Abstract
Colorectal cancer (CRC) is the third most commonly diagnosed cancer worldwide. Hereditary CRC syndromes account for approximately 5–10% of all CRC, with a lifetime risk of CRC that approaches 50–80% in the absence of endoscopic or surgical treatment. Hereditary CRC syndromes can be phenotypically divided into polyposis and non-polyposis syndrome, mainly according to the conditions of polyps. The typical representatives are familial adenomatous polyposis (FAP) and Lynch syndromes (LS), respectively. Over the past few decades, molecular genetics enhanced the discovery of cancer-predisposing genes and revolutionized the field of clinical oncology. Hereditary CRC syndromes have been a key part of this effort, with data showing that pathogenic variants are present in up to 10% of cases. Molecular phenotypes of tumors can not only help identify individuals with genetic susceptibility to CRC but also guide the precision prevention and treatment for the development of CRC. This review emphasizes the molecular basis and prevention strategies for hereditary CRC syndromes.
1. Introduction
Hereditary colorectal cancer (CRC) syndromes exhibit different inheritance patterns and phenotypic features. The expansion of family registries and advances in genomic technologies have led to the discovery of multiple genes for specific hereditary syndromes, facilitating clinical diagnosis and treatment. Genetic susceptibility is associated with 2–8% of all CRCs and 1/5 of CRCs diagnosed before the age of 50 due to pathogenic germline variation in high-risk cancer genes [1,2,3,4,5]. In hereditary CRC syndromes, adenomatous polyposis syndrome accounts for ~1% and is strongly associated with APC (dominant inheritance) and MUTYH (recessive inheritance); Lynch syndrome (LS) accounts for 2–3% and is associated with germline or epistatic mutations in the DNA mismatch repair (MMR) genes MLH1, MSH2, MSH6, and PMS2; hamartomatous polyposis syndrome (<0.1%) is caused by mutations in SMAD4, BMPR1A, STK11, and PTEN. Nowadays, the understanding of genetic susceptibility to CRC is more extensive. Continued molecular genetic studies help to understand the nature of mutations in cancer susceptibility genes and facilitate the therapeutic task of individualized medicine. Therefore, the following article outlines the characteristics of hereditary CRC syndromes, the susceptibility genes proposed in recent years, and the associated chemopreventive and immunotherapeutic strategies.
2. Overview of Hereditary CRC Syndromes
2.1. Adenomatous Polyposis Syndromes
Familial adenomatous polyposis (FAP) is the most common adenomatous polyposis syndrome. FAP is a rare autosomal dominant disorder characterized by multiple adenomatous polyps of the gastrointestinal mucosa and various extraintestinal lesions, with a 100% lifetime risk of CRC if not treated [6] (Table 1). FAP can be classified into classic and attenuated types. Attenuated FAP (AFAP) is a milder form, with a delayed age of onset, fewer polyps (0–100), a lower overall risk of CRC (60–80%), and mostly in the right hemicolon. Early identification and management of FAP are essential due to the nearly 100% lifetime risk of CRC without intervention. Germline mutations in the APC (MIM: 611731) gene can be detected in more than 90% of FAP cases. APC testing should be taken into consideration when one of the following occurs: (1) more than 20 adenomas in the colorectum over the course of the patient’s lifetime, (2) a family history of FAP, or (3) 10 cumulative adenomas discovered after a colonoscopy. Screening colonoscopy has been proven to lower the risk of CRC in patients with FAP until the polyp burden is unmanageable by endoscopic methods. In families with classic FAP, a high-quality colonoscopy is recommended beginning between the ages of 10–15 years and repeated every year if an APC variant is identified. Early colonoscopy initiation may be taken into consideration for patients who have a family history of CRC with very early onset. Due to the attenuated character of AFAP, colonoscopy can be started at a slightly later age (late teens) and repeated every 1–2 years [6,7]. Extraintestinal manifestations in FAP involve duodenal adenomas, desmoids, thyroid tumors, and central nervous system tumors [8,9]. Consequently, monitoring and treating extracolonic lesions is also extremely crucial.

Table 1.
Risk of hereditary colorectal cancer syndromes.
Polymerase proofreading-associated polyposis (PPAP) has been identified as a novel adenomatous polyposis syndrome, which is an autosomal dominant diseasecaused by germline pathogenic variants in the exonuclease (proofreading) domains of POLE (MIM: 174762) and POLD1 (MIM: 174761) [10]. Although the clinical phenotype has not been established, the available evidence showed that PPAP has a high-penetrant susceptibility to polyposis, CRCs, and other extracolonic tumors.
MUTYH-associated polyposis (MAP) is considered to be the second most common adenomatous polyposis syndrome. MAP is an autosomal recessive condition with high penetration and is linked to biallelic germline variants in the DNA glycosylase gene MUTYH (MIM: 604933). The identification of MUTYH lays the foundation for the base excision repair (BER) pathway in polyposis and CRC. Carriers of biallelic MUTYH variants exhibit a high lifetime risk of CRC, while it is debatable whether patients with a monoallelic mutation have an increased genetic susceptibility to CRC (1.5–2 fold) [11,12]. Patients with MAP display a very broad and diverse clinical spectrum, which is generally more compared to AFAP. Therefore, endoscopic surveillance is recommended from age 18 with a 1–2 years interval, according to the findings [13].
Recently, two novel adenomatous polyposis-predisposing syndromes with autosomal recessive inheritance have been proposed. NTHL1 (MIM: 602656) is the second DNA glycosylase gene identified for the BER pathway [14]. Biallelic mutations of MSH3 (MIM: 600887), an MMR gene not linked to LS, were detected in families with polyposis and gastrointestinal malignancy, thereby identifying as another recessive subtype of colorectal adenomatous polyposis syndromes [15].
Constitutional MMR deficiency syndrome (CMMRD) is an uncommon, inherited cancer predisposition syndrome caused by biallelic germline pathogenic variants in four MMR genes, MLH1 (MIM: 120436), MSH2 (MIM: 609309), MSH6 (MIM: 600678), and PMS2 (MIM: 600259). Such cases with biallelic germline mutations in the MMR gene are distinct from classic LS. Patients with CMMRD have a higher risk of developing cancers as early as childhood and adolescence. It was estimated that 80% of patients experience their first cancer before 18 years old [16].
2.2. Hamartomatous Polyposis Syndromes
A hamartoma is a disordered and uncontrolled proliferation of normal cells. Based on this, the hamartomatous polyposis syndromes are distinguished from the adenomatous polyposis syndromes. Even though there is no direct evidence, hamartomatous polyposis syndromes have an estimated prevalence of 1/100,000 [17]. The types of gastrointestinal hereditary hamartomatous syndromes mainly include Peutz–Jeghers syndrome (PJS), PTEN-hamartoma tumor syndrome (PHTS), and juvenile polyposis syndrome (JPS). The evaluation criteria have been created by National Comprehensive Cancer Network (NCCN). The patient’s medical history, a skin-focused physical examination, and the feature of gastrointestinal polyps should be taken into consideration during the diagnostic process. Surprisingly, colonoscopy almost reduces the risk of CRC to the level of the general population.
Peutz–Jeghers Syndrome (PJS) is a rare inherited autosomal dominant syndrome known as mucocutaneous pigmentations because nearly all individuals exhibit melanotic macules or “spots” on their lips, buccal mucosa, fingertips, and toe tips. Hamartomatous polyps are mainly found in the small intestine (60–90%), colon (50–64%), or extraintestinal organs such as the gallbladder or bladder [18]. PJS is linked to an increased risk of CRC, and the majority of cases result from mutations in the tumor suppressor gene LKB1/STK11 (MIM: 602216).
Juvenile polyposis syndrome (JPS) is also an inherited autosomal dominant syndrome driven by mutations in either BMPR1A (MIM: 601299) or SMAD4 (MIM: 600993) [19]. Patients with JPS are characterized by multiple hamartomatous polyps in the gastrointestinal tract, primarily colon polyps, occasionally stomach polyps, and infrequently small intestine polyps. There is no extra risk of non-gastrointestinal cancer reported in JPS.
PTEN-hamartoma tumor syndrome (PHTS) is a disorder caused by mutations in the tumor suppressor gene PTEN (MIM: 601728) and is linked to increased risk for cancer in multiple organs, including the breast, thyroid, kidney, and skin. The gastrointestinal manifestations of PHTS are mainly gastrointestinal hamartomatous polyps. Although there is a moderate risk of CRC, colonoscopic surveillance can be delayed until after the age of 35. Notably, although most cases of PHTS correspond to Cowden syndrome (CS), PHTS also includes Bantayan–Riley–Ruvalcaba syndrome (BRRS).
2.3. Other Polyposis Syndromes
Serrated polyposis syndrome (SPS) is a hereditary condition known as multiple serrated polyps throughout the colorectum, including hyperplastic polyps, sessile serrated lesions, sessile serrated lesion with dysplasia, and conventional serrated adenomas [20]. Patients with SPS have an increased risk (16–29%) of CRC [21]. RNF43 (MIM: 612482), along with BRAF (MIM: 164757) mutation, has been identified in rare cases of serrated polyposis [22]. However, high-penetrance germline mutations have not been found yet.
Mixed polyposis syndrome (MPS) is a rare autosomal dominant disease and is characterized by the presence of multiple histologic polyps, including adenomas, hamartomas, and serrated lesions, with an increased risk of CRC. Some affected people have been found to have GREM1 (MIM: 603054) germline variants [23,24], while the genetic basis of most MPS families is still unclear, and there are insufficient data to prove the optimal monitoring interval.
2.4. Non-Polyposis Syndromes
LS is the most common hereditary non-polyposis syndrome, accounting for 2–4% of all CRCs, and was initially identified by Lynch in 1996 [25,26]. LS is an autosomal dominant condition caused by a germline mutation in one of the DNA MMR genes MLH1, MSH2, MSH6, and PMS2, which preserve genome integrity by postreplicative proofreading and editing. LS has a significantly higher risk of CRC, and an estimated 60–80% of MMR mutations will lead to CRC progression [27]. Patients with LS also have an increased risk of developing extra-colon cancers such as endometrial, ovarian, stomach, and small intestine cancers, especially if they have mutations in the MLH1, MSH2, and MSH6 genes.
In contrast to polyposis syndromes, LS does not exhibit any characteristic endoscopic signs, making it a particularly difficult condition to diagnose. The Amsterdam criteria, which had been developed in 1991, served as the basis for the initial diagnosis [28]. These criteria concentrated on age at onset and family history, which could help to select families for genetic identification research. Following the discovery of a germline mutation in an MMR gene, Bethesda guidelines further use microsatellite instability (MSI) to select patients for genetic testing [29]. Neither of these criteria is optimal in identifying LS cases, prompting the recommendation to offer genetic testing to all newly diagnosed CRC patients [30,31]. All people with a molecular diagnosis of LS should have a colonoscopy starting at 20–25 [7]. Vasen et al. tracked 205 LS families and found a decreased incidence of CRC with colonoscopy intervals of 1–2 years vs. 2–3 years [32]. NCCN recommendations urge surveillance colonoscopy every 1–2 years for mutation carriers [33]. Given the decreased cancer risk in MSH6 and PMS2 mutation carriers, surveillance at age 25–30 may be considered [34].
Familial Colorectal Cancer Type X (FCCTX) is a kind of hereditary non-polyposis CRC with autosomal dominant inheritance, which also meets Amsterdam criteria Ⅱ. Unlike LS, its MMR gene has no germline mutation and shows microsatellite stability (MSS) [35]. This genetic difference is important because it determines the management strategies. The age at diagnosis of FCCTX is later than that of LS (approximately 40 years), and they do have a 2-fold increased risk compared to the general population. Although there is currently no standard follow-up protocol for monitoring and intervention, patients are recommended to get screening at the age of 40, but not before.
3. Predisposing Genes for Hereditary CRC Syndromes
Linkage analysis was the dominant tool of gene discovery until the Millenium shift and was used to discover genes of hereditary CRC syndromes [36]. Most of the more than 100 known cancer susceptibility genes were found by a mendelian-type family linkage approach, and the majority of the 10 high-risk genes for CRC susceptibility have also been identified by this method [36,37]. To this day, technologies such as whole-genome sequencing (WGS) and whole-exome sequencing (WES) have not only revolutionized tumor genomics but also made it an essential component of precision cancer therapy. The number of genes involved with inherited CRC syndrome predisposition is constantly growing.
3.1. Adenomatous Polyposis Syndromes
APC, located on chromosome 5q21, is a tumor suppressor gene for CRC [8,38]. The main pathogenic mechanism is disturbing the Wnt/β-catenin pathway. Mutated APC protein lacks the ability to degrade the β-catenin, and its cytoplasmic accumulation has been observed in FAP patients, which can be inhibited by the non-steroid anti-inflammatory drug (NSAIDs) sulindac [39] (Figure 1). Another consequence of APC mutations is chromosomal instability (CIN), which can lead to polyps being more likely to acquire a second strike [40]. Even though APC is a key gene for FAP or AFAP, about 10–30% of patients do not have APC variants [41]. Possible explanations for this may be mutations in other genes that have not yet been identified. As a result, numerous research sought to identify new genes that predispose to adenomatous polyposis syndromes.
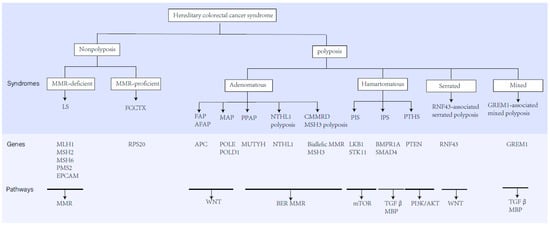
Figure 1.
The classification, predisposing genes, inheritance, and related molecular pathways of hereditary CRC syndromes.
In 2002, it was discovered that certain variations of the MUTHY gene were associated with an increased risk of developing numerous colorectal adenomas and carcinomas [42]. The MUTHY gene, located on chromosomes 1p32.1–p34.3, performs an important role in BER and has the ability to defend against DNA damage under oxidative stress. Mutations in MUTYH may lead to G:C→T: A reversal in the replication process [42,43]. In this way, MUTYH mutations may contribute the tumor formation and development. Next, some studies have made efforts to search for other variants in genes involved in BER. NTHL1 is the second DNA glycosylase gene identified in the BER pathway and is thought to be a predisposing gene for polyposis and CRC [14]. The p.Gln90* homozygous nonsense variant in NTHL1 was detected in initial families, and additional research has reported the compound heterozygous mutations. The prevalence of NTHL1-associated syndrome is still unclear, but it was estimated at 1:114,770 according to the prevalence in the European population, which is only one-fifth of MAP (1:19,079) [44]. As the deficiency of NTHL1 can lead to polyposis and carcinoma, and the tumor phenotypes are broad spectrum, Grolleman et al. suggested that biallelic NTHL1 mutation should be included in the surveillance guidelines for MAP [45]. Breast cancer screening is also recommended because of the moderate risk.
With the development of next-generation sequencing (NGS) technologies, WES and WGS have become the primary detecting method for susceptibility genes, and new candidate genes for CRC are constantly proposed. POLE and POLD1 were the first two variants found by the combination of linkage analysis and WES [10]. Germline pathogenic mutations in POLE and POLD1 were found to be linked to multiple polyposis and CRC [46]. POLE and POLD1 are members of the B family of replicative and repair DNA polymerases and have a proofreading function. A Chinese study investigated 1392 patients mainly affected by CRC and proposed a potential carcinogenesis hypothesis mediated by POLE/POLD1. The results showed that POLE/POLD1 variants carriers had a significantly high frequency of MMR variants and tumor mutational burden (TMB). POLE variants may affect cancer development through MMR, TGFβ, and RTK/RAS/RAF signaling pathways, and POLD1 through MMR pathways [46].
To uncover further genes with high-penetrance causative mutations, Adam et al. employed exome sequencing leukocyte DNA from 102 individuals with unexplained adenomatous polyposis and found a recessive variant of colorectal adenomatous polyposis caused by biallelic MSH3 germline mutations, an MMR gene unrelated to LS [15]. Similar to this, Olkinuora et al. discovered that the biallelic nonsense variant of DNA MMR gene MLH3 (MIM: 604395) underlies a novel syndrome with susceptibility to FAP or AFAP [47]. The AXIN2 (MIM:604025) gene is also a key regulator of β-catenin degradation in the Wnt pathway. Rivera et al. identified a novel missense variant in AXIN2 in one family with AFAP, which lays the foundation for screening AXIN2 in FAP patients negative for alterations in APC and MUTYH [48]. With the molecular genetics of hereditary CRC syndromes becoming increasingly clear, the moderate penetrance genes have become a new research hotspot. GALNT12 (MIM#610290) controlled the initiation of mucin-type O-linked glycosylation, which plays an important role in adhesion, migration, and immune surveillance. Recent studies have demonstrated the relationship between GALNT12 as a moderate penetrance gene for CRC susceptibility [48,49,50,51].
3.2. Non-Adenomatous Polyposis Syndromes
Germline mutations in the LKB1/STK11 gene, located on chromosome 19p13.3, have been detected in 50–70% of patients with PJS [52,53]. LKB1 can regulate cell polarity through PAR proteins and cell metabolism by the AMPK pathway. Additionally, LKB1 deficiency can inhibit serine biosynthesis and lead to DNA damage. In this way, LKB1 mutation may contribute to tumor formation and development. However, LKB1 is no clear mutation hotspot because of many missense mutations and deletions occurring through the whole LKB1 gene. For 80% of CS cases, a germline mutation in the PTEN gene has been identified as the culprit [54]. The PTEN gene is located on chromosome 10q23.3, and the PTEN protein has phosphatase activity. Since it is involved in the DNA repair and cell cycle checkpoint, PTEN deficiency has been reported to result in chromosomal instability. PTEN can negatively regulate the activity of the PI3K/AKT signaling pathway, thus inhibiting the occurrence and development of tumors. In total, 50–60% of JPS cases are caused by mutations in SMAD4 and BMPR1A, and both of them are implicated in TGFβ signaling, which regulates cell proliferation and differentiation [19]. Although there are no clear mutations detected in the remaining 40%–50% of patients, the mutation in ENG (MIM: 131195) and SMAD9 (MIM:603295) has been reported in a small number of patients, which are also involved in the TGFβ signaling. Furthermore, contiguous deletion of BMPR1A and PTEN is associated with severe JPS in infancy.
The genetic mechanism of MPS remains unclear, but duplication of 40 kb in the 3′ regions of the SCG5 (MIM: 173120) gene and upstream region of GREM1, has been identified as the pathogenic mutation in families of Ashkenazi descent [23]. There is also evidence to suggest that the ENG genes are involved in hyperplasic mixed polyposis [55]. In 2014, Gala et al. performed WES in 20 SPS families, and first identified germline RNF43 pathogenic variants as causative for SPS [56]. RNF43 was a negative Wnt regulator, and the dependency of SPS on the Wnt signaling pathway was also demonstrated in the organoid cultures [22]. In these serrated polyps, somatic alteration including BRAF mutations and a high level of promoter methylation has been reported [22]. In spite of the fact that GREM1 and MUTYH have also been reported for SPS, genetic testing is typically inconclusive [57].
3.3. Non-Polyposis Syndromes
For non-polyposis cases, this genetic susceptibility to CRC has traditionally been linked to germline mutations or epimutations in the DNA MMR genes. The MMR system corrects base mismatches, insertions, and losses that occur during DNA replication or exogenous damage. According to the genetic and clinical level, DNA MMR deficiency (dMMR) vs. proficiency (pMMR) classified hereditary non-polyposis syndromes into LS and FCCTX, respectively. More than 90% of LS-associated CRCs are associated with germline mutations in the DNA MMR genes, including MLH1 (42%), MSH2 (33%), MSH6 (18%), and PMS2 (7.5%) [58]. The dMMR would result in DNA repair defects and high microsatellite instability (MSI-H). Thus, MSI can be a genetic marker for tumors with dMMR system. EPCAM (MIM: 185535) gene mutation was also regarded as the main cause of LS, and EPCAM gene mutation accounts for 1–3% of all LS patients [59]. The loss of the EPCAM gene will result in the methylation of the MSH2 promoter, which will eventually render MSH2 inactive and cause LS.
Although the genetic basis of FCCTX is still unclear compared to LS, several candidate genes have been proposed, including GALNT12, RPS20 (MIM: 603682) and BRF1 (MIM: 604902), SEMA4A (MIM: 607292), and FAN1 (MIM: 613534) [60,61,62]. These genes may participate in protein glycosylation, ribosome biosynthesis, semaphoring signaling, DNA repair, or various other biological pathways.
4. Precision Medicine in Hereditary CRC Syndromes
4.1. Prophylactic Surgery
Surgery is the cornerstone of treatment for hereditary polyposis and non-polyposis syndromes and should be performed when CRC occurs or when the polyp burden cannot be managed by colonoscopy. In patients with FAP and other polyp syndromes, prophylactic surgery remains the gold standard for CRC prevention, but the timing varies. The most common surgical alternatives are total abdominal colectomy with an ileorectal anastomosis (IRA) or proctocolectomy with end ileostomy or restoration via an ileal pouch-anal anastomosis [9]. The determination of the surgery method is mainly based on the burden of colorectal polyps and the options of the patients. Patients with IRA retain an excessive risk of rectal cancer. Although studies prophylactic surgery has been shown to significantly reduce the risk of CRC in FAP, prophylactic surgery for LS is controversial. Some scholars believe that the risk of cancer in LS is as high as 80% and that prophylactic surgery can reduce the risk of CRC, whereas others argue against prophylactic surgery for MMR carriers due to incomplete dominant inheritance, the fact that 15–20% of LS patients do not develop CRC, and the fact that 6–20% of patients still develop CRC after colectomy. Therefore, prophylactic surgery may be considered for those who have difficulty with or refuse to undergo colonoscopic follow-up. For people with hamartomatous polyposis syndrome, preventative surgical resection is not advised because colonoscopic therapy may bring the risk of CRC down to that of the normal population.
4.2. Chemoprevention
The primary objective of hereditary CRC syndromes is cancer prevention. Research into the chemoprevention of hereditary CRC syndromes has been encouraged by the need for frequent surveillance procedures, necessary surgical intervention, and ongoing risk of disease progression. This treatment offers an alluring alternative to preventing the development of cancer in high-risk cancer syndromes. Although chemoprevention has been reported to be effective for duodenal and colonic polyps in FAP and for reducing the risk of cancer in LS, data from clinical trials are still sparse. An ideal preventive medication should be low toxicity, long-time effective, and easily accessible. Current research mainly focused on NSAIDs, such as sulindac, aspirin, and celecoxib.
4.2.1. Sulindac
Sulindac, a less selective cyclooxygenase inhibitor, has long been prescribed to FAP patients with colorectal polyps. In the first randomized controlled trial, which was carried out in 1993 by Giardiello et al., 22 patients with FAP were randomly assigned to receive sulindac 150 mg or placebo twice daily for 9 months [63]. Data revealed a 56% decrease in polyp count and a 65% reduction in polyp size in patients using sulindac. However, no patient achieved complete polyp clearance, and the number and size of polyps increased after 3 months of discontinuing sulindac, suggesting the necessity for ongoing treatment.
Subsequently, Giardiello et al. carried out another randomized study with 41 patients who had the APC mutation, but the findings revealed that sulindac at regular doses did not stop the progression of adenomas [64]. The focus of subsequent research moved to COX-2 inhibitor (celecoxib) and its critical contribution to the development of gastrointestinal polyps.
4.2.2. Celecoxib
Colonic adenomas have elevated COX-2 expression, which indicates a higher likelihood of malignant transformation [65]. In the beginning, Steinbach et al. discovered that the polyp burden in patients with FPA was lower in the celecoxib group (400 mg daily) compared to the placebo group (p = 0.001) and that there was no difference in adverse events between the groups [66]. Similarly, Phillips et al. discovered further qualitative improvement and quantitative reduction in duodenal polyposis when patients received celecoxib 400 mg twice daily [67]. However, the duration of these studies was short. To solve this problem, Burke et al. performed a study to assess the efficacy of celecoxib in kids with FAP over a 5-year period. The findings demonstrated that fewer patients in the celecoxib group (12.7% vs. 25.5%) reached the primary endpoint (≥20 polyps or >2 mm in size or a diagnosis of CRC) [68]. Celecoxib, the first chemopreventive medicine approved by the Food and Drug Administration (FDA) for the treatment of colonic polyps in patients with FAP, seems to be safe and effective based on the studies mentioned above. However, drug development was eventually halted due to unacceptable cardiovascular toxicity in the general population [69,70].
4.2.3. Aspirin
Since aspirin can simultaneously inhibit the activity of COX-1 and COX-2, it has been a research hotspot. Evidence suggests that aspirin lowers the risk of CRC in both FAP patients and the general population. To determine the efficacy of aspirin (600 mg/d) and/or resistant starch (30 g/d) in treating young persons with FAP, the Concerted Action Polyp Prevention (CAPP) group undertook a multicenter, randomized, controlled trial (CAPP1). The results showed that patients taking 600 mg of aspirin daily tend to have lower polyp burden (both in terms of size and number), but resistant starch does not seem to have any clinical effect on adenomas [71]. In the subsequent CAPP2 study, researchers discovered that giving patients with LS 600 mg of aspirin daily for 25 months significantly decreased the risk of CRC [72]. Ait Ouakrim et al. observed similar results, indicating that LS patients who used aspirin had a lower risk for both 1 month–4.9 years (HR, 0.49; 95% CI, 0.27–0.90; p = 0.02) and 5 years (HR, 0.25; 95% CI, 0.10–0.62; p= 0.003), compared with less than 1 month of use [73]. The American College of Gastroenterology advises aspirin use for patients with LS to prevent CRC based on the aforementioned research; however, the ideal dosage is yet unknown. Because of this, the CAAPP3 study is being carried out to examine various trial dosages of aspirin (100, 300, or 600 mg daily) in patients with LS, which may eventually provide more conclusive evidence.
4.2.4. Combination Therapy
Combination therapy might be a way to deal with the issue of dose-related side effects. Meyskens et al. demonstrated that the combination of sulindac 150 mg and difluoromethylornithine (DMFO) 500 mg can lower the risk of recurrent adenomatous polyps with few side effects [74]. Similar to this, it has been demonstrated that in adult FAP patients, the combination of sulindac 150 mg/DMFO 750 mg daily is more beneficial than placebo/DMFO 750 mg daily or placebo/sulindac 150 mg daily [75]. Lynch et al. showed that FAP patients with celecoxib/DMFO experienced more significant declines in adenoma burden (32% vs. 22%, p = 0.17) and polyp count (11% vs. 1%, p = 0.76) than celecoxib monotherapy [76]. In addition, a double-blind, randomized, placebo-controlled research found that 75 mg of erlotinib once a day and 150 mg of sulindac twice a day dramatically reduced the number of duodenal polyps in FAP patients [77].
4.2.5. Other Agents
Certain free fatty acids(FFA) were also linked to a decrease in arachidonic acid levels and COX-2 expression. West et al. discovered a 22.4% reduction in the number of polyps (p = 0.012) and a 29.8% reduction in polyp size (p = 0.027) in FAP patients treated with EPA-FFA compared to the placebo group, with results similar to those in the COX-2 inhibitor study [78]. The efficacy of Curcumin on the mean number and size of adenomas in FAP patients was also evaluated; however, there was no significant difference compared to the placebo [79]. Furthermore, four patients with FAP received six months of sirolimus treatment, showing promising effects, especially on the number of polyps in the rectal remnant and ileal pouch, although at the cost of numerous adverse events [80]. Ascorbic acid has also been studied because of its antioxidant and antitumor properties [81,82]. Nevertheless, no studies have convincingly demonstrated the clinical benefit of ascorbic acid in reducing the risk of CRC in patients with FAP.
Overall, the optimal chemopreventive agent has not been found in patients with hereditary CRC syndromes, and further exploration of novel chemoprevention strategies is still required.
4.3. Immuno-Prevention in Hereditary CRC Syndromes
In recent years, with a better understanding of the mechanisms of tumor immune response, immunotherapy has exploded in the field of oncology treatment, and more precise therapeutic strategies have been developed. CRC with DNA dMMR is characterized by TMB, which can produce abundant neoantigens, thereby activating the antitumor immune response. On the one hand, well-established approaches, namely immune checkpoint blockade, have been developed to generally support the host’s antitumor response capacity. On the other hand, tumor cell-specific or overexpressed neoantigens have opened up prospects for vaccine development.
4.3.1. Immune Checkpoint Inhibitors (ICIs) Therapy
ICIs are a specific type of immunotherapy that can improve antitumor immune responses by inhibiting T-cell negative regulatory receptors, such as cytotoxic T lymphocyte antigen 4 (CTLA4) and programmed cell death 1 (PD-1). ICIs have been demonstrated to be effective among patients with metastatic CRC with MSI-H and/or dMMR. Compared to traditional chemotherapy, ICIs can produce longer progression-free survival with fewer adverse events. An anti-CTLA-4 antibody (ipilimumab) and two anti-PD-1 antibodies (pembrolizumab and nivolumab) were approved by US FDA for the treatment of patients with MSI-H or dMMR metastatic CRC in 2011 and 2014, respectively [83]. Furthermore, clinical trials have repeatedly shown that inhibiting immune checkpoints with pembrolizumab ornivolumab) are effective in treating MSI cancers originating from the colorectum and other organs [83,84,85,86,87]. MSI-H and dMMR are associated with highly immune infiltrative tumor microenvironments, and both of them are the features of LS. Hence, ICIs are being studied as immune-preventive agents in LS [88]. POLE-mutant CRCs show increased CD8+ lymphocyte infiltration and high TMB, which are associated with ultramutated phenotype. Thus, CRC patients with POLE alterations may also benefit from immunotherapy [89,90]. As a result of these advancements, hereditary CRC syndromes have emerged as a central focus in the field of precision medicine and immune-oncology in the past years.
4.3.2. Vaccines
The responsiveness to ICIs is determined by several important parameters, such as the TMB (high amounts of tumor neoantigens), tumor-infiltrating lymphocytes, and regulatory checkpoint receptors [91]. Certain tumors have lower T-cell infiltration than others. This could be because of deficiencies in priming or the absence of high-affinity T cells. At this time, vaccinations may prove to be beneficial solutions in combination with ICIs, because they target particular mutant antigens. In contrast to the mostof other tumor types, LS-associated dMMR cancers produce mutational neoantigens and constitute a substantial pool of prospective vaccine targets [92]. These factors will help researchers better understand how vaccines affect patients’ prognoses and survival rates. Dendritic cells (DC) known as the strongest antigen-presenting cells play a key role in initiating the immune system’s response to exogenous antigens. Thus, DC vaccine adjuvant can stimulate DC antigen submission, which can induce therapeutic CD4+T-cell and CD8+T-cell responses and facilitate the conversion of naive T cells to cytotoxic T cells [93].
In addition, several clinical and pre-clinical trials for LS and dMMR cancer are underway to test the safety and immunogenicity of frameshift peptide (FSP) neoantigen-based vaccine [94,95,96]. In a phase I/Iia clinical trial, 22 patients with advanced dMMR CRC received FSP neoantigen-based vaccine, and the results showed that vaccine-induced humoral and cellular immune responses were observed in all patients and no vaccination-induced severe adverse events occurred [95]. Thus, vaccines may be a promising novel approach for the treatment and even prevention of dMMR cancer, even though it is necessary to evaluate vaccines using more appropriate molecular markers, larger randomized trials, and more patients with early-stage CRC, which will be the goal researchers are working towards.
5. Conclusions
Hereditary CRC syndromes are a prominent example of how our knowledge of the human disease has advanced over the past 30 years. Effective targeted therapies are now available for some of the affected signaling pathways, such as the NSAIDs sulindac and celecoxib that significantly reduce adenoma load in patients with APC germline mutations, probably because of the typical inhibition of the WNT/β-catenin signaling pathway. In addition, breakthroughs in LS vaccination have been achieved using FSP neoantigens induced by dMMR mechanisms. Nevertheless, additional research is required to improve our understanding of the genotype–phenotype variety of hereditary CRC syndromes as well as the most effective management strategies. The cost of conducting genetic research techniques is continuing to decline, which is pushing the concept of precision medicine closer and closer to being a reality.
Author Contributions
L.C. reviewed the literature and drafted the manuscript; L.Y. revised the manuscript; B.H. approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Health Commission of Sichuan Province (GB2018001) and China Postdoctoral Science Foundation (2022M712265).
Conflicts of Interest
All authors declare that they have no conflict of interest.
References
- AlDubayan, S.H.; Giannakis, M.; Moore, N.D.; Han, G.C.; Reardon, B.; Hamada, T.; Mu, X.J.; Nishihara, R.; Qian, Z.; Liu, L.; et al. Inherited DNA-Repair Defects in Colorectal Cancer. Am. J. Hum. Genet. 2018, 102, 401–414. [Google Scholar] [CrossRef] [PubMed]
- DeRycke, M.S.; Gunawardena, S.; Balcom, J.R.; Pickart, A.M.; Waltman, L.A.; French, A.J.; McDonnell, S.; Riska, S.M.; Fogarty, Z.C.; Larson, M.C.; et al. Targeted sequencing of 36 known or putative colorectal cancer susceptibility genes. Mol. Genet. Genom. Med. 2017, 5, 553–569. [Google Scholar] [CrossRef]
- Pearlman, R.; Frankel, W.L.; Swanson, B.; Zhao, W.; Yilmaz, A.; Miller, K.; Bacher, J.; Bigley, C.; Nelsen, L.; Goodfellow, P.J.; et al. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients with Early-Onset Colorectal Cancer. JAMA Oncol. 2017, 3, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, E.M.; Koeppe, E.; Everett, J.; Ulintz, P.; Kiel, M.; Osborne, J.; Williams, L.; Hanson, K.; Gruber, S.B.; Rozek, L.S. Germline Genetic Features of Young Individuals with Colorectal Cancer. Gastroenterology 2018, 154, 897–905.e1. [Google Scholar] [CrossRef] [PubMed]
- Mork, M.E.; You, Y.N.; Ying, J.; Bannon, S.A.; Lynch, P.M.; Rodriguez-Bigas, M.A.; Vilar, E. High Prevalence of Hereditary Cancer Syndromes in Adolescents and Young Adults with Colorectal Cancer. J. Clin. Oncol. 2015, 33, 3544–3549. [Google Scholar] [CrossRef]
- Samadder, N.J.; Baffy, N.; Giridhar, K.V.; Couch, F.J.; Riegert-Johnson, D. Hereditary Cancer Syndromes-A Primer on Diagnosis and Management, Part 2: Gastrointestinal Cancer Syndromes. Mayo Clin. Proc. 2019, 94, 1099–1116. [Google Scholar] [CrossRef]
- Kanth, P.; Grimmett, J.; Champine, M.; Burt, R.; Samadder, N.J. Hereditary Colorectal Polyposis and Cancer Syndromes: A Primer on Diagnosis and Management. Am. J. Gastroenterol. 2017, 112, 1509–1525. [Google Scholar] [CrossRef] [PubMed]
- Dinarvand, P.; Davaro, E.P.; Doan, J.V.; Ising, M.E.; Evans, N.R.; Phillips, N.J.; Lai, J.; Guzman, M.A. Familial Adenomatous Polyposis Syndrome: An Update and Review of Extraintestinal Manifestations. Arch. Pathol. Lab. Med. 2019, 143, 1382–1398. [Google Scholar] [CrossRef]
- Hampel, H.; Kalady, M.F.; Pearlman, R.; Stanich, P.P. Hereditary Colorectal Cancer. Hematol. Oncol. Clin. North Am. 2022, 36, 429–447. [Google Scholar] [CrossRef]
- Palles, C.; Cazier, J.B.; Howarth, K.M.; Domingo, E.; Jones, A.M.; Broderick, P.; Kemp, Z.; Spain, S.L.; Guarino, E.; Salguero, I.; et al. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat. Genet. 2013, 45, 136–144. [Google Scholar] [CrossRef]
- Win, A.K.; Dowty, J.G.; Cleary, S.P.; Kim, H.; Buchanan, D.D.; Young, J.P.; Clendenning, M.; Rosty, C.; MacInnis, R.J.; Giles, G.G.; et al. Risk of colorectal cancer for carriers of mutations in MUTYH, with and without a family history of cancer. Gastroenterology 2014, 146, 1208–1211.e1–5. [Google Scholar] [CrossRef]
- Patel, R.; McGinty, P.; Cuthill, V.; Hawkins, M.; Clark, S.K.; Latchford, A. Risk of colorectal adenomas and cancer in monoallelic carriers of MUTYH pathogenic variants: A single-centre experience. Int. J. Color. Dis. 2021, 36, 2199–2204. [Google Scholar] [CrossRef] [PubMed]
- Vasen, H.F.; Tomlinson, I.; Castells, A. Clinical management of hereditary colorectal cancer syndromes. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Weren, R.D.; Ligtenberg, M.J.; Kets, C.M.; de Voer, R.M.; Verwiel, E.T.; Spruijt, L.; van Zelst-Stams, W.A.; Jongmans, M.C.; Gilissen, C.; Hehir-Kwa, J.Y.; et al. A germline homozygous mutation in the base-excision repair gene NTHL1 causes adenomatous polyposis and colorectal cancer. Nat. Genet. 2015, 47, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Spier, I.; Zhao, B.; Kloth, M.; Marquez, J.; Hinrichsen, I.; Kirfel, J.; Tafazzoli, A.; Horpaopan, S.; Uhlhaas, S.; et al. Exome Sequencing Identifies Biallelic MSH3 Germline Mutations as a Recessive Subtype of Colorectal Adenomatous Polyposis. Am. J. Hum. Genet. 2016, 99, 337–351. [Google Scholar] [CrossRef]
- Aronson, M.; Colas, C.; Shuen, A.; Hampel, H.; Foulkes, W.D.; Baris Feldman, H.; Goldberg, Y.; Muleris, M.; Wolfe Schneider, K.; McGee, R.B.; et al. Diagnostic criteria for constitutional mismatch repair deficiency (CMMRD): Recommendations from the international consensus working group. J. Med. Genet. 2022, 59, 318–327. [Google Scholar] [CrossRef]
- Boland, C.R.; Idos, G.E.; Durno, C.; Giardiello, F.M.; Anderson, J.C.; Burke, C.A.; Dominitz, J.A.; Gross, S.; Gupta, S.; Jacobson, B.C.; et al. Diagnosis and Management of Cancer Risk in the Gastrointestinal Hamartomatous Polyposis Syndromes: Recommendations from the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2022, 162, 2063–2085. [Google Scholar] [CrossRef]
- Gilad, O.; Rosner, G.; Fliss-Isakov, N.; Aharon-Kaspi, S.; Strul, H.; Gluck, N.; Kariv, R. Clinical and Histologic Overlap and Distinction Among Various Hamartomatous Polyposis Syndromes. Clin. Transl. Gastroenterol. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Dal Buono, A.; Gaiani, F.; Poliani, L.; Laghi, L. Juvenile polyposis syndrome: An overview. Best Pract. Res. Clin. Gastroenterol. 2022, 58–59, 101799. [Google Scholar] [CrossRef] [PubMed]
- Fousekis, F.S.; Mitselos, I.V.; Christodoulou, D.K. Diagnosis, epidemiology and management of serrated polyposis syndrome: A comprehensive review of the literature. Am. J. Transl. Res. 2021, 13, 5786–5795. [Google Scholar] [PubMed]
- Je, I.J.; Rana, S.A.; Atkinson, N.S.; van Herwaarden, Y.J.; Bastiaansen, B.A.; van Leerdam, M.E.; Sanduleanu, S.; Bisseling, T.M.; Spaander, M.C.; Clark, S.K.; et al. Clinical risk factors of colorectal cancer in patients with serrated polyposis syndrome: A multicentre cohort analysis. Gut 2017, 66, 278–284. [Google Scholar]
- Yan, H.H.N.; Lai, J.C.W.; Ho, S.L.; Leung, W.K.; Law, W.L.; Lee, J.F.Y.; Chan, A.K.W.; Tsui, W.Y.; Chan, A.S.Y.; Lee, B.C.H.; et al. RNF43 germline and somatic mutation in serrated neoplasia pathway and its association with BRAF mutation. Gut 2017, 66, 1645–1656. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, E.; Leedham, S.; Lewis, A.; Segditsas, S.; Becker, M.; Cuadrado, P.R.; Davis, H.; Kaur, K.; Heinimann, K.; Howarth, K.; et al. Hereditary mixed polyposis syndrome is caused by a 40-kb upstream duplication that leads to increased and ectopic expression of the BMP antagonist GREM1. Nat. Genet. 2012, 44, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Rohlin, A.; Eiengård, F.; Lundstam, U.; Zagoras, T.; Nilsson, S.; Edsjö, A.; Pedersen, J.; Svensson, J.; Skullman, S.; Karlsson, B.G.; et al. GREM1 and POLE variants in hereditary colorectal cancer syndromes. Genes Chromosomes Cancer 2016, 55, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; Shaw, M.W.; Magnuson, C.W.; Larsen, A.L.; Krush, A.J. Hereditary factors in cancer. Study of two large midwestern kindreds. Arch. Intern. Med. 1966, 117, 206–212. [Google Scholar] [CrossRef]
- Aaltonen, L.A.; Salovaara, R.; Kristo, P.; Canzian, F.; Hemminki, A.; Peltomäki, P.; Chadwick, R.B.; Kääriäinen, H.; Eskelinen, M.; Järvinen, H.; et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N. Engl. J. Med. 1998, 338, 1481–1487. [Google Scholar] [CrossRef]
- Moreira, L.; Balaguer, F.; Lindor, N.; de la Chapelle, A.; Hampel, H.; Aaltonen, L.A.; Hopper, J.L.; Le Marchand, L.; Gallinger, S.; Newcomb, P.A.; et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA 2012, 308, 1555–1565. [Google Scholar] [CrossRef]
- Vasen, H.F.; Watson, P.; Mecklin, J.P.; Lynch, H.T. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 1999, 116, 1453–1456. [Google Scholar] [CrossRef]
- Umar, A.; Boland, C.R.; Terdiman, J.P.; Syngal, S.; de la Chapelle, A.; Rüschoff, J.; Fishel, R.; Lindor, N.M.; Burgart, L.J.; Hamelin, R.; et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J. Natl. Cancer Inst. 2004, 96, 261–268. [Google Scholar] [CrossRef]
- Palomaki, G.E.; McClain, M.R.; Melillo, S.; Hampel, H.L.; Thibodeau, S.N. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet. Med. 2009, 11, 42–65. [Google Scholar] [CrossRef]
- Berg, A.O.; Armstrong, K.; Botkin, J.; Calonge, N.; Haddow, J.; Hayes, M.; Teutsch, S. Recommendations from the EGAPP Working Group: Genetic testing strategies in newly diagnosed individuals; with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet. Med. 2009, 11, 35–41. [Google Scholar]
- Vasen, H.F.; Abdirahman, M.; Brohet, R.; Langers, A.M.; Kleibeuker, J.H.; van Kouwen, M.; Koornstra, J.J.; Boot, H.; Cats, A.; Dekker, E.; et al. One to 2-year surveillance intervals reduce risk of colorectal cancer in families with Lynch syndrome. Gastroenterology 2010, 138, 2300–2306. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.M.; Gupta, S.; Burke, C.A.; Axell, L.; Chen, L.M.; Chung, D.C.; Clayback, K.M.; Dallas, S.; Felder, S.; Gbolahan, O.; et al. NCCN Guidelines® Insights: Genetic/Familial High-Risk Assessment: Colorectal, Version 1. J. Natl. Compr. Cancer Netw. 2021, 19, 1122–1132. [Google Scholar]
- Syngal, S.; Brand, R.E.; Church, J.M.; Giardiello, F.M.; Hampel, H.L.; Burt, R.W. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am. J. Gastroenterol. 2015, 110, 223–262. [Google Scholar] [CrossRef]
- Xu, Y.; Li, C.; Zhang, Y.; Guo, T.; Zhu, C.; Xu, Y.; Liu, F. Comparison Between Familial Colorectal Cancer Type X and Lynch Syndrome: Molecular, Clinical, and Pathological Characteristics and Pedigrees. Front. Oncol. 2020, 10, 1603. [Google Scholar] [CrossRef]
- Valle, L.; de Voer, R.M.; Goldberg, Y.; Sjursen, W.; Försti, A.; Ruiz-Ponte, C.; Caldés, T.; Garré, P.; Olsen, M.F.; Nordling, M.; et al. Update on genetic predisposition to colorectal cancer and polyposis. Mol. Aspects Med. 2019, 69, 10–26. [Google Scholar] [CrossRef]
- Turnbull, C.; Sud, A.; Houlston, R.S. Cancer genetics, precision prevention and a call to action. Nat. Genet. 2018, 50, 1212–1218. [Google Scholar] [CrossRef]
- Nielsen, M.; Hes, F.J.; Nagengast, F.M.; Weiss, M.M.; Mathus-Vliegen, E.M.; Morreau, H.; Breuning, M.H.; Wijnen, J.T.; Tops, C.M.; Vasen, H.F.; et al. Germline mutations in APC and MUTYH are responsible for the majority of families with attenuated familial adenomatous polyposis. Clin. Genet. 2007, 71, 427–433. [Google Scholar] [CrossRef]
- Boon, E.M.; Keller, J.J.; Wormhoudt, T.A.; Giardiello, F.M.; Offerhaus, G.J.; van der Neut, R.; Pals, S.T. Sulindac targets nuclear beta-catenin accumulation and Wnt signalling in adenomas of patients with familial adenomatous polyposis and in human colorectal cancer cell lines. Br. J. Cancer 2004, 90, 224–229. [Google Scholar] [CrossRef]
- Pino, M.S.; Chung, D.C. The chromosomal instability pathway in colon cancer. Gastroenterology 2010, 138, 2059–2072. [Google Scholar] [CrossRef]
- Grover, S.; Kastrinos, F.; Steyerberg, E.W.; Cook, E.F.; Dewanwala, A.; Burbidge, L.A.; Wenstrup, R.J.; Syngal, S. Prevalence and phenotypes of APC and MUTYH mutations in patients with multiple colorectal adenomas. JAMA 2012, 308, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Al-Tassan, N.; Chmiel, N.H.; Maynard, J.; Fleming, N.; Livingston, A.L.; Williams, G.T.; Hodges, A.K.; Davies, D.R.; David, S.S.; Sampson, J.R.; et al. Inherited variants of MYH associated with somatic G:C→T:A mutations in colorectal tumors. Nat. Genet. 2002, 30, 227–232. [Google Scholar] [CrossRef]
- Jones, S.; Emmerson, P.; Maynard, J.; Best, J.M.; Jordan, S.; Williams, G.T.; Sampson, J.R.; Cheadle, J.P. Biallelic germline mutations in MYH predispose to multiple colorectal adenoma and somatic G:C→T:A mutations. Hum. Mol. Genet. 2002, 11, 2961–2967. [Google Scholar] [CrossRef] [PubMed]
- Weren, R.D.; Ligtenberg, M.J.; Geurts van Kessel, A.; De Voer, R.M.; Hoogerbrugge, N.; Kuiper, R.P. NTHL1 and MUTYH polyposis syndromes: Two sides of the same coin? J. Pathol. 2018, 244, 135–142. [Google Scholar] [CrossRef]
- Grolleman, J.E.; de Voer, R.M.; Elsayed, F.A.; Nielsen, M.; Weren, R.D.A.; Palles, C.; Ligtenberg, M.J.L.; Vos, J.R.; Ten Broeke, S.W.; de Miranda, N.F.C.C.; et al. Mutational Signature Analysis Reveals NTHL1 Deficiency to Cause a Multi-tumor Phenotype. Cancer Cell 2019, 35, 256–266.e5. [Google Scholar] [CrossRef]
- Yao, J.; Gong, Y.; Zhao, W.; Han, Z.; Guo, S.; Liu, H.; Peng, X.; Xiao, W.; Li, Y.; Dang, S.; et al. Comprehensive analysis of POLE and POLD1 Gene Variations identifies cancer patients potentially benefit from immunotherapy in Chinese population. Sci. Rep. 2019, 9, 15767. [Google Scholar] [CrossRef] [PubMed]
- Olkinuora, A.; Nieminen, T.T.; Mårtensson, E.; Rohlin, A.; Ristimäki, A.; Koskenvuo, L.; Lepistö, A. Biallelic germline nonsense variant of MLH3 underlies polyposis predisposition. Genet. Med. 2019, 21, 1868–1873. [Google Scholar] [CrossRef]
- Rivera, B.; Perea, J.; Sánchez, E.; Villapún, M.; Sánchez-Tomé, E.; Mercadillo, F.; Robledo, M.; Benítez, J.; Urioste, M. A novel AXIN2 germline variant associated with attenuated FAP without signs of oligondontia or ectodermal dysplasia. Eur. J. Hum. Genet. 2014, 22, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Clarke, E.; Green, R.C.; Green, J.S.; Mahoney, K.; Parfrey, P.S.; Younghusband, H.B.; Woods, M.O. Inherited deleterious variants in GALNT12 are associated with CRC susceptibility. Hum. Mutat. 2012, 33, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Lorca, V.; Rueda, D.; Martín-Morales, L.; Poves, C.; Fernández-Aceñero, M.J.; Ruiz-Ponte, C.; Llovet, P.; Marrupe, D.; García-Barberán, V.; García-Paredes, B.; et al. Role of GALNT12 in the genetic predisposition to attenuated adenomatous polyposis syndrome. PLoS ONE 2017, 12, e0187312. [Google Scholar] [CrossRef]
- Evans, D.R.; Venkitachalam, S.; Revoredo, L.; Dohey, A.T.; Clarke, E.; Pennell, J.J.; Powell, A.E.; Quinn, E.; Ravi, L.; Gerken, T.A.; et al. Evidence for GALNT12 as a moderate penetrance gene for colorectal cancer. Hum. Mutat. 2018, 39, 1092–1101. [Google Scholar] [CrossRef]
- Jenne, D.E.; Reimann, H.; Nezu, J.; Friedel, W.; Loff, S.; Jeschke, R.; Müller, O.; Back, W.; Zimmer, M. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat. Genet. 1998, 18, 38–43. [Google Scholar] [CrossRef]
- Hemminki, A.; Markie, D.; Tomlinson, I.; Avizienyte, E.; Roth, S.; Loukola, A.; Bignell, G.; Warren, W.; Aminoff, M.; Höglund, P.; et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature 1998, 391, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Liaw, D.; Marsh, D.J.; Li, J.; Dahia, P.L.; Wang, S.I.; Zheng, Z.; Bose, S.; Call, K.M.; Tsou, H.C.; Peacocke, M.; et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat. Genet. 1997, 16, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Sweet, K.; Willis, J.; Zhou, X.P.; Gallione, C.; Sawada, T.; Alhopuro, P.; Khoo, S.K.; Patocs, A.; Martin, C.; Bridgeman, S.; et al. Molecular classification of patients with unexplained hamartomatous and hyperplastic polyposis. JAMA 2005, 294, 2465–2473. [Google Scholar] [CrossRef] [PubMed]
- Gala, M.K.; Mizukami, Y.; Le, L.P.; Moriichi, K.; Austin, T.; Yamamoto, M.; Lauwers, G.Y.; Bardeesy, N.; Chung, D.C. Germline mutations in oncogene-induced senescence pathways are associated with multiple sessile serrated adenomas. Gastroenterology 2014, 146, 520–529. [Google Scholar] [CrossRef]
- Valle, L.; Vilar, E.; Tavtigian, S.V.; Stoffel, E.M. Genetic predisposition to colorectal cancer: Syndromes, genes, classification of genetic variants and implications for precision medicine. J. Pathol. 2019, 247, 574–588. [Google Scholar] [CrossRef]
- Olkinuora, A.P.; Peltomäki, P.T.; Aaltonen, L.A.; Rajamäki, K. From APC to the genetics of hereditary and familial colon cancer syndromes. Hum. Mol. Genet. 2021, 30, R206–R224. [Google Scholar] [CrossRef]
- Cini, G.; Quaia, M.; Canzonieri, V.; Fornasarig, M.; Maestro, R.; Morabito, A.; D’Elia, A.V.; Urso, E.D.; Mammi, I.; Viel, A. Toward a better definition of EPCAM deletions in Lynch Syndrome: Report of new variants in Italy and the associated molecular phenotype. Mol. Genet. Genom. Med. 2019, 7, e587. [Google Scholar] [CrossRef]
- Schulz, E.; Klampfl, P.; Holzapfel, S.; Janecke, A.R.; Ulz, P.; Renner, W.; Kashofer, K.; Nojima, S.; Leitner, A.; Zebisch, A.; et al. Germline variants in the SEMA4A gene predispose to familial colorectal cancer type X. Nat. Commun. 2014, 5, 5191. [Google Scholar] [CrossRef]
- Valle, L. Recent Discoveries in the Genetics of Familial Colorectal Cancer and Polyposis. Clin. Gastroenterol. Hepatol. 2017, 15, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Peltomaki, P.; Olkinuora, A.; Nieminen, T.T. Updates in the field of hereditary nonpolyposis colorectal cancer. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Giardiello, F.M.; Hamilton, S.R.; Krush, A.J.; Piantadosi, S.; Hylind, L.M.; Celano, P.; Booker, S.V.; Robinson, C.R.; Offerhaus, G.J. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N. Engl. J. Med. 1993, 328, 1313–1316. [Google Scholar] [CrossRef] [PubMed]
- Giardiello, F.M.; Yang, V.W.; Hylind, L.M.; Krush, A.J.; Petersen, G.M.; Trimbath, J.D.; Piantadosi, S.; Garrett, E.; Geiman, D.E.; Hubbard, W.; et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. N. Engl. J. Med. 2002, 346, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- McLean, M.H.; Murray, G.I.; Fyfe, N.; Hold, G.L.; Mowat, N.A.; El-Omar, E.M. COX-2 expression in sporadic colorectal adenomatous polyps is linked to adenoma characteristics. Histopathology 2008, 52, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, G.; Lynch, P.M.; Phillips, R.K.; Wallace, M.H.; Hawk, E.; Gordon, G.B.; Wakabayashi, N.; Saunders, B.; Shen, Y.; Fujimura, T.; et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med. 2000, 342, 1946–1952. [Google Scholar] [CrossRef]
- Phillips, R.K.; Wallace, M.H.; Lynch, P.M.; Hawk, E.; Gordon, G.B.; Saunders, B.P.; Wakabayashi, N.; Shen, Y.; Zimmerman, S.; Godio, L.; et al. A andomized, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut 2002, 50, 857–860. [Google Scholar] [CrossRef]
- Burke, C.A.; Phillips, R.; Berger, M.F.; Li, C.; Essex, M.N.; Iorga, D.; Lynch, P.M. Children’s International Polyposis (CHIP) study: A randomized, double-blind, placebo-controlled study of celecoxib in children with familial adenomatous polyposis. Clin. Exp. Gastroenterol. 2017, 10, 177–185. [Google Scholar] [CrossRef]
- Arber, N.; Eagle, C.J.; Spicak, J.; Rácz, I.; Dite, P.; Hajer, J.; Zavoral, M.; Lechuga, M.J.; Gerletti, P.; Tang, J.; et al. Celecoxib for the prevention of colorectal adenomatous polyps. N. Engl. J. Med. 2006, 355, 885–895. [Google Scholar] [CrossRef]
- Bertagnolli, M.M. Chemoprevention of colorectal cancer with cyclooxygenase-2 inhibitors: Two steps forward, one step back. Lancet Oncol. 2007, 8, 439–443. [Google Scholar] [CrossRef]
- Burn, J.; Bishop, D.T.; Chapman, P.D.; Elliott, F.; Bertario, L.; Dunlop, M.G.; Eccles, D.; Ellis, A.; Evans, D.G.; Fodde, R.; et al. A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer Prev. Res. 2011, 4, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Burn, J.; Gerdes, A.M.; Macrae, F.; Mecklin, J.P.; Moeslein, G.; Olschwang, S.; Eccles, D.; Evans, D.G.; Maher, E.R.; Bertario, L.; et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: An analysis from the CAPP2 randomised controlled trial. Lancet 2011, 378, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Ait Ouakrim, D.; Dashti, S.G.; Chau, R.; Buchanan, D.D.; Clendenning, M.; Rosty, C.; Winship, I.M.; Young, J.P.; Giles, G.G.; Leggett, B.; et al. Aspirin, Ibuprofen, and the Risk of Colorectal Cancer in Lynch Syndrome. J. Natl. Cancer Inst. 2015, 107, djv170. [Google Scholar] [CrossRef] [PubMed]
- Meyskens, F.L., Jr.; McLaren, C.E.; Pelot, D.; Fujikawa-Brooks, S.; Carpenter, P.M.; Hawk, E.; Kelloff, G.; Lawson, M.J.; Kidao, J.; McCracken, J.; et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: A randomized placebo-controlled, double-blind trial. Cancer Prev. Res. 2008, 1, 32–38. [Google Scholar] [CrossRef]
- Burke, C.A.; Dekker, E.; Samadder, N.J.; Stoffel, E.; Cohen, A. Efficacy and safety of eflornithine (CPP-1X)/sulindac combination therapy versus each as monotherapy in patients with familial adenomatous polyposis (FAP): Design and rationale of a randomized, double-blind, Phase III trial. BMC Gastroenterol. 2016, 16, 87. [Google Scholar] [CrossRef]
- Lynch, P.M.; Burke, C.A.; Phillips, R.; Morris, J.S.; Slack, R.; Wang, X.; Liu, J.; Patterson, S.; Sinicrope, F.A.; Rodriguez-Bigas, M.A.; et al. An international randomised trial of celecoxib versus celecoxib plus difluoromethylornithine in patients with familial adenomatous polyposis. Gut 2016, 65, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Samadder, N.J.; Kuwada, S.K.; Boucher, K.M.; Byrne, K.; Kanth, P.; Samowitz, W.; Jones, D.; Tavtigian, S.V.; Westover, M.; Berry, T.; et al. Association of Sulindac and Erlotinib vs Placebo with Colorectal Neoplasia in Familial Adenomatous Polyposis: Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018, 4, 671–677. [Google Scholar] [CrossRef]
- West, N.J.; Clark, S.K.; Phillips, R.K.; Hutchinson, J.M.; Leicester, R.J.; Belluzzi, A.; Hull, M.A. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut 2010, 59, 918–925. [Google Scholar] [CrossRef]
- Cruz-Correa, M.; Hylind, L.M.; Marrero, J.H.; Zahurak, M.L.; Murray-Stewart, T.; Casero, R.A., Jr.; Montgomery, E.A.; Iacobuzio-Donahue, C.; Brosens, L.A.; Offerhaus, G.J.; et al. Efficacy and Safety of Curcumin in Treatment of Intestinal Adenomas in Patients with Familial Adenomatous Polyposis. Gastroenterology 2018, 155, 668–673. [Google Scholar] [CrossRef]
- Roos, V.H.; Meijer, B.J.; Kallenberg, F.G.J.; Bastiaansen, B.A.J.; Koens, L.; Bemelman, F.J.; Bossuyt, P.M.M.; Heijmans, J.; van den Brink, G.; Dekker, E. Sirolimus for the treatment of polyposis of the rectal remnant and ileal pouch in four patients with familial adenomatous polyposis: A pilot study. BMJ Open Gastroenterol. 2020, 7, e000497. [Google Scholar] [CrossRef]
- DeCosse, J.J.; Miller, H.H.; Lesser, M.L. Effect of wheat fiber and vitamins C and E on rectal polyps in patients with familial adenomatous polyposis. J. Natl. Cancer Inst. 1989, 81, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Bussey, H.J.; DeCosse, J.J.; Deschner, E.E.; Eyers, A.A.; Lesser, M.L.; Morson, B.C.; Ritchie, S.M.; Thomson, J.P.; Wadsworth, J. A randomized trial of ascorbic acid in polyposis coli. Cancer 1982, 50, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable Clinical Benefit with Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, Y.; Ijichi, H.; Koike, K. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 373, 1979. [Google Scholar]
- Wang, F.; Zhao, Q.; Wang, Y.N.; Jin, Y.; He, M.M.; Liu, Z.X.; Xu, R.H. Evaluation of POLE and POLD1 Mutations as Biomarkers for Immunotherapy Outcomes Across Multiple Cancer Types. JAMA Oncol. 2019, 5, 1504–1506. [Google Scholar] [CrossRef]
- Mehnert, J.M.; Panda, A.; Zhong, H.; Hirshfield, K.; Damare, S.; Lane, K.; Sokol, L.; Stein, M.N.; Rodriguez-Rodriquez, L.; Kaufman, H.L.; et al. Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer. J. Clin. Investig. 2016, 126, 2334–2340. [Google Scholar] [CrossRef]
- Lichtenstern, C.R.; Ngu, R.K.; Shalapour, S.; Karin, M. Immunotherapy, Inflammation and Colorectal Cancer. Cells 2020, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Sanchez, A.; Grossman, M.; Yeung, K.; Sei, S.S.; Lipkin, S.; Kloor, M. Vaccines for immunoprevention of DNA mismatch repair deficient cancers. J. Immunother. Cancer 2022, 10, e004416. [Google Scholar] [CrossRef] [PubMed]
- Westdorp, H.; Fennemann, F.L.; Weren, R.D.; Bisseling, T.M.; Ligtenberg, M.J.; Figdor, C.G.; Schreibelt, G.; Hoogerbrugge, N.; Wimmers, F.; de Vries, I.J. Opportunities for immunotherapy in microsatellite instable colorectal cancer. Cancer Immunol. Immunother. 2016, 65, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Katsnelson, A. Preventive Cancer Vaccine Based on Neoantigens Gets Put to the Test. ACS Cent. Sci. 2021, 7, 1288–1291. [Google Scholar] [CrossRef] [PubMed]
- Kloor, M.; Reuschenbach, M.; Pauligk, C.; Karbach, J.; Rafiyan, M.R.; Al-Batran, S.E.; Tariverdian, M.; Jäger, E.; von Knebel Doeberitz, M. A Frameshift Peptide Neoantigen-Based Vaccine for Mismatch Repair-Deficient Cancers: A Phase I/IIa Clinical Trial. Clin. Cancer Res. 2020, 26, 4503–4510. [Google Scholar] [CrossRef]
- Solomon, A.; Alteber, Z.; Bassan, D.; Sharbi-Yunger, A.; Esbit, S.; Tzehoval, E.; Eisenbach, L. On the development of a neoantigen vaccine for the prevention of Lynch Syndrome. Int. J. Cancer 2022, 151, 107–119. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).