Abstract
Heme is a member of the porphyrins family of cyclic tetrapyrroles and influences various cell processes and signalling pathways. Enzyme deficiencies in the heme biosynthetic pathway provoke rare human inherited metabolic diseases called porphyrias. Protein levels and activity of enzymes involved in the heme biosynthetic pathway and especially 5′-Aminolevulinate Synthase 1 are featured by 24-h rhythmic oscillations driven by the biological clock. Heme biosynthesis and circadian pathways intermingle with mutual modulatory roles. Notably, heme is a ligand of important cogs of the molecular clockwork, which upon heme binding recruit co-repressors and inhibit the transcription of numerous genes enriching metabolic pathways and encoding functional proteins bringing on crucial cell processes. Herein, we assessed mRNA levels of circadian genes in patients suffering from porphyrias and found several modifications of core clock genes and clock-controlled genes expression, associated with metabolic and electrolytic changes. Overall, our results show an altered expression of circadian genes accompanying heme biosynthesis disorders and confirm the need to deepen the knowledge of the mechanisms through which the alteration of the circadian clock circuitry could take part in determining signs and symptoms of porphyria patients and then again could represent a target for innovative therapeutic strategies.
1. Introduction
Deficiency of enzymes of the heme biosynthetic pathway causes a category of human metabolic diseases called porphyrias, characterized by different modes of inheritance and clinical manifestations, with signs and symptoms manifesting in different organ systems [1,2,3]. Porphyrias are associated with build-up and undue excretion of heme pathway intermediates, such as the heme precursors 5-aminolevulinic acid (ALA) and porphobilinogen (PBG) and their oxidized products, such as porphirins [4,5]. They are classified in acute porphyrias (affecting nervous system and skin) and cutaneous porphyrias (affecting mainly the skin) [6]. Acute porphyrias comprise acute intermittent porphyria, variegate porphyria, hereditary coproporphyria, and delta-aminolevulinic acid dehydratase deficiency porphyria [6]. Cutaneous porphyrias include porphyria cutanea tarda, erythropoietic protoporphyria, X-linked protoporphyria, congenital erythropoietic porphyria, and hepatoerythropoietic porphyria [6]. Acute intermittent porphyria (AIP) is inherited in an autosomal dominant manner and mutations cause partial deficiency of PBG deaminase, the third enzyme of the heme biosynthetic pathway performing the polymerization of the monopyrrole PBG into hydroxymethylbilane. ALA and PBG accumulate during overt crises, which manifests with neuro-visceral symptoms intensified by some drugs, hormones, and nutritional changes. AIP treatment relies on intravenous injection of ferric chloride salt of heme (hemin) or carbohydrate loading for mild attacks or when hemin is unobtainable [7,8]. In general, porphyrias are inherited diseases, while porphyria cutanea is caused by an acquired enzymatic deficiency in liver, although an inherited deficiency is a predisposing factor in some cases (Yasuda 2018) [9]. ALAS1 (5′-Aminolevulinate Synthase 1), the rate limiting enzyme in heme biosynthesis, oscillates with circadian rhythmicity driven by the biological clock [10,11,12,13]. Biological processes show variations in the temporal dimension that may be sporadic or recurrent and, in this case, may be rhythmic or arrhythmic. Periodic variations recurring with a frequency of one cycle in approximately 24 h are defined as circadian. Circadian rhythmicity characterizes the function of body systems that allow homeostasis maintenance, and among these, the metabolic pathways are preeminent. Derangement of customary circadian rhythmicity underlies pathophysiological mechanisms in several metabolic [14,15,16,17,18,19,20,21,22]. Circadian rhythms are endogenous cycles driven by molecular clockworks ticking in mammals through transcriptional/translational feedback loops operated by a positive limb, working through the transcriptional activators ARNTL/ARTL2 and CLOCK (or NPAS2) which are complex and activate a negative limb, with Period (PER1, PER2, PER3) and Cryptochrome (CRY1, CRY2) proteins that in turn inhibit the transactivation complex and close the loop [23,24]. The circadian proteins must be post-translationally modified, mainly phosphorylated by caseinkinases, and tagged for proteasomal degradation or acetylated/deacetylated by sirtuins to allow unrelenting and properly self-sustained clock functioning [25,26]. The ARNTL/ARNTL2-CLOCK complex activates also the expression of the nuclear receptors RORs (α,β,γ) and REV-ERBs (α,β), which drive the expression of ARNTL. Besides, the ARNTL/ARTL2-CLOCK (or NPAS2) complex also activates first order clock-controlled genes (DBP, HLF, TEF, NFIL3), driving the expression of thousands of genes involved in metabolic pathways and crucial cell processes [27].
Heme modulates numerous cell processes related to oxygen sensing/transport, electron transfer-mediated biochemical reactions, and signalling pathways [28]. Remarkably, heme is a ligand of REV-ERBs, which upon heme binding recruit the co-repressor NCoR with REV-ERB-NCoR complex stabilization, inhibit the transcription of target genes involved in glucose and lipid metabolism, and modulate ARNTL expression as well as the expression of ARNTL-driven circadian genes and their encoded proteins [29]. Besides, heme works as a regulatory ligand in humans through a heme-regulatory SC(841)PA motif within the C terminus of Per2, and, interacting with its stabilizing counterpart cryptochrome, reduces Per2 stability binding solely to ferric heme and consequently performing as a redox sensor capable to modulate circadian gene expression and period length [30].
On these premises, we aimed to evaluate the expression of core clock genes and clock-controlled genes in humans affected by disorders due to enzymatic deficiency/dysfunction in the pathway of heme biosynthesis.
2. Materials and Methods
2.1. Patients
In the present study, thirty-four patients with different forms of porphyria were enrolled: twenty-one patients affected by acute intermittent porphyria (AIP), seven patients affected by hereditary coproporphyria (HCP), four patients affected by porphyria cutanea tarda (PCT), and two patients affected by congenital erythropoietic porphyria (CEP). Some of them (seventeen) presented clinical symptoms and alterations of biochemical analysis, and for some of them, diagnosis was confirmed by genetic analysis, while seventeen asymptomatic family members were enrolled for genetic testing. In addition, fifteen healthy blood donors were enrolled as controls, as they are considered appropriate candidates for studies of genetic respect to a random sample of the population [31].
Several laboratory tests are available for diagnosis of porphyria [32]. In the present study, the laboratory diagnostic procedures were performed using two important sequential steps: biochemical analysis and molecular analysis.
2.2. Biochemical Analysis
The first step for establishing the diagnosis of porphyria comprises the following biochemical analyses: plasma porphyrin scan, ALA and PBG determination, measurement of urine porphyrins, and porphobilinogen deaminase (PBGD) enzyme activity measurement. The fluorometric emission scanning (using excitation at 405 nm) of plasma samples, simply diluted five-fold in phosphate-buffered saline, allows the differentiation of three conditions according to their porphyrin content. The emission maximum at 626–628 nm is a specific finding in variegate porphyria, while in erythropoietic protoporphyria a characteristic peak is found at 636 nm. A fluorescence emission maximum at 618–622 nm corresponds to a third group that includes normal subjects, non-porphyria patients and patients suffering from acute intermittent porphyria, hereditary coproporphyria, congenital erythropoietic porphyria, and porphyria cutanea tarda [33].
ALA and PBG are commonly quantified after purification, from a spot urine sample, using the commercially available anion-exchange and cation-exchange columns for photometric determination of urinary 5-ALA and PBG (ClinEasy® Complete Kit for ALA/PBG in Urine, Recipe GmbH, Munich, Germany). This test is revealing for AIP diagnosis. The measurement of urine porphyrins was performed using commercially available anion-exchange and cation-exchange columns for photometric determination of urinary porphyrins (ClinEasy® Complete Kit for Total Porphyrins in Urine, Recipe GmbH, Munich, Germany). Although various methods have been developed for the analysis of porphyrins, reverse-phase high-pressure liquid chromatography (HPLC) coupled with fluorescence detection has become the gold standard method in this regard to determine URO, HEPTA, HEXA, PENTA, and COPRO I and III concentrations in urine. The complete separation of these heme synthesis intermediates is important for diagnosing certain types of porphyrias. The PBGD enzyme activity measurement is an enzymatic assay used for suspected diagnosis AIP and in our laboratory is performed for the biochemical confirmation of this form of porphyria. The measurement of PBGD activity is based on the measurement of the rate of synthesis of uroporphyrin from PBG in incubated, lysed erythrocytes (PBGDW Porphobilinogen Deaminase, Washed Erythrocytes, Mayo Clinic Laboratories, Rochester, MN, USA).
2.3. Molecular Analyses
According to clinical symptoms and the routine biochemical procedure, the diagnosis of one of the different forms of porphyria is subsequently confirmed by the sequence analysis of one of the responsible genes for each type. On the basis of diagnostic suspicion, the propositus and successively the first-degree relatives were analyzed. In particular, the HMBS gene was analyzed in twenty-one patients suspected for AIP, seven patients with CPO were analyzed for CPOX gene mutations, four CTP patients were screened for UROD mutations, and the gene UROS was sequenced in two propositus with both clinical and biochemical evidence of PEC. For each gene, all the coding regions and exon/intron boundaries were analyzed using direct sequencing by Sanger method. A sequence analysis of HMBS (NM_000190.3), CPOX (NM_000097.5), UROD (NM_000374.4) and UROS (NM_000375.2) genes allowed to determinate three previously described variant and eight novel variations.
2.4. HMBS Variants
In twenty-one AIP patients enrolled in this study, a five-point mutation in HMBS gene was identified in twelve propositus and family members. No variation was found in the remaining nine propositus.
A well-represented guanine deletion at position 181 in exon 5 of HMBS gene, causative of premature truncation of the protein (96 aminoacids instead of 361 aminoacids) was identified in a symptomatic woman. Two splicing variations in HMBS gene were identified: c.652-2delA (IVS12-2delA) and c.772-3C > G (IVS 13-3C > G). A previously described deletion of an adenine in position -2 of acceptor site of exon 12 was detected in a propositus and in his two siblings and in another unrelated proband. In the acceptor site of exon 13 a cytosine to guanine substitution c.772-3C > G was detected in a woman with severe symptoms of porphyria.
In the family of AIP 09-018 a previously described missense variation (c.580C > T) was detected in the propositus and in three asymptomatic family members.
The two brothers enrolled in this study, with only one of them symptomatic, presented a novel six-nucleotide TACCCG deletion in exon 3 of HMBS gene at positions 72 and 77 of the cDNA (numbered from the translation initiation codon ATG), that results in a deletion of two amino acids in the protein which causes the p.Thr25_Arg26del, inherited from the father (not enrolled in this study). The same mutation was not found in healthy unrelated individuals. Threonine-Arginine at position 25–26 is highly conserved among species, and the p. Thr25_Arg26del was predictive of a significant conformational change.
2.5. CPOX Variants
Three missense substitutions in CPOX gene were identified in three hereditary porphyrias propositus. A guanine to timine substitution was detected at position 613 in exon 2 leading to the amino acid substitution p.205V > L in two symptomatic brothers and in their asymptomatic sister and mother. The c.395C > T transition in exon 1 of CPOX gene was responsible for the p.132A > V substitution in a twenty-year-old proband. This variation was maternally inherited from the maternal grandmother. A c.148C > T transition causative for p.50P > S was detected in only symptomatic women. It is difficult to determine, in the absence of functional data, whether p.205V > L, p.132A > V and p.50P > S represent pathogenic mutations. However, the three missense mutations affect residues that are highly conserved across species and were absent in 100 control chromosomes, therefore these substitutions likely determine a conformational change affecting the signaling pathway.
2.6. UROD Variant
In UROD gene, two novel variants are described: an adenine to guanine substitution at position 1000 and a deletion of three nucleotide in exon 4. In exon 10 of UROD gene, a missense variation causative of p.I334V was found in three asymptomatic siblings of a patient. Three nucleotide deletions found in exon 4 of UROD gene, c. 246-248delCAT, is causative of deletion of isoleucine at position 82 of the protein.
Finally, a diagnosis of PEC, a rare and severe recessively transmitted porphyria, was confirmed in two Pakistani brothers by identification of a splicing site variation c.660+4delA in donor splice site of exon 9. The deletion at homozygous status was inherited from consanguineous parents. The characterization of splicing by reverse transcription and sequencing allowed to demonstrate the deletion of 99 nucleotides corresponding to UROS gene exon 9 removing during mRNA transcription.
2.7. Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction
We used snapshot mRNA extracted from peripheral blood mononuclear cells (PBMC) sampled at the same time-of-day (09:00 a.m.). Total RNA from patients and controls was extracted using the RNeasy® Mini Kit (QIAGEN) and subsequently digested by DNase I. cDNA was synthesized from 100 ng total RNA with Quantifast RT-PCR kit (QIAGEN). For real-time PCR, we used the Human QuantiTec Primers Assay (SYBR Green QuantiTect Primers Assay; QIAGEN) (Supplementary Table S1). All qPCRs were performed in a 10 μL final volume, with three replicates per sample. Reactions were set up in 96-well plates using a 7900HT Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Expression levels of the target genes were normalized using the housekeeping control gene GAPDH. The mRNA amount of each target gene relative to control gene was calculated through the comparative Ct method (i.e., the 2(−ΔΔCt) method).
2.8. Statistical Analysis
Porphyrias are rare diseases (defined as a condition that affects less than 1 in 2000 people in the European Union or less than 200.000 people in the USA) [34], hence, we planned to enroll and test a small sample size [35]. Sample size estimates for hypothesis testing based on achieving statistical power = 0.8 with effect size = 0.8, β = 0.2 and α = 0.05 projected as a minimum 15 subjects per group [36]. To upkeep statistical power and numerosity, we unified patients with CEP and PCT in one group to confront AIP patients, HCP patients, and control subjects. We report categorical variables as frequencies and percentages, and continuous variables as median and interquartile ranges. Circadian gene expression levels were calculated using the 2-ΔΔCt formula and reported as median, 25th percentile (Q1) and 75th percentile (Q3). For patients with multiple evaluations of clinical/biochemical data at different acquisition timings, we considered arithmetic means of the reported values for statistical evaluation. For our analyses, we used nonparametric statistics. Differences between groups for categorical variables were tested with the Fisher exact test and Cochran’s Q test. Hypotheses regarding differences among two groups for continuous variables were tested by means of the Mann–Whitney rank sum test; we used the Kruskal–Wallis one-way analysis of variance to test differences among multiple groups for continuous variables. For the evaluation of the relationships between circadian gene expression levels and clinical/biochemical variables of patients affected by porphyrias, we calculated Spearman’s rank correlation coefficients (r) considering the gene expression level IQRs of controls as reference values, and we tested differences between patients affected by porphyrias with gene expression levels equal to or higher than the 75th percentile (over-expression) and patients affected by porphyrias with gene expression levels equal to or lower than the 25th percentile (down-regulation). Correlations among circadian gene expression levels were evaluated calculating Spearman’s rank correlation coefficients (r).
We performed a retrospective study and some clinical and laboratory data could be lost. We reported data and a univariate analysis for all the variables included in the study. List-wise deletion of missing values was performed. We did not test differences between variables that had the number of tested cases inferior to 60% of the total cases. For all the analyses, a p-value below 0.05 was considered statistically significant. All the statistical analyses were performed using MedCalc statistical software (MedCalc Software, Acacialaan 22, Ostend, 8400, Belgium).
3. Results
Of the 34 patients affected by porphyrias and consecutively enrolled in our study, 14 (41%) were males, and the median age was 46 (IQR 29–54) years. Table 1 shows characteristics and clinical/biochemical features and Table 2 shows gene and protein mutations found in the patients affected by porphyrias enrolled in our study. Most patients had values in the normal range for the tested variables, with the predictable exception of blood, urinary, and fecal porphyrins and metabolites, which resulted as elevated in almost all the tested patients. Only one patient had a positive urine culture for Escherichia coli; another one was positive for S antigen of Hepatitis B and one for Hepatitis C antibodies. One patient was obese and three were overweight, and five had hypercholesterolemia. One patient showed subclinical Hashimoto thyroiditis. Three patients showed elevated transaminases values. Three patients were anemic and five had low ferritin levels; two had a low platelets count. Four patients showed a mild degree of hyperparathyroidism.

Table 1.
Laboratory characteristics of patients affected by porphyrias.

Table 2.
Genes and proteins mutations found in the enrolled patients affected by porphyrias.
Differences of circadian genes expression tested with the Kruskal–Wallis one-way analysis of variance were detailed in Table 3 and distributions are shown in Figure 1 and Figure 2. With the exception of ARNTL and TEF, all genes appeared significantly over-expressed in patients affected by all the tested types of porphyrias (both acute and cutaneous forms) with respect to controls. In addition, DBP and NFIL3 showed a significantly higher expression in patients affected by CEP and PCT with respect to AIP. No gene appeared down-regulated with respect to the controls. As shown in Table 4, ARNTL, CLOCK, CRY1, CRY2, CSNK1E, DBP NFIL3, NR1D1, PER2, and SIRT1 showed significantly lower expression in symptomatic patients. No significant difference was found for ARNTL2, HLF, PER1, PER3, RORA, TEF, and TIMELESS. Considering the control subjects, ARNTL (p = 0.57), CLOCK (p = 0.06), NFIL3 (p = 0.06), and PER2 (p = 0.128) showed no statistical difference in expression between symptomatic patients and controls, while CRY1 (p < 0.001), CRY2 (p = 0.011), CSNK1E (p = 0.029), DBP (p = 0.003), NR1D1 (p = 0.027), and SIRT1 (p = 0.001) showed a significantly higher expression in symptomatic patients in respect to the controls.

Table 3.
Circadian genes expression in controls and patients affected by porphyrias and differences between the groups.
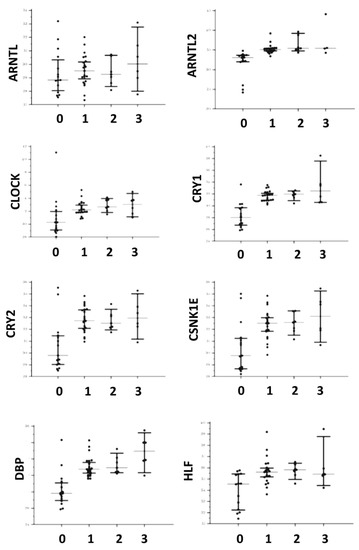
Figure 1.
Distributions of gene expression in both controls and affected patients for ARNTL, ARNTL2, CLOCK, CRY1, CRY2, CSNK1E, DBP, and HLF. The central line corresponds to the median, upper, and lower bars to the IQR. For the X axis label, 0 corresponds to controls, 1 to AIP, 2 to HCP, and 3 to CEP and PCT.
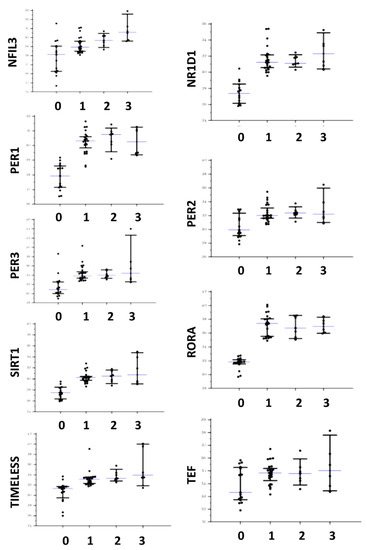
Figure 2.
Distributions of gene expression in both controls and affected patients for NFIL3, NR1D1, PER1, PER2, PER3, RORA, SIRT1, TEF, and TIMELESS. The central line corresponds to the median, upper, and lower bars to the IQR. For the X axis label, 0 corresponds to controls, 1 to AIP, 2 to HCP, and 3 to CEP and PCT.

Table 4.
Differences in circadian genes expression between patients with signs and symptoms of porphyrias and asymptomatic porphyria patients.
Over-expression (over the 75th percentile of controls) of circadian genes was associated with many significant differences in some clinical and biochemical variables, even if they lingered in their normal ranges. All the significant differences were detailed in Table 5. No gene appeared down-regulated, so we did not test the differences between patients with normal and reduced (under the 25th percentile) expression.

Table 5.
Laboratory characteristics found significantly different between porphyria patients with normal expression and porphyria patients with over-expression of circadian genes.
Lower levels of total amylases were found for patients with over-expression of ARTNL, ARTNL2, CLOCK, and CRY2, while lower levels of lipases were found associated with over-expression of CLOCK, CRY1, PER1, PER2, SIRT1, and TEF. ARNTL over-expression was associated also with lower levels of CRP. Some differences in hepatic function markers were found, as higher values of INR were found associated with the over-expression of ARTNL2 and CRY2, higher values of AST with ARTNL2, higher levels of total bilirubin with CLOCK and CSNK1E, higher values of phosphatases with CSNK1E, and higher levels of gamma glutamyl transpeptidase (GGT) were associated with higher expression of PER1. Lower iron blood levels were found in patients with a higher expression of ARNTL2, while higher iron blood levels were found in patients with over-expression of CSNK1E, and higher ferritin values were found in patients with an over-expression of CLOCK.
Higher values of alpha-fetoprotein correlated with over-expression of CLOCK and CRY1; lower values of CA 19-9 were found in patients with a higher expression of CLOCK, CRY1 and PER2. Higher values of CA 125 were associated with a higher expression of CRY1 and higher values of CA 15.3 were associated with PER1 over-expression. Lower values of PBG were found in patients with an over-expression of CLOCK and higher values of total porphyrins were found associated with an over-expression of TIMELESS. Higher values of uric acid correlated with over-expression of CRY2, PER2, and TEF. Lower values of chloride were associated with a higher expression of TIMELESS and CSNK1E (associated also with lower values of sodium), while reduced calcium levels were associated with over-expression of PER2. Higher expression of PER1 was associated with no production of anti-thyroid peroxidase (anti-TPO) antibodies. Lower values of urine-specific weight were associated with over-expression of PER2 and TIMELESS, while PER3, SIRT1 and TEF showed an association with higher urine pH values. TIMELESS was also associated with lower levels of urea and parathyroid hormone. Over-expression of DBP, HLF, NFIL3, and NDRD1 was not associated with significant differences in all the tested clinical/biochemical variables. RORA was not tested as all the patients showed an over-expression of this gene. The analysis of the correlation matrices (Figure 3) highlights different profiles when comparing the controls with the global group of patients and even more if only symptomatic patients are considered. In particular, the levels of expression of RORA and TIMELESS show very different correlation patterns compared to the groups of patients, considered globally or only if symptomatic.
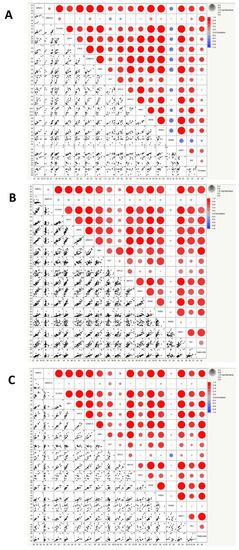
Figure 3.
Correlation matrices showing correlation values between the expression of circadian genes in controls (A), grouped patients affected by porphyrias (B), symptomatic patients affected by porphyrias (C). The heat maps show the correlation coefficients, which measure the degree of the linear relationship between each pair of variables. The correlation values can fall between −1 and +1. Positive correlation is measured on a 0.1 to 1.0 scale. The range from 0.1 to 0.3 indicates weak positive correlation, the range from 0.3 to 0.5 indicates a moderate positive correlation, and the range from 0.5 to 1.0 indicates a strong positive correlation. Bubble size represents statistical significance expressed as −Log “p-value” (log-rank test), where the larger the size, the greater the significance.
4. Discussion
Heme is a member of the porphyrins family of cyclic tetrapyrroles, comprising macrocycles composed of four pyrrole-derived rings hinged by methine bridges and undertaking vital roles in various bio-systems. All hemes comprise a central iron ion, which is combined by the four pyrrole nitrogen atoms [37]. In hemoproteins such as hemoglobin and myoglobin, heme is the prosthetic group essential for oxygen transport and storage and is needed in various cytochromes for electron transport and for mixed function oxidases in cytochrome P450. Furthermore, heme is a cofactor of peroxidase for hydrogen peroxide production and of catalase for hydrogen peroxide decomposition. Heme also plays a regulatory role in the sensing of diatomic gases and signal transduction, gene transcription/translation, microRNA processing, protein stability, mitochondrial protein import, metabolic pathways, drug detoxification, and functioning of the molecular clockwork [38]. Defects of heme synthesis can cause various disorders for instance anemia and porphyrias [37]. The levels and activity of enzymes involved in the heme biosynthetic pathway and in particular ALAS1 (5′-Aminolevulinate Synthase 1) show 24-h cyclic fluctuations driven by the molecular clockwork [10]. In turn, heme interacts with REV-ERBs and modulates transcriptional processes in the TTFL, mainly with inhibitory effects on the expression of circadian genes through NCoR-HDAC3 corepressor complex recruitment [29]. Moreover, by means of Rev-erbα–mediated repression of metabolic genes transcription, heme restrains the expression of genes managing gluconeogenesis and glucose output in the liver, so that Rev-erbα works as a heme sensor and harmonizes the biological clock ticking with glucose homeostasis, and energy metabolism [39,40]. Interestingly, in all patients affected by both acute and cutaneous forms of porphyria, heme biosynthesis failure was associated with increased expression levels of the considered circadian genes when compared with the controls, except for ARNTL and TEF. In the patients affected by CEP and PCT the expression levels of DBP and NFIL3 were significantly higher with regard to the patients affected by AIP, while significantly lower expression levels of ARNTL, CLOCK, CRY1, CRY2, CSNK1E, DBP NFIL3, NR1D1, PER2, and SIRT1 were found in symptomatic patients when compared to asymptomatic patients. In the whole group of patients, lower values of PBG were found to be associated with high expression levels of CLOCK and higher values of total porphyrins were found to be associated with high expression levels of TIMELESS. Heme impacts the molecular clockwork binding to different circadian proteins. In particular, heme directly interacts with CLOCK protein in the nucleus of human cells binding to the PAS-A and PAS-B domains, with Histidine residue at position 144 as a ligand, and disrupts CLOCK interactions with the E-boxes in the promoters of target genes, as evidenced by DNA binding assays. Flexibility in the heme pocket scaffolds an additional Histidine and Cystidine coordination and this conformationally mobile CLOCK protein structural framework brings about heme-dependent transcriptional regulation [41].
Regarding blood electrolytes balance, lower calcium values were found to be associated with high expression levels of PER2, lower values of chloride and sodium were found to be associated with high expression levels of CSNK1E, and lower values of chloride were found to be associated with high expression levels of TIMELESS. A different pattern of correlation among circadian gene expression levels was found for RORA and TIMELESS expression levels when confronting the controls with the global group of patients and the differences were even more evident when considering exclusively the symptomatic patients. TIMELESS is encompassed in the molecular clockwork and plays a role in embryonic development, cell cycle progression, DNA damage response and cooperates in the replication fork protection complex safeguarding fork integrity and genome stability during DNA replication [42].
Our results are partially in agreement with a study performed in six asymptomatic Caucasian postmenopausal women suffering from AIP with and without biochemical activity and four sex-matched controls. The serum levels of cortisol, melatonin, ALA, and PBG were evaluated at 3-h intervals for 21 h and mRNA levels of the circadian genes CRY1, PER2, NR1D1, and genes involved in heme synthesis ALAS1, ALAS2, and PBGD were evaluated in peripheral blood mononuclear cells at 6-h intervals for 24 h. Interestingly, in AIP patients with biochemical activity, the CRY1 expression level was increased and was accompanied by lower serum levels of cortisol with dampened early morning increase and lack of 24-h rhythmicity, suggesting a significant alteration of the circadian clock circuitry [43].
5. Conclusions
Overall, the results of our study highlight a global alteration of the expression of circadian genes in the presence of deficits of enzymes involved in the heme biosynthetic pathway. Heme deficiency has direct implications in the pathophysiological characteristics of porphyrias, determining the semeiological and symptomatological picture detectable in the patients suffering from porphyrias. On the other hand, the altered expression of circadian genes involves a widespread modification of metabolic pathways and electrolyte balance that could play a key role in determining the clinical picture found in patients suffering from these rare diseases. In our study, we performed a preliminary investigation to confirm alterations of circadian genes expression in the context of heme synthesis disorders. We analyzed snapshot samples of mRNA extracted from PBMC collected at the same time-of-day, so that the results regarding differential expression of circadian genes are consistent, but we have no time-series data on behavioral patterns or other physiological parameters. Consequently, we cannot make any statement regarding alterations of circadian rhythmicity in patients suffering from porphyrias. We could only hypothesize changes of rhythmic patterns in line with altered clock genes mRNA levels, but this issue could be appropriately addressed in future studies conducted on normal subjects and porphyria patients in a controlled environment and with specific parameters (i.e., temperature, light, nutrition, rest/activity, and segregation). An in-depth knowledge of the mechanisms underlying the altered expression of circadian genes in heme synthesis disorders and a better definition of the pathophysiological alterations consequent to the derangement of the circadian clock circuitry in this context could shed light on important signaling pathways and could represent, in the near future, a therapeutic target in the hope of increasing the effectiveness of pharmacological treatments and improving the quality of life of patients suffering from porphyrias.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines10123198/s1. Table S1: List of human QuantiTec Primers used for RT-PCR.
Author Contributions
G.M. (Gianluigi Mazzoccoli), M.S. and C.C.G. conceived the study; G.M. (Gianluigi Mazzoccoli), R.T. and F.C. performed statistical and bioinformatics analysis; M.S., C.C.G., G.M. (Giuseppe Merla), M.N., A.F.S. and B.A. enrolled the patients, performed in vitro experiments, and acquired data; G.M. (Gianluigi Mazzoccoli), M.S., E.M., R.T., F.C., S.D.C., F.A. and C.C.G. analyzed data; G.M. (Gianluigi Mazzoccoli), M.S. and C.C.G. wrote the manuscript. All authors have read and approved the journal’s authorship agreement. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the “5x1000” voluntary contribution and by a grant to G.M. (Gianluigi Mazzoccoli) from the Italian Ministry of Health (RC2022-2024).
Institutional Review Board Statement
The study was approved by the local Ethical Committee (Mo/CSS/C.I.TestGen, 10/01/2020, Lab.Immunogenetics).
Informed Consent Statement
All the enrolled subjects gave their written informed consent to participate.
Data Availability Statement
Data will be made available on request.
Acknowledgments
We thank Francesco Mazzarelli and Annalucia Carbone for technical support. We thank all the porphyria patients who voluntarily participated in the study.
Conflicts of Interest
The authors declare that there are no conflicts of interest with respect to the authorship and/or publication of this article.
References
- Puy, H.; Gouya, L.; Deybach, J.-C. Porphyrias. Lancet 2010, 375, 924–937. [Google Scholar] [CrossRef]
- Bissell, D.M.; Anderson, K.E.; Bonkovsky, H.L. Porphyria. N. Engl. J. Med. 2017, 377, 862–872. [Google Scholar] [CrossRef]
- de Souza, P.V.S.; Badia, B.D.M.L.; Farias, I.B.; Pinto, W.B.V.D.R.; Oliveira, A.S.B. Acute Hepatic Porphyria: Pathophysiological Basis of Neuromuscular Manifestations. Front. Neurosci. 2021, 15, 715523. [Google Scholar] [CrossRef]
- Szlendak, U.; Bykowska, K.; Lipniacka, A. Clinical, Biochemical and Molecular Characteristics of the Main Types of Porphyria. Adv. Clin. Exp. Med. 2016, 25, 361–368. [Google Scholar] [CrossRef]
- Phillips, J.D. Heme biosynthesis and the porphyrias. Mol. Genet. Metab. 2019, 128, 164–177. [Google Scholar] [CrossRef]
- Stein, P.E.; Badminton, M.N.; Rees, D.C. Update review of the acute porphyrias. Br. J. Haematol. 2016, 176, 527–538. [Google Scholar] [CrossRef]
- Bustad, H.; Kallio, J.; Vorland, M.; Fiorentino, V.; Sandberg, S.; Schmitt, C.; Aarsand, A.; Martinez, A. Acute Intermittent Porphyria: An Overview of Therapy Developments and Future Perspectives Focusing on Stabilisation of HMBS and Proteostasis Regulators. Int. J. Mol. Sci. 2021, 22, 675. [Google Scholar] [CrossRef]
- Wylie, K.; Testai, F.D. Neurological Manifestations of Acute Porphyrias. Curr. Neurol. Neurosci. Rep. 2022, 22, 355–362. [Google Scholar] [CrossRef]
- Yasuda, M.; Chen, B.; Desnick, R.J. Recent advances on porphyria genetics: Inheritance, penetrance & molecular heterogeneity, including new modifying/causative genes. Mol. Genet. Metab. 2018, 128, 320–331. [Google Scholar] [CrossRef]
- Chen, H.; Isayama, K.; Kumazawa, M.; Zhao, L.; Yamauchi, N.; Shigeyoshi, Y.; Hashimoto, S.; Hattori, M.-A. Integration of the nuclear receptor REV-ERBα linked with circadian oscillators in the expressions ofAlas1, Ppargc1a, andIl6genes in rat granulosa cells. Chronobiol.-Int. 2015, 32, 739–749. [Google Scholar] [CrossRef]
- Zheng, B.; Albrecht, U.; Kaasik, K.; Sage, M.; Lu, W.; Vaishnav, S.; Li, Q.; Sun, Z.S.; Eichele, G.; Bradley, A.; et al. Nonredundant Roles of the mPer1 and mPer2 Genes in the Mammalian Circadian Clock. Cell 2001, 105, 683–694. [Google Scholar] [CrossRef]
- Kaasik, K.; Lee, C.C. Reciprocal regulation of haem biosynthesis and the circadian clock in mammals. Nature 2004, 430, 467–471. [Google Scholar] [CrossRef]
- Rogers, P.M.; Ying, L.; Burris, T.P. Relationship between circadian oscillations of Rev-erbα expression and intracellular levels of its ligand, heme. Biochem. Biophys. Res. Commun. 2008, 368, 955–958. [Google Scholar] [CrossRef]
- Albrecht, U. Timing to Perfection: The Biology of Central and Peripheral Circadian Clocks. Neuron 2012, 74, 246–260. [Google Scholar] [CrossRef]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef]
- Maywood, E.S.; O’Neill, J.S.; Reddy, A.B.; Chesham, J.E.; Prosser, H.M.; Kyriacou, C.P.; Godinho, S.I.H.; Nolan, P.M.; Hastings, M.H. Genetic and Molecular Analysis of the Central and Peripheral Circadian Clockwork of Mice. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 85–94. [Google Scholar] [CrossRef]
- Bass, J.; Takahashi, J.S. Circadian Integration of Metabolism and Energetics. Science 2010, 330, 1349–1354. [Google Scholar] [CrossRef]
- Partch, C.L.; Green, C.B.; Takahashi, J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2013, 24, 90–99. [Google Scholar] [CrossRef]
- Hastings, M.H.; Reddy, A.B.; Maywood, E.S. A clockwork web: Circadian timing in brain and periphery, in health and disease. Nat. Rev. Neurosci. 2003, 4, 649–661. [Google Scholar] [CrossRef]
- Reddy, A.B. Genome-Wide Analyses of Circadian Systems; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2013; Volume 217, pp. 379–388. [Google Scholar] [CrossRef]
- Reddy, A.B.; O’Neill, J. Healthy clocks, healthy body, healthy mind. Trends Cell Biol. 2010, 20, 36–44. [Google Scholar] [CrossRef]
- Vinciguerra, M.; Tevy, M.F.; Mazzoccoli, G. A ticking clock links metabolic pathways and organ systems function in health and disease. Clin. Exp. Med. 2013, 14, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2016, 18, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.H.; Takahashi, J.S. Circadian clock genes and the transcriptional architecture of the clock mechanism. J. Mol. Endocrinol. 2019, 63, R93–R102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Fitzpatrick, K.; Olker, C.; Vitaterna, M.H.; Turek, F.W. Casein kinase 1 epsilon and circadian misalignment impact affective behaviours in mice. Eur. J. Neurosci. 2021, 55, 2939–2954. [Google Scholar] [CrossRef] [PubMed]
- Bellet, M.M.; Orozco-Solis, R.; Sahar, S.; Eckel-Mahan, K.; Sassone-Corsi, P. The Time of Metabolism: NAD+, SIRT1, and the Circadian Clock. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 31–38. [Google Scholar] [CrossRef]
- Asher, G.; Schibler, U. Crosstalk between Components of Circadian and Metabolic Cycles in Mammals. Cell Metab. 2011, 13, 125–137. [Google Scholar] [CrossRef]
- Mense, S.M.; Zhang, L. Heme: A versatile signaling molecule controlling the activities of diverse regulators ranging from transcription factors to MAP kinases. Cell Res. 2006, 16, 681–692. [Google Scholar] [CrossRef]
- Raghuram, S.; Stayrook, K.R.; Huang, P.; Rogers, P.M.; Nosie, A.K.; McClure, D.B.; Burris, L.L.; Khorasanizadeh, S.; Burris, T.; Rastinejad, F. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBα and REV-ERBβ. Nat. Struct. Mol. Biol. 2007, 14, 1207–1213. [Google Scholar] [CrossRef]
- Yang, J.; Kim, K.D.; Lucas, A.; Drahos, K.E.; Santos, C.S.; Mury, S.P.; Capelluto, D.G.S.; Finkielstein, C.V. A Novel Heme-Regulatory Motif Mediates Heme-Dependent Degradation of the Circadian Factor Period 2. Mol. Cell. Biol. 2008, 28, 4697–4711. [Google Scholar] [CrossRef]
- Golding, J.; Northstone, K.; Miller, L.L.; Smith, G.D.; Pembrey, M. Differences between blood donors and a population sample: Implications for case–control studies. Int. J. Epidemiol. 2013, 42, 1145–1156. [Google Scholar] [CrossRef]
- Di Pierro, E.; De Canio, M.; Mercadante, R.; Savino, M.; Granata, F.; Tavazzi, D.; Nicolli, A.; Trevisan, A.; Marchini, S.; Fustinoni, S. Laboratory Diagnosis of Porphyria. Diagnostics 2021, 11, 1343. [Google Scholar] [CrossRef]
- Salamanca, R.D.E.; Sepulveda, P.; Moran, M.J.; Santos, J.L.; Fontanellas, A.; Hernandez, A. Clinical utility of fluorometric scanning of plasma porphyrins for the diagnosis and typing of porphyrias. Clin. Exp. Dermatol. 1993, 18, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Elder, G.; Harper, P.; Badminton, M.; Sandberg, S.; Deybach, J.-C. The incidence of inherited porphyrias in Europe. J. Inherit. Metab. Dis. 2012, 36, 849–857. [Google Scholar] [CrossRef]
- Hee, S.W.; Willis, A.; Smith, C.T.; Day, S.; Miller, F.; Madan, J.; Posch, M.; Zohar, S.; Stallard, N. Does the low prevalence affect the sample size of interventional clinical trials of rare diseases? An analysis of data from the aggregate analysis of clinicaltrials.gov. Orphanet, J. Rare Dis. 2017, 12, 44. [Google Scholar] [CrossRef]
- Guo, Y.; Logan, H.L.; Glueck, D.H.; Muller, K.E. Selecting a sample size for studies with repeated measures. BMC Med. Res. Methodol. 2013, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Ogun, A.S.; Joy, N.V.; Valentine, M. Biochemistry, Heme Synthesis; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Adachi, Y.; Umeda, M.; Kawazoe, A.; Sato, T.; Ohkawa, Y.; Kitajima, S.; Izawa, S.; Sagami, I.; Taketani, S. The novel heme-dependent inducible protein, SRRD regulates heme biosynthesis and circadian rhythms. Arch. Biochem. Biophys. 2017, 631, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wu, N.; Curtin, J.C.; Qatanani, M.; Szwergold, N.R.; Reid, R.A.; Waitt, G.M.; Parks, D.J.; Pearce, K.H.; Wisely, G.B.; et al. Rev-erbα, a Heme Sensor That Coordinates Metabolic and Circadian Pathways. Science 2007, 318, 1786–1789. [Google Scholar] [CrossRef]
- Yin, L.; Wu, N.; Lazar, M.A. Nuclear Receptor Rev-Erbα: A Heme Receptor that Coordinates Circadian Rhythm and Metabolism. Nucl. Recept. Signal. 2010, 8, e001. [Google Scholar] [CrossRef]
- Freeman, S.L.; Kwon, H.; Portolano, N.; Parkin, G.; Girija, U.V.; Basran, J.; Fielding, A.J.; Fairall, L.; Svistunenko, D.A.; Moody, P.C.E.; et al. Heme binding to human CLOCK affects interactions with the E-box. Proc. Natl. Acad. Sci. USA 2019, 116, 19911–19916. [Google Scholar] [CrossRef]
- Mazzoccoli, G.; Laukkanen, M.; Vinciguerra, M.; Colangelo, T.; Colantuoni, V. A Timeless Link Between Circadian Patterns and Disease. Trends Mol. Med. 2015, 22, 68–81. [Google Scholar] [CrossRef]
- Larion, S.; Caballes, F.R.; Hwang, S.-I.; Lee, J.-G.; Rossman, W.E.; Parsons, J.; Steuerwald, N.; Li, T.; Maddukuri, V.; Groseclose, G.; et al. Circadian rhythms in acute intermittent porphyria—A pilot study. Eur. J. Clin. Investig. 2013, 43, 727–739. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).