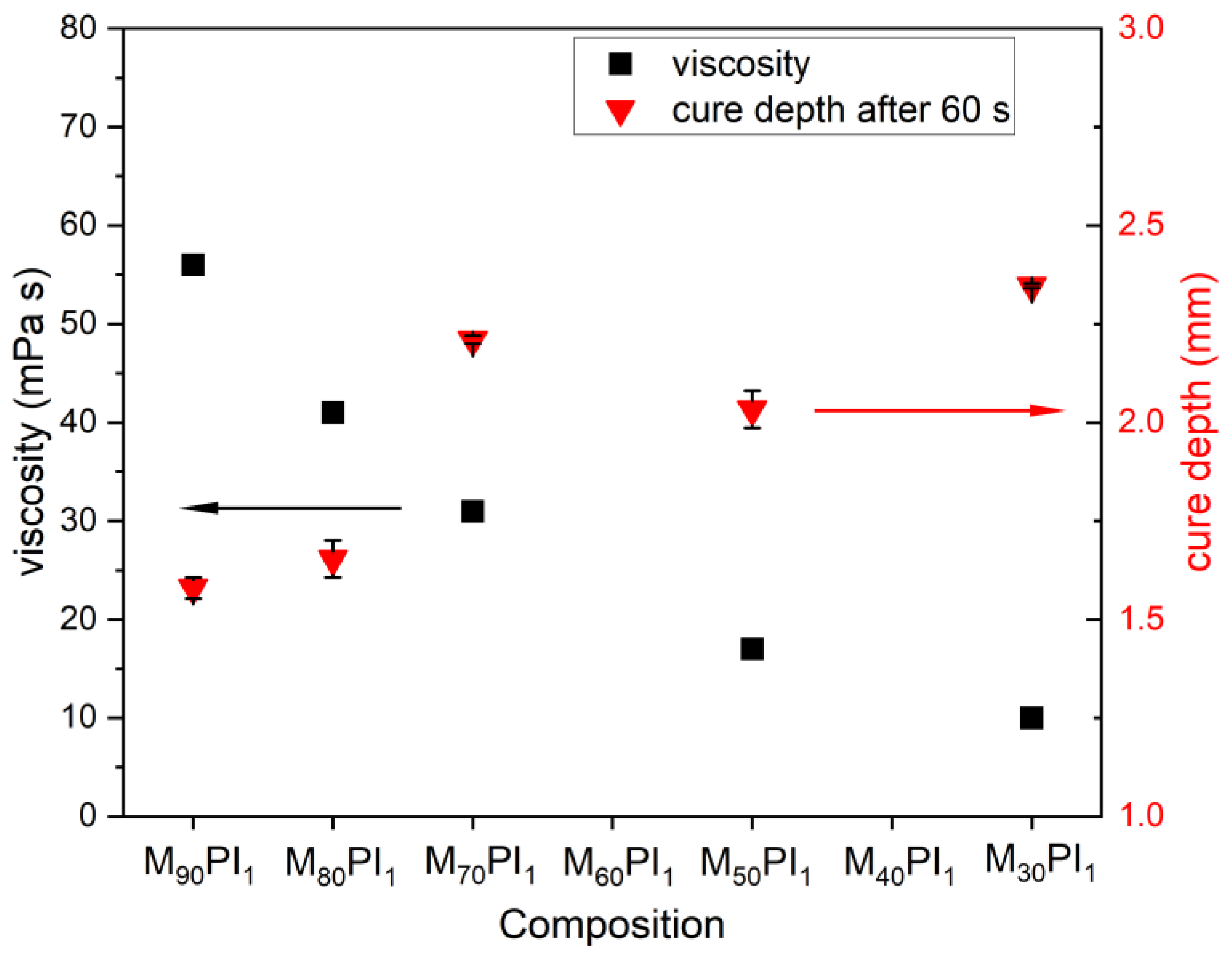
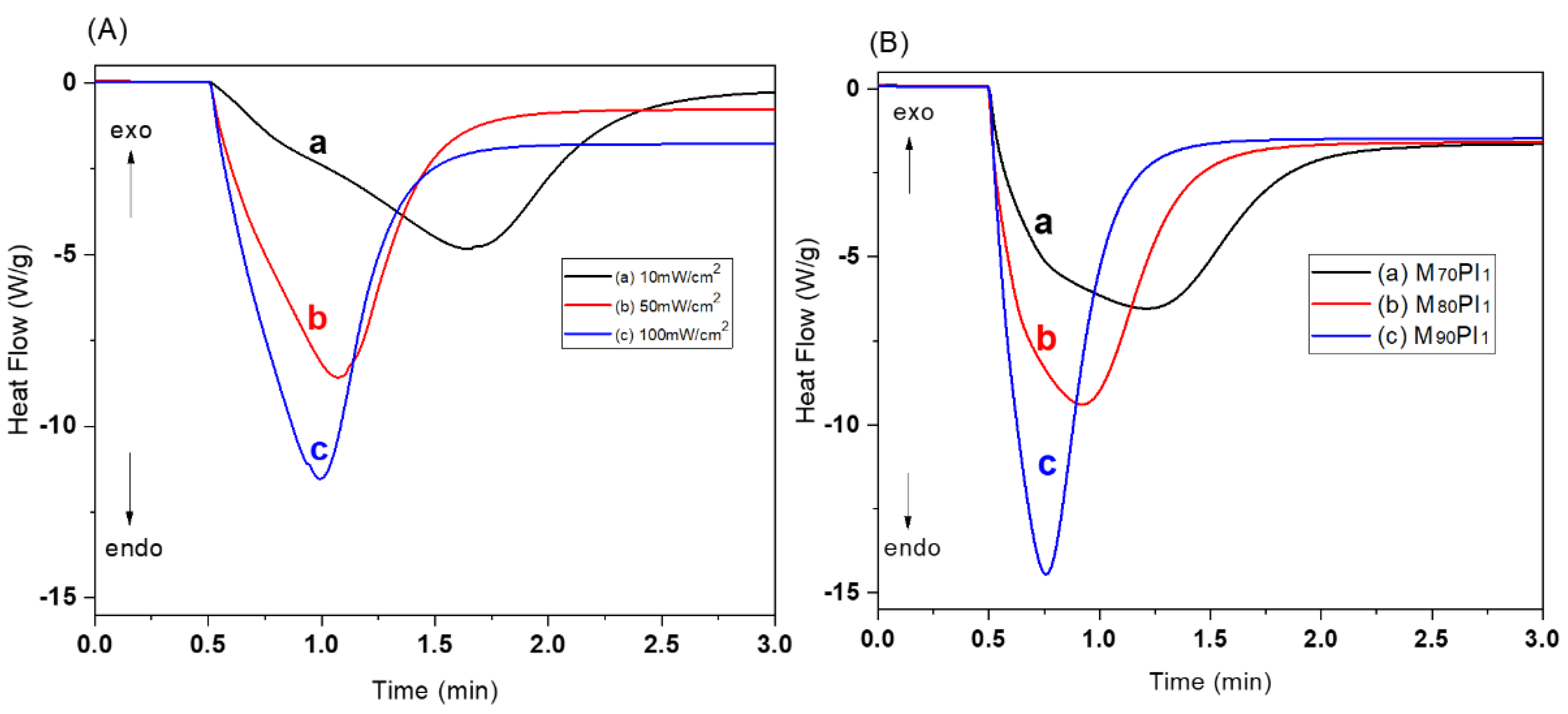
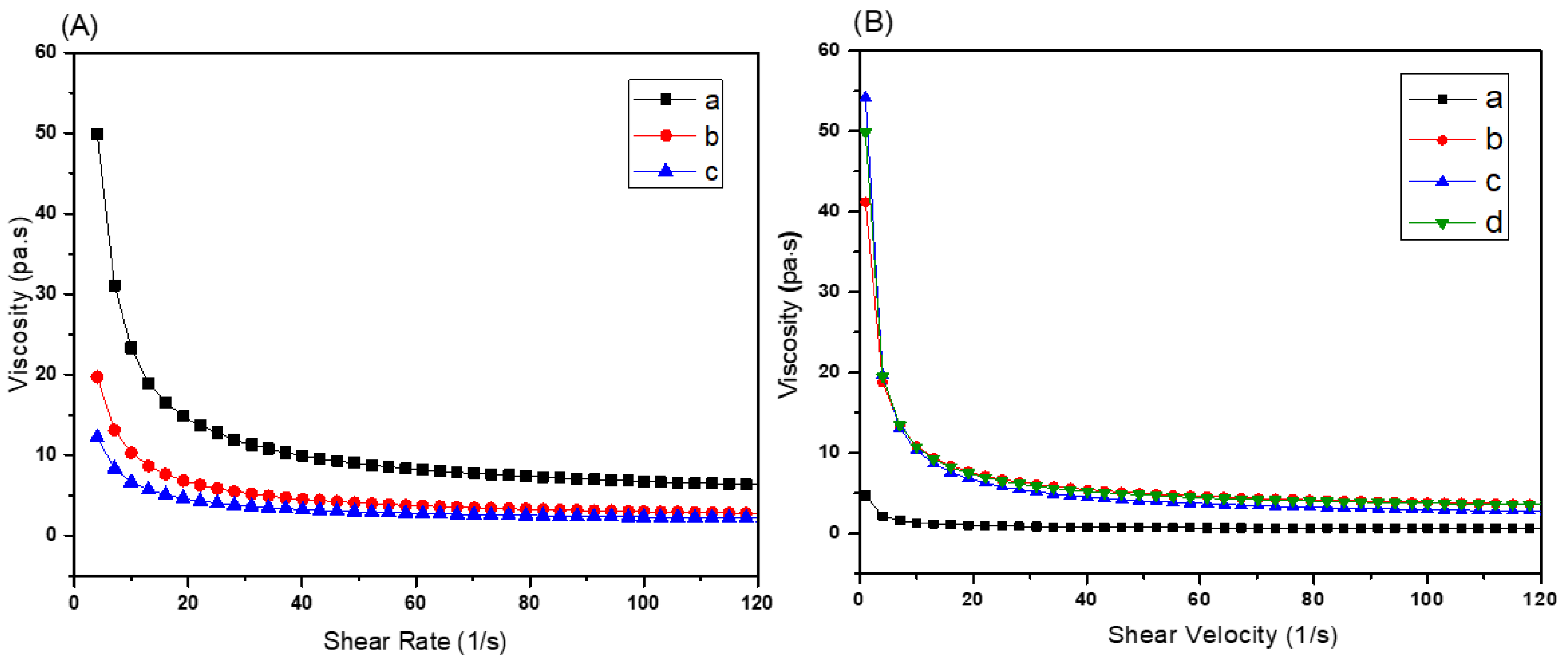
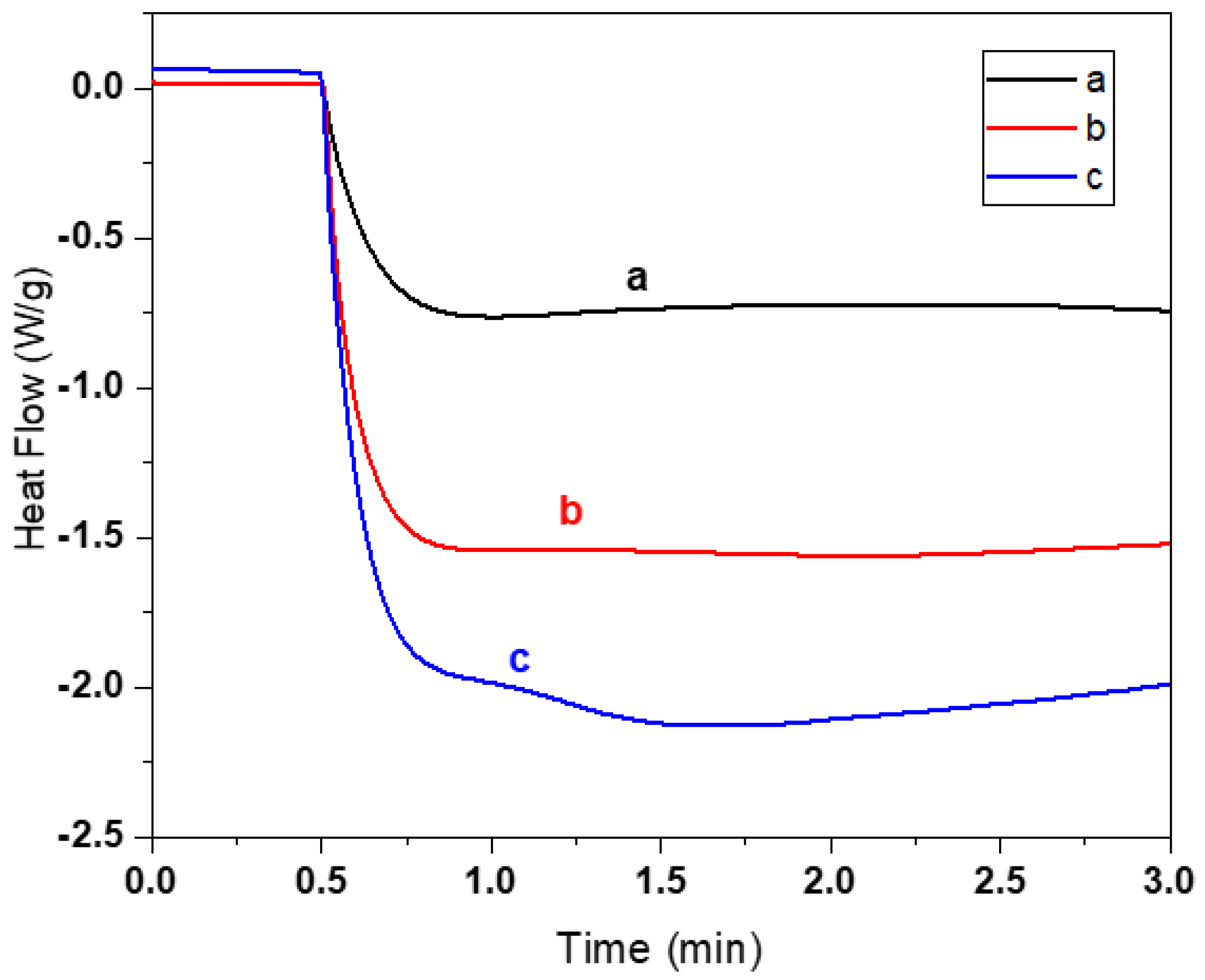
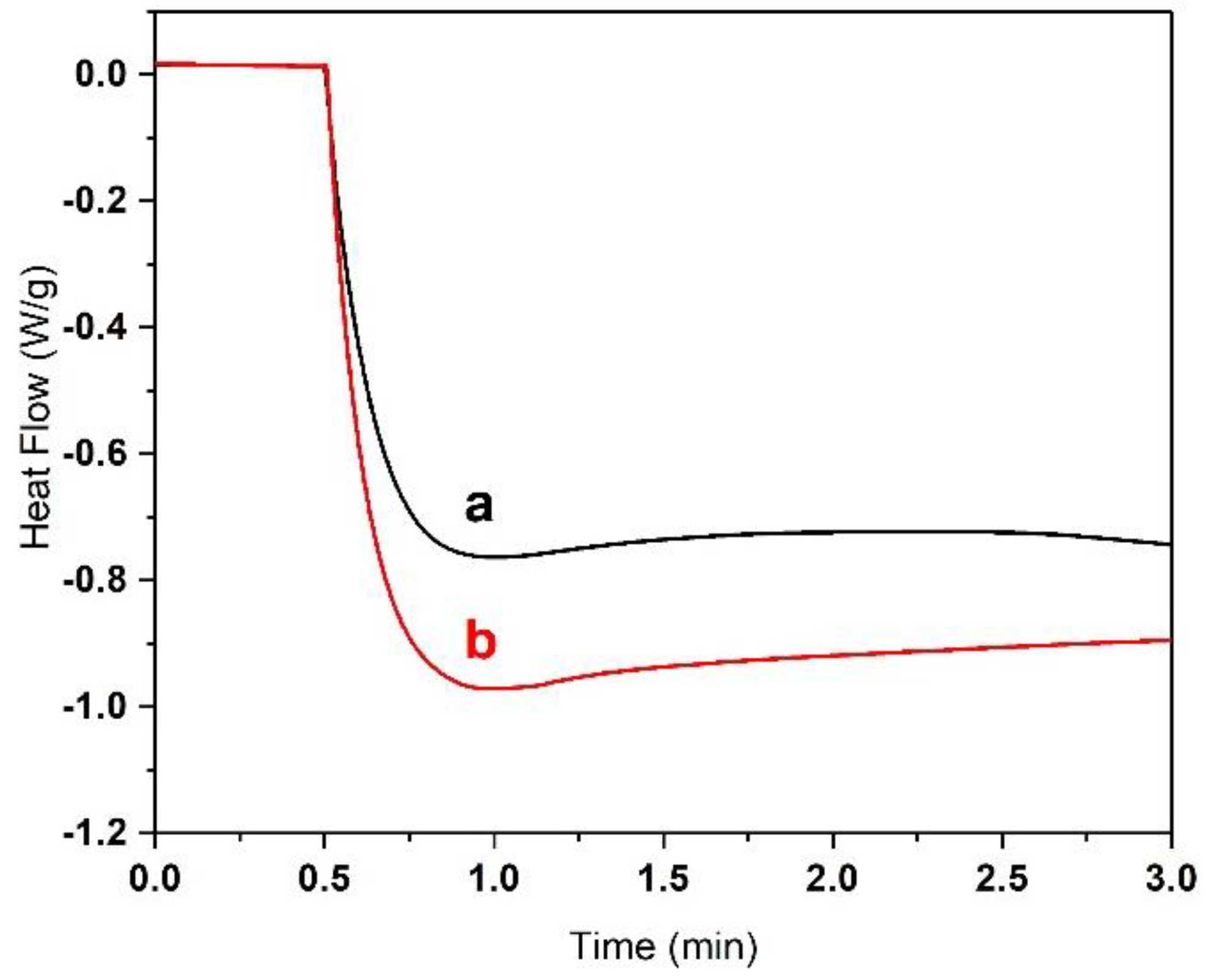
3.1. Selection of the Acrylate Composition and Dye Content in the Resin
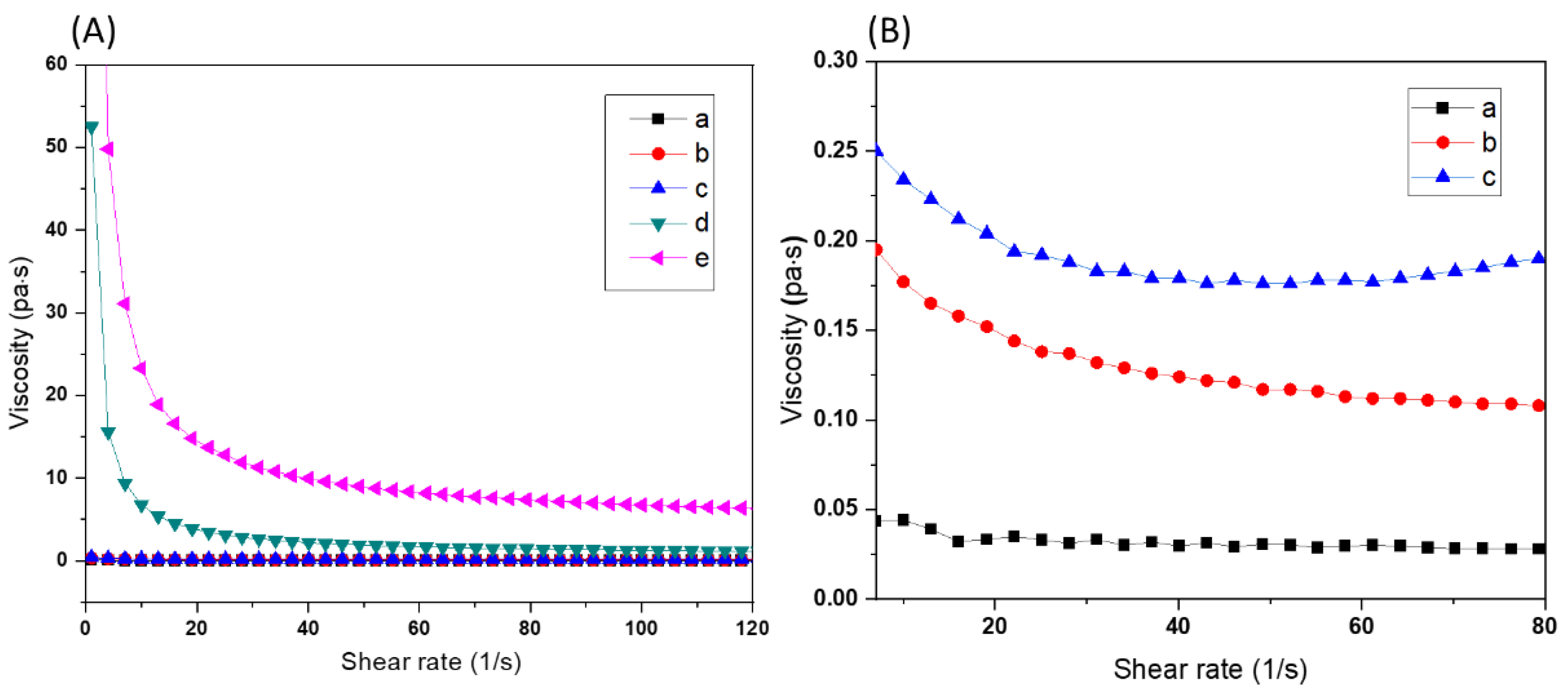
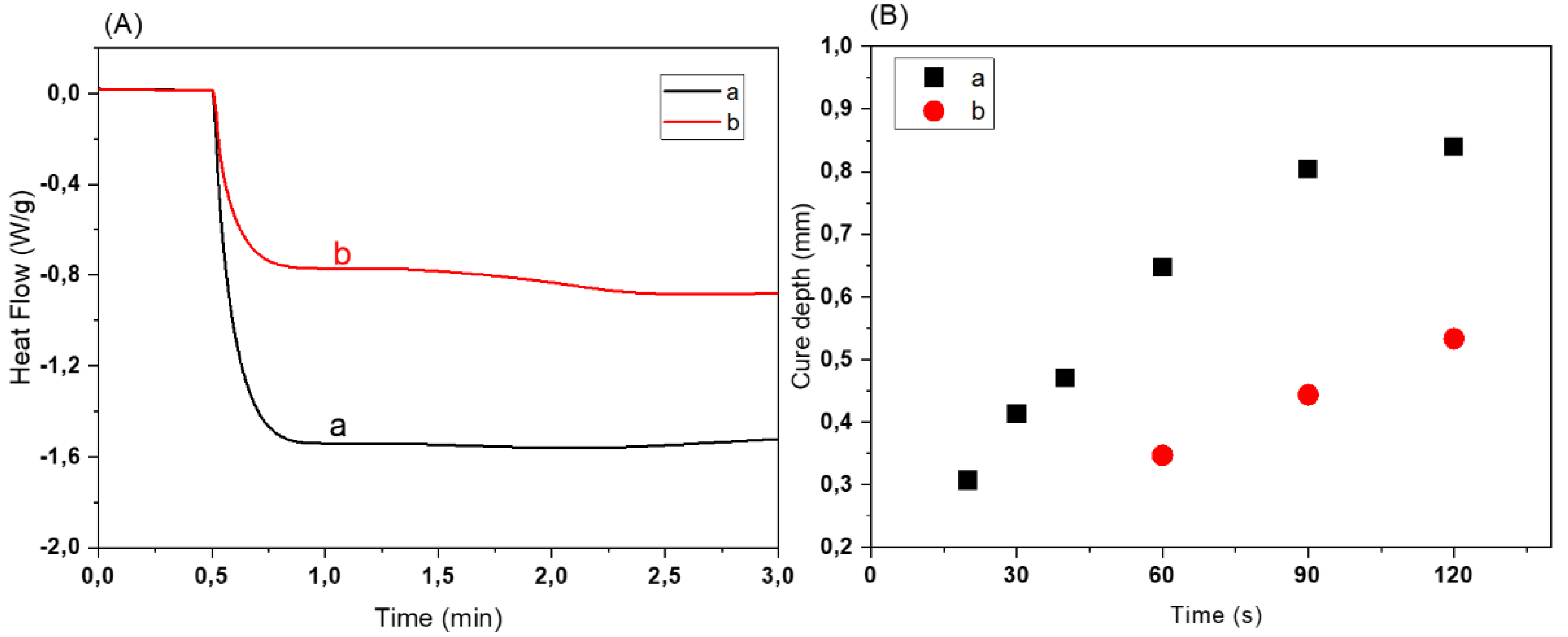
3.2. Optimization of Ceramic Resin Compositions
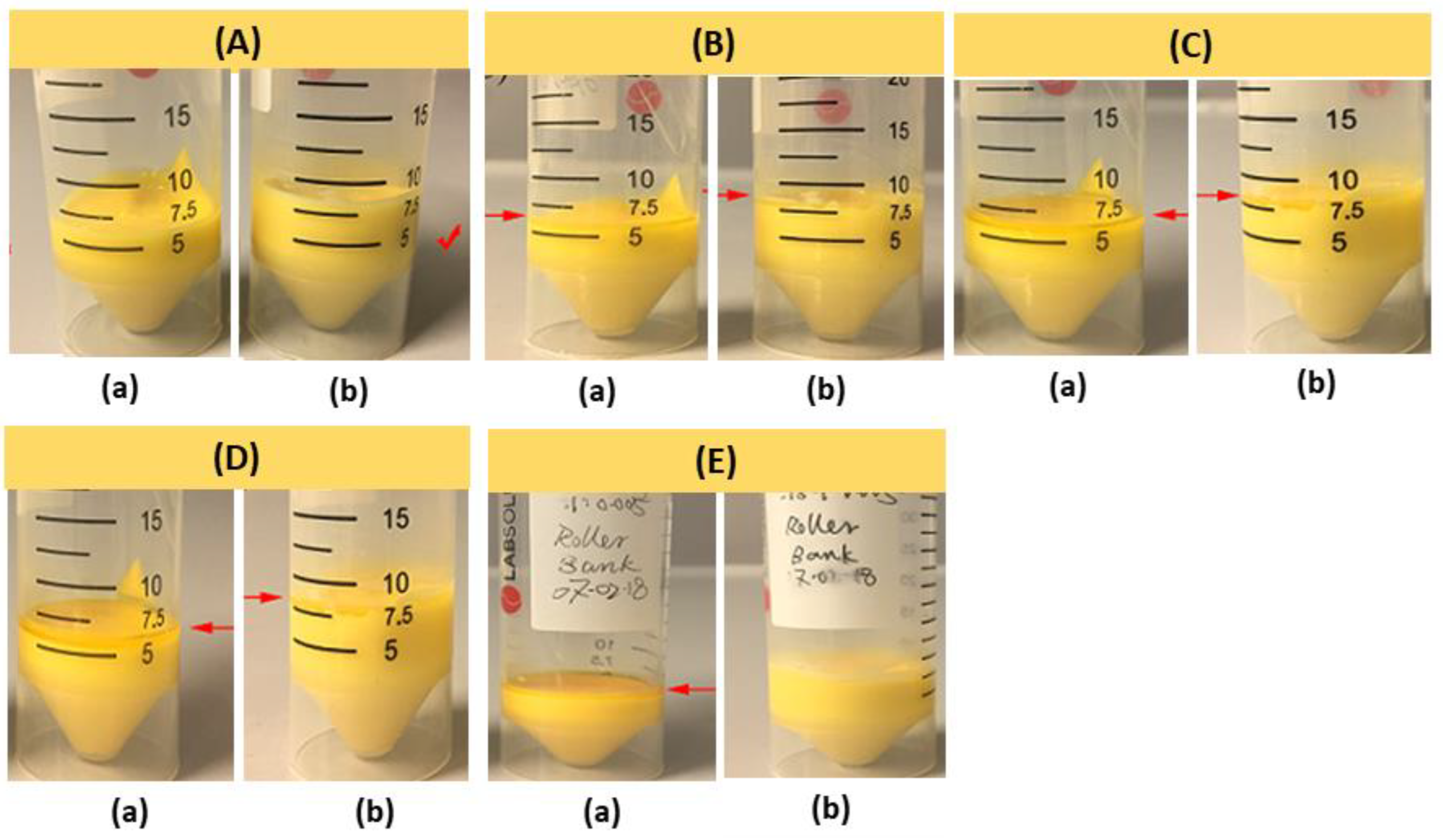
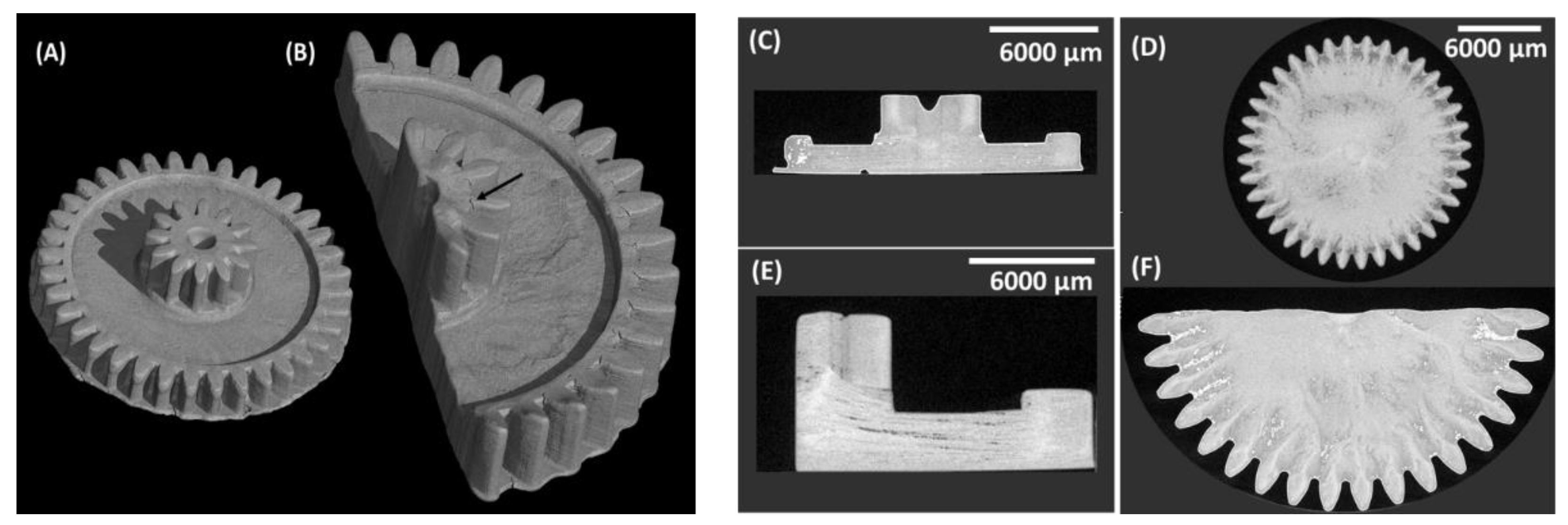
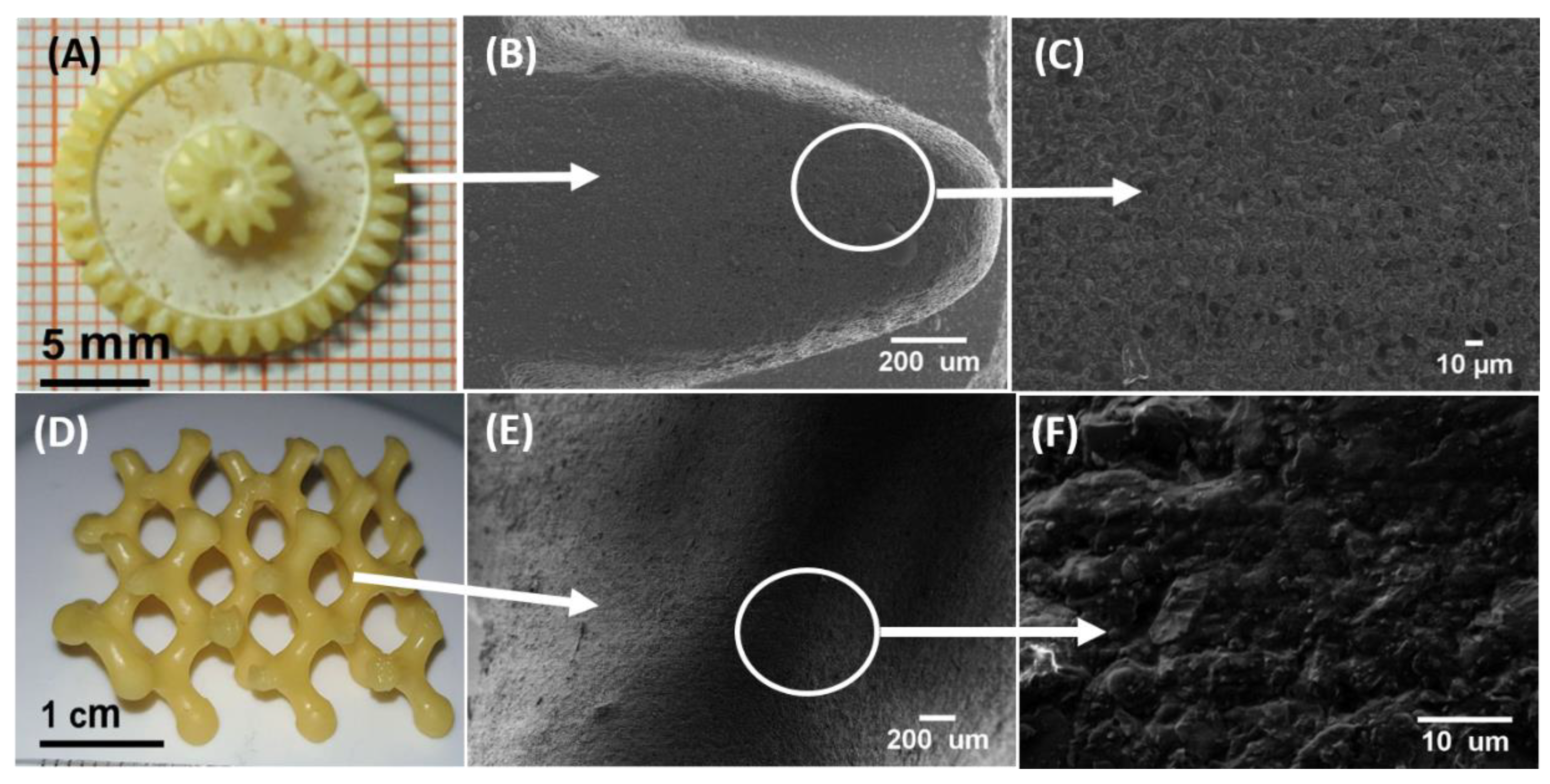
3.3. Fabrication of 3D Scaffolds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Shah, A.T.; Ain, Q.; Chaudhry, A.A.; Khan, A.F.; Iqbal, B.; Ahmad, S.; Siddiqi, S.A.; Rehman, I.U. A study of the effect of precursors on physical and biological properties of mesoporous bioactive glass. J. Mater. Sci. 2015, 50, 1794–1804. [Google Scholar] [CrossRef]
- Zahid, S.; Shah, A.T.; Jamal, A.; Chaudhry, A.A.; Khan, A.S.; Khan, A.F.; Muhammad, N.; Rehman, I.U. Biological behavior of bioactive glasses and their composites. RSC Adv. 2016, 6, 70197–70214. [Google Scholar] [CrossRef]
- Shah, A.T.; Batool, M.; Chaudhry, A.A.; Iqbal, F.; Javaid, A.; Zahid, S.; Ilyas, K.; Qasim, S.B.; Khan, A.F.; Khan, A.S. Effect of calcium hydroxide on mechanical strength and biological properties of bioactive glass. J. Mech. Behav. Biomed. Mater. 2016, 61, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Madrid, A.P.M.; Vrech, S.M.; Sanchez, M.A.; Rodriguez, A.P. Advances in additive manufacturing for bone tissue engineering scaffolds. Mater. Sci. Eng. C 2019, 100, 631–644. [Google Scholar] [CrossRef]
- Gmeiner, R.; Mitteramskogler, G.; Stampfl, J.; Boccaccini, A.R. Stereolithographic ceramic manufacturing of high strength bioactive glass. Int. J. Appl. Ceram. Technol. 2015, 12, 38–45. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.; Hui, D. Additive manufacturing, 3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Baino, F.; Magnaterra, G.; Fiume, E.; Schiavi, A.; Tofan, L.P.; Schwentenwein, M.; Verné, E. Digital light processing stereolithography of hydroxyapatite scaffolds with bone-like architecture, permeability, and mechanical properties. J. Am. Ceram. Soc. 2022, 105, 1648–1657. [Google Scholar] [CrossRef]
- Harun, W.; Kamariah, M.; Muhamad, N.; Ghani, S.; Ahmad, F.; Mohamed, Z. A review of powder additive manufacturing processes for metallic biomaterials. Powder Technol. 2018, 327, 128–151. [Google Scholar] [CrossRef]
- Huang, Y.; Leu, M.C.; Mazumder, J.; Donmez, A. Additive manufacturing: Current state, future potential, gaps and needs, and recommendations. J. Manuf. Sci. Eng. 2015, 137, 014001. [Google Scholar] [CrossRef] [Green Version]
- Tosto, C.; Pergolizzi, E.; Blanco, I.; Patti, A.; Holt, P.; Karmel, S.; Cicala, G. Epoxy based blends for additive manufacturing by liquid crystal display, LCD, printing: The effect of blending and dual curing on daylight curable resins. Polymers 2020, 12, 1594. [Google Scholar] [CrossRef]
- Obst, P.; Riedelbauch, J.; Oehlmann, P.; Rietzel, D.; Launhardt, M.; Schmölzer, S.; Osswald, T.A.; Witt, G. Investigation of the influence of exposure time on the dual-curing reaction of RPU 70 during the DLS process and the resulting mechanical part properties. Addit. Manuf. 2020, 32, 101002. [Google Scholar] [CrossRef]
- Halloran, J.W. Ceramic stereolithography: Additive manufacturing for ceramics by photopolymerization. Annu. Rev. Mater. Res. 2016, 46, 19–40. [Google Scholar] [CrossRef]
- Wang, X.; Schmidt, F.; Hanaor, D.; Kamm, P.H.; Li, S.; Gurlo, A. Additive manufacturing of ceramics from preceramic polymers: A versatile stereolithographic approach assisted by thiol-ene click chemistry. Addit. Manuf. 2019, 27, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Bae, C.-J.; Ramachandran, A.; Chung, K.; Park, S. Ceramic stereolithography: Additive manufacturing for 3D complex ceramic structures. J. Korean Ceram. Soc. 2017, 54, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Komissarenko, D.A.; Sokolov, P.S.; Evstigneeva, A.D.; Shmeleva, I.A.; Dosovitsky, A.E. Rheological and curing behavior of acrylate-based suspensions for the DLP 3D printing of complex zirconia parts. Materials 2018, 11, 2350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, L.; Ding, S.; Wen, C. Additive manufacturing technology for porous metal implant applications and triple minimal surface structures: A review. Bioact. Mater. 2019, 4, 56–70. [Google Scholar] [CrossRef]
- Thavornyutikarn, B.; Tesavibul, P.; Sitthiseripratip, K.; Chatarapanich, N.; Feltis, B.; Wright, P.F.; Turney, T.W. Porous 45S5 Bioglass®-based scaffolds using stereolithography: Effect of partial pre-sintering on structural and mechanical properties of scaffolds. Mater. Sci. Eng. C 2017, 75, 1281–1288. [Google Scholar] [CrossRef]
- Alvim, H.H.; Alecio, A.C.; Vasconcellos, W.A.; Furlan, M.; de Oliveira, J.E.; Saad, J.R. Analysis of camphorquinone in composite resins as a function of shade. Dent. Mater. 2007, 23, 1245–1249. [Google Scholar] [CrossRef]
- Schuster, M.; Turecek, C.; Kaiser, B.; Stampfl, J.; Liska, R.; Varga, F. Evaluation of biocompatible photopolymers I: Photoreactivity and mechanical properties of reactive diluents. J. Macromol. Sci. Part A 2007, 44, 547–557. [Google Scholar] [CrossRef]
- Lee, J.H.; Prud’Homme, R.K.; Aksay, I.A. Cure depth in photopolymerization: Experiments and theory. J. Mater. Res. 2001, 16, 3536–3544. [Google Scholar] [CrossRef] [Green Version]
- Hyun, H.-K.; Ferracane, J.L. Influence of biofilm formation on the optical properties of novel bioactive glass-containing composites. Dent. Mater. 2016, 32, 1144–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brady, G.; Halloran, J. Differential photo-calorimetry of photopolymerizable ceramic suspensions. J. Mater. Sci. 1998, 33, 4551–4560. [Google Scholar] [CrossRef]
- Ronca, A.; Ambrosio, L.; Grijpma, D.W. Preparation of designed poly, D, L-lactide)/nanosized hydroxyapatite composite structures by stereolithography. Acta Biomater. 2013, 9, 5989–5996. [Google Scholar] [CrossRef] [PubMed]
- Mahnam, N.; Beheshty, M.H.; Barmar, M.; Shervin, M. Modification of dicyandiamide-cured epoxy resin with different molecular weights of polyethylene glycol and its effect on epoxy/glass prepreg characteristics. High Perform. Polym. 2013, 25, 705–713. [Google Scholar] [CrossRef]
- Gherman, T.; Moldovan, M.; Filip, M.; Ancuța, T.; Râpă, M.; Cuc, S. Effect of PEG Content on the Mechanical Properties of Bis-GMA/TEGDMA/UDMA Dental Resin Composites. In Key Engineering Materials; Trans Tech Publications Ltd.: Bäch, Switzerland, 2017; Volume 752, pp. 3–10. [Google Scholar] [CrossRef]
- Tomeckova, V.; Halloran, J.W. Cure depth for photopolymerization of ceramic suspensions. J. Eur. Ceram. Soc. 2010, 30, 3023–3033. [Google Scholar] [CrossRef]
- Halloran, J.W.; Tomeckova, V.; Gentry, S.; Das, S.; Cilino, P.; Yuan, D.; Guo, R.; Rudraraju, A.; Shao, P.; Wu, T. Photopolymerization of powder suspensions for shaping ceramics. J. Eur. Ceram. Soc. 2011, 31, 2613–2619. [Google Scholar] [CrossRef]

| Samples | mTMPE, [%] | mHEA, [%] | PI, mPI/macr, [%] | Dye, mdye/macr, [%] |
|---|---|---|---|---|
| M90PI1 | 90 | 10 | 1 | - |
| M80PI1 | 80 | 20 | 1 | - |
| M70PI1 | 70 | 30 | 1 | - |
| M50PI1 | 50 | 50 | 1 | - |
| M30PI1 | 30 | 70 | 1 | - |
| M90PI1D0.005 | 90 | 10 | 1 | 0.005 |
| M90PI1D0.010 | 90 | 10 | 1 | 0.010 |
| M90PI1D0.015 | 90 | 10 | 1 | 0.015 |
| M80PI1D0.005 | 80 | 20 | 1 | 0.005 |
| Samples | mBAG/macr + mBAG, [%] | mTMPE, [%] | mHEA, [%] | mPI/macr, [%] | mdye/macr, [%] | mPEG/macr + mPEG, [%] |
|---|---|---|---|---|---|---|
| 20BM90PI1D0.005 | 20 | 90 | 10 | 1 | 0.005 | - |
| 30BM90PI1D0.005 | 30 | 90 | 10 | 1 | 0.005 | - |
| 40BM90PI1D0.005 | 40 | 90 | 10 | 1 | 0.005 | - |
| 50BM90PI1D0.005 | 50 | 90 | 10 | 1 | 0.005 | - |
| 60BM90PI1D0.005 | 60 | 90 | 10 | 1 | 0.005 | - |
| 60BM90PI1D0.005E10 | 60 | 90 | 10 | 1 | 0.005 | 10 |
| 60BM90PI1D0.005E15 | 60 | 90 | 10 | 1 | 0.005 | 15 |
| 45BM90PI1D0.005E10 | 45 | 90 | 10 | 1 | 0.005 | 10 |
| 55BM90PI1D0.005E10 | 55 | 90 | 10 | 1 | 0.005 | 10 |
| 55BM90PI1D0.005E15 | 55 | 90 | 10 | 1 | 0.005 | 15 |
| 65BM90PI1D0.005E10 | 65 | 90 | 10 | 1 | 0.005 | 10 |
| 55BM80PI1D0.005 | 55 | 80 | 20 | 1 | 0.005 | - |
| 55BM80PI1D0.005E10 | 55 | 80 | 20 | 1 | 0.005 | 10 |
| 40BM80PI1D0.01E10 | 40 | 80 | 20 | 1 | 0.01 | 10 |
| 55BM80PI1D0.01E10 | 55 | 80 | 20 | 1 | 0.01 | 10 |
| 60BM80PI1D0.01E10 | 60 | 80 | 20 | 1 | 0.01 | 10 |
| 55BM80PI2D0.01E10 | 55 | 80 | 20 | 2 | 0.01 | 10 |
| 55BM80PI1D0.015E10 | 55 | 80 | 20 | 1 | 0.015 | 10 |
| 60BM80PI1D0.015E10 | 60 | 80 | 20 | 1 | 0.015 | 10 |
| 50BM90PI1D0 | 50 | 90 | 10 | 1 | - | - |
| 50BM90PI1D0.01 | 50 | 90 | 10 | 1 | 0.01 |
| Samples | 55BM90PI1D0.005E0 | 55BM90PI1D0.005E10 | 55BM90PI1D0.005E15 | 60BM90PI1D0.005E10 | 60BM90PI1D0.005E15 |
|---|---|---|---|---|---|
| Shore-D | 71.4 ± 0.6 | 66.5 ± 0.4 | 61.4 ± 0.3 | 60.6 ± 0.5 | 57.1 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Schmidt, F.; Görke, O.; Asif, A.; Weinhold, J.; Aghaei, E.; Rehman, I.u.; Gurlo, A.; Shah, A.T. Ceramic Stereolithography of Bioactive Glasses: Influence of Resin Composition on Curing Behavior and Green Body Properties. Biomedicines 2022, 10, 395. https://doi.org/10.3390/biomedicines10020395
Chen Q, Schmidt F, Görke O, Asif A, Weinhold J, Aghaei E, Rehman Iu, Gurlo A, Shah AT. Ceramic Stereolithography of Bioactive Glasses: Influence of Resin Composition on Curing Behavior and Green Body Properties. Biomedicines. 2022; 10(2):395. https://doi.org/10.3390/biomedicines10020395
Chicago/Turabian StyleChen, Qirong, Franziska Schmidt, Oliver Görke, Anila Asif, Joachim Weinhold, Erfan Aghaei, Ihtesham ur Rehman, Aleksander Gurlo, and Asma Tufail Shah. 2022. "Ceramic Stereolithography of Bioactive Glasses: Influence of Resin Composition on Curing Behavior and Green Body Properties" Biomedicines 10, no. 2: 395. https://doi.org/10.3390/biomedicines10020395
APA StyleChen, Q., Schmidt, F., Görke, O., Asif, A., Weinhold, J., Aghaei, E., Rehman, I. u., Gurlo, A., & Shah, A. T. (2022). Ceramic Stereolithography of Bioactive Glasses: Influence of Resin Composition on Curing Behavior and Green Body Properties. Biomedicines, 10(2), 395. https://doi.org/10.3390/biomedicines10020395








