Zinc in Prostate Health and Disease: A Mini Review
Abstract
1. Introduction
2. Method
3. Results
3.1. Overview of Zn Proteins and Circumstances for Prostate Malignant Transformation
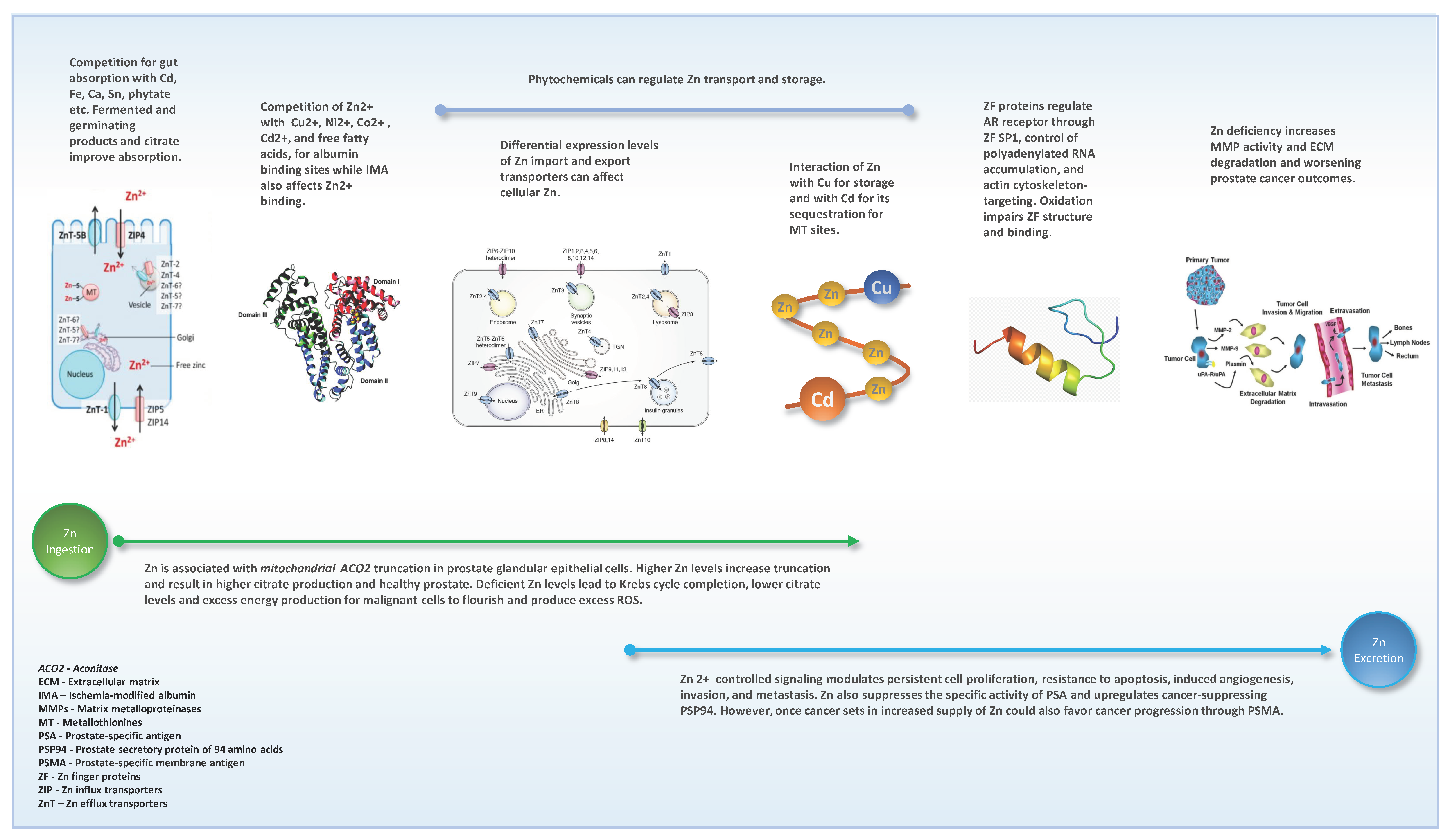
3.2. Zn Absorption from Gut and Blood Circulation
3.3. Zn Tissue Accumulation and Functions
3.4. Cellular Zn Moieties
3.4.1. Zn Cellular and Sub-Cellular Transporters
Zn Influx (Import) Transporters
Zn Efflux (Export) Transporters
3.4.2. Metallothionines
3.4.3. Matrix Metalloproteinases
3.4.4. Zn Finger Proteins
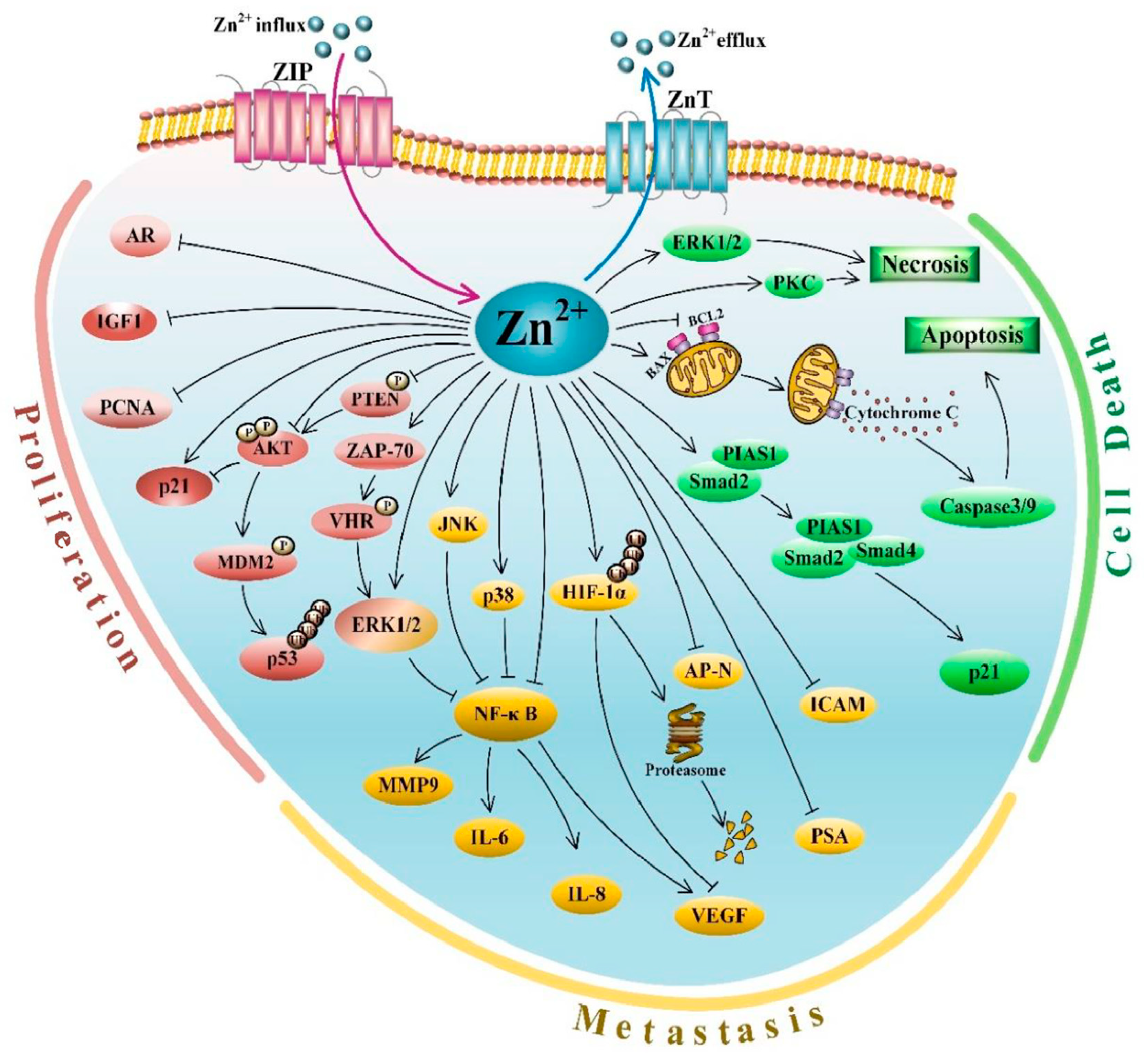
3.5. Zn Signaling
3.6. Prostatic Fluid Proteins and Zn
3.7. Related Genetic Associations
3.8. Zn Supplementation Effects
3.9. Interactions of Zn
3.9.1. Interaction with Other Elements
3.9.2. Interaction of Zn with Demographic, Lifestyle, and Health Factors
3.10. Transgender Women and Potential Impact on Zn Homeostasis
3.11. Zn-Dependent Technologies Associated with Prostate Cancer
4. Discussion and Commentary
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKT | Akt serine/threonine kinase family |
| AP | N Aminopeptidase N |
| AR | Androgen receptor |
| CA1 | Carbonic anhydrase 1 |
| C15orf39 | Chromosome 15 Open Reading Frame 39 |
| ECM | Extracellular matrix |
| EMT | Epithelial-to-mesenchymal transformation |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| HIF-1 | Hypoxia-inducible factor-1_ |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IGF-1 | Insulin-like growth factor 1 |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| JNK c | Jun N-terminal kinase |
| MDM2 | Murine double minute 2 |
| MMP-9 | Matrix metallopeptidase 9 |
| mpMRI | Multiparametric Magnetic Resonance Imaging |
| MSMB | Microseminoprotein β |
| MT | Metallothionines |
| MT2A | Metallothionine 2A |
| mtDNA | Mitochondrial DNA |
| mTOR | Mechanistic target of rapamycin kinase |
| NF ĸ-B | Nuclear factor kappa B |
| p21 | Inhibitor of cyclin-dependent kinase |
| p38 | p38 mitogen-activated protein kinases |
| p53 | p53 tumor suppressor protein |
| PCNA | Proliferating cell nuclear antigen |
| PHI | Prostate Health Index |
| PIAS1 | Protein inhibitor of activated STAT 1 |
| PI-RADS | Prostate Imaging Reporting & Data System |
| PKC | Protein kinase C |
| PLZF | Promyelocytic leukemia zinc finger |
| PPCDC | Phosphopantothenoylcysteine decarboxylase |
| PSA | Prostate-specific antigen |
| PSP94 | Prostate secretory protein of 94 amino acids |
| PSMA | Prostate-specific membrane antigen |
| PSMB4 | Proteasome Subunit Beta 4 |
| PTEN | Phosphatase and tensin homolog |
| RNF217 | Ring finger protein 217 |
| SELENBP1 | Selenium Binding Protein 1 |
| SgI | Semenogelin I |
| SMAD2 | SMAD Family Member 2 |
| SMAD4 | SMAD Family Member 4 |
| SMIM1 | Small Integral Membrane Protein 1 |
| SP1 | Specificity protein 1 |
| TRAMP | Transgenic adenocarcinoma of the mouse prostate |
| VEGF | Vascular endothelial growth factor |
| VHR | Vaccinia H1-related phosphatase |
| ZAP | 7 0 Zeta chain-associated protein-70 |
| ZFs | Zinc fingers |
| ZFC3H1 | C3H1 domain-containing protein |
| ZIPs | Zn-regulated, Iron-regulated transporter-like proteins (Zn influx proteins) |
| ZnTs | Zinc efflux transporter proteins |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Leitzmann, M.F.; Rohrmann, S. Risk factors for the onset of prostatic cancer: Age, location, and behavioral correlates. Clin. Epidemiol 2012, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.M.; Mucci, L.A. Diet and Lifestyle in Prostate Cancer. Adv. Exp. Med. Biol. 2019, 1210, 1–27. [Google Scholar] [PubMed]
- Harryman, W.L.; Warfel, N.A.; Nagle, R.B.; Cress, A.E. The Tumor Microenvironments of Lethal Prostate Cancer. Adv. Exp. Med. Biol. 2019, 1210, 149–170. [Google Scholar] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P. Nutrients and Oxidative Stress: Friend or Foe? Oxidative Med. Cell. Longev. 2018, 2018, 9719584. [Google Scholar] [CrossRef]
- Estevez, M.; Li, Z.; Soladoye, O.P.; Van-Hecke, T. Health Risks of Food Oxidation. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2017; Volume 82, pp. 45–81. [Google Scholar]
- Maret, W. Zinc biochemistry: From a single zinc enzyme to a key element of life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef]
- Vallee, B.L.; Falchuk, K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [CrossRef]
- Prasad, A.S.; Schulert, A.R.; Miale, A., Jr.; Farid, Z.; Sandstead, H.H. Zinc and iron deficiencies in male subjects with dwarfism and hypogonadism but without ancylostomiasis, schistosomiasis or severe anemia. Am. J. Clin. Nutr. 1963, 12, 437–444. [Google Scholar]
- Fang, L.; Watkinson, M. Subcellular localised small molecule fluorescent probes to image mobile Zn(2). Chem. Sci. 2020, 11, 11366–11379. [Google Scholar] [CrossRef]
- Franklin, R.B.; Costello, L.C. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch. Biochem. Biophys. 2007, 463, 211–217. [Google Scholar] [CrossRef]
- Franklin, R.B.; Milon, B.; Feng, P.; Costello, L.C. Zinc and zinc transporters in normal prostate and the pathogenesis of prostate cancer. Front. Biosci. 2005, 10, 2230–2239. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: Connecting the dots. Mol. Cancer 2006, 5, 17. [Google Scholar] [CrossRef][Green Version]
- Costello, L.C.; Franklin, R.B. Zinc: The Wonder Drug for the Treatment of Carcinomas. Acta Sci. Cancer Biol. 2020, 4, 33–39. [Google Scholar] [CrossRef]
- Hainaut, P.; Mann, K. Zinc binding and redox control of p53 structure and function. Antioxid. Redox Signal. 2001, 3, 611–623. [Google Scholar] [CrossRef]
- Ha, J.H.; Prela, O.; Carpizo, D.R.; Loh, S.N. p53 and Zinc: A Malleable Relationship. Front. Mol. Biosci. 2022, 9, 895887. [Google Scholar] [CrossRef]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]
- Institute of Medicine (U.S.) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Maares, M.; Haase, H. A Guide to Human Zinc Absorption: General Overview and Recent Advances of In Vitro Intestinal Models. Nutrients 2020, 12, 762. [Google Scholar] [CrossRef]
- Foote, J.W.; Delves, H.T. Albumin bound and alpha 2-macroglobulin bound zinc concentrations in the sera of healthy adults. J. Clin. Pathol. 1984, 37, 1050–1054. [Google Scholar] [CrossRef]
- Stewart, A.J.; Blindauer, C.A.; Berezenko, S.; Sleep, D.; Sadler, P.J. Interdomain zinc site on human albumin. Proc. Natl. Acad. Sci. USA 2003, 100, 3701–3706. [Google Scholar] [CrossRef]
- Yamanaka, Y.; Matsugano, S.; Yoshikawa, Y.; Orino, K. Binding Analysis of Human Immunoglobulin G as a Zinc-Binding Protein. Antibodies 2016, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Orino, K. Functional binding analysis of human fibrinogen as an iron- and heme-binding protein. Biometals 2013, 26, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Babaeva, E.E.; Vorobyova, U.A.; Denisova, E.A.; Medvedeva, D.A.; Cheknev, S.B. Binding of zinc cations to human serum gamma-globulin. Bull. Exp. Biol. Med. 2006, 141, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Mocchegiani, E.; Costarelli, L.; Giacconi, R.; Cipriano, C.; Muti, E.; Malavolta, M. Zinc-binding proteins (metallothionein and alpha-2 macroglobulin) and immunosenescence. Exp. Gerontol. 2006, 41, 1094–1107. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.J.; Bradwell, A.R. Identification of the serum binding proteins for iron, zinc, cadmium, nickel, and calcium. Clin. Chem. 1983, 29, 629–633. [Google Scholar] [CrossRef]
- Chilvers, D.C.; Dawson, J.B.; Bahreyni-Toosi, M.H.; Hodgkinson, A. Identification and determination of copper--and zinc--protein complexes in blood plasma after chromatographic separation on DEAE-Sepharose CL-6B. Analyst 1984, 109, 871–876. [Google Scholar] [CrossRef]
- Handing, K.B.; Shabalin, I.G.; Kassaar, O.; Khazaipoul, S.; Blindauer, C.A.; Stewart, A.J.; Chruszcz, M.; Minor, W. Circulatory zinc transport is controlled by distinct interdomain sites on mammalian albumins. Chem. Sci. 2016, 7, 6635–6648. [Google Scholar] [CrossRef]
- Sokolowska, M.; Wszelaka-Rylik, M.; Poznanski, J.; Bal, W. Spectroscopic and thermodynamic determination of three distinct binding sites for Co(II) ions in human serum albumin. J. Inorg. Biochem. 2009, 103, 1005–1013. [Google Scholar] [CrossRef]
- Bal, W.; Christodoulou, J.; Sadler, P.J.; Tucker, A. Multi-metal binding site of serum albumin. J. Inorg. Biochem. 1998, 70, 33–39. [Google Scholar] [CrossRef]
- Lu, J.; Stewart, A.J.; Sleep, D.; Sadler, P.J.; Pinheiro, T.J.; Blindauer, C.A. A molecular mechanism for modulating plasma Zn speciation by fatty acids. J. Am. Chem. Soc. 2012, 134, 1454–1457. [Google Scholar] [CrossRef]
- Regan-Smith, S.; Fritzen, R.; Hierons, S.J.; Ajjan, R.A.; Blindauer, C.A.; Stewart, A.J. Strategies for Therapeutic Amelioration of Aberrant Plasma Zn(2+) Handling in Thrombotic Disease: Targeting Fatty Acid/Serum Albumin-Mediated Effects. Int. J. Mol. Sci. 2022, 23, 10302. [Google Scholar] [CrossRef]
- Ha, X.; Wang, J.; Chen, K.; Deng, Y.; Zhang, X.; Feng, J.; Li, X.; Zhu, J.; Ma, Y.; Qiu, T.; et al. Free Fatty Acids Promote the Development of Prostate Cancer by Upregulating Peroxisome Proliferator-Activated Receptor Gamma. Cancer Manag. Res. 2020, 12, 1355–1369. [Google Scholar] [CrossRef]
- Aslan, R.; Eryılmaz, R.; Sevim, M.; Demir, M.; Taken, K. The Diagnostic Value of Ischemia-modified Albumin in Prostate Cancer. Med. Bull. Haseki. 2020, 58, 42–47. [Google Scholar] [CrossRef]
- Da Silveira, R.A.; Hermes, C.L.; Almeida, T.C.; Bochi, G.V.; De Bona, K.S.; Moretto, M.B.; Moresco, R.N. Ischemia-modified albumin and inflammatory biomarkers in patients with prostate cancer. Clin. Lab. 2014, 60, 1703–1708. [Google Scholar] [CrossRef]
- Kirmit, A.; Kader, S.; Aksoy, M.; Bal, C.; Nural, C.; Aslan, O. Trace elements and oxidative stress status in patients with psoriasis. Postępy Dermatol. Alergol. 2020, 37, 333–339. [Google Scholar] [CrossRef]
- Serinkan Cinemre, F.B.; Cinemre, H.; Bahtiyar, N.; Kahyaoglu, B.; Agac, M.T.; Shundo, H.; Sevinç, L.; Aydemir, B. Apelin, Omentin-1, and Vaspin in patients with essential hypertension: Association of adipokines with trace elements, inflammatory cytokines, and oxidative damage markers. Ir. J. Med. Sci. 2021, 190, 97–106. [Google Scholar] [CrossRef]
- Shevtsova, A.; Gordiienko, I.; Tkachenko, V.; Ushakova, G. Ischemia-Modified Albumin: Origins and Clinical Implications. Dis. Markers 2021, 2021, 9945424. [Google Scholar] [CrossRef]
- Kelleher, S.L.; McCormick, N.H.; Velasquez, V.; Lopez, V. Zinc in specialized secretory tissues: Roles in the pancreas, prostate, and mammary gland. Adv. Nutr. 2011, 2, 101–111. [Google Scholar] [CrossRef]
- Kambe, T.; Matsunaga, M.; Takeda, T.A. Understanding the Contribution of Zinc Transporters in the Function of the Early Secretory Pathway. Int. J. Mol. Sci. 2017, 18, 2179. [Google Scholar] [CrossRef]
- Jouybari, L.; Kiani, F.; Akbari, A.; Sanagoo, A.; Sayehmiri, F.; Aaseth, J.; Chartrand, M.S.; Sayehmiri, K.; Chirumbolo, S.; Bjørklund, G. A meta-analysis of zinc levels in breast cancer. J. Trace Elem. Med. Biol. 2019, 56, 90–99. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Q.; Hu, X.; Dong, X.; Wang, L.; Liu, Q.; Long, Z.; Li, L. Comparative study of serum zinc concentrations in benign and malignant prostate disease: A Systematic Review and Meta-Analysis. Sci. Rep. 2016, 6, 25778. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.T.; Martins, A.F.; Jordan, V.C.; Sherry, A.D. Zinc as an Imaging Biomarker of Prostate Cancer. Isr. J. Chem. 2017, 57, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch. Biochem. Biophys. 2016, 611, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B. Concepts of citrate production and secretion by prostate 1. Metabolic relationships. Prostate 1991, 18, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Liu, Y.; Franklin, R.B.; Kennedy, M.C. Zinc inhibition of mitochondrial aconitase and its importance in citrate metabolism of prostate epithelial cells. J. Biol. Chem. 1997, 272, 28875–28881. [Google Scholar] [CrossRef]
- Xue, Y.N.; Liu, Y.N.; Su, J.; Li, J.L.; Wu, Y.; Guo, R.; Yu, B.-B.; Yan, X.-Y.; Zhang, L.-C.; Sun, L.-K.; et al. Zinc cooperates with p53 to inhibit the activity of mitochondrial aconitase through reactive oxygen species accumulation. Cancer Med. 2019, 8, 2462–2473. [Google Scholar] [CrossRef]
- Franz, M.C.; Anderle, P.; Burzle, M.; Suzuki, Y.; Freeman, M.R.; Hediger, M.A.; Kovacs, G. Zinc transporters in prostate cancer. Mol. Asp. Med. 2013, 34, 735–741. [Google Scholar] [CrossRef]
- Zaichick, V. A Systematic Review of the Zinc Content of the Normal Human Prostate Gland. Biol. Trace Elem. Res. 2021, 199, 3593–3607. [Google Scholar] [CrossRef]
- Costello, L.C.; Liu, Y.; Zou, J.; Franklin, R.B. Evidence for a zinc uptake transporter in human prostate cancer cells which is regulated by prolactin and testosterone. J. Biol. Chem. 1999, 274, 17499–17504. [Google Scholar] [CrossRef]
- Liang, J.Y.; Liu, Y.Y.; Zou, J.; Franklin, R.B.; Costello, L.C.; Feng, P. Inhibitory effect of zinc on human prostatic carcinoma cell growth. Prostate 1999, 40, 200–207. [Google Scholar] [CrossRef]
- Johnson, L.A.; Kanak, M.A.; Kajdacsy-Balla, A.; Pestaner, J.P.; Bagasra, O. Differential zinc accumulation and expression of human zinc transporter 1 (hZIP1) in prostate glands. Methods 2010, 52, 316–321. [Google Scholar] [CrossRef]
- Li, D.; Stovall, D.B.; Wang, W.; Sui, G. Advances of Zinc Signaling Studies in Prostate Cancer. Int. J. Mol. Sci. 2020, 21, 667. [Google Scholar] [CrossRef]
- Chyan, W.; Zhang, D.Y.; Lippard, S.J.; Radford, R.J. Reaction-based fluorescent sensor for investigating mobile Zn2+ in mitochondria of healthy versus cancerous prostate cells. Proc. Natl. Acad. Sci. USA 2014, 111, 143–148. [Google Scholar] [CrossRef]
- Vickram, S.; Rohini, K.; Anbarasu, K.; Dey, N.; Jeyanthi, P.; Thanigaivel, S.; Issac, P.K.; Arockiaraj, J. Semenogelin, a coagulum macromolecule monitoring factor involved in the first step of fertilization: A prospective review. Int. J. Biol. Macromol. 2022, 209 Pt A, 951–962. [Google Scholar] [CrossRef]
- Kambe, T.; Taylor, K.M.; Fu, D. Zinc transporters and their functional integration in mammalian cells. J. Biol. Chem. 2021, 296, 100320. [Google Scholar] [CrossRef]
- Liuzzi, J.P.; Cousins, R.J. Mammalian zinc transporters. Annu. Rev. Nutr. 2004, 24, 151–172. [Google Scholar] [CrossRef]
- Eide, D.J. The SLC39 family of metal ion transporters. Pflügers Arch. 2004, 447, 796–800. [Google Scholar] [CrossRef]
- Palmiter, R.D.; Huang, L. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflügers Arch. 2004, 447, 744–751. [Google Scholar] [CrossRef]
- Kimura, T.; Kambe, T. The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective. Int. J. Mol. Sci. 2016, 17, 336. [Google Scholar] [CrossRef]
- Kury, S.; Dreno, B.; Bezieau, S.; Giraudet, S.; Kharfi, M.; Kamoun, R.; Moisan, J.-P. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat. Genet. 2002, 31, 239–240. [Google Scholar] [CrossRef]
- Ahmad, F.; Cherukuri, M.K.; Choyke, P.L. Metabolic reprogramming in prostate cancer. Br. J. Cancer 2021, 125, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.K.; Vela, H.; Vela, G.; Stark, P.; Barrera-Juarez, E.; Grabrucker, A.M. Zinc Deficiency in Men Over 50 and Its Implications in Prostate Disorders. Front. Oncol. 2020, 10, 1293. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, A.L.; Somji, S.; Sens, M.A.; Sens, D.A.; Garrett, S.H. Zinc transporter mRNA expression in the RWPE-1 human prostate epithelial cell line. Biometals 2008, 21, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Bulldan, A.; Bartsch, J.W.; Konrad, L.; Scheiner-Bobis, G. ZIP9 but not the androgen receptor mediates testosterone-induced migratory activity of metastatic prostate cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1857–1868. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.B.; Feng, P.; Milon, B.; Desouki, M.M.; Singh, K.K.; Kajdacsy-Balla, A.; Bagasra, O.; Costello, L.C. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol. Cancer 2005, 4, 32. [Google Scholar] [CrossRef]
- Huang, L.; Kirschke, C.P.; Zhang, Y. Decreased intracellular zinc in human tumorigenic prostate epithelial cells: A possible role in prostate cancer progression. Cancer Cell Int. 2006, 6, 10. [Google Scholar] [CrossRef]
- Chen, Q.G.; Zhang, Z.; Yang, Q.; Shan, G.Y.; Yu, X.Y.; Kong, C.Z. The role of zinc transporter ZIP4 in prostate carcinoma. Urol. Oncol. 2012, 30, 906–911. [Google Scholar] [CrossRef]
- To, P.K.; Do, M.H.; Cho, J.H.; Jung, C. Growth Modulatory Role of Zinc in Prostate Cancer and Application to Cancer Therapeutics. Int. J. Mol. Sci. 2020, 21, 2991. [Google Scholar] [CrossRef]
- Taylor, K.M.; Muraina, I.A.; Brethour, D.; Schmitt-Ulms, G.; Nimmanon, T.; Ziliotto, S.; Kille, P.; Hogstrand, C. Zinc transporter ZIP10 forms a heteromer with ZIP6 which regulates embryonic development and cell migration. Biochem. J. 2016, 473, 2531–2544. [Google Scholar] [CrossRef]
- Lao, L.; Franklin, R.B.; Costello, L.C. High-affinity L-aspartate transporter in prostate epithelial cells that is regulated by testosterone. Prostate 1993, 22, 53–63. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, C.; Wu, W.; Shi, L.; Jiang, C.; Wang, L.; Ding, Z.; Liu, Y. Zinc transporter ZIP12 maintains zinc homeostasis and protects spermatogonia from oxidative stress during spermatogenesis. Reprod. Biol. Endocrinol. 2022, 20, 17. [Google Scholar] [CrossRef]
- Matsui, C.; Takatani-Nakase, T.; Hatano, Y.; Kawahara, S.; Nakase, I.; Takahashi, K. Zinc and its transporter ZIP6 are key mediators of breast cancer cell survival under high glucose conditions. FEBS Lett. 2017, 591, 3348–3359. [Google Scholar] [CrossRef]
- Nordberg, M.; Nordberg, G.F. Metallothionein and Cadmium Toxicology-Historical Review and Commentary. Biomolecules 2022, 12, 360. [Google Scholar] [CrossRef]
- Hinata, N.; Fujisawa, M. Racial Differences in Prostate Cancer Characteristics and Cancer-Specific Mortality: An Overview. World J. Men’s Health 2022, 40, 217–227. [Google Scholar] [CrossRef]
- Rishi, I.; Baidouri, H.; Abbasi, J.A.; Bullard-Dillard, R.; Kajdacsy-Balla, A.; Pestaner, J.P.; Skacel, M.; Tubbs, R.; Bagasra, O. Prostate cancer in African American men is associated with downregulation of zinc transporters. Appl. Immunohistochem. Mol. Morphol. 2003, 11, 253–260. [Google Scholar] [CrossRef]
- Bosomworth, H.J.; Thornton, J.K.; Coneyworth, L.J.; Ford, D.; Valentine, R.A. Efflux function, tissue-specific expression and intracellular trafficking of the Zn transporter ZnT10 indicate roles in adult Zn homeostasis. Metallomics 2012, 4, 771–779. [Google Scholar] [CrossRef]
- Chimienti, F.; Devergnas, S.; Favier, A.; Seve, M. Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes 2004, 53, 2330–2337. [Google Scholar] [CrossRef]
- Kirschke, C.P.; Huang, L. ZnT7, a novel mammalian zinc transporter, accumulates zinc in the Golgi apparatus. J. Biol. Chem. 2003, 278, 4096–4102. [Google Scholar] [CrossRef]
- Huang, L.; Kirschke, C.P.; Gitschier, J. Functional characterization of a novel mammalian zinc transporter, ZnT6. J. Biol. Chem. 2002, 277, 26389–26395. [Google Scholar] [CrossRef]
- Kambe, T.; Narita, H.; Yamaguchi-Iwai, Y.; Hirose, J.; Amano, T.; Sugiura, N.; Sasaki, R.; Mori, K.; Iwanaga, T.; Nagao, M. Cloning and characterization of a novel mammalian zinc transporter, zinc transporter 5, abundantly expressed in pancreatic beta cells. J. Biol. Chem. 2002, 277, 19049–19055. [Google Scholar] [CrossRef]
- Huang, L.; Gitschier, J. A novel gene involved in zinc transport is deficient in the lethal milk mouse. Nat. Genet. 1997, 17, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.B.; Wenzel, H.J.; Kafer, K.E.; Schwartzkroin, P.A.; Palmiter, R.D. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc. Natl. Acad. Sci. USA 1999, 96, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D.; Cole, T.B.; Findley, S.D. ZnT-2, a mammalian protein that confers resistance to zinc by facilitating vesicular sequestration. EMBO J. 1996, 15, 1784–1791. [Google Scholar] [CrossRef] [PubMed]
- Henshall, S.M.; Afar, D.E.; Rasiah, K.K.; Horvath, L.G.; Gish, K.; Caras, I.; Ramakrishnan, V.; Wong, M.; Jeffry, U.; Kench, J.G.; et al. Expression of the zinc transporter ZnT4 is decreased in the progression from early prostate disease to invasive prostate cancer. Oncogene 2003, 22, 6005–6012. [Google Scholar] [CrossRef] [PubMed]
- Kirschke, C.P.; Huang, L. Expression of the ZNT (SLC30) family members in the epithelium of the mouse prostate during sexual maturation. J. Mol. Histol. 2008, 39, 359–370. [Google Scholar] [CrossRef]
- Langmade, S.J.; Ravindra, R.; Daniels, P.J.; Andrews, G.K. The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. J. Biol. Chem. 2000, 275, 34803–34809. [Google Scholar] [CrossRef]
- Palmiter, R.D.; Findley, S.D. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 1995, 14, 639–649. [Google Scholar] [CrossRef]
- Singh, C.K.; Malas, K.M.; Tydrick, C.; Siddiqui, I.A.; Iczkowski, K.A.; Ahmad, N. Analysis of Zinc-Exporters Expression in Prostate Cancer. Sci. Rep. 2016, 6, 36772. [Google Scholar] [CrossRef]
- Hasumi, M.; Suzuki, K.; Matsui, H.; Koike, H.; Ito, K.; Yamanaka, H. Regulation of metallothionein and zinc transporter expression in human prostate cancer cells and tissues. Cancer Lett. 2003, 200, 187–195. [Google Scholar] [CrossRef]
- Otsuka, T.; Iguchi, K.; Fukami, K.; Ishii, K.; Usui, S.; Sugimura, Y.; Hirano, K. Androgen receptor W741C and T877A mutations in AIDL cells, an androgen-independent subline of prostate cancer LNCaP cells. Tumour Biol. 2011, 32, 1097–1102. [Google Scholar] [CrossRef]
- Iguchi, K.; Otsuka, T.; Usui, S.; Ishii, K.; Onishi, T.; Sugimura, Y.; Hirano, K. Zinc and metallothionein levels and expression of zinc transporters in androgen-independent subline of LNCaP cells. J. Androl. 2004, 25, 154–161. [Google Scholar] [CrossRef]
- Song, Y.; Elias, V.; Wong, C.P.; Scrimgeour, A.G.; Ho, E. Zinc transporter expression profiles in the rat prostate following alterations in dietary zinc. Biometals 2010, 23, 51–58. [Google Scholar] [CrossRef]
- Kukic, I.; Kelleher, S.L.; Kiselyov, K. Zn2+ efflux through lysosomal exocytosis prevents Zn2+-induced toxicity. J. Cell Sci. 2014, 127 Pt 14, 3094–3103. [Google Scholar]
- Inoue, K.; Matsuda, K.; Itoh, M.; Kawaguchi, H.; Tomoike, H.; Aoyagi, T.; Nagai, R.; Hori, M.; Nakamura, Y.; Tanaka, T. Osteopenia and male-specific sudden cardiac death in mice lacking a zinc transporter gene, Znt5. Hum. Mol. Genet. 2002, 11, 1775–1784. [Google Scholar] [CrossRef]
- Johnson, E.D.; Butler, K.; Gupta, S. Bone Health in Patients with Prostate Cancer: An Evidence-Based Algorithm. Fed. Pract. 2021, 38 (Suppl. S3), S20–S26. [Google Scholar] [CrossRef]
- Wang, A.; Karunasinghe, N.; Plank, L.; Zhu, S.; Osborne, S.; Bishop, K.; Brown, C.; Schwass, T.; Masters, J.; Holmes, M.; et al. Effect of Androgen Deprivation Therapy on Bone Mineral Density in a Prostate Cancer Cohort in New Zealand: A Pilot Study. Clin. Med. Insights Oncol. 2017, 11, 1179554917733449. [Google Scholar] [CrossRef]
- Tepaamorndech, S.; Huang, L.; Kirschke, C.P. A null-mutation in the Znt7 gene accelerates prostate tumor formation in a transgenic adenocarcinoma mouse prostate model. Cancer Lett. 2011, 308, 33–42. [Google Scholar] [CrossRef]
- Vasak, M. Advances in metallothionein structure and functions. J. Trace Elem. Med. Biol. 2005, 19, 13–17. [Google Scholar] [CrossRef]
- Thomas, E.A.; Bailey, L.B.; Kauwell, G.A.; Lee, D.Y.; Cousins, R.J. Erythrocyte metallothionein response to dietary zinc in humans. J. Nutr. 1992, 122, 2408–2414. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Abern, M.R.; Jagai, J.S.; Kajdacsy-Balla, A. Observational Study of the Association between Air Cadmium Exposure and Prostate Cancer Aggressiveness at Diagnosis among a Nationwide Retrospective Cohort of 230,540 Patients in the United States. Int. J. Environ. Res. Public Health 2021, 18, 8333. [Google Scholar] [CrossRef]
- McDonald, A.C.; Gernand, J.; Geyer, N.R.; Wu, H.; Yang, Y.; Wang, M. Ambient air exposures to arsenic and cadmium and overall and prostate cancer-specific survival among prostate cancer cases in Pennsylvania, 2004 to 2014. Cancer 2022, 128, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Gumulec, J.; Raudenska, M.; Adam, V.; Kizek, R.; Masarik, M. Metallothionein—immunohistochemical cancer biomarker: A meta-analysis. PLoS ONE 2014, 9, e85346. [Google Scholar] [CrossRef] [PubMed]
- Loffek, S.; Schilling, O.; Franzke, C.W. Series “matrix metalloproteinases in lung health and disease”: Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Woessner, J.F.J. Matrix metalloproteinase inhibition. From the Jurassic to the third millennium. Ann. N. Y. Acad. Sci. 1999, 878, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (MMPs): Chemical-biological functions and (Q)SARs. Bioorganic Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef]
- Mustafa, S.; Koran, S.; AlOmair, L. Insights into the Role of Matrix Metalloproteinases in Cancer and its Various Therapeutic Aspects: A Review. Front. Mol. Biosci. 2022, 9, 896099. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Swetha, R.; Gayen, C.; Kumar, D.; Singh, T.D.; Modi, G.; Singh, S.K. Biomolecular basis of matrix metallo proteinase-9 activity. Future Med Chem. 2018, 10, 1093–1112. [Google Scholar] [CrossRef]
- Tezvergil-Mutluay, A.; Agee, K.A.; Hoshika, T.; Carrilho, M.; Breschi, L.; Tjaderhane, L.; Nishitani, Y.; Carvalho, R.M.; Looney, S.; Tay, F.R.; et al. The requirement of zinc and calcium ions for functional MMP activity in demineralized dentin matrices. Dent. Mater. 2010, 26, 1059–1067. [Google Scholar] [CrossRef]
- Seltzer, J.L.; Jeffrey, J.J.; Eisen, A.Z. Evidence for mammalian collagenases as zinc ion metalloenzymes. Biochim. Biophys. Acta 1977, 485, 179–187. [Google Scholar] [CrossRef]
- Nosrati, R.; Kheirouri, S.; Ghodsi, R.; Ojaghi, H. The effects of zinc treatment on matrix metalloproteinases: A systematic review. J. Trace Elem. Med. Biol. 2019, 56, 107–115. [Google Scholar] [CrossRef]
- Kang, M.; Zhao, L.; Ren, M.; Deng, M.; Li, C. Zinc mediated hepatic stellate cell collagen synthesis reduction through TGF-beta signaling pathway inhibition. Int. J. Clin. Exp. Med. 2015, 8, 20463–20471. [Google Scholar]
- Binnebosel, M.; Grommes, J.; Koenen, B.; Junge, K.; Klink, C.D.; Stumpf, M.; Öttinger, A.P.; Schumpelick, V.; Klinge, U.; Krones, C.J. Zinc deficiency impairs wound healing of colon anastomosis in rats. Int. J. Colorectal Dis. 2010, 25, 251–257. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; Del Rio Hernandez, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef]
- Binder, M.J.; Ward, A.C. The Role of the Metzincin Superfamily in Prostate Cancer Progression: A Systematic-Like Review. Int. J. Mol. Sci. 2021, 22, 3608. [Google Scholar] [CrossRef]
- Neuhaus, D. Zinc finger structure determination by NMR: Why zinc fingers can be a handful. Prog. Nucl. Magn. Reason. Spectrosc. 2022, 130–131, 62–105. [Google Scholar] [CrossRef]
- Mackeh, R.; Marr, A.K.; Fadda, A.; Kino, T. C2H2-Type Zinc Finger Proteins: Evolutionarily Old and New Partners of the Nuclear Hormone Receptors. Nucl. Recept. Signal. 2018, 15, 1550762918801071. [Google Scholar] [CrossRef]
- Safe, S.; Kim, K. Nuclear receptor-mediated transactivation through interaction with Sp proteins. Prog. Nucleic Acid. Res. Mol. Biol. 2004, 77, 1–36. [Google Scholar]
- Augello, M.A.; Den, R.B.; Knudsen, K.E. AR function in promoting metastatic prostate cancer. Cancer Metastasis Rev. 2014, 33, 399–411. [Google Scholar] [CrossRef]
- Tan, M.H.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.L. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef]
- Huang, H.; Xu, H.; Li, P.; Ye, X.; Chen, W.; Chen, W.; Huang, X. Zinc finger C3H1 domain-containing protein (ZFC3H1) evaluates the prognosis and treatment of prostate adenocarcinoma (PRAD): A study based on TCGA data. Bioengineered 2021, 12, 5504–5515. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Mackenzie, G.G. Zinc, oxidant-triggered cell signaling, and human health. Mol. Asp. Med. 2005, 26, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Orlov, A.P.; Orlova, M.A.; Trofimova, T.P.; Kalmykov, S.N.; Kuznetsov, D.A. The role of zinc and its compounds in leukemia. J. Biol. Inorg. Chem. 2018, 23, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Kroncke, K.D. Zinc finger proteins as molecular targets for nitric oxide-mediated gene regulation. Antioxid. Redox Signal. 2001, 3, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Heiss, N.S.; Gloeckner, G.; Bachner, D.; Kioschis, P.; Klauck, S.M.; Hinzmann, B.; Rosenthal, A.; Herman, G.E.; Poustka, A. Genomic structure of a novel LIM domain gene (ZNF185) in Xq28 and comparisons with the orthologous murine transcript. Genomics 1997, 43, 329–338. [Google Scholar] [CrossRef][Green Version]
- Smirnov, A.; Cappello, A.; Lena, A.M.; Anemona, L.; Mauriello, A.; Di Daniele, N.; Annicchiarico-Petruzzelli, M.; Melino, G.; Candi, E. ZNF185 is a p53 target gene following DNA damage. Aging 2018, 10, 3308–3326. [Google Scholar] [CrossRef]
- Vanaja, D.K.; Cheville, J.C.; Iturria, S.J.; Young, C.Y. Transcriptional silencing of zinc finger protein 185 identified by expression profiling is associated with prostate cancer progression. Cancer Res. 2003, 63, 3877–3882. [Google Scholar]
- Liang, W.; Chen, W.; Wei, J.; Yao, H.; Shi, J.; Hou, X.; Deng, Y.; Ou, M. Zinc finger C3H1-type containing serves as a novel prognostic biomarker in human pan-cancer. Gene 2022, 820, 146251. [Google Scholar] [CrossRef]
- Hogstrand, C.; Kille, P.; Nicholson, R.I.; Taylor, K.M. Zinc transporters and cancer: A potential role for ZIP7 as a hub for tyrosine kinase activation. Trends Mol. Med. 2009, 15, 101–111. [Google Scholar] [CrossRef]
- Maret, W. Redox biochemistry of mammalian metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1079–1086. [Google Scholar] [CrossRef]
- Miyai, T.; Hojyo, S.; Ikawa, T.; Kawamura, M.; Irie, T.; Ogura, H.; Hijikata, A.; Bin, B.-H.; Yasuda, T.; Kitamura, H.; et al. Zinc transporter SLC39A10/ZIP10 facilitates antiapoptotic signaling during early B-cell development. Proc. Natl. Acad. Sci. USA 2014, 111, 11780–11785. [Google Scholar] [CrossRef]
- Taylor, K.M.; Hiscox, S.; Nicholson, R.I.; Hogstrand, C.; Kille, P. Protein kinase CK2 triggers cytosolic zinc signaling pathways by phosphorylation of zinc channel ZIP7. Sci. Signal. 2012, 5, ra11. [Google Scholar] [CrossRef]
- Yamashita, S.; Miyagi, C.; Fukada, T.; Kagara, N.; Che, Y.S.; Hirano, T. Zinc transporter LIVI controls epithelial-mesenchymal transition in zebrafish gastrula organizer. Nature 2004, 429, 298–302. [Google Scholar] [CrossRef]
- Fukada, T.; Yamasaki, S.; Nishida, K.; Murakami, M.; Hirano, T. Zinc homeostasis and signaling in health and diseases: Zinc signaling. J. Biol. Inorg. Chem. 2011, 16, 1123–1134. [Google Scholar] [CrossRef]
- Zhang, A.; Gupte, A.A.; Chatterjee, S.; Li, S.; Ayala, A.G.; Miles, B.J.; Hamilton, D.J. Enhanced Succinate Oxidation with Mitochondrial Complex II Reactive Oxygen Species Generation in Human Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 12168. [Google Scholar] [CrossRef]
- Ishida, T.; Takechi, S. Nrf2-ARE-Dependent Alterations in Zinc Transporter mRNA Expression in HepG2 Cells. PLoS ONE 2016, 11, e0166100. [Google Scholar] [CrossRef]
- O’Keefe, D.S.; Bacich, D.J.; Huang, S.S.; Heston, W.D.W. A Perspective on the Evolving Story of PSMA Biology, PSMA-Based Imaging, and Endoradiotherapeutic Strategies. J. Nucl. Med. 2018, 59, 1007–1013. [Google Scholar] [CrossRef]
- Karunasinghe, N.; Bishop, K.; Murray, P.; Xu, Y.; Goudie, M.; Ng, L.; Zhu, S.; Han, J.Y.; Ferguson, L.R.; Masters, J.; et al. Role of β-microseminoprotein from prostate cancer initiation to recurrence: A mini-review. World J. Clin. Urol. 2014, 3, 20–30. [Google Scholar] [CrossRef]
- Webber, M.M.; Waghray, A.; Bello, D. Prostate-specific antigen, a serine protease, facilitates human prostate cancer cell invasion. Clin. Cancer Res. 1995, 1, 1089–1094. [Google Scholar]
- Ishii, K.; Otsuka, T.; Iguchi, K.; Usui, S.; Yamamoto, H.; Sugimura, Y.; Yoshikawa, K.; Hayward, S.W.; Hirano, K. Evidence that the prostate-specific antigen (PSA)/Zn2+ axis may play a role in human prostate cancer cell invasion. Cancer Lett. 2004, 207, 79–87. [Google Scholar] [CrossRef]
- Karunasinghe, N.; Ng, L.; Wang, A.; Vaidyanathan, V.; Zhu, S.; Ferguson, L.R. Selenium Supplementation and Prostate Health in a New Zealand Cohort. Nutrients 2019, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Knee, R.A.; Hickey, D.K.; Beagley, K.W.; Jones, R.C. Transport of IgG across the blood-luminal barrier of the male reproductive tract of the rat and the effect of estradiol administration on reabsorption of fluid and IgG by the epididymal ducts. Biol. Reprod. 2005, 73, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Z.; Niu, N.; Chang, Q.; Deng, R.; Korteweg, C.; Gu, J. IgG gene expression and its possible significance in prostate cancers. Prostate 2012, 72, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Hojyo, S.; Fukada, T. Roles of Zinc Signaling in the Immune System. J. Immunol. Res. 2016, 2016, 6762343. [Google Scholar] [CrossRef] [PubMed]
- Krzeslak, A.; Forma, E.; Chwatko, G.; Jozwiak, P.; Szymczyk, A.; Wilkosz, J.; Różański, W.; Bryś, M. Effect of metallothionein 2A gene polymorphism on allele-specific gene expression and metal content in prostate cancer. Toxicol. Appl. Pharmacol. 2013, 268, 278–285. [Google Scholar] [CrossRef]
- Forma, E.; Krzeslak, A.; Wilkosz, J.; Jozwiak, P.; Szymczyk, A.; Rozanski, W.; Brys, M. Metallothionein 2A genetic polymorphisms and risk of prostate cancer in a Polish population. Cancer Genet. 2012, 205, 432–435. [Google Scholar] [CrossRef]
- Kayaalti, Z.; Aliyev, V.; Soylemezoglu, T. The potential effect of metallothionein 2A-5A/G single nucleotide polymorphism on blood cadmium, lead, zinc and copper levels. Toxicol. Appl. Pharmacol. 2011, 256, 1–7. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, X. Association between matrix-metalloproteinase polymorphisms and prostate cancer risk: A meta-analysis and systematic review. Cancer Manag. Res. 2018, 10, 5247–5259. [Google Scholar] [CrossRef]
- Kodali, H.P.; Pavilonis, B.T.; Schooling, C.M. Effects of copper and zinc on ischemic heart disease and myocardial infarction: A Mendelian randomization study. Am. J. Clin. Nutr. 2018, 108, 237–242. [Google Scholar] [CrossRef]
- Ng, E.; Lind, P.M.; Lindgren, C.; Ingelsson, E.; Mahajan, A.; Morris, A.; Lind, L. Genome-wide association study of toxic metals and trace elements reveals novel associations. Hum. Mol. Genet. 2015, 24, 4739–4745. [Google Scholar] [CrossRef]
- Prasad, A.S.; Mukhtar, H.; Beck, F.W.; Adhami, V.M.; Siddiqui, I.A.; Din, M.; Hafeez, B.B.; Kucuk, O. Dietary zinc and prostate cancer in the TRAMP mouse model. J. Med. Food 2010, 13, 70–76. [Google Scholar] [CrossRef]
- Yan, M.; Song, Y.; Wong, C.P.; Hardin, K.; Ho, E. Zinc deficiency alters DNA damage response genes in normal human prostate epithelial cells. J. Nutr. 2008, 138, 667–673. [Google Scholar] [CrossRef]
- Epstein, M.M.; Kasperzyk, J.L.; Andren, O.; Giovannucci, E.L.; Wolk, A.; Hakansson, N.; Andersson, S.-O.; Johansson, J.-E.; Fall, K.; Mucci, L.A. Dietary zinc and prostate cancer survival in a Swedish cohort. Am. J. Clin. Nutr. 2011, 93, 586–593. [Google Scholar] [CrossRef]
- Leitzmann, M.F.; Stampfer, M.J.; Wu, K.; Colditz, G.A.; Willett, W.C.; Giovannucci, E.L. Zinc supplement use and risk of prostate cancer. J. Natl. Cancer Inst. 2003, 95, 1004–1007. [Google Scholar] [CrossRef]
- Wong, P.F.; Abubakar, S. Comparative transcriptional study of the effects of high intracellular zinc on prostate carcinoma cells. Oncol. Rep. 2010, 23, 1501–1516. [Google Scholar]
- Pompano, L.M.; Boy, E. Effects of Dose and Duration of Zinc Interventions on Risk Factors for Type 2 Diabetes and Cardiovascular Disease: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 141–160. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Patel, A.; Chang, H.; Ahmad, N. Dietary Phytochemicals in Zinc Homeostasis: A Strategy for Prostate Cancer Management. Nutrients 2021, 13, 1867. [Google Scholar] [CrossRef]
- Singh, C.K.; Pitschmann, A.; Ahmad, N. Resveratrol-zinc combination for prostate cancer management. Cell Cycle 2014, 13, 1867–1874. [Google Scholar] [CrossRef]
- Gray, M.A.; Centeno, J.A.; Slaney, D.P.; Ejnik, J.W.; Todorov, T.; Nacey, J.N. Environmental exposure to trace elements and prostate cancer in three New Zealand ethnic groups. Int. J. Environ. Res. Public Health 2005, 2, 374–384. [Google Scholar] [CrossRef]
- Wu, H.; Wang, M.; Raman, J.D.; McDonald, A.C. Association between urinary arsenic, blood cadmium, blood lead, and blood mercury levels and serum prostate-specific antigen in a population-based cohort of men in the United States. PLoS ONE 2021, 16, e0250744. [Google Scholar] [CrossRef]
- van Wijngaarden, E.; Singer, E.A.; Palapattu, G.S. Prostate-specific antigen levels in relation to cadmium exposure and zinc intake: Results from the 2001–2002 National Health and Nutrition Examination Survey. Prostate 2008, 68, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.G.; Ahmed, K.; Zaidi, S.F.; Muhammad, J.S. Ins and outs of cadmium-induced carcinogenesis: Mechanism and prevention. Cancer Treat Res. Commun. 2021, 27, 100372. [Google Scholar] [CrossRef] [PubMed]
- Lossow, K.; Schwarz, M.; Kipp, A.P. Are trace element concentrations suitable biomarkers for the diagnosis of cancer? Redox Biol. 2021, 42, 101900. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.A.K.; Adly, H.M.; Abdelkhaliq, A.A.; Nassir, A.M. Serum Levels of Selenium, Zinc, Copper, Manganese, and Iron in Prostate Cancer Patients. Curr. Urol. 2020, 14, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Ogunlewe, J.O.; Osegbe, D.N. Zinc and cadmium concentrations in indigenous blacks with normal, hypertrophic, and malignant prostate. Cancer 1989, 63, 1388–1392. [Google Scholar] [CrossRef]
- Nordberg, G.F.; Akesson, A.; Nogawa, K.; Nordberg, M. Cadmium. In Handbook on the Toxicology of Metals, 5th ed.; Academic Press: London, UK, 2022; Volume 2, pp. 141–196. [Google Scholar]
- Kim, K.; Melough, M.M.; Vance, T.M.; Kim, D.; Noh, H.; Koo, S.I.; Chun, O.K. The relationship between zinc intake and cadmium burden is influenced by smoking status. Food Chem. Toxicol. 2019, 125, 210–216. [Google Scholar] [CrossRef]
- Clark, L.C.; Combs, G.F.J.; Turnbull, B.W.; Slate, E.H.; Chalker, D.K.; Chow, J.; Davis, L.S.; Glover, R.A.; Graham, G.F.; Gross, E.G.; et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA 1996, 276, 1957–1963. [Google Scholar] [CrossRef]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Karunasinghe, N.; Zhu, S.; Wang, A.H. Selenium and its’ role in the maintenance of genomic stability. Mutat. Res. 2012, 733, 100–110. [Google Scholar] [CrossRef]
- Karunasinghe, N.; Zhu, S.; Ferguson, L.R. Benefits of Selenium Supplementation on Leukocyte DNA Integrity Interact with Dietary Micronutrients: A Short Communication. Nutrients 2016, 8, 249. [Google Scholar] [CrossRef]
- Karunasinghe, N.; Han, D.Y.; Zhu, S.; Duan, H.; Ko, Y.J.; Yu, J.F.; Triggs, C.M.; Ferguson, L.R. Effects of supplementation with selenium, as selenized yeast, in a healthy male population from New Zealand. Nutr. Cancer. 2013, 65, 355–366. [Google Scholar] [CrossRef]
- Karunasinghe, N.; Han, D.Y.; Goudie, M.; Zhu, S.; Bishop, K.; Wang, A.; Duan, H.; Lange, K.; Ko, S.; Medhora, R.; et al. Prostate Disease Risk Factors among a New Zealand Cohort. Nutrigenet Nutrigenomics 2013, 5, 339–351. [Google Scholar] [CrossRef]
- Karunasinghe, N.; Han, D.Y.; Zhu, S.; Yu, J.; Lange, K.; Duan, H.; Medhora, R.; Singh, N.; Kan, J.; Alzaher, W.; et al. Serum selenium and single-nucleotide polymorphisms in genes for selenoproteins: Relationship to markers of oxidative stress in men from Auckland, New Zealand. Genes Nutr. 2012, 7, 179–190. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, J.; Li, H. Selenium, aging and aging-related diseases. Aging Clin. Exp. Res. 2019, 31, 1035–1047. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Del Giovane, C.; Dennert, G.; Zwahlen, M.; Brinkman, M.; Zeegers, M.P.; Horneber, M.; D’Amico, R.; Crespi, C.M. Selenium for preventing cancer. Cochrane Database Syst Rev. 2018, 1, CD005195. [Google Scholar] [CrossRef]
- Institute of Medicine (U.S.) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; The National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- Waters, D.J.; Chiang, E.C. Five threads: How U-shaped thinking weaves together dogs, men, selenium, and prostate cancer risk. Free Radic. Biol. Med. 2018, 127, 36–45. [Google Scholar] [CrossRef]
- Tan, C.; Chen, H. Screening of prostate cancer by analyzing trace elements in hair and chemometrics. Biol. Trace Elem. Res. 2011, 144, 97–108. [Google Scholar] [CrossRef]
- Guo, J.; Deng, W.; Zhang, L.; Li, C.; Wu, P.; Mao, P. Prediction of prostate cancer using hair trace element concentration and support vector machine method. Biol. Trace Elem. Res. 2007, 116, 257–272. [Google Scholar] [CrossRef]
- Zaichick, V.; Zaichick, S. Significance of trace element quantities in the prostatic secretion of patients with benign prostatic hyperplasia and prostate cancer. J. Cancer Metastasis Treat 2019, 5, 48. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Karunasinghe, N.; Zhu, S.; Han, D.Y.; Triggs, C.M.; Wang, A.H.; Masters, J.G. Understanding Heterogeneity in Supplementation Effects of Selenium in Men: A Study of Stratification Variables and Human Genetics in a Prospective Sample from New Zealand. Curr. Pharm. Pers. Med. 2012, 10, 204–216. [Google Scholar] [CrossRef]
- Skalny, A.V.; Serebryansky, E.P.; Korobeinikova, T.V.; Tsatsakis, A.; Vardavas, C.; Paoliello, M.M.B.; Sotnikova, T.I.; Aschner, M.; Tinkov, A.A. Smoking is associated with altered serum and hair essential metal and metalloid levels in women. Food Chem. Toxicol. 2022, 167, 113249. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, M.T.; Nezami, H.; Nakhaee, S.; Aaseth, J.; Mehrpour, O. Assessing Heavy Metal Burden Among Cigarette Smokers and Non-smoking Individuals in Iran: Cluster Analysis and Principal Component Analysis. Biol. Trace Elem. Res. 2021, 199, 4036–4044. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Fernandez, M.; Powers, M.; Grau-Perez, M.; Domingo-Relloso, A.; Lolacono, N.; Goessler, W.; Zhang, Y.; Fretts, A.; Umans, J.G.; Maruthur, N.; et al. Urinary Zinc and Incident Type 2 Diabetes: Prospective Evidence From the Strong Heart Study. Diabetes Care 2022, 45, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Chirayil, S.; Jordan, V.C.; Martins, A.F.; Paranawithana, N.; Ratnakar, S.J.; Sherry, A.D. Manganese(II)-Based Responsive Contrast Agent Detects Glucose-Stimulated Zinc Secretion from the Mouse Pancreas and Prostate by MRI. Inorg. Chem. 2021, 60, 2168–2177. [Google Scholar] [CrossRef] [PubMed]
- Khalighinejad, P.; Parrott, D.; Jordan, V.C.; Chirayil, S.; Preihs, C.; Rofsky, N.M.; Xi, Y.; Sherry, A.D. Magnetic Resonance Imaging Detection of Glucose-Stimulated Zinc Secretion in the Enlarged Dog Prostate as a Potential Method for Differentiating Prostate Cancer From Benign Prostatic Hyperplasia. Investig. Radiol. 2021, 56, 450–457. [Google Scholar] [CrossRef]
- Crowley, F.; Mihalopoulos, M.; Gaglani, S.; Tewari, A.K.; Tsao, C.K.; Djordjevic, M.; Kyprianou, N.; Purohit, R.S.; Lundon, D.J. Prostate cancer in transgender women: Considerations for screening, diagnosis and management. Br. J. Cancer 2022. [Google Scholar] [CrossRef]
- Nik-Ahd, F.; Jarjour, A.; Figueiredo, J.; Anger, J.T.; Garcia, M.; Carroll, P.R.; Cooperberg, M.R.; Vidal, A.C.; Freedland, S.J. Prostate-Specific Antigen Screening in Transgender Patients. Eur. Urol. 2023, 83, 48–54. [Google Scholar] [CrossRef]
- Bertoncelli Tanaka, M.; Sahota, K.; Burn, J.; Falconer, A.; Winkler, M.; Ahmed, H.U.; Rashid, T.G. Prostate cancer in transgender women: What does a urologist need to know? BJU Int. 2022, 129, 113–122. [Google Scholar] [CrossRef]
- Gaglani, S.; Purohit, R.S.; Tewari, A.K.; Kyprianou, N.; Lundon, D.J. Embryologic and hormonal contributors to prostate cancer in transgender women. Am. J. Clin. Exp. Urol. 2022, 10, 63–72. [Google Scholar]
- Zhang, P.; Schatz, A.; Adeyemi, B.; Kozminski, D.; Welsh, J.; Tenniswood, M.; Wang, W.-L.W. Vitamin D and testosterone co-ordinately modulate intracellular zinc levels and energy metabolism in prostate cancer cells. J. Steroid Biochem. Mol. Biol. 2019, 189, 248–258. [Google Scholar] [CrossRef]
- Thomas, P.; Pang, Y.; Dong, J.; Berg, A.H. Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: II. Role of human ZIP9 in testosterone-induced prostate and breast cancer cell apoptosis. Endocrinology 2014, 155, 4250–4265. [Google Scholar] [CrossRef]
- Herzberg, M.; Lusky, A.; Blonder, J.; Frenkel, Y. The effect of estrogen replacement therapy on zinc in serum and urine. Obstet. Gynecol. 1996, 87, 1035–1040. [Google Scholar] [CrossRef]
- O’Connor, J.P.; Kanjilal, D.; Teitelbaum, M.; Lin, S.S.; Cottrell, J.A. Zinc as a Therapeutic Agent in Bone Regeneration. Materials 2020, 13, 2211. [Google Scholar] [CrossRef]
- Verroken, C.; Collet, S.; Lapauw, B.; T’Sjoen, G. Osteoporosis and Bone Health in Transgender Individuals. Calcif. Tissue Int. 2022, 110, 615–623. [Google Scholar] [CrossRef]
- Chan Swe, N.; Ahmed, S.; Eid, M.; Poretsky, L.; Gianos, E.; Cusano, N.E. The effects of gender-affirming hormone therapy on cardiovascular and skeletal health: A literature review. Metabol. Open. 2022, 13, 100173. [Google Scholar] [CrossRef]
- Cortesi, M.; Fridman, E.; Volkov, A.; Shilstein, S.; Chechik, R.; Breskin, A.; Vartsky, D.; Raviv, G.; Ramon, J. New prospective for non-invasive detection, grading, size evaluation, and tumor location of prostate cancer. Prostate 2010, 70, 1701–1708. [Google Scholar] [CrossRef]
- Maret, W. Analyzing free zinc(II) ion concentrations in cell biology with fluorescent chelating molecules. Metallomics 2015, 7, 202–211. [Google Scholar] [CrossRef]
- Schilling, K.; Moore, R.E.T.; Sullivan, K.V.; Capper, M.S.; Rehkamper, M.; Goddard, K.; Ion, C.; Coombes, R.C.; Vesty-Edwards, L.; Lamb, A.D.; et al. Zinc stable isotopes in urine as diagnostic for cancer of secretory organs. Metallomics 2021, 13, mfab020. [Google Scholar] [CrossRef]
- Hastuti, A.; Costas-Rodriguez, M.; Matsunaga, A.; Ichinose, T.; Hagiwara, S.; Shimura, M.; Vanhaecke, F. Cu and Zn isotope ratio variations in plasma for survival prediction in hematological malignancy cases. Sci. Rep. 2020, 10, 16389. [Google Scholar] [CrossRef]
- Kazi Tani, L.S.; Gourlan, A.T.; Dennouni-Medjati, N.; Telouk, P.; Dali-Sahi, M.; Harek, Y.; Sun, Q.; Hackler, J.; Belhadj, M.; Schomburg, L.; et al. Copper Isotopes and Copper to Zinc Ratio as Possible Biomarkers for Thyroid Cancer. Front. Med. 2021, 8, 698167. [Google Scholar] [CrossRef]
- Ferro, M.; Crocetto, F.; Bruzzese, D.; Imbriaco, M.; Fusco, F.; Longo, N.; Napolitano, L.; La Civita, E.; Cennamo, M.; Liotti, A.; et al. Prostate Health Index and Multiparametric MRI: Partners in Crime Fighting Overdiagnosis and Overtreatment in Prostate Cancer. Cancers 2021, 13, 4723. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Structure of the SP1 Protein. Based on PyMOL Rendering of PDB 1sp1 2010. Available online: https://en.wikipedia.org/wiki/Sp1_transcription_factor#/media/File:Protein_SP1_PDB_1sp1.png (accessed on 12 October 2022.).
- Dasgupta, S.; Srinidhi, S.; Vishwanatha, J.K. Oncogenic activation in prostate cancer progression and metastasis: Molecular insights and future challenges. J. Carcinog. 2012, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Strom, P.; Nordstrom, T.; Aly, M.; Egevad, L.; Gronberg, H.; Eklund, M. The Stockholm-3 Model for Prostate Cancer Detection: Algorithm Update, Biomarker Contribution, and Reflex Test Potential. Eur. Urol. 2018, 74, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Karunasinghe, N.; Minas, T.Z.; Bao, B.Y.; Lee, A.; Wang, A.; Zhu, S.; Masters, J.; Goudie, M.; Huang, S.-P.; Jenkins, F.J.; et al. Assessment of factors associated with PSA level in prostate cancer cases and controls from three geographical regions. Sci. Rep. 2022, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Shames, D.M.; Woodhouse, L.R. Zinc homeostasis in humans. J. Nutr. 2000, 130 (Suppl. S5), 1360S–1366S. [Google Scholar] [CrossRef]
- Cagliari, M.; Bressi, B.; Bassi, M.C.; Fugazzaro, S.; Prati, G.; Iotti, C.; Costi, S. Feasibility and Safety of Physical Exercise to Preserve Bone Health in Men With Prostate Cancer Receiving Androgen Deprivation Therapy: A Systematic Review. Phys. Ther. 2022, 102, pzab288. [Google Scholar] [CrossRef]
- Jones, J.M.; Tsang, D.S.; Zheng, S.; Yeheskel, A.; Catton, C.N.; Cheung, A.M.; Hamilton, R.; Alibhai, S.M.H. Implementing and Evaluating the Impact of BoneRx: A Healthy Bone Prescription for Men with Prostate Cancer Initiating Androgen Deprivation Therapy. J. Clin. Med. 2022, 11, 2703. [Google Scholar] [CrossRef]
- Wang, A.; Obertova, Z.; Brown, C.; Karunasinghe, N.; Bishop, K.; Ferguson, L.; Lawrenson, R. Risk of fracture in men with prostate cancer on androgen deprivation therapy: A population-based cohort study in New Zealand. BMC Cancer 2015, 15, 837. [Google Scholar] [CrossRef]
- Shao, Y.H.; Moore, D.F.; Shih, W.; Lin, Y.; Jang, T.L.; Lu-Yao, G.L. Fracture after androgen deprivation therapy among men with a high baseline risk of skeletal complications. BJU Int. 2013, 111, 745–752. [Google Scholar] [CrossRef]
- Fukunaka, A.; Fujitani, Y. Role of Zinc Homeostasis in the Pathogenesis of Diabetes and Obesity. Int. J. Mol. Sci. 2018, 19, 476. [Google Scholar] [CrossRef]
- Ndovi, T.T.; Parsons, T.; Choi, L.; Caffo, B.; Rohde, C.; Hendrix, C.W. A new method to estimate quantitatively seminal vesicle and prostate gland contributions to ejaculate. Br. J. Clin. Pharmacol. 2007, 63, 404–420. [Google Scholar] [CrossRef]
- Rider, J.R.; Wilson, K.M.; Sinnott, J.A.; Kelly, R.S.; Mucci, L.A.; Giovannucci, E.L. Ejaculation Frequency and Risk of Prostate Cancer: Updated Results with an Additional Decade of Follow-up. Eur. Urol. 2016, 70, 974–982. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karunasinghe, N. Zinc in Prostate Health and Disease: A Mini Review. Biomedicines 2022, 10, 3206. https://doi.org/10.3390/biomedicines10123206
Karunasinghe N. Zinc in Prostate Health and Disease: A Mini Review. Biomedicines. 2022; 10(12):3206. https://doi.org/10.3390/biomedicines10123206
Chicago/Turabian StyleKarunasinghe, Nishi. 2022. "Zinc in Prostate Health and Disease: A Mini Review" Biomedicines 10, no. 12: 3206. https://doi.org/10.3390/biomedicines10123206
APA StyleKarunasinghe, N. (2022). Zinc in Prostate Health and Disease: A Mini Review. Biomedicines, 10(12), 3206. https://doi.org/10.3390/biomedicines10123206





